Summary of Investigation: Assisted reproduction technologies (ART) are now commonly used to conceive. ART is associated with higher incidence of negative birth outcomes which may be due to altered cytokine signaling. Materials and Methods: This pilot study evaluated the suppressors of cytokine signaling SOCS and levels of proinflammatory cytokines ART and non-ART placentas (n=14 each) matched for maternal and gestational age, delivery method, pregnancy weight gain, and body mass index. Comparisons of advanced maternal age (AMA), with or without pre-term birth (PTB) were included. SOCS1, 2, and 3 levels were evaluated with immunohistochemistry and IFN-γ, IL1-β, IL-6, IL-8, IL-10, and TNF-α with ELISA. Results: ART was associated with significantly lower SOCS3. Although SOCS1/IL-10 and SOCS2 and 3/IFN-γ significantly associated in normal conception, associations were lost in ART. In AMA, placental SOCS1 and 2 were associated with IFN-γ, and SOCS3 with IL-6, but under 35 these associations were lost. Term birth was associated with placental SOCS1 inhibition of IL-8 and SOCS2 induction of IL-10, but PTB was not. Conclusion: Cytokine signaling is dysregulated in human placentas by ART which might be a cause of negative reproductive outcomes in ART.
Assisted reproductive techniques (ART), are a commonly used procedure to help couples conceive. First established as a viable technique in the 1970s, ART is becoming ever more popular for couples with the most recent data indicating over 180,000 ART procedures that resulted in 71,152 live born infants in 2015 in the United States [1]. While most children born through ART are healthy, it has been noted that they have higher rates of low birth weight, preterm delivery, pediatric cancers and perinatal mortality when compared to naturally conceived children [1, 2]. Currently it is not known whether these outcomes are related to ART or due to other causes such as the underlying infertility [2].
The placenta is a transient organ of pregnancy that develops from the blastocyst and is entirely of fetal origin apart from one layer in contact with the maternal uterine lining (the decidua) that is of maternal origin. It plays an important role in fetal development by regulating delivery of gases and nutrients and removing wastes between the mother and fetus [3]. One of the most important classes of molecules in the placenta are the suppressors of cytokine signaling (SOCS), which are involved in placental implantation, placental and fetal growth, and parturition (birth) [4, 5]. Because pregnancy is a delicate balance between inflammation and suppression of immune responses (i.e. the mother must not reject the baby), the interplay of cytokines and their expression are tightly regulated in pregnancy. Our laboratory has previously demonstrated that there are differences in placental SOCS1 and SOCS3, as well as IL-6 and TNF-α in a mouse model of assisted reproduction, which could lead to dysregulated inflammatory responses [6, 7]. The mouse placenta is quite different in structure from the human placenta [6] and mouse pregnancy has many different attributes to human; despite this, both ourselves and others have demonstrated placental differences in mouse placentas when ART was used for conception that are both structural and functional [7-12]. With this preponderance of evidence from the mouse model, we wished to determine if similar effects on the human placenta occur. In this study we determined whether altered placental inflammatory responses might contribute to adverse pregnancy outcomes commonly associated with ART such as small-for-gestational age (SGA), intra-uterine growth restriction (IUGR), premature rupture of the membranes, and pre-term birth [13, 14].
Our primary hypothesis is that there are differences in SOCS proteins between ART and normally conceived placentas in humans, as we have previously observed in mice. Because the tissues are matched, we also aimed to determine differences in SOCS and cytokines observed with maternal and gestational age. We studied 28 maternal age, gestational age and ethnically matched ART and normal placentas. The placentas were screened for SOCS1, 2, and 3 using immunohistochemistry (IHC). We also determined levels of pro- and anti-inflammatory cytokines in the placentas to see whether changes in SOCS proteins are also reflected by changes in inflammatory status.
