Radiofrequency ablation (RFA) has been proposed as an alternative to hysterectomy for treatment of uterine fibroids and adenomyosis. Until now, there have been no prospective studies published investigating fertility and pregnancy outcomes after RFA of myomas. A 31-weeks pregnant woman who had undergone laparoscopic RFA about 3 years ago was admitted to our hospital with abdominal pain. During conservative treatment, the patient complained of very painful abdominal gas and recurrent variable deceleration was observed without uterine contractions in the cardiotocography. An emergency cesarean was performed, and placenta percreta with active bleeding on the posterior wall of the uterus was found, along with multiple placental percreta lesions. She was discharged from the hospital on the seventh day after conservative surgery with a good overall condition. To our knowledge, this is the first case of placenta percreta after RFA for adenomyosis.
Adenomyosis is a common nonneoplastic gynecologic disease that has been on the rise in pregnant women and is attributable to late marriages, delayed conception of first pregnancies, and the progress of infertility treatments [1]. In recent decades, the demand has increased for alternative uterine preserving methods when treating adenomyosis. Radiofrequency ablation (RFA) has been proposed as an alternative to hysterectomy for treatment of uterine fibroids and adenomyosis [2-3], as reports and experiences of pregnancy after RFA treatment are mostly related to uterine fibroids rather than adenomyosis. However, here we report a case of placenta percreta in a pregnant woman with a history of laparoscopic RFA for adenomyosis three years ago.
A 34-year-old woman, was 31 weeks and 4 days pregnant when she visited the emergency room with chief symptoms of abdominal pain and diarrhea for 3 days. She had undergone laparoscopic RFA for adenomyosis via M-1004 (RF Medical, Seoul, Korea) at a local hospital about three years prior. The patient succeeded in pregnancy after receiving in vitro fertilization and embryo transfer at a local infertility clinic and received regular checkups at another local hospital. She had abdominal pain three days before she visited and was taking a medication for enteritis because no special abnormal signs were found. She took the medicine; however, her symptoms gradually worsened. Her pain persisted, and she had a soft stool almost every hour. When she arrived at the emergency room, her vital signs were blood pressure 118/82 mmHg, body temperature 36.7 ℃, heart rate 101 beats/min, and respiratory rate 20 breaths/min. A blood test showed Hb 12.9 g/dL, WBC 7830/μL, platelet 173,000/μL, and CRP slightly increased to 1.0 mg/dL. There was no electrolyte imbalance, and otherwise no unusual test results. A fetal sonography showed the fetus presenting as vertex and the expected fetal weight was 1457 grams. The amniotic fluid index (AFI) was 9.7, an appropriate value, and the cervical length was 3.3 cm, with a T-shape. There was no leakage or pooling observed during the pelvic examination, and both the nitrazine and premature rupture of membrane (PROM) kit tests were negative. The fetal heartbeat was active, and uterine contractions appeared every 2 to 3 minutes during fetal cardiotoco-monitoring. After administration of the tocolytics, the patient symptoms improved; however, the patient complained of abrupt abdominal pain. The entire abdomen was tender; however, subplacental hematoma via ultrasound was not observed. Recurrent variable deceleration was observed without a uterine contraction in the cardiotocography (Figure 1); therefore, we performed an emergency cesarean section under the diagnosis of fetal distress. A large amount of blood and clots in the abdominal cavity was observed. A female baby was born, weighting 1.43 kg, with a 1 minute Apgar score of 4 and 5 minute Apgar score of 7. Placenta percreta with active bleeding was observed on the posterior wall of the uterus, and multiple lesions of placental percreta were also observed on the fundus, anterior, and posterior uterine wall (Figure 2). We experienced difficulty completely removing the placental invasion tissue, first considering that the severe adhesion was detected on the posterior uterine wall and the intestine. Therefore, we partially removed the placenta tissues and placed hemostatic sutures at the percreta sites, with two layers. The bleeding decreased, and the operation was completed while preserving the uterus. The patient was discharged from the hospital with a good overall condition on the seventh day after surgery. In the outpatient follow-up 6 months and 8 months after discharge, the posterior wall of the uterus had recovered, as shown via ultrasonography (Figure 3).
