The present study aimed to elucidate the mechanisms by which chorioamnionitis affects the intrauterine development of the human eye at the molecular level, utilising immunohistochemistry. The expression of six different proteins and glycoproteins in human foetal eyes was examined after labelling with specific antibodies. Fifteen out of 87 specimens had a normal appearance without inflammatory lesions. The rest showed chorioamnionitis of various degrees. Some of the foetuses had Down syndrome, toxoplasma or CMV (cytomegalovirus) infection, or a hydatidiform mole (partial mole). The expression of vimentin, myosin, desmin, fibronectin, tenascin-C and tenascin-R was detected and quantified. The results demonstrate that, while the expression of all five proteins and glycoproteins was entirely normal in foetuses with a low grade or no inflammation in the chorion and amnion, expression was significantly impaired in cases of at least moderate chorioamnionitis. This was especially marked in the case of previous intrauterine toxoplasma or CMV infection or Down syndrome. Our findings suggest that chorioamnionitis itself and not preterm birth alone may be, at least to some extent, directly responsible for the structural abnormalities often found in the eyes of children who survive. This is compatible with the existence of a possible direct mechanism, by which this condition might contribute to the manifestation of retinopathy of prematurity.
Chorioamnionitis (or intra-amniotic infection) is a relatively common occurrence in pregnancy and a significant concern for the obstetrician. The present study sought to provide foundational knowledge on its effects on the development of the human eye at the molecular level, employing immunohistochemistry. It also aimed to explore possible direct mechanisms that may play a role in the pathogenesis of retinopathy of prematurity (ROP) in the setting of intra-amniotic infection.
The particular proteins studied here (fibronectin, tenascin-C, tenascin-R, vimentin, desmin and myosin) were chosen because they are among the first to appear during mammalian foetal development. They exhibit highly dynamic patterns of expression during foetal growth, playing an important role in the development of the nervous system, vasculogenesis and skeletogenesis. The glycoproteins fibronectin, tenascin-C and tenascin-R constitute the main components of the extracellular matrix (ECM). The ECM is a substrate involved in foetal tissue morphogenesis and the transmission of intracellular signals through specialised cell surface receptors. The intermediate filaments (IF) vimentin and desmin, as well as myosin, are proteins found in the cytoskeleton of muscle tissue, and are implicated in the process of mechanotransduction. Aberrant expression of these proteins can, therefore, be expected to affect the future development of the eye.
Chorioamnionitis is an intrauterine inflammation, secondary to bacterial infection of the choriodecidual space or the foetal membranes and is a common finding in cases of premature birth. It is generally the result of a polymicrobial infection, with Ureaplasma urealyticum, Mycoplasma hominis and Gram-negative anaerobes being frequent isolates. In chorioamnionitis, the mother has typically no signs of systemic infection. Histological examination of the placenta is the gold standard for diagnosis [1].
The implications of intra-amniotic infection for the neonate have been long known and studied and include premature birth, neonatal sepsis and intraventricular haemorrhage. The eye is a well-known target organ, with ROP being a leading manifestation. The latter has been linked with the presence of specific cytokines [2, 3] that induce neovascularisation. Preterm children are also likely to develop other ocular morbidities, such as refractive error, strabismus, cerebral visual impairment, colour vision deficits, reduced contrast sensitivity (CS), visual field defects and decreased visual acuity (VA) [4, 5].
Although the aetiological links between chorioamnionitis and ROP have been extensively documented, it has been argued that the involvement of the former in the pathogenesis of the latter may be at least partly an indirect consequence. The mechanism could be explained by the role of chorioamnionitis in the induction of very preterm birth [6]. Immunohistochemistry, which was the method employed here, uses specific antibodies for the in situ detection of antigens in a cross-section of tissue. These antibodies produce a characteristic stain in tissues containing the antigen. The degree of staining is graded during examination by microscope.