The human placentas used in this study were collected between 2012 and 2016 at birth with informed consent from mothers for inclusion of their tissues into the Hawaii Biorepository, including consent for future investigation. Placentas were from ART or normal conception matched at baseline for maternal age, gestational age, delivery method, BMI, and pregnancy weight gain (Table 1). For ethnicity, only Caucasians, Asians, and Native Hawaiian and Pacific Island people are represented in the cohort, with significantly fewer Native Hawaiian and Pacific Island women in the ART group, which is expected as traditionally indigenous communities have lower uptake of ART services [15]. Within the ART and normal conception groups a nested study determining the effects of advanced maternal age and preterm birth were included, and these tissues were matched only for maternal age and preterm birth due to small numbers (Table 1).
Placentas were archived whole frozen at -80°C until request, whereupon, samples 0.1-0.5 grams of tissue were cut frozen and transported on dry ice to the University of British Columbia. Before use, placenta tissues were washed then homogenized 1:3 by weight in Tris-HCl pH 7.4 containing 5 mM MgCl2. Protein was determined using the Bicinchoninic Acid Assay and normalized to 1 mg/mL before ELISA assay [16].
Placental tissue blocks were also available upon request and were microtomed to a thickness of 8-10 µm and mounted on poly lysine coated glass slides at the University of Hawaii Medical School Histology Core, and shipped to the University of British Columbia.
The Biorepository in Hawaii has approval to consent women for donation of placental and other reproductive tissues, to be archived in the repository for later use, including genetic studies. This individual study was approved by the Review Ethics Board at the University of British Columbia (H14-00092) and tissues transferred from the Biorepository in Hawaii to the University of British Columbia under executed material transfer agreement M14-01051.
Immunohistochemistry was undertaken for SOCS1, 2, and 3 on placental slides (n = 28: 14 ART and 14 normal conception). The protocol for immunohistochemistry was as previously published by us with minor modifications for optimizing SOCS antibodies [17].
Briefly, slides were de-paraffinized by placing the slides in xylene for five minutes, then rehydrated through an ethanol gradient, dipped in milliQ water and quenched in 0.3% H2O2 for five minutes. Antigen retrieval was performed in citrate buffer solution at ~95 °C for 40 minutes. Following antigen retrieval, slides were washed 2×2 min in TNT. Slides were then incubated with SOCS1, 2, or 3 antibodies (SOCS1: sc-7005-R; SOCS2 sc-7007; SOCS3: sc-7009). SOCS1 and 2 were used at 1:50 dilution in 5% TNB, and SOCS3 used at 1:100 dilution. Incubation occurred overnight at 4 °C for 16-20 hours, then slides were washed 3 × 5 minutes in TNT, followed by secondary incubation for one hour at room temperature with donkey-anti-rabbit HRP for SOCS1 and 2 or donkey-anti-sheep HRP for SOCS3 at 1:200 dilution, then in TNT 3 × 5 minutes. Slides were developed utilizing DAB, development time was ten minutes, 30 seconds, and 15 minutes for SOCS1, 2, and 3, respectively. Following DAB, slides were washed 3 × 15 minutes in TNT. Slides were then counter-stained with Gills Hematoxylin, acid-ethanol destained, washed 2 × 15 minutes in TNT, then dehydrated through an ethanol gradient before mounting with Shur Mount.
Slides were visualized with bright field microscopy using a DMLB Microscope at ×100 power. Pictures were taken under constant focus and light exposure utilizing an camera. All files were saved as TIFF files in 24-bit coloured images and images were analyzed with Image J v. 1.52a program. Analysis was performed at ×100 resolution. Each image was inverted, converted to an RGB Stack, and then adjusted for a red threshold. Once the red threshold had occurred, they were measured for area density. The area number was then subtracted from 100% to give the percentage of area that is stained. Representative slides are shown in Figure 1.
The levels of the cytokines IFN-γ, IL1-β, IL-6, IL-8, IL-10, and TNF-α were determined simultaneously in placental lysates prepared as above using a commercial multiplex cytokine ELISA as per the manufacturer’s instructions and read on a SQ120 instrument. The commercial ELISAs were first optimized for placental lysate concentration and levels of quantitation determined with a spiking assay (recovery for all cytokines was 96 ± 11 %). Then, with absolute quantitation confirmed; levels of cytokines were quantitated in placenta lysate from the standard curves.
