Purpose of Investigation: Retained products of conception (RPOC) is the leading cause of postpartum or post-abortion hemorrhage. RPOC showing high vascularity at ultrasound assessment (HV-RPOC) showed an enhanced hemorrhagic risk following blind surgical uterine emptying. The authors describe the clinical outcome of patients suffering from HV-RPOC undergoing hysteroscopic removal. Material and Methods: Cohort of symptomatic patients suffering from HV-RPOC following miscarriages, pregnancy terminations, and term deliveries. After ultrasound selection based on color-Doppler showing HV-RPOC, all patients underwent hysteroscopic resection. Results: Twenty-seven patients met the selection criteria. HV-RPOC were found in 14 and six women after first and second trimester pregnancy termination, respectively. In seven women RPOC were found after term delivery. In 18 women (66.6%) RPOC were found firmly adherent to myometrium. Neither intra- nor postoperative complications were recorded. Hysteroscopy follow-up was carried-out in 16 patients. Normal findings were found in 15 women, in one case a mild adhesion was observed. Conclusion: HV-RPOC are often characterized by abnormally adherent placenta, suggesting that an accreta can be an underlying etiology. A reliable management can be accomplished by hysteroscopic resection.
Retained products of conception (RPOC) are described as placental tissue that remains in the uterus following delivery, miscarriage or pregnancy termination. RPOC complicate about 2% of term deliveries and from 17% up to 40% of first and second trimester pregnancies ending, respectively [1, 2]. RPOC are a leading cause of secondary post-partum or post-abortion hemorrhages, due to vascular connections between breached intervillous spaces of residual placental tissue and uterine vessels supplying the placental site. A correct diagnosis of RPOC is necessary to address the patient’s safest therapy, mainly to avoid the sequelae associated with unnecessary surgery [3]. Clinical signs such as uterine bleeding, uterine tenderness, and fever are not specific, whereas the addition of ultrasound techniques and color Doppler application has proven to be effective in improving the diagnosis of RPOC [4-7]. Moreover, the scoring of vascular signal within a retained endometrial mass may be of value to address the most reliable management [8-10]. The traditional therapy of RPOC is represented by surgical uterine emptying by dilatation and curettage (D&C). Nevertheless, the current literature contains many reports of patients with RPOC showing high vascularity (HV-RPOC) which include warnings that management by D&C might entail a risk of extensive bleeding and even the need for hysterectomy [8, 11]. In these cases, uterine artery embolization (UAE), medical therapies, and expectant management have been proposed as alternative primary treatments [12-14]. Hysteroscopic resection (HR) is an accepted surgical option for the management of RPOC. Several retrospective studies suggested that hysteroscopy should be preferred with respect to D&C in the surgical management of RPOC, due to the lower prevalence of incomplete uterine emptying and intrauterine adhesion formation [15]. Nevertheless, to date no trial focused on the reliability of hysteroscopy in the management of HV- RPOC. Herein, the authors present an observational study aimed at evaluating the perioperative outcomes of a historical cohort of patients with uterine bleeding caused by HV- RPOC and managed by HR.
The study population included a consecutive series of women admitted to two Public Obstetrics and Gynecology Departments (Hospital of Lodi and Palagi Hospital of Florence, Italy) from May 2013 to May 2018 because of HV-RPOC diagnosed after miscarriages, voluntary termination of pregnancy, and term deliveries. All women received an active management due to an ongoing persistent or excessive uterine bleeding. Clinical, ultrasonographic, hysteroscopic, and pathologic records were obtained through the authors’ gynecological database. The patient’s selection was based on gray-scale and qualitative color Doppler transvaginal ultrasound imaging by the use of 5-7 MHz vaginal probes reporting an endometrial heterogeneous mass distinct from the surrounding endometrium, measured in two orthogonal planes and showing marked vascularity. Marked vascularity was defined as type 3, according to Kamaya classification and identifying an endometrial vascular signal greater than that observed in the myometrium in the same image section (Figure 1) [8]. All women were scheduled to HR under conscious sedation and each of them gave a tailored written informed consent. Because of the lack of guidelines in the management of HV-RPOC, the retrospective nature of the study and the custom of both participating Centers to treat RPOC by hysteroscopy surgery, an Institutional Review Board approval was not need. After cervical dilatation the authors entered uterine cavity by a 27-Fr resectoscope fitted with a 4-mm or 2-mm bipolar loop, connected to a Versapoint Bipolar System generator set at 160W power. Continuous flow of 400 mL/min of saline was delivered as distension medium at usual working pressure of 80 mm/Hg, controlled by an electronic irrigation-suction device. When such pressure resulted insufficient in maintaining uterine distension, due to fluid leakage between the external sheath of hysteroscope and endocervix, it was increased to 100-120 mm/Hg. After the full clearance of blood clots and tissue debris, teh authors identified the uterine fundus and tubal ostia landmarks. Progressing outward with the hysteroscope, the topography and boundaries of residual placenta implantation were identified. Hysteroscopic interventions were conducted using of the cold loop to separate placental tissue from basal decidua and the underlying myometrium at implantation site. Electrified loop was used with high and low frequency currents to slice bulky placental tissue or organized blood clots far from uterine walls and to coagulate bleeding vessels encountered during placental separation, respectively. Near myometrium, a careful use of cutting currents was adopted to slice adherent placental tissue not cleavable by the cold action of the loop. During interventions, the liquid distending medium absorption was monitored. All removed specimens were sent for pathological evaluation. At the end of surgery, based on the surgeon’s judgement, a three-way balloon catheter inflated within the uterine cavity and/or intra-operative intramuscular administration of 0.4 mg of methylergometrine or 5U of oxytocin was used as prophylactic measures to limit further bleeding. All women were scheduled for a transvaginal ultrasonography or office hysteroscopy re-assessment two months after surgery.