 Figure 1.
Figure 1.— Cardiotocography performed on complaining severe abdominal pain. Recurrent variable decelerations (arrows) were observed without uterine contraction.
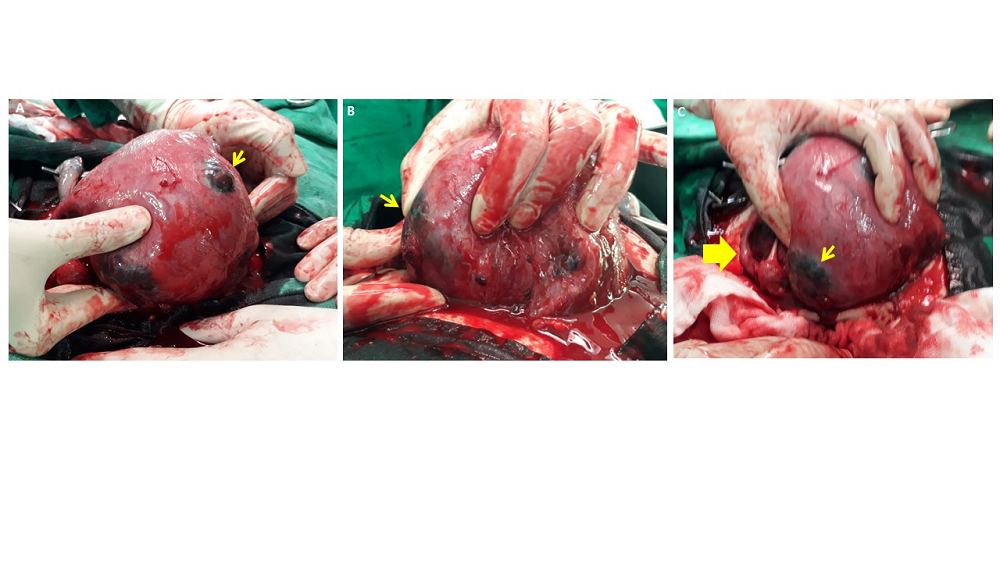 Figure 2.
Figure 2.— Placental percreta was observed on the fundus (A), anterior (B) and posterior wall(C) of Uterus, and uterine perforation(C, thick arrow) with active bleeding was observed on the posterior wall.
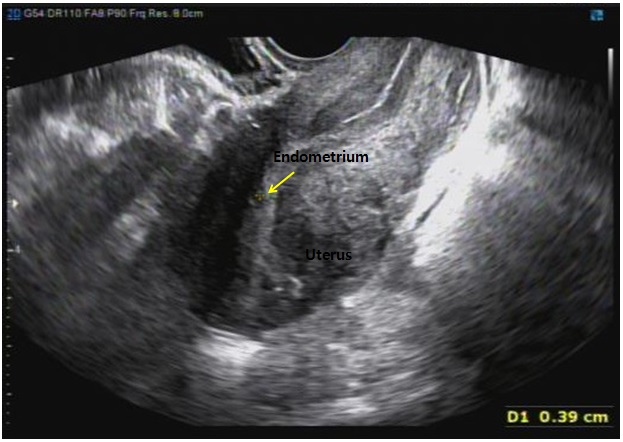 Figure 3.
Figure 3.— The endometrium (arrow) was observed in normal form on ultrasound, which was performed six months.
RFA has been proposed as an alternative to hysterectomy for treatment of uterine fibroids [2-3]. Current RFA protocols differ from electro myolysis in that the current radio signal equipment operates at a frequency of 3 to 4 MHz. Although new therapeutic options for adenomyosis have been introduced, there are no evidence-based guidelines and experience is mostly based on the treatment of uterine fibroids rather than adenomyosis; published studies typically have a short follow up [4-5] and numerous endometrial ablations [6-7]. Scarperi et al. showed that laparoscopic radiofrequency thermal ablation reduced uterine adenomyosis-related symptoms and volume [8].