Normality of the data was determined by DíAgostino-Pearson Omnibus test for normality. For binary clinical outcomes and demographics (e.g. sex, abruption, delivery method, etc.), data were analyzed with Student’s t-tests. For continuous variables (e.g. diabetes mellitus, age, etc.), correlations were performed using Spearman’s rank-sum test or Mann Whitney U test. All analysis was performed on GraphPad Prism 6.0.
The demographics of the entire cohort were matched at baseline for maternal age, gestational age, pregnancy weight gain, delivery method, and ethnicity; with the exception that there were more Asians in the control group than in the assisted reproduction group (Table 1).
| Normal conception (n = 14) | Assisted reproductive technology (n = 14) | p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal age (years) | 33.77 ± 2.02 | 34.85 ± 1.24 | 0.55 | ||||||||
| Gestational age (days) | 248.7 ± 30.9 | 248.2 ± 26.0 | 0.95 | ||||||||
| Delivery method | |||||||||||
| Caesarian | 64 % (n = 9) | 64 % (n = 9) | 1 | ||||||||
| Vaginal | 36 % (n = 5) | 36 % (n = 5) | |||||||||
| Pregnancy weight gain (kg) | 11.2 ± 8.7 | 9.4 ± 3.9 | 0.57 | ||||||||
| Ethnicity | |||||||||||
| Asian | 61 % | 48 % | 0.12 | ||||||||
| Caucasian | 28 % | 48 % | 0.008 | ||||||||
| Native Hawaiian/Pacific Islander | 10 % | 4 % | 1 | ||||||||
| Nested study | |||||||||||
| AMA - PT |
AMA - T |
NMA - PT |
NMA - T |
AMA - PT |
AMA - T |
NMA - PT |
NMA - T |
||||
| Maternal age (years) | 38.0 ± 2.0 | 42.3 ± 4.0 | 26.5 ± 4.9 | 30.7 ± 2.1 | 40.6 ± 3.1 | 37.0 ± 2.7 | 31.8 ± 1.7 | 31.0 ± 1.0 | |||
| Gestational age (weeks) | 33.5 ± 1.1 | 38.9 ± 0.7 | 30.4 ± 5 | 39.4 ± 0.9 | 34.2 ± 1.7 | 38.1 ± 1.2 | 32.3 ± 4.7 | 38.3 ± 0.9 | |||
Independent of ART, in the entire cohort; pre-term birth was associated with higher maternal weight pre-gravida (p = 0.02), higher weight gain in pregnancy (p = 0.02), greater occurrence of PPROM (p = 0.01), lower delivered baby weight (p < 0.001), shorter length (p = 0.002), and decreased head circumference (p < 0.001). In contrast, term infants were more likely to be induced (p = 0.05), all of which are consistent with the literature [18, 19]. Additionally, advanced maternal age in the cohort (AMA, i.e. ≥ 35 years) was associated with increased rates of severe preeclampsia (p = 0.04), also consistent with the literature [20].
When stratified into normal conception vs. ART, there was a higher rate of gestational diabetes in ART group than the natural conception group (p = 0.04), consistent with a previously published study [21]. There were also trends towards lower incidence of preterm rupture of the membranes (p = 0.06), higher incidence of severe preeclampsia (p = 0.08), and greater need for augmentation of labour with ART (p = 0.09).
Taken together, these results demonstrate that this cohort is representative of the population as a whole with respect to pregnancy complications and birth outcomes. The novel data from clinical outcomes is that ART may be associated with gestational diabetes and irregularities of parturition.
When SOCS levels were compared between normal conception and ART, there were no differences in SOCS1 and SOCS2, but levels of SOCS3 were significantly lower in ART placentas as compared to normal conception (p = 0.03, Mann-Whitney U test, Figure 2C).