All 87 foetuses were obtained from the Obstetrics and Gynaecology Department of the Alexandroupolis University General Hospital in Greece. At the moment of miscarriage or the termination of pregnancy following prenatal control and prenatal decision, the foetuses were into the second trimester of intrauterine development (19th-24th week). The foetuses were subjected to autopsy and dissection at the Histology-Embryology laboratory of the hospital. The study was conducted after receiving approval by the local Bioethics and Human Investigations Committee (Ref Num. 45/27/HMB/16.11.2009), which ruled that it was in conformity with the local legislation, as well as with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Fifteen foetuses of normal appearance. There were no apparent lesions in the amnio chorionic membrane when subjected to histological assessment.
Fifteen foetuses of normal appearance. Histological evaluation revealed acute chorioamnionitis of a mild degree.
Fifteen foetuses of normal appearance. Acute chorioamnionitis of a moderate degree was revealed in the histological assessment.
Fifteen foetuses of normal appearance, with acute chorioamnionitis of marked degree, as shown by histology.
Fifteen foetuses of normal appearance. Histological examination revealed hydatidiform mole (partial mole), as well as chorioamnionitis of various degrees.
Eight foetuses with Down syndrome (trisomy 21), all of which had lesions consistent with marked chorioamnionitis.
Two foetuses with Toxoplasma gondii infection, all of which had lesions consistent with marked chorioamnionitis.
Two foetuses with CMV infection, all of which had lesions consistent with marked chorioamnionitis.
All tissue samples were first fixed in a neutral 10% formol solution and then placed in permanent paraffin blocks. A Leica microtome was used to produce 3 μm thick sections, which were then placed in a water bath and afterwards on glass slides. The latter was put in a laboratory oven at 75 ℃ for 30 minutes, and subsequently a haematoxylin-eosin stain was obtained by an automated process.
The immunohistological method employed was the streptavidin-biotin-peroxidase technique. Initially, the sections underwent gradual paraffin removal and hydration in ethanol solutions. Then the internal peroxidase was neutralised in a methanol/3% H2O2 solution for 30 minutes in complete darkness, then the sections were rinsed with tap water, followed by distilled water. The antigen loci were exposed by placing the sections in a microwave oven in a 0.001 M citric acid solution for three four-minute sessions. They were subsequently rinsed with distilled water, submerged in 0.25 M phosphate buffered saline (PBS, pH 7.4) and incubated for 20 minutes with 20% rabbit or pig serum solution (NRS, DAKO, Denmark) in PBS.
This was followed by incubation with primary antibodies for 1 hour at room temperature. The sections were then rinsed with PBS before being incubated with the secondary antibody for approximately 30 minutes at room temperature. The sections were rinsed again with PBS and incubated for 30 minutes with the streptavidin-biotin-peroxidase complex (DAKO, Denmark) diluted 1 : 100 in PBS. Sections were again rinsed with PBS.
Finally, the sections were submerged in a PBS solution of 3.3 quadhydrochloric biaminobenzidine (2.5 g/100 mL, 50 μL of H2O) for 10 seconds. The slides were then microscopically assessed in terms of immunostaining and underwent additional 10-second incubations when necessary. After rinsing with tap water, they were successively placed in haematoxylin for 15 seconds, dehydrated in ethanol solutions, submerged in xylol and processed with DPX. Paraffin sections from embryonic adrenal glands were used as positive controls.
The specimens were submitted to immunohistochemical assessment in terms of the expression of six proteins and glycoproteins. Desmin, myosin, fibronectin, tenascin-C, tenascin-R and vimentin were detected with their respective specific antibodies.
Cells with detectable cytoplasmic immunostaining were considered as positive. The result was quantified by measuring the proportion of positive cells in an area of ten high magnification (× 40) visual fields in three successive tissue sections, as illustrated in Table 1.
| Grading | Proportion of cells with |
|---|---|
| 0 | 0-5% |
| 1 + | < 25% |
| 2 + | 25-50% |
| 3 + | > 50% |
The following antibodies were used:
1. Mouse monoclonal antibodies
Anti-vimentin, in 1 : 400 dilution (Thermo Fisher Scientific), anti-tenascin-C (1 : 100 dilution, Thermo Fisher Scientific, UK), anti-fibronectin (1 : 400 dilution Thermo Fisher Scientific, UK), anti-myosin (1 : 150 dilution, Thermo Fisher Scientific, UK), anti-desmin (1 : 100 dilution, Thermo Fisher Scientific, UK).
