Systemic lupus erythematosus (SLE) is a diffuse connective tissue disease with autoimmune mediating, multiple positive autoantibodies, multiple-system, and organ involvement. SLE can involve the skin mucosa, joint muscles, kidneys, ingenious organs, blood systems, and nervous systems. SLE occurs most frequently in women of childbearing age and rarely occurs before puberty or after menopause [1]. Past studies have shown that the pathogenesis of SLE is closely related to sex hormones, which are mainly estrogen and prolactin. During pregnancy, in order to adapt to the growth and development of embryo and fetus, the level of sex hormones in the body changes, especially estrogen, progestin, and prolactin [2-4]. Therefore, pregnancy has the risk of aggravating the activity of SLE. SLE itself does not affect women’s fertility, unless combined with severe kidney damage or amenorrhea induced by cytotoxic drugs such as cyclophosphamide [5]. The pregnancy is no longer a taboo in SLE women, and the fetal loss rate in SLE women has drastically dropped nowadays. However, the risk of pregnancy-related complications SLE women is still two to four times higher than that of the healthy population. These complications include spontaneous abortion, stillbirth, premature delivery, preeclampsia, intrauterine growth restriction, neonatal lupus, and neonatal death [6]. At present, there is no precise guide to pregnancy test and management for SLE patients, but the importance of close follow-up by the rheumatology and obstetrics doctors is the world consensus. This study investigated the pregnancy outcomes and risk factors in pregnant women with SLE. The objective was to provide a reference for clinically guiding SLE women to successfully complete the pregnancy.
This study collected 78 SLE patients with 82 pregnancies hospitalized in Affiliated Hospital of Jining Medical College from February 2012 to January 2017. This study was approved by the ethics committee of Jining Medical College and written informed consent was obtained from all participants.
Diagnosis of SLE was in line with the criteria established by the American Rheumatism Society in 2000 [7]. The pre-pregnancy SLE activity was assessed by SLE disease activity index (SLEDAI) as follows: 0-4 points: inactivity; ≥ 5 points: activity (5-9 points: mild activity; 10-14 points: moderate activity; ≥ 15 points: severe activity). The activity of SLE during pregnancy was assessed by the lupus activity index in pregnancy (LAI-P). The increase of LAI-P by more than 0.25 was defined as the SLE deterioration during pregnancy [8].
Fetal outcome was defined as follows: 1) spontaneous abortion: the pregnancy was spontaneously interrupted before the 20th week of gestation; 2) stillbirth: the fetal died within 20 weeks of gestation; 3) therapeutic abortion: the pregnancy was terminated due to illness; 4) fetal loss: spontaneous abortion, stillbirth or therapeutic abortion; 5) premature delivery: live birth before the 37th week of gestation.
General information of SLE patients, maternal history data, clinical symptoms and laboratory examination data one to three months before pregnancy, during pregnancy, at delivering and after childbirth, drug treatment, and pregnancy outcome were collected.
All statistical analysis was carried out using SPSS 22.0 software. The single factor analysis was performed for compare the variables between SLE with deterioration group and SLE without deterioration group, and between fetal loss group live birth group. The enumeration data were presented as number, and were compared using χ2 test. The measurement data were pre-sented as mean ± SD, and were compared using the t-test. The logistic regression analysis was conducted for further analyzing the significant variables in single factor analysis. The odds ratio (OR) value was calculated, and the receiver operating characteristic (ROC) curves of significant variables for predicting SLE condition and fetal outcome were made. P < 0.05 was considered as statistically significant.
There were 82 pregnancies in 78 SLE patients. The age of patients was 20-37 years, with average of 25.74 ± 4.67 years. The course of SLE before pregnancy was 1-16 years, with an average of 5.85 ± 2.42 years. In 78 SLE patients, there were 45 (57.69%) cases of pregnancy once, 19 (24.36%) cases of pregnancy twice, eight (10.26%) cases of pregnancy for three times, and six (7.69%) cases of pregnancy for four times. In 82 pregnancies, there were 31 (37.80) cases of SLE activity before pregnancy (SLEDAI ≥ 5 points), in which there were 30 (36.59 %) cases of mild activity and one (1.22%) case of moderate activity. The laboratory examination before pregnancy showed that, there were 21 (25.61 %) cases with positive anti-dsDNA antibody, and 33 (40.24 %) cases with positive anti-CL antibody.
According to LAI-P, there were 31 (37.80%) cases of SLE with deterioration and 51 (62.20%) cases of SLE without deterioration during pregnancy. The single-factor analysis of factors for SLE with deterioration during pregnancy showed that there was significant difference in complement 3, 24-hour urinary protein (24h UP), anti-dsDNA antibody, anti-CL antibody, pre-pregnancy prednisone dose, and SLEDAI between SLE with deterioration group and SLE without deterioration group, respectively (p < 0.05 or p < 0.01) (Table 1).