When stratified for preterm birth regardless of conception method, both SOCS1 and SOCS3 were significantly elevated compared to term births (p = 0.01 and 0.005 respectively, Figures 2D and 2F). There were no apparent effects of advanced maternal age on SOCS proteins, irrespective of ART status (Figure 2G - I, respectively). These data point to dysregulation of SOCS signaling in disorders of parturition such as preterm labour, that are associated with ART.
 Figure 1.
Figure 1.— Representative immunohistochemistry micrographs for SOCS 1, 2, and 3. Left column is negative control, right column is the ART slide (positive). Black arrows indicate positive DAB staining (brown) localized to the syncytium. Endothelial staining is not apparent. Counterstaining of nuclei (blue) is with Gill’s Hematoxylin. Despite quenching, slight non-specific staining of erythrocytes is noticeable.
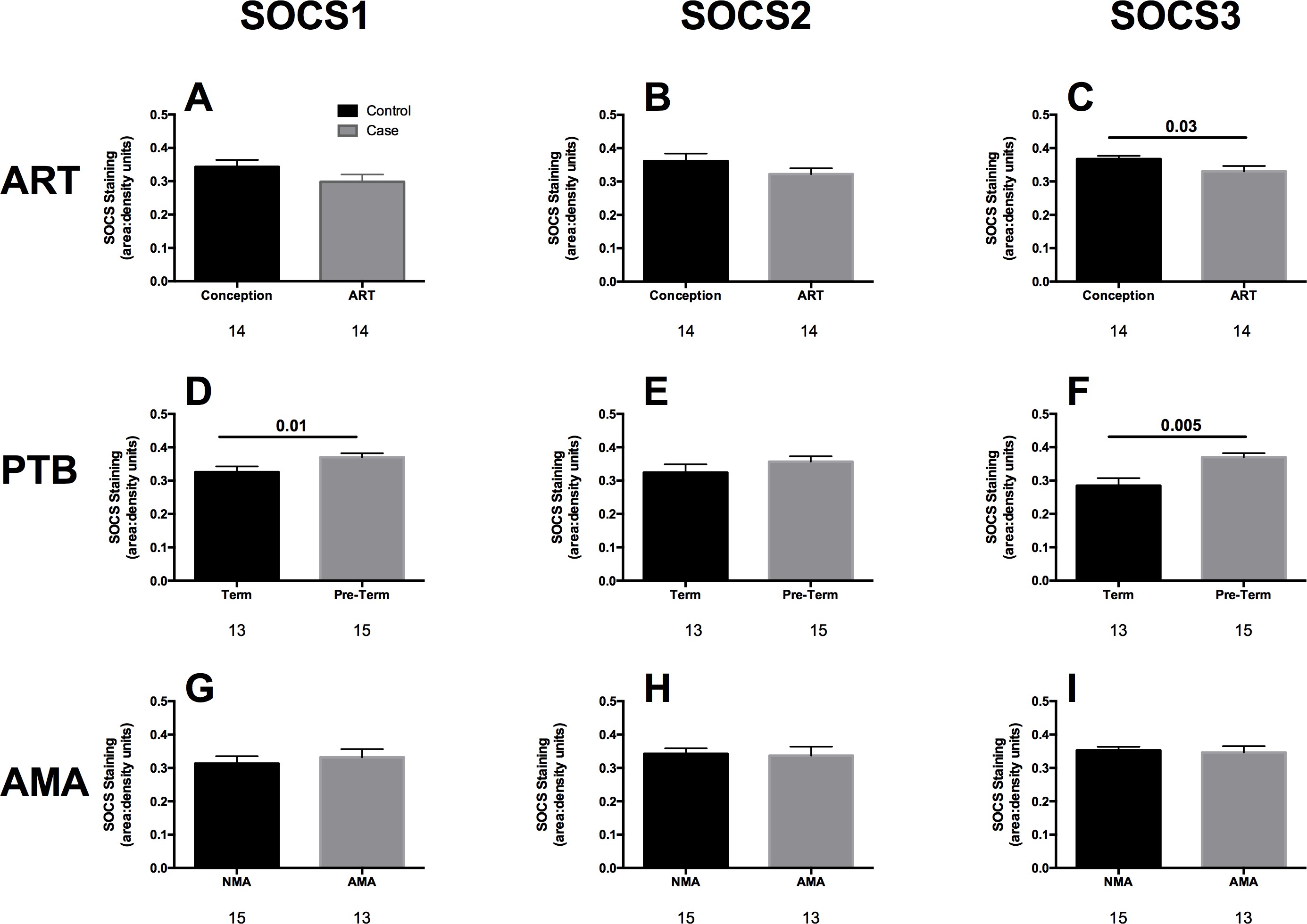 Figure 2.
