1 Department of Obstetrics and Gynecology, Faculty of Medicine Istanbul, Istanbul University, 34452 Istanbul, Turkey
2 Department of Anatomy, Faculty of Medicine, Gaziosmanpasa University, 60250 Tokat, Turkey
3 Department of Obstetrics and Gynecology, Faculty of Medicine, Pamukkale University, 20160 Denizli, Turkey
4 Department of Obstetrics and Gynecology, Faculty of Medicine, Afyonkarahisar University of Health Sciences, 03200 Afyonkarahisar, Turkey
5 Department of Pathology, Faculty of Medicine, Gaziosmanpasa University, 60250 Tokat, Turkey
6 Faculty of Health Sciences and Central Research Laboratory, Mardin Artuklu University, 47200 Mardin, Turkey
Abstract
Objective: This study aims to determine the protective effects of
apocynin, a NADPH oxidase inhibitor, and melatonin, an endogenous anti-oxidant,
in an animal model of ovarian ischemia/reperfusion (I/R) injury. Materials/Methods: Thirty-five female rats were randomly divided into
five groups, namely group I (sham), group II (I/R), group III (I/R
Keywords
- Apocynin
- Ischemia
- Melatonin
- Ovary
- Reperfusion injury
Ovarian torsion is a gynecological emergency that mostly affects adolescents and young women [1]. The rotation of an ovary around its own axis leads to biochemical and histological alterations that ultimately result in ovarian dysfunction [2]. Ovarian torsion impairs blood flow to tissues and causes ovarian ischemia. Ischemia eventually triggers the degradation of adenosine triphosphate and increases hypoxanthine. After ovarian torsion is reversed, tissues begin to receive blood flow again, and hypoxanthine is converted into superoxide anions, hydroxyl radicals, and peroxynitrite within an oxygenated environment. The phase related to torsion/detorsion damage corresponds to ovarian ischemia/reperfusion (I/R) injury [3, 4]. Experimental studies have attempted to prevent oxidative damage in ovaries by using a number of anti-oxidant agents [5, 6]. Additionally, various treatments, such as pre-treatment with losartan, granulocyte colony-stimulating factor, and methylene blue, have also been found to be useful [7, 8, 9].
Apocynin (4-hydroxy-3methoxy-acetophenone) is naturally found in the roots of Apocynum cannabinum (Canadian hemp) and Picrorhiza kurroa (Scrophulariaceae). This molecule inhibits NADPH oxidase, which catalyzes the synthesis of superoxide anion from oxygen [10]. It has been reported that apocynin suppresses apoptosis by decreasing myeloperoxidase activity and supporting anti-oxidant defense mechanisms in animal models of cerebral artery occlusion [11], renal I/R injury [12], and testicular torsion/detorsion damage [13].
Melatonin (N-acetyl-5-methyl-tryptamine), which is secreted by the pineal gland, is one of the most powerful anti-oxidant agents. Melatonin scavenges hydroxyl radicals, peroxyl radicals, singlet oxygen molecules, peroxynitrite anions, and superoxide anions and inhibits anti-oxidant enzymes, including glucose-6-phosphate dehydrogenase, glutathione peroxidase, and superoxide dismutase (SOD) [14, 15]. Melatonin has also been reported to exert anti-oxidant effects in cardiac I/R injury [14, 16] and testicular torsion/detorsion damage [15, 16, 17, 18].
This study aims to determine the protective effects of apocynin and melatonin in an animal model of ovarian I/R injury.
This study was approved by the local ethics committee (2015 HADYEK-29).
Thirty-five adult female Wistar albino rats (body weight, 200-250 g) were
randomly divided into five groups, namely group I (control, n = 7),
group II (I/R, n = 7), group III (I/R
I/R injury was not induced in group I rats. Rats underwent a sham operation and received 1 mL of 1% ethanol. I/R injury was induced in group II rats, followed by the administration of 1 mL of normal saline. I/R injury was induced in group III rats, followed by the intraperitoneal injection of 10 mg/kg apocynin 30 minutes before reperfusion. I/R injury was induced in group IV rats, followed by the intraperitoneal injection of 20 mg/kg apocynin before reperfusion. Apocynin was dissolved in normal saline (Sigma Chemical Co., St. Louis, MO, USA) [19, 20]. I/R injury was induced in group V rats, followed by the intraperitoneal injection of 10 mg/kg melatonin 30 minutes before reperfusion. Melatonin was dissolved in 1% ethanol (Sigma Chemical Co.) [21, 22].