The authors identified 27 women that met the inclusion criteria, ranging in age from 21 to 44 years (average 33.8 years). Eighteen women recorded one or more term vaginal deliveries while seven of them were nulliparous. Previous uterine surgery, consisting of one or more D&C (12 patients) and cesarean section (two patient), was recorded in 51.8% of cases. Clinical data concerning current pregnancies complicated by HV-RPOC are summarized in Table 1. In 14 women HV-RPOC complicated first trimester pregnancies, due to miscarriage and voluntary pregnancy termination in 11 and three cases, respectively. In six patients, second trimester uterine emptying was indicated because of pregnancy termination due to fetal malformations and miscarriage in five and one case, respectively. In seven women HV-RPOC was diagnosed after term vaginal delivery. The average time elapsed from pregnancy termination and HR was 54.5 (range, 12-96) days. Average hemoglobin level before surgery was 10.7 (range 6.0-13.8) gr/dL. Perioperative hysteroscopy findings and follow-up data are shown in Table 2. In all cases hysteroscopy imaging was consistent with RPOC showing eutopic endometrial implantations. In 18 out of 27 (66.6%) patients, the authors found firm residual placenta adhesions to underlying myometrium, requiring an electrosurgical slicing of the tissue to obtain the full clearance of placental bed (Figure 2). The average operating time was 33 (range 8-60) minutes and the average deficit of saline was 80 (range 0-300) cc. Methylergometrine or oxytocin were administered to 12 patients, and in five of them the uterine cavity was fitted with a three-way catheter balloon. Neither intranor early postoperative complications were recorded. All women were discharged within 72 hours from surgery. In all cases pathological reports confirmed the diagnosis of RPOC, based on the finding of chorionic villi in the surgical specimens. Hysteroscopy follow-up was accomplished in 16 out of 27 patients and in one of them (6.2%), the authors found an abnormally shaped endometrium, consisting of a mild adhesion.
| Patients | Gestational age (weeks) | Pregnancy termination | Primary uterine emptying | Time elapsed to hysteroscopy (days) | β-hCG (UI/mL) | hGB (gr/dL) |
|---|---|---|---|---|---|---|
| 1 | 10 | M | Gemeprost | 44 | 2300 | 11.7 |
| 2 | 37 | TVD | Manual placenta removal, 2 D&C | 61 | 0 | 10.2 |
| 3 | 15 | M | Gemeprost | 74 | 43 | 10.1 |
| 4 | 7 | VPT | RU-486 Misoprostol | 32 | 2176 | 13.4 |
| 5 | 9 | M | Gemeprost | 18 | 301 | 6.0 |
| 6 | 39 | TVD | Manual placenta removal, 1 D&C | 55 | 67 | 11.4 |
| 7 | 39 | TVD | Manual placenta removal, 2 D&C | 38 | 128 | 13.8 |
| 8 | 9 | M | 2 D&C | 12 | 82 | 8.5 |
| 9 | 9 | M | Gemeprost | 61 | 0 | 10.2 |
| 10 | 20 | VPT | Misoprostol | 23 | 0 | 7.4 |
| 11 | 14 | VPT | RU-486 Gemeprost | 67 | 36 | 11.2 |
| 12 | 10 | M | Misoprostol, 1 D&C | 90 | 0 | 13.9 |
| 13 | 10 | M | Misoprostol | 26 | 121 | 11.0 |
| 14 | 8 | VPT | RU-486 Misoprostol | 46 | 48 | 11.8 |
| 15 | 14 | VPT | RU-486 Misoprostol | 60 | 35 | 9.6 |
| 16 | 19 | VPT | RU-486 Misoprostol | 60 | 0 | 12.3 |
| 17 | 40 | TVD | Normal deliverance | 90 | 0 | 10.5 |
| 18 | 38 | TVD | Normal deliverance, 1 D&C | 57 | 0 | 11.0 |
| 19 | 40 | TVD | Normal deliverance | 38 | 0 | 11.0 |
| 20 | 37 | TVD | Normal deliverance, 1 D&C | 90 | 25 | 9.9 |
| 21 | 9 | VPT | D&C | 73 | 60 | 10.2 |
| 22 | 10 | M | D&C | 60 | 0 | 11.5 |
| 23 | 7 | M | Misoprostol | 68 | 0 | 10.9 |
| 24 | 9 | M | D&C | 34 | 0 | 11.1 |
| 25 | 17 | VPT | D&C | 96 | 35 | 10.0 |
| 26 | 8 | M | Misoprostol | 70 | 0 | 12.2 |
| 27 | 8 | M | D&C | 30 | 0 | 11.9 |
M = miscarriage, TVD = term vaginal delivery, VPT = voluntary pregnancy termination, RU-486 = mifepristone, D&C = uterine dilatation and curettage.