Figure 2.— SOCS expression levels in human placenta. A-C: Normal conception vs. assisted reproduction (ART); D-F: Term vs. preterm birth (PTB) regardless of conception method. G-I Advanced maternal age (AMA ≥ 35 years) vs. normal maternal age (< 35 years), regardless of conception method. Bars are means ± SEM. N values for each bar are listed underneath.
When confounding factors (maternal age and gestational age) were stratified there were significantly lower levels of SOCS3 at term in ART placentas from AMA women (p = 0.01), and also in pre-term placentas (p = 0.01). Similarly, in normal maternal age (i.e. < 35 years), SOCS3 was lower in placentas from ART pregnancies than normal conception (p = 0.02) and in ART pre-term deliveries than normal conception (p = 0.01). This indicates that SOCS3 dysregulation is not a function of AMA because the significance of the data does not change with stratification ñ rather SOCS3 dysregulation appears to be an underlying mechanism in pre-term birth, that is even more pronounced in ART.
The levels of IFN-γ, IL1-β, IL-6, IL-8, IL1-0, and TNF-α did not differ significantly with ART (vs. normal conception), pre-term birth (vs. term) and AMA (vs. normal age).
Similarly, when stratified for ART, gestational age and maternal age status, no significant differences in cytokines were observed, likely due to small sample size and effects of other clinical variable confounders on cytokine expression.
Correlating levels of SOCS proteins to levels of cytokines in the placental tissues regardless of the method of conception, placental SOCS3 was significantly positively associated with IFN-γ (p = 0.04, r = 0.39).
When stratified for ART or normal conception, normal conception placentas showed a strong significant associations between SOCS1 and IL-10 (p = 0.002, r = 0.77, Figure 3A). In comparison, the SOCS IL-10 relationship is lost in ART placentas (p = 0.53, r = 0.18, Figure 3B). Both normal conception and ART placentas showed correlations between both SOCS2 and SOCS3 and IFN-γ (p = 0.02, r = 0.69, Figure 3C and p = 0.005, r = 0.75, Figure 3E). However, in ART the SOCS2/ IFN-γ association was lost (p = 0.7279, r = 0.08 Figure 3D) as was the SOCS3/IFN-γ association (p = 0.56, r = 0.17, Figure 3F). This may indicate a switching of mixed signaling pathways between SOCS1 JAK/STAT and SOCS 2/MAPK signaling in normal conception to a greater involvement of SOCS2/MAPK signaling in ART placental tissues.
When stratified for AMA or normal maternal age, normal pregnancy age was not associated with any SOCS/cytokine correlations. However, with AMA SOCS 1 was significantly positively correlated with TNF-α (p = 0.04, r = 0.60, Figure 4A) but not in placentas from normal aged mothers (Figure 4B), SOCS 2 was significantly correlated with TNF-α (p = 0.003, r = 0.80 Figure 4C), again lost in placentas from normal aged mothers (Figure 4D) while SOCS 3 was positively associated with IL-6 (p = 0.046, r = 0.42 Figure 4E) which was not seen in placentas from normal aged mothers (Figure 4F). Taken together this indicated that AMA is associated with increased placental inflammation.