The rat ovarian I/R model was established as previously described by Ozsoy et
al. [23]. Rats were starved 12 hours before laparotomy and anesthetized with 50
mg/kg ketamine hydrochloride (Ketalar
Blood samples were obtained by direct cardiac puncture under anesthesia and
stored at -80
Oxidative stress in ovarian tissue homogenates was determined by measuring the
activities of SOD and CAT, as well as the levels of MDA and PC, respectively.
Analyses of all samples were performed at ambient temperature. All tissues were
rinsed with ice-cold isotonic saline solution and homogenized by using a
homogenizer (IKA Ultra-Turrax t 25 Basic, Stanfen, Germany). After filtration and
centrifugation of the homogenates, supernatants were used to determine enzymatic
activities. SOD activity was specified by inhibiting the nitroblue tetrazolium
(NBT) reduction, and one unit of SOD was defined as the amount of enzyme causing
50% inhibition of the NBT reduction rate [24]. Catalase (CAT) activity was
specified by determining the rate constant of the H
After fixation in 10% formalin solution, ovarian tissues were washed,
dehydrated, and embedded in paraffin wax. Paraffin blocks were cut to generate
5-
Histological changes were evaluated according to their severity: score 0
indicated an absence of pathological findings, and scores 1, 2, and 3 indicated
an involvement of
Immunohistochemistry was performed as previously described [27]. Briefly,
5-
Evaluation of the immunohistochemical labeling was performed using H-score
analysis as previously described [27]. Semi-quantitative assessments of
immunoreactivity for caspase 3, Bax, and iNOS were classified as follows: 0 (no
staining), 1
Collected data were analyzed by using Statistical Package for Social Sciences
version 20.0 (SPSS IBM, Armonk, NY, USA). Results were expressed as means
Ovarian tissue and serum SOD, CAT, MDA, and PC activities in all groups are
given in Table 1,2. Tissue and serum SOD and CAT activities were decreased
in the I/R group compared with the control group. Compared with the I/R group,
melatonin and apocynin treatment significantly increased SOD and CAT activities,
but this increase was more prominent in I/R
| SOD | CAT | MDA | PC | |
| Control | 50.61 |
0.39 |
1.79 |
768 |
| I/R | 25.06 |
0.20 |
3.84 |
1885 |
| IR |
35.50 |
0.27 |
2.97 |
694 |
| IR |
33.46 |
0.33 |
2.62 |
576 |
| IR |
36.01 |
0.36 |
2.52 |
652 |
*P MDA: malondialdehyde; PC: protein carbonyl. | ||||
| SOD | CAT | MDA | PC | |
| Control | 6.26 |
0.10 |
0.77 |
160 |
| I/R | 4.39 |
0.05 |
1.14 |
461 |
| IR |
5.27 |
0.09 |
0.67 |
223 |
| IR |
6.83 |
0.12 |
0.78 |
219 |
| IR |
6.64 |
0.12 |
0.66 |
205 |
SOD: superoxide dismutase; CAT: catalase; MDA: malondialdehyde; PC: protein carbonyl; I/R: ischemia/reperfusion; APC: apocynin; MEL: melatonin. | ||||
Microscopic images of histopathological findings in all groups are presented in Fig. 1. Ovaries of rats in the control group had a normal structure. However, in the I/R group, severe histopathological changes were observed. Follicular degeneration, inflammatory cell infiltration, interstitial edema, massive hemorrhage, and diffuse congestion were noted in the ovaries of rats with I/R injury. Melatonin and apocynin reversed the cellular defects in follicles and reduced hemorrhage and congestion.