| Patients | Size of RPOC (cm) | Operating times (minutes) | Adherent placenta | Additional therapies |
|---|---|---|---|---|
| 1 | 2.0 | 19 | yes | None |
| 2 | 2.0 | 26 | yes | None |
| 3 | 4.5 | 21 | yes | Methylergometrine, uterine balloon |
| 4 | 3.8 | 25 | no | None |
| 5 | 3.5 | 40 | yes | Methylergometrine, uterine balloon, 5U PRBC, 1U FFP |
| 6 | 5.5 | 22 | yes | None |
| 7 | 3.8 | 22 | yes | None |
| 8 | 2.5 | 18 | yes | None |
| 9 | 2.5 | 32 | yes | Methylergometrine, uterine balloon |
| 10 | 4.0 | 45 | yes | Methylergometrine, uterine balloon, 5U PRBC, 1U FFP |
| 11 | 4.5 | 51 | yes | Methylergometrine |
| 12 | 3.8 | 40 | yes | None |
| 13 | 2.0 | 18 | yes | None |
| 14 | 3.0 | 20 | no | None |
| 15 | 1.8 | 8 | yes | None |
| 16 | 3.5 | 26 | yes | Methylergometrine |
| 17 | 1.7 | 30 | no | None |
| 18 | 4.0 | 75 | yes | Oxytocin, uterine balloon |
| 19 | 2.5 | 45 | no | Oxytocin |
| 20 | 2.0 | 35 | no | None |
| 21 | 3.5 | 60 | no | Oxytocin |
| 22 | 1.5 | 20 | no | None |
| 23 | 1.5 | 30 | no | None |
| 24 | 2.0 | 35 | yes | None |
| 25 | 4.0 | 60 | yes | Oxytocin |
| 26 | 2.0 | 40 | no | Oxytocin |
| 27 | 1.5 | 30 | yes | Oxytocin |
PRBC= packed red blood cells, FFP= fresh frozen plasma, RPOC= retained products of Conception.
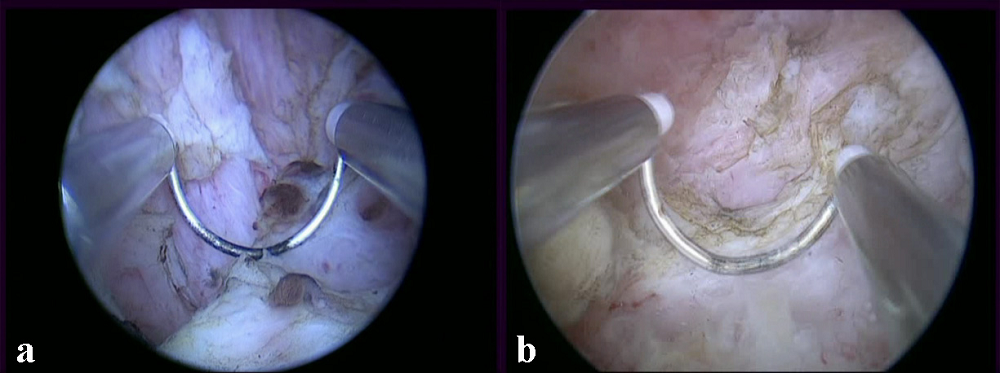 Figure 2.
Figure 2.— After the removal of RPOCs bulging within endometrial cavity, adherent placenta residuals (whitish tissue) spreading to inner myometrial tissue (pinkish tissue) and not cleavable by the cold action of the loop are shown at implantation site. Their electrosurgical separation from underlying myometrium is in progress in patient 10 (showing also some thrombosed spiral vessels between residual placenta and myometrium) (Figure 2a) and patient 2 (Figure 2b).