When stratified for pre-term or term, placentas from children born at term showed a good positive correlation between SOCS2 and IL-10 (p = 0.03, r = 0.62, Figure 5A) this correlation was not observed from placentas from preterm birth (Figure 5B) and a negative association between SOCS3 and IL-8 (p = 0.03, r = -0.59, Figure 5C), a correlation that was lost in pre-term birth (Figure 5D). As these associations were lost for pre-term birth, this indicates that at term SOCS signaling is associated with anti-inflammatory effects through increased SOCS causing greater IL-10 signaling and less IL-8 signaling, however with pre-term birth losing these associations indicates an inflammatory state.
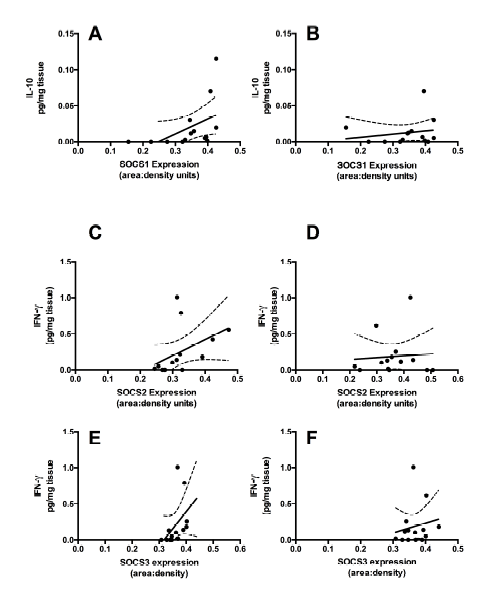 Figure 3.
Figure 3.— Correlations between SOCS and cytokines in placentas from normally conceived and ART pregnancies. A and B: SOCS1 correlates strongly with IL-10 in normally conceived placentas (p = 0.002, r = 0.77) but not in ART (p = 0.52, r = 0.19). C and D: SOCS2 correlates well with IFN-γ in normally conceived placentas (p = 0.02, r = 0.60) but not in ART (p = 0.80, r = 0.08), and E and F: SOCS3 correlates strongly with IFN-γ in normally conceived placentas (p = 0.02, r = 0.48) but not in ART (p = 0.56, r = 0.17). Solid line is the correlation fit, dashed line is the 95% confidence interval.
 Figure 4.
Figure 4.— Significant associations between SOCS and cytokines in placental tissue from AMA pregnancies ñ these associations did not occur placentas from mothers under 35. A and B: SOCS1 is positively correlated with TNF-α (p = 0.04, r = 0.60) in placentas from AMA mothers, but the correlation does not occur in normal maternal age (p = 0.38, r = 0.28). B: SOCS2 is strongly correlated with TNF-α (p = 0.003, r = 0.80) in AMA, but not in normal maternal age (p = 0.93, r = -0.03). C: SOCS3 is strongly correlated with IL6 (p = 0.009, r = 0.73), but this is not observed in placentas from normal maternal aged women (p = 0.89, r = -0.04). Solid line is correlation fit, dashed line is 95% confidence interval.
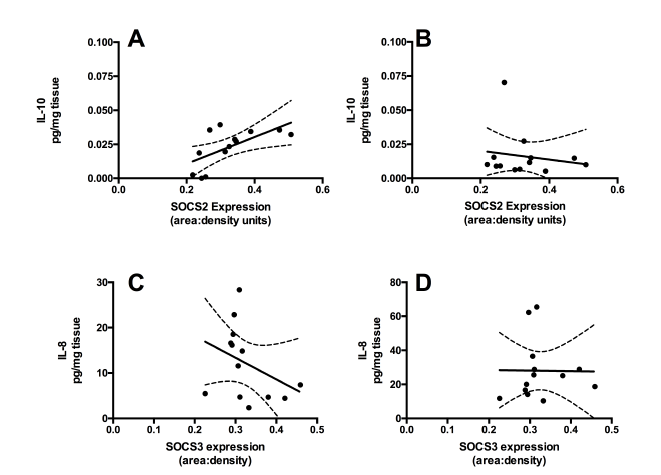 Figure 5.