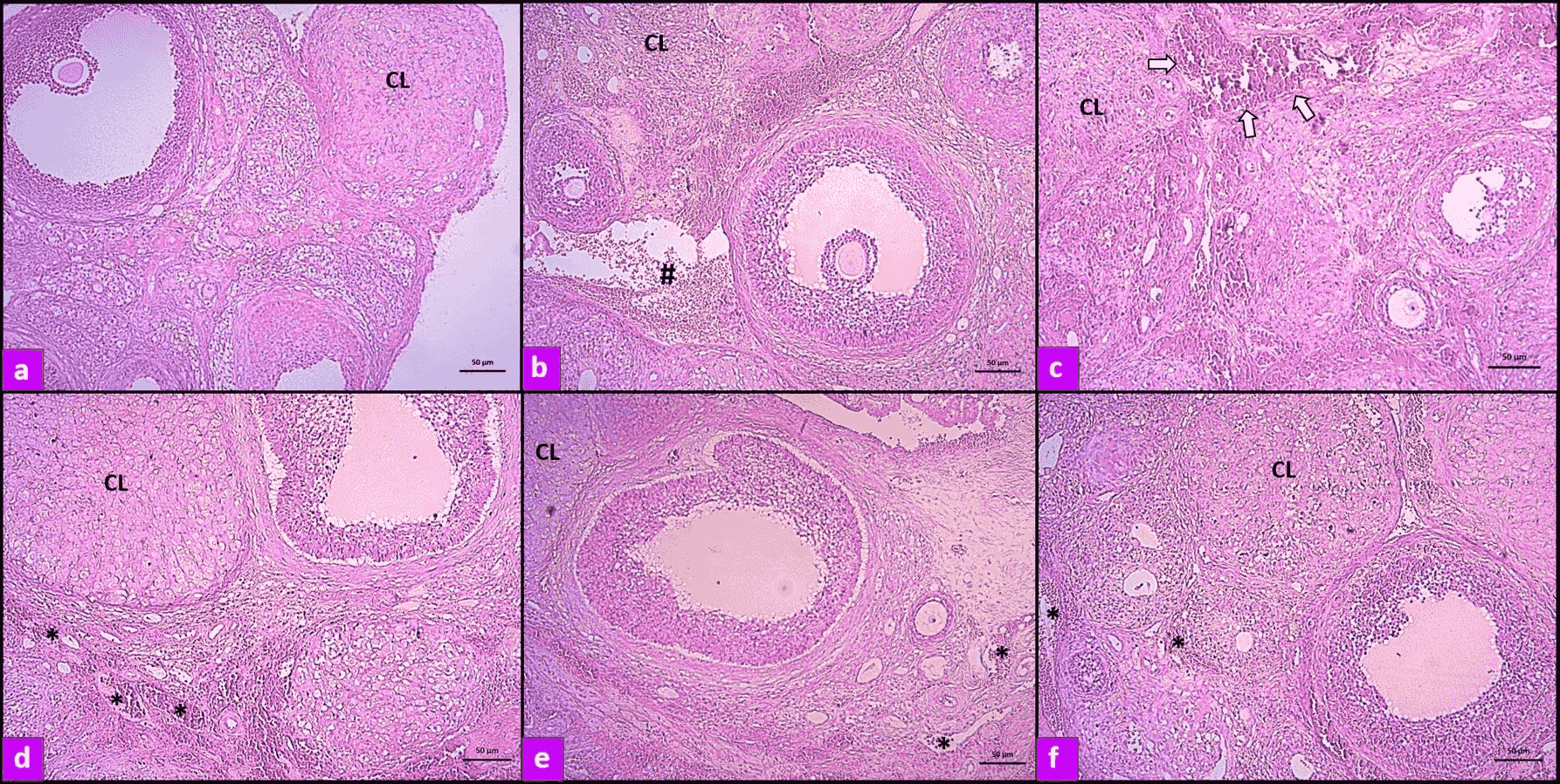 Fig. 1.