After RFA, myometrial scar healing may be variable and this, along with reduced myometrial volume, may eventually jeopardize fertility [3-5, 9-10]. Until now, there have been no prospective studies published investigating fertility and pregnancy outcomes after RFA of myoma. However, there are 20 reported case series of pregnancies after RFA treatment. Nine of the 12 full term live births were delivered by cesarean section (75%) [11-14]. Berman et al reported abnormal placentation, uterine rupture, scarring, or uterine thinning, as well as one complication with expulsion of a degenerated myoma and delayed postpartum hemorrhage after delivery by cesarean section at 37 weeks gestation [11]. Reports of pregnancy after RFA treatment are mainly for uterine myoma and rarely for uterine adenomyosis. To the best of our knowledge, this is the first report of a complication related to pregnancy after RFA for adenomyosis.
Morbidly adherent placentas (MAPs) are unusually adhered to the implantation site and are associated with absent or scanty decidua and a physiological line of cleavage. Based on histologic diagnosis, MAPs include placenta accrete (chorionic villi attach to myometrium rather than decidua), placenta increta (chorionic villi penetrate into the myometrium), and placenta percreta (chorionic villi penetrate through the myometrium to the uterine serosa or adjacent organs) and are a cause of major morbidity and mortality in pregnant women [15].
Adenomyosis is the presence of endometrial tissue within the myometrium and below the endomyometrial junction. The ectopically located endometrium of adenomyosis is generally inactive or proliferative; however, functional changes may be seen in some cases and a stromal decidual reaction may be encountered when the patient is pregnant [16]. Decidualized adenomyosis during pregnancy should differentiate from uterine leiomyoma with degeneration [17] and MAPs [18]. One should remember that the typical characteristics of adenomyosis may become atypical or bizarre during pregnancy secondary to decidualization caused by hormonal effects or increased vascularization, especially when adenomyosis is confined to the placental area [19]. Tongsong et al. reported and illustrated a false-positive diagnosis of adherent placenta attributable to underlying adenomyosis [18], highlighting the importance of well documented pre-existing lesions of the uterus, including adenomyosis. Also, scar healing of adenomyosis after RFA might cause absent or scanty decidua in decidualized adenomyosis in pregnant women, which is a suggested cause of MAP as in this case.
An optimal management plan for life-threatening MAPs has yet to be defined. Total hysterectomy is considered in the case of life-threatening severe bleeding or insufficient hemostasis [20-21]. Two strategies for the management of placenta percreta have evolved and include surgical removal of the uterus and involved tissues or conservative therapy with the placenta left in situ after delivery of the fetus [22]. Conservative treatment is possible only when hemodynamic conditions are stable and with adequate technical support. Conservative treatment for placenta percreta-induced uterine rupture has been reported, such as uterine curettage along with packing, leaving the placenta in situ, adjuvant chemotherapy, and bilateral uterine vessel occlusion [22-24]. In order to preserve fertility, we propose leaving the adherent placenta (or the adherent portions of the placenta) in situ at the time of the caesarean section.
To our knowledge, this is the first case of placenta percreta after RFA for adenomyosis. Prior to RFA, clinicians should inform the patient about the risk of MPA. Further, clinicians should consider the possibility of placental problems or uterine rupture as the pregnancy progresses or when pregnant women with a history of RFA for adenomyosis complain of abdominal pain. In addition, control of potentially life-threatening hemorrhage is the first priority; however, the patient’s desire for future fertility should be considered.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Ilsan Paik Hospital (IRB 2018-10-022-001).
We would like to express my gratitude to all those who helped me during the writing of this manuscript.
The authors declare no conflict of interest.