Figure 5.— Significant associations between SOCS and cytokines in term placental tissue, that were lost in pre-term birth. A and B: SOCS2 is correlated with placental IL-10 at term (p = 0.03, r = 0.62), but not in pre-term placentas (p = 0.01, r = 0.95). C and D: SOCS3 is negatively correlated with IL-8 (p = 0.03, r = -0.59) at term, but not in pre-term placentas (p = 0.59, r = -0.17). Solid line is correlation fit, dashed line is 95% confidence interval.
This is the first study, to our knowledge, demonstrating dysregulation of human placental SOCS and associated effects on cytokine signaling in ART. Based on the results described above, there is a significant decrease in SOCS3 in ART placentas when compared to placentas from normal conception. Additionally, the associations between SOCS1 and IL10 and SOCS2 and 3 with IFN-γ in placentas from naturally conceived pregnancies were lost in ART placentas. Correct SOCS3 production is required for placentation, placental development, parturition, and fetal development [7]. Because SOCS3 acts directly on the JAK/STAT3 pathway as a negative inhibitor of self, placental proteins that are not normally present, including NmL, Bcl-XL, p21, MYC, and NOS2 maybe upregulated [22]. These data also confirm our previously published data showing that ART can downregulate placental SOCS3 signaling in the mouse model of reproduction [7]. Hence this study also validates the mouse model of ART as appropriate for studying placental SOCS and cytokine signaling in the context of human pregnancy. Deregulation of placental SOCS/cytokine signaling in AMA and pre-term birth, regardless of conception method are also significant, secondary findings.
Several significant positive correlations between placental cytokines and SOCS protein levels were observed in placentas from normally conceived pregnancies namely: SOCS1 positively correlated with IL10, and both SOCS2 and SOCS3 with IFN-γ. These associations were lost in placentas from pregnancies conceived with ART, implying that ART is dysregulating SOCS/cytokine interactions at the SOCS protein level. It has been shown that SOCS1 is associated with attenuating IL-10-mediated immune signaling and that IL10-induced upregulation of SOCS1 is a critical part of immune signaling pathway regulation [23]. These authors reported that IL10-induced SOCS1 directly inhibits IFN-γ signaling and therefore dampens inflammation. The consequences of loss of the IL10-SOCS1 interaction in ART, would be more placental inflammation. Similarly, in ART the loss of the direct positive correlations of SOCS2 and SOCS3 with IFN-γ observed in normal conception, could also have serious consequences. Yu et al. showed that SOCS2 impairs IFN/JAK/STAT signaling by reducing the stability of tyrosine kinase 2, downregulating IFN receptors, STAT1 phosphorylation and nuclear translocation [24]. Similarly, the SOCS3-dependent expression of IFN-γ has been shown to inhibit IL-6 effects on STAT3 phosphorylation and other IL6-mediated transcriptional responses in human microvascular endothelial cells [25]. This has obvious parallels to placental endothelial function. The cytokine IL-6 is involved in endothelial dysfunction and has pro- and anti-inflammatory effects. The anti-inflammatory effects of IL6 are modulated by SOCS3 signaling at STAT3, and as noted above, SOCS3 is significantly lower in ART as compared to normal conception, in addition to the loss of the natural association with IFN-γ. Taken together these results suggest that SOCS dysregulation, particularly SOCS3, and down-stream effects on cytokine regulation may be a mechanism for placental dysfunction and/or adverse pregnancy outcomes in ART. Moreover, in advanced maternal age placental SOCS1 and 2 expression were positively correlated with levels of TNF-α, while SOCS3 correlated with IL6, but this did not occur in placentas from pregnancy where women were under 35 years of age (“normal maternal age”). This indicates that in AMA, the placenta has a greater pro-inflammatory environment due to loss of the natural SOCS feedback mechanism. AMA is associated with higher risk of chromosomal abnormalities, miscarriage, and very pre-term birth [26], which are also complications of ART [1, 2] and these complications are also associated with increased levels of pro-inflammatory cytokines [27-29]. Taken together, these data imply that a combined mechanism for negative outcomes is the intersection of ART effects on cytokine signaling and independent effects of AMA on the same inflammatory cytokine signaling pathways. Furthermore, negative associations between SOCS3 