Fig. 1.Photomicrographs of ovarian sections demonstrated by hematoxylin
and eosin staining. (a) Normal tissue; (b, c) Ovarian tissue from rats with I/R
injury. The number symbol [#] and arrow indicate hemorrhage and vascular
congestion, respectively; (d) Ovarian tissue from rats with I/R injury and
treatment with 10 mg/kg apocynin; (e) Ovarian tissue from rats with I/R injury
and treatment with 20 mg/kg apocynin; (f) Ovarian tissue from rats with I/R
injury and treatment with melatonin. The asterisks [*] indicate that apocynin and
melatonin treatment reduces vascular congestion. (10
Histopathological findings in ovaries are given in Table 3. Tissue injury scores
demonstrating follicular degeneration, inflammatory cell infiltration, edema,
hemorrhage, and vascular congestion were significantly higher in the I/R group
compared with the control group (P
| Control | I/R | I/R |
IR |
IR | |
| Follicular degeneration | 0 | 1.3 | 0.8 | 0.6 | 0.5 |
| Inflammatory cell infiltration | 0.2 | 1.1 | 0.9 | 0.75 | 0.5 |
| Edema | 0.25 | 1.5 | 0.75 | 0.6 | 0.8 |
| Hemorrhage | 0.2 | 1.9 | 0.9 | 1.3 | 1.1 |
| Vascular congestion | 0.3 | 2.1 | 1.4 | 1.2 | 1.2 |
| Tissue injury score | 0.95 | 7.9* | 4.75 |
4.45 |
4.1 |
*P APC: apocynin; MEL: melatonin. | |||||
Bax, caspase 3, and iNOS were immunohistochemically stained in ovarian tissues
(Figs. 2-4). H-score analysis revealed that bax, caspase 3, and iNOS
immunoreactivities were significantly increased in the I/R group compared with
the control group (P
| Control | I/R | I/R |
I/R |
I/R | |
| Bax | 12 |
85 |
56 |
45 |
47 |
| Caspase-3 | 15 |
132 |
87 |
73 |
62 |
| iNOS | 10 |
74 |
60 |
42 |
36 |
*P iNOS: inducible nitric oxide synthase. | |||||
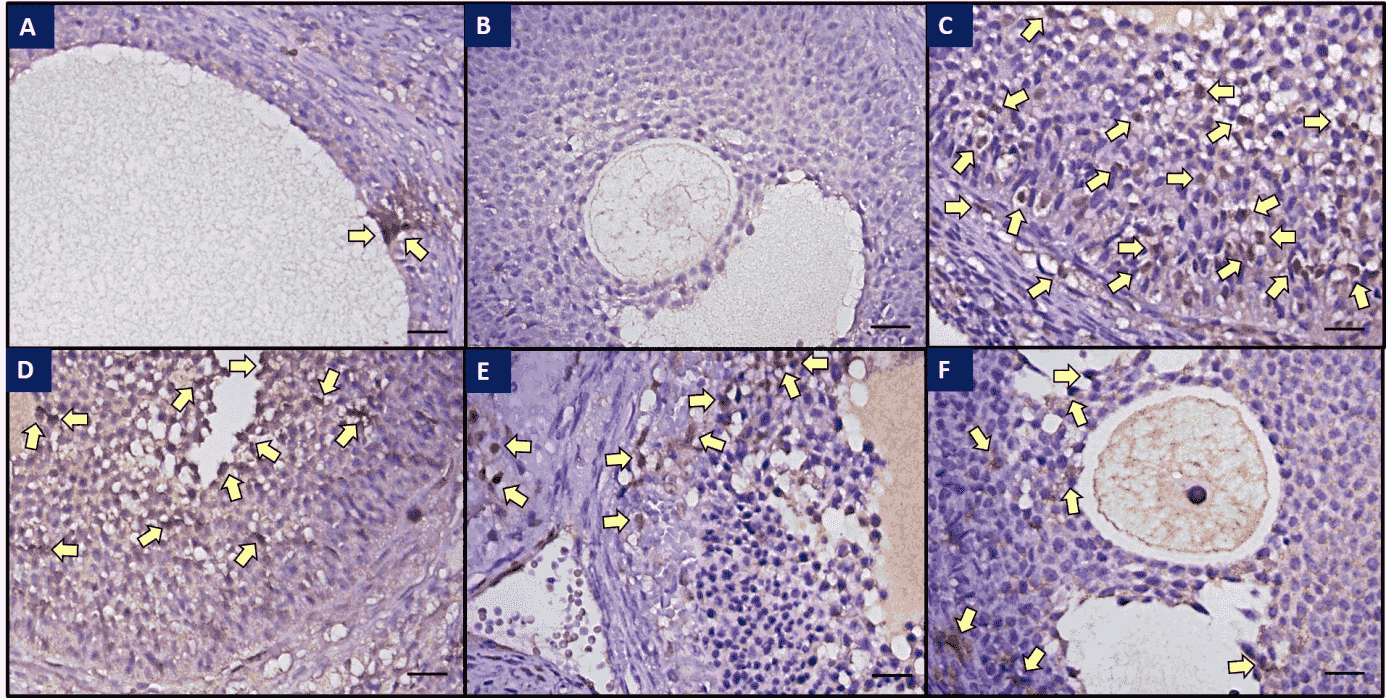 Fig. 2.