2. Rabbit polyclonal antibodies
Anti-tenascin R (1 : 60 dilution, Thermo Fisher Scientific, UK).
All eyes appeared normal. Macroscopic examination of the eyes of the foetuses did not show changes suggestive of aberrant development, such as membranes, adhesions or proliferation of vessels. Representative pictograms of the immunohistochemical staining are shown in Figure 1.
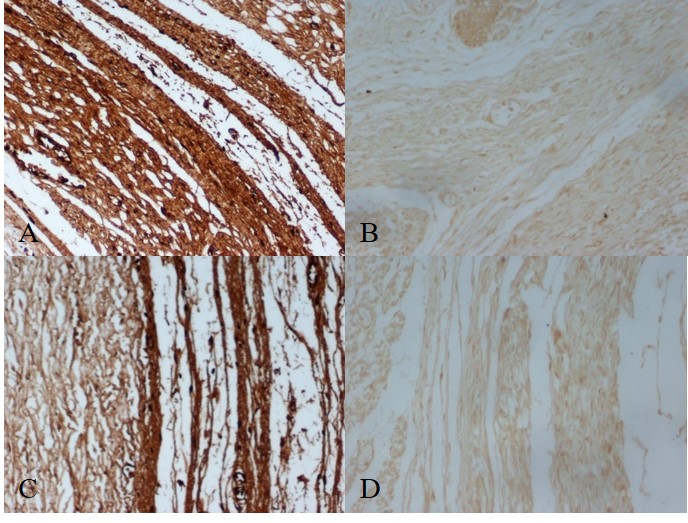 Figure 1.
Figure 1.— Representative pictograms of immunohistochemical staining from various parts of the eye. (A) Representative immunohistochemical staining from the white outer layer of the eye (sclera) for fibronectin in a foetus at 20 weeks of gestation without chorioamnionitis (× 200). (B) Representative immunohistochemical staining from the white outer layer of the eye (sclera) for fibronectin in a foetus at 20 weeks of gestation with chorioamnionitis (× 200). (C) Representative immunohistochemical staining from the vascular layer of the eye between the sclera and the retina (choroid) for fibronectin in a foetus at 20 weeks of gestation without chorioamnionitis (× 200). (D) Representative immunohistochemical staining from the vascular layer of the eye between the sclera and the retina (choroid) for fibronectin in a foetus at 20 weeks of gestation with chorioamnionitis (× 200).
In all foetuses of normal appearance and with mild or no chorioamnionitis lesions, the expression of all molecules was found to be completely normal (Figure 2).
 Figure 2.
Figure 2.— Results from embryos of normal appearance and chorioamnionitis of various degrees.
Subjects of normal appearance and moderate to marked chorioamnionitis lesions showed impaired expression of all molecules in various proportions (Figure 2). The same was true for foetuses with hydatidiform chorionic villi (Figure 3).
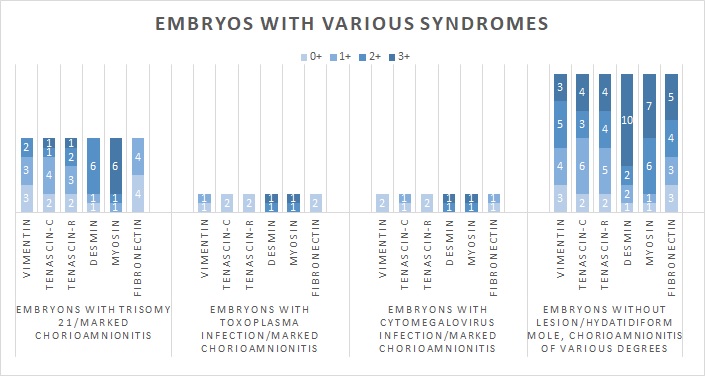 Figure 3.