and IL-8 (such that SOCS3 expression is suppressing IL-8-mediated inflammation) and a strong positive correlation between SOCS2 and IL-10, meaning that SOCS2 is upregulating the anti-inflammatory IL10, were lost for pre-term birth. Due to a loss of these associations in pre-term birth (regardless of natural conception or ART) the natural anti-inflammatory effects of SOCS proteins are attenuated and a placental inflammatory state occurs. These data are supported by previous studies demonstrating that inflammation of the fetal membranes is associated with pre-term birth [30], chronic inflammation of the placental tissues is associated with severe retinopathy of pregnancy, LBW babies and pre-term birth [31], and clinical placental correlates of inflammation (e.g. lymphocyte invasion of placental tissue, amniotic fluid infection) are associated with pre-term birth [32]. However, the vast majority of published studies use clinical or histopathological measures of inflammation. Here we add significantly to the conversation around placental inflammation and preterm birth, by providing mechanistic data showing that normal placental SOCS signaling that inhibits IL-8 and increases IL-10 in placentas at term, is lost in pre-term birth. Hence re-establishing SOCS regulation of anti-inflammatory signaling could be a useful target in preventing pre-term birth.
Because our clinical findings are supported by other publications (e.g. preterm babies have more LBW and SGA) [15, 18-21], this provides evidence that our cohort is broadly representative of the general population and we do not have underlying confounding in pregnancy outcomes. Despite this, we acknowledge that the sample size was small, in part due to this being a pilot study and in part, due to the difficulty of procuring human placenta specifically from ART pregnancies. Sample size effects were partially mitigated by base-line matching the tissues to maternal and gestational age, BMI, and pregnancy weight gain. Additionally, the semi-quantitation approach taken using photomicrographs and immunohistochemistry may not be as sensitive as a completely quantitative approach using e.g. ELISA or mass spectrometry. This being said, there are multiple published examples of immunohistochemistry approaches providing reliable estimations of placental protein levels [33-35]. In some ways the semi-quantitative approach strengthens our results because it is less sensitive than other quantitative methodologies, meaning that our data contain higher type II error (false negative) for SOCS, and making our significant findings conservative with respect to significance.
In conclusion, this is the first report of SOCS dysregulation in human placentas attributable to ART, independent of maternal age, gestational age, ethnicity, pregnancy weight gain or delivery method. Additionally, using a stratified nested design, we also determined interactive effects of AMA and ART on placental SOCS and cytokine dysregulation, whereby the interaction together increases inflammatory cytokine signaling. Finally, regardless of ART status, we demonstrated that in pre-term birth SOC-mediated anti-inflammatory effects are attenuated, thereby providing new mechanistic data on the involvement of placental signaling and pre-term birth. The use of ART in increasing world-wide due to lifestyle factors such as delayed decisions around reproduction in working women [36] and increasing levels of both male and female infertility worldwide [37, 38]. These techniques have allowed thousands of people to successfully conceive that would otherwise not have been able to, they are a net positive good. ART is, however not without significantly increased risk of negative pregnancy outcomes. Studying ART effects on reproductive tissues can be valuable for understanding the underlying mechanisms of negative outcomes in ART in an attempt to mitigate these. In the context of this first report in humans, as well as previous studies in mice [7, 9, 10, 39, 40], mitigating placental SOCS deregulation and/or reestablishing correct inflammatory and anti-inflammatory signaling may be a fruitful avenue for preventing negative outcomes in ART.
The University of Hawaii Human Reproductive Biospecimen Repository was funded by National Institutes of Health RMATRIX - 3U54MD007584-03S1. Dr. Kim is supported by a Canadian Institutes of Health Research (CIHR) Clinician-Scientist Salary Award (MC2-127872). Dr. Collier and Dr. Kim received a joint grant from the Associate Deans, Research of the Faculties of Dentistry and Pharmaceutical Sciences, UBC to support this research.