Fig. 2.Immunohistochemical staining of caspase-3 in ovaries of all
groups. (a) Weak caspase-3 immunoreactivity was observed in the control group;
(b) Control tissue, no immunohistochemical staining was observed; (c) Strong
caspase-3 immunoreactivity was observed in the I/R group; (d) Immunoreactivity
was decreased in the I/R
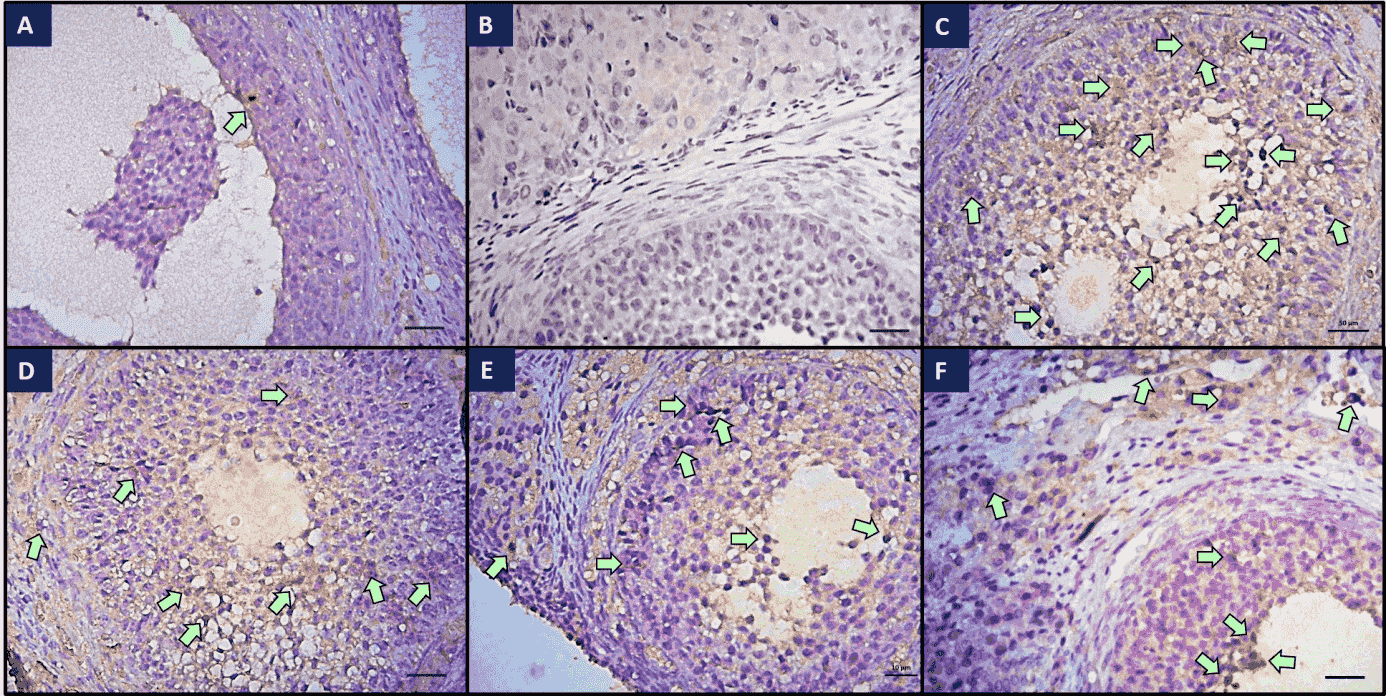 Fig. 3.
Fig. 3.Immunohistochemical staining of Bax in ovaries of all groups.