Figure 3.— Results from embryos with various syndromes and chorioamnionitis of various degrees.
As a general trend, the expression of fibronectin and the tenascins was the most severely affected in the groups mentioned above. Thus, for foetuses of normal appearance with moderate chorioamnionitis, five cases had a 0 or 1 + expression of fibronectin, which would correspond to one-third of the total. For those of normal appearance with marked chorioamnionitis, ten cases were found to stain likewise.
The data for fibronectin concurred with that for tenascin-C and tenascin-R, as seen in the graphs. It must be pointed out, however, that even in the case of the aforementioned molecules, there was a substantial proportion of cells with normal expression. For instance, 3 + fibronectin expression was demonstrated in 6 foetuses in the normal foetus with moderate chorioamnionitis category and 2 foetuses in the case of marked chorioamnionitis. The individual results for tenascin-C were 6 and 4 cases.
Least prone to impairment was the expression of desmin and myosin. Even among phenotypically normal foetuses with marked chorioamnionitis (15 cases), there was only one specimen with 1 + expression of desmin, all the rest displaying 2 + (7 cases) or 3 + (7 cases) desmin expression (Figure 2).
The results for myosin were also better than those for fibronectin and the tenascins, with 9 subjects having 3 + expression of this macromolecule among phenotypically normal foetuses with marked chorioamnionitis (Figure 2). Among cases of hydatidiform moles, 10 foetuses had normal (3 +) desmin levels and 7 specimens had + 3 myosin (Figure 3).
Foetuses with either Down syndrome, toxoplasmosis or CMV infection had in all cases extensive lesions in the amniochorionic membrane, when subjected to histological assessment, as expected (Figure 3). Here again, desmin and myosin seemed to have been relatively well-preserved, in sharp contrast to the other molecules. Among the 8 subjects with Down syndrome, 6 cases had 2 + desmin and 7 cases had 2 + or 3 + myosin expression. One of the two foetuses with Toxoplasma infection had full desmin expression, while the other one was graded as 2 +, while the results for myosin were precisely the same. As far as fibronectin, vimentin, tenascin-C and tenascin-R were concerned, these were severely inhibited. In all foetuses with toxoplasma or CMV, the expression of each of these molecules was 0 or 1 +. In subjects with trisomy 21, all eight cases were found to have an expression of + 1 or less for fibronectin, while the same held true for the majority of subjects concerning vimentin (6 cases), tenascin-C (6 cases) and tenascin-R (5 cases).
Our results were subjected to statistical analysis through the Mann-Whitney U-test, using IBM SPSS Statistics software V23.
The group of foetuses without lesions/embryonic villi without lesions was used as the control group. The distributions between this group and each of the other groups were compared for every single protein separately. A two-tailed p-value < 0.05 was considered sufficient to reject the null hypothesis and thus demonstrate statistical significance.
As expected, the group of foetuses without lesions/embryonic villi with low-grade chorioamnionitis confirmed the null hypothesis, as its distribution was literally identical to that of the control group.
In every other group, the p values were considerably lower than 0.05, thus demonstrating that the distributions for every protein in all groups were stochastically unequal with that of the normal controls. The only slight exception to this trend was found for myosin in the trisomy 21 group. Even in this case, statistical significance was found, albeit by a narrow margin (U = 45.00, N1 = 15, N2 = 8, p = 0.048, Mann-Whitney).
There has been comparatively little research in terms of elucidating the early mechanisms and alterations that affect the human eye at the molecular level. We hope that this study contributes to that end.
As neonatal care is steadily improving, thus leading to a decline in newborn mortality, the incidence of ROP is increasing [6]. This stresses even more the need to better understand the mechanisms that trigger this potentially blinding disorder. We focus on chorioamnionitis as a causal factor of both stillbirth and premature delivery, whose primary threat to the eye is through increased risk of the development of ROP.