The therapy of RPOC varies according to clinical presentation and evolution. An expectant management or medical therapy based on prostaglandins’ administration are alternative measures with respect to surgery in asymptomatic patients. Nevertheless, in women with uterine bleeding an active management based on surgical uterine emptying is recommended [16]. HV-RPOC occur in 18% of women suffering from placenta residuals [8]. In these cases, it is generally believed that blind surgical removal by D&C may elicit or worse uterine bleeding, due to the mechanical leakage produced to the walls of sub-involved and patent placental vessels [11, 13, 14]. In a series of 18 women selected by color Doppler spectral analysis and treated by ultrasound-guided D&C, Van den Bosch et al. reported a significant blood loss in 33% of cases [11]. Based on qualitative color Doppler selection, Groszmann et al. suggested the safety of D&C in 31 women, reporting a blood loss exceeding 100 cc in only three cases [17]. In a series of 31 patients managed by first-line UAE, Bazeries et al. reported a reliable procedure in 90% of cases although two of them (6.4%) underwent a hysterectomy due to refractory hemorrhage [12]. In recent years many observational trials suggested clinical improvements by the use of hysteroscopy surgery with respect to D&C in the management of RPOC. Allowing for a selective resection of uterine retentions under direct vision, hysteroscopy seems less traumatic for the healthy endometrium as well as less likely to generate synechiae than blind aspiration [15]. Until now, HV-RPOC have been managed and correlated with hysteroscopic findings in only one retrospective trial. This study was conducted in 16 women with RPOC showing an abnormally adherent placenta, requiring hysteroscopic electrosurgical slicing to obtain its full separation from myometrium. In all patients preoperative ultrasound demonstrated a hypervascular endometrial mass [18]. The present results agree with that finding, showing a high prevalence (66.6%) of HV-RPOC characterized by an abnormally adherent placenta detected during hysteroscopic surgery. In the first series pioneering HR in the treatment of RPOC, their separation from implantation site was reported as easily accomplished by the cold action of the loop, using the tip of the electrode as a curette, by following a safe anatomical cleavage between residual trophoblast and basal decidua [19-21]. More rarely, due to the missing of that cleavage plane, an electrosurgical activation of the loop has been reported as necessary to clear the placental site and inner myometrium from adherent trophoblastic tissue [22, 23]. Therefore, based on surgical hysteroscopy experience RPOC can present either “non-adherent” and “adherent” patterns. This latter and less frequent condition is characterized by enhanced vascularity by color Doppler application and by hysteroscopy detection of abnormally adherent placenta residuals, suggesting that an accreta may represent the underlying etiology. In cases of accreta, the exceeding placental deepening within myometrium is shared either by villous and endovascular non-villous trophoblast, this latter leading to a remodeling not only of spiral vessels but also of more peripheral uterine arterial branches, such as radial and even arcuate ones [24]. Indeed, it is not surprising the association between adherent RPOC caused by focal placenta accreta and its enhanced vascularity, often extended also to myometrium [8]. Although conducted on a small number of women, the present authors’ experience suggests that HR of HV-RPOC is safe and effective. All patients were treated by a single step surgery allowing to control bleeding symptoms without the need of further medical intervention. This finding agrees with the meta-analysis of Hooker et al., reporting a rate of persistent RPOC after a single HR in only 1.4% of women [15]. Although in larger series a significant rate of early major complications such as uterine hemorrhage or perforations was reported in up to 15% of women undergoing HR of RPOC [25]. In the present study no intra-operative complication was noticed, according to the low complication rate (1.1%) summarized by Smorgick et al. in a recent review [26]. After interventions, the present authors accomplished a hysteroscopic reassessment in 16 out of 27 women and in only one case (6.2%) the authors found a scarred endometrial lining, confirming the low prevalence of adhesions formation, ranging from 5.7% to 12.8% following HR [15, 26]. Operative hysteroscopy is usually considered not indicated in women with ongoing bleeding, due to the assumption that an impaired visualization hampers a safe and effective surgery. Based on the presented results the authors suggest that current uterine bleeding due to HV-RPOC does not represent a counter-indication to hysteroscopic surgery. Nevertheless, the focal nature of pathology, the precise identification of its landmarks and the continuous maintenance of good visualization in the surgical field, represent essential assumptions to pursue a safe HR, whereas the continuous availability of hysteroscopy set-up and an experienced surgical team is needed.
HV-RPOC are often characterized by an abnormally adherent placenta residual at implantation site, suggesting that an accreta may be a frequent underlying etiology. Their removal can be reliably and safely accomplished by HR even in patients with ongoing bleeding.
The present authors thanks Mrs Caroline Calnan for supporting the English language review of the manuscript.
The authors declare no conflict of interest.