(a) Weak Bax immunoreactivity was observed in the control group; (b) Control
tissue, no immunohistochemical staining was observed; (c) Strong Bax
immunoreactivity was observed in the I/R group; (d) Immunoreactivity was
decreased in the I/R
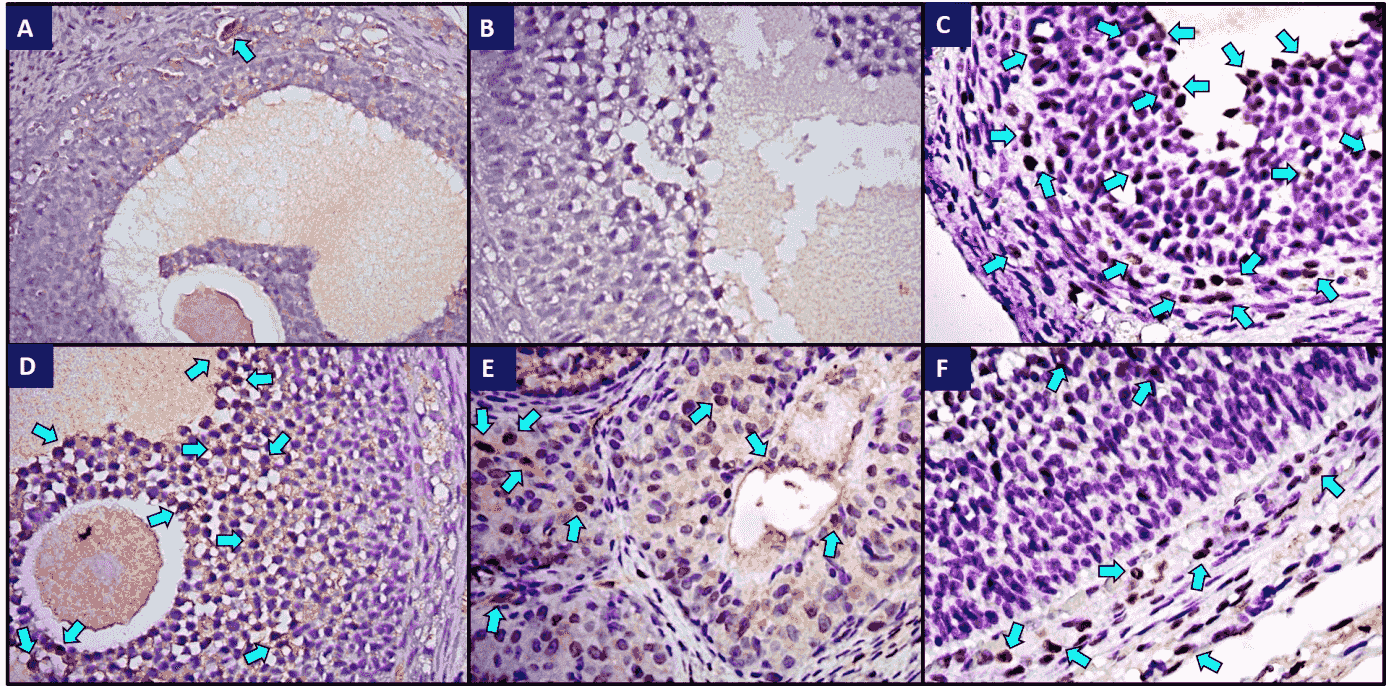 Fig. 4.
Fig. 4.Immunohistochemical staining of iNOS in ovaries of all groups.
(a) Weak iNOS immunoreactivity was observed in the control group; (b) Control
tissue, no immunohistochemical staining was observed; (c) Strong iNOS
immunoreactivity was observed in the I/R group. The arrows indicate iNOS
immunopositive granulosa cells in secondary follicles; (d) Immunoreactivity was
decreased in the I/R
Adnexal torsion may cause permanent damage in ovaries and defects in folliculogenesis, which may eventually result in female infertility. The mechanism of injury is usually attributed to the generation of reactive oxygen species (ROS) during both ischemia and reperfusion. Thus, it would be prudent to sustain anti-oxidative defense mechanisms by using anti-oxidant agents so that ovaries can overcome ROS-mediated I/R injury after adnexal detorsion is achieved [1, 2, 3, 4].
This study was successful in establishing an animal model of ovarian I/R injury
as evidenced by increasing MDA and PC levels and decreasing SOD and CAT
activities in both ovarian tissue and serum. MDA is an index of lipid
peroxidation by which ROS-induced oxidative damage is monitored within tissues.