There is abundant clinical evidence underpinning the connection between chorioamnionitis during pregnancy and the development of ROP in infants [7-9]. This does not imply that the former is a necessary and sufficient condition for the latter, even though the correlation is significantly strong. Altered expression of vasculogenesis-mediating proteins, resulting from intrauterine inflammation, can, therefore, provide a molecular explanation of the above link.
There has been an extensive discussion as to whether intra-amniotic infection has a direct role in the development of ROP, or if it constitutes an indirect risk factor through the induction of premature birth. Pre-term delivery, before the attainment of full vascularisation by the embryonic retina, is believed to be the main cause of hypoxia and therefore cytokine production in the neonatal retina. However, the role of chorioamnionitis itself as a contributing factor has been left open for discussion. The main evidence used so far tends to be derived from statistical data [10]. A similar discussion is being held regarding other adverse effects of chorioamnionitis, such as its impact on the neurodevelopmental outcome in preterm infants [11, 12]. We suggest that immunohistochemistry can help detect possible early lesions to the cytoskeleton and thus provide more direct evidence concerning the mechanisms that are potentially implicated in this process.
The results of the present study indicate that, in foetuses with signs of chorioamnionitis, there was a statistically significant reduction in the expression of a host of connective tissue molecules, which moreover seems to be related to the degree of inflammation. These findings suggest that intrauterine inflammation itself may have a direct effect on the unravelling of processes such as ROP, as malformations in the connective tissue cytoskeleton can be expected in their turn to inhibit vascularisation and affect tissue structure.
Individuals with Down syndrome are known to suffer from various ocular abnormalities, such as myopia, hyperopia, astigmatism, nystagmus, cataract and glaucoma [13-15]. According to our findings, the expression of at least vimentin, fibronectin and the tenascins was dramatically reduced in these foetuses, and this could possibly help explain the occurrence of refractive errors, such as myopia and astigmatism, at the molecular level. Since these refractive errors are due to irregularities in ocular axial length or corneal shape, the connection appears to be highly plausible.
Patients infected with congenital toxoplasmosis tend to have various ocular abnormalities, including chorioretinal scars and may develop dragging of the macula secondary to a peripheral lesion, retinal detachment, optic atrophy, cataract, amblyopia and phthisis [16]. As in the case of Down syndrome, vimentin, fibronectin and the tenascins seemed to have been significantly impaired. This might be due to the presence of extensive chorioretinal atrophy, as demonstrated earlier by Brézin et al. [17]. Interestingly, desmin and myosin do not seem to have been affected to a high degree.
Finally, patients with a congenital CMV infection may develop chorioretinitis, optic atrophy, pigmentary retinopathy and strabismus [18]. This pathology is also reflected in our immunohistochemical study.
According to the results of this study, there seems to be a correlation between a reduction in the expression of cytoskeleton structural proteins and the degree of histologically noted intra-amniotic infection. All molecules were affected by the presence of inflammation, though not to the same degree.
This seems to imply that chorioamnionitis itself and not preterm birth alone may be, at least to some extent, directly responsible for the structural ocular lesions often found in children with prior exposure to it. Our findings suggest the plausibility of a direct mechanism by which this condition might contribute to the manifestation of ROP.
By all accounts, the paramount importance of prevention, detection and treatment of chorioamnionitis is underpinned by these data. Further research should elucidate the possibility of similar trends in other organs or systems.
The study took place at the Laboratory of Histology-Embryology of the Alexandroupolis University General Hospital in Greece. The materials studied, the antibodies and other consumables were provided by the Faculty of Medicine of Democritus University of Thrace, in Alexandroupolis. We would like to express our gratitude to the peer reviewers and editors for their opinions and suggestions. The authors also thank Proof-Reading-Service.com for scientific English editing and proofreading.
The authors declare no conflict of interest.