On the other hand, PC is a product of protein denaturation that appears as a
result of I/R injury [25]. Both SOD and CAT are anti-oxidant enzymes
participating in defense mechanisms in tissues. SOD catalyzes the transformation
of superoxide into H
To date, various pharmaceuticals and chemicals have been experimentally used to eliminate the hazards of torsion related I/R injury in ovaries [3, 4]. A thorough review of the literature has yielded no studies on the protective effects of apocynin and melatonin on ovarian I/R injury and, to the best of our knowledge, this is the first study to investigate the possible benefits of preoperative apocynin and melatonin treatment on I/R injury associated with surgical correction of adnexal torsion.
Apocynin inhibits the generation of ROS by blocking NADPH oxidase. This enzyme
is considered to be the major source of ROS-mediated I/R injury, as activated
NADPH oxidase helps to transport electrons to oxygen and subsequently produce ROS
molecules. Moreover, apocynin stimulates
Both low (20 mg/kg) and high (50-100 mg/kg) doses of apocynin have been used to suppress oxidative stress in renal, cerebral, and endothelial tissues [11, 12, 13]. In another study, diabetes-induced testicular damage could be effectively treated by apocynin administered at a dose of 16 mg/kg for four weeks. Low dose apocynin treatment was able to reverse all histopathological changes caused by I/R injury in testes [29]. On the other hand, a high dose of apocynin was significantly more effective in restoring biochemical and histological parameters to normal levels in testicular tissue. Both low and high doses of apocynin could trigger the spermatogenic process, which was disrupted by I/R injury [30]. In this study, two doses (10 and 20 mg/kg) of apocynin were used, and we found that these regimens were similar in terms of efficacy. Moreover, both apocynin doses significantly improved biochemical, histopathological, and immunohistochemical parameters of oxidative damage in ovaries that underwent I/R injury.
Melatonin, as a major neuroregulatory hormone, is mainly secreted from the pineal gland within the circadian rhythm. Because of its lipophilic and hydrophilic properties, melatonin can easily pass through all biological membranes, enter cells, and distribute to subcellular compartments. In this way, melatonin protects cells from oxidative damage in both lipid and aqueous environments. Furthermore, melatonin impairs leukocyte migration, inhibits leukocyte adhesion to endothelial cells, and improves blood flow in oxidative processes [21, 22].
An animal study reported that the administration of melatonin caused a significant decrease in lipid peroxidation content and a significant improvement in SOD and CAT activities in testes that underwent torsion-related I/R injury. On the contrary, changes in enzyme activities of the contralateral testis were insignificant [31]. A similar Iranian study indicated that either melatonin or its combination with metformin restored SOD activity, as well as MDA and myeloperoxidase levels in rat testes that had been exposed to I/R injury. No significant difference was found between the administration of melatonin or melatonin and another compound. The beneficial effects of melatonin were also demonstrated by means of histopathological recovery [32]. As for the present study, melatonin could reverse I/R injury in terms of biochemical, histopathological, and immunohistochemical alterations in ovaries.
In conclusion, conservative detorsion surgery may not provide adequate protection to ovaries. Our results suggest that preoperative apocynin and melatonin administration can be used to protect ovaries from ROS-mediated I/R injury after adnexal detorsion is performed surgically. Both apocynin and melatonin are powerful as therapeutic anti-oxidant agents with considerable bioavailability and safety. In clinical practice, the application of apocynin and melatonin to treat I/R injury as an alternative therapeutic approach may help to rescue ovaries from subsequent subfertility after adnexal detorsion. However, these findings should be interpreted carefully as their power is limited by a relatively small study cohort and a lack of longitudinal data on fertility. The clinical availability of these compounds merits application in I/R injury studies following adnexal torsion. Further experimental animal and clinical studies are warranted to clarify the protective effects and to develop shared protocols for the clinical use (dosage, duration, method of administration) of apocynin and melatonin treatment in ovarian torsion-related I/R injury before conservative surgery.
CKİ, MU contributed to the concept and designed of the study; CKİ, MU, AA, VU contributed to data collection and /or processing; CKİ, MU, MKP, AA analyzed and /or interpreted data; CKİ, MU, ÖKC, MKP contributed to the literature review and writing of the manuscript and all authors read and approved the final manuscript.
We are grateful to Erkut Somak and Yılmaz Özcan for their contribution to the study.
All authors declare that they have no conflict of interests. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.