Using SLE with deterioration as a dependent variable and complement 3, 24h UP, anti-dsDNA antibody, anti-CL antibody, pre-pregnancy prednisone dose, and SLEDAI as independent variables, the binary logistic regression analysis was performed. Results found that, the complement 3 and SLEDAI were the main risk factors of SLE with deterioration during pregnancy. The OR values of complement 3 and SLEDAI were 208.771 (3.681-11841.894) and 71.045 (2.674-1887.362), respectively (Table 2). The logistic regression equation was as follows: p = Exp (-14.828 + 5.341×complement 3 + 4.263 × SLEDAI). The receiver operating characteristic (ROC) curves of complement 3 and SLEDAI for predicting the SLE condition during pregnancy were shown in Figure 1. The area under curve (AUC), sensitivity and specificity of complement 3 for predicting SLE with deterioration during pregnancy were 0.876, 89.5% and 85.7%, respectively. The AUC, sensitivity, and specificity of SLEDAI for predicting SLE with deterioration during pregnancy were 0.878, 94.7%, and 81.0%, respectively.
For the fetal outcome, there were 27 (32.93%) cases of fetal loss and 55 (67.07%) cases of live births. In 27 (32.93%) cases of fetal loss, there were 22 (26.83%) cases of therapeutic abortions, four (4.88%) cases of spontaneous abortion, and one (1.22%) case of stillbirth. In 55 cases of live birth, there were 29 (35.37%) cases of full-term delivery and 26 (31.71%) cases of premature delivery. The single-factor analysis of factors for fetal outcome showed that, there was significant difference in complement 3, combined antiphospholipid syndrome (APS), SLEDAI, and SLE deterioration during pregnancy between fetal loss group and live birth group (p < 0.01) (Table 3).
Using fetal loss as a dependent variable and complement 3, combined APS, SLEDAI, and SLE deterioration as independent variables, the binary logistic regression analysis was performed. Results found that, the combined APS and SLEDAI were the main risk factors of fetal loss. The OR values of combined APS and SLE deterioration were 26.296 (1.330-519.958) and 79.612 (3.728-1699.958), respectively (Table 4). The logistic regression equation was as follows: p = Exp (-8.178 + 3.269 × combined APS + 4.377 × SLEDAI). The ROC curves of combined APS and SLEDAI for predicting the SLE condition during pregnancy are shown in Figure 2. The AUC, sensitivity, and specificity of combined APS for predicting the fetal loss were 0.850, 85.0%, and 85.0%, respectively. The AUC, sensitivity, and specificity of SLEDAI for predicting the fetal loss were 0.900, 85.0%, and 95.0%, respectively.
| Index | SLE with deterioration | SLE without deterioration | t/χ2 | p |
|---|---|---|---|---|
| n | 31 | 51 | ||
| Age (years) | 25.23 ± 3.14 | 26.12 ± 3.46 | -1.169 | 0.246 |
| Disease course (years) | 5.45 ± 2.37 | 4.67 ± 1.98 | 1.604 | 0.113 |
| WBC (× 109 /L) | 7.13 ± 2.45 | 7.67 ± 1.64 | -1.196 | 0.235 |
| Hemoglobin (g/L) | 92.59 ± 26.03 | 88.12 ± 19.26 | 0.890 | 0.376 |
| Platelets (× 109 /L) | 156.33 ± 34.58 | 163.73 ± 28.52 | -1.050 | 0.297 |
| ESR (mm/h) | 21.68 ± 5.78 | 19.56 ± 5.18 | 1.720 | 0.089 |
| Serum albumin (g/L) | 28.93 ± 5.12 | 30.94 ± 6.27 | -1.505 | 0.136 |
| Serum globulin (g/L) | 30.93± 5.68 | 32.45 ± 4.33 | -1.368 | 0.175 |
| CRP (mg/L) | 3.15 ± 0.67 | 2.87 ± 0.78 | 1.660 | 0.101 |
| IgG (g/L) | 16.94 ± 3.78 | 17.33 ± 2.56 | -0.557 | 0.580 |
| IgA (g/L) | 1.87 ± 0.56 | 1.99 ± 0.58 | -0.920 | 0.360 |
| IgM (g/L) | 1.11 ± 0.31 | 1.25 ± 0.38 | -1.730 | 0.088 |
| Complement 3 (g/L) | 0.81 ± 0.26 | 0.98 ± 0.22 | -3.166 | 0.002 |
| Complement 4 (g/L) | 0.13 ± 0.05 | 0.15 ± 0.06 | -1.555 | 0.124 |
| 24h UP (g/24h) | 0.99 ± 0.34 | 0.74 ± 0.38 | 3.003 | 0.004 |
| Positive anti-dsDNA antibody [n(%)] | 13 (41.94) | 8 (15.67) | 6.9732 | 0.008 |
| Positive anti-CL antibody [n(%)] | 18 (58.06) | 15 (29.41) | 6.5822 | 0.010 |
| Pre-pregnancy prednisone dose [n(%)] | 6.762 | 0.009 | ||
| ≥ 10 mg/day | 16 (51.61) | 12 (23.53) | ||
| < 10 mg/day | 15 (48.39) | 39 (76.47) | ||
| SLEDAI [n(%)] | 8.701 | 0.003 | ||
| ≥ 5 points | 18 (58.06) | 13 (25.49) | ||
| < 5 points | 13 (41.94) | 38 (74.51) |
SLE: systemic lupus erythematosus; WBC: white blood cell; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; IgG: immunoglobulin G; IgA: immunoglobulin A; IgM: immunoglobulin M; 24h UP: 24-hour urine protein; SLEDAI: systemic lupus erythematosus disease activity index.
| Variable | B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. | for Lower EXP(B) |
|---|---|---|---|---|---|---|---|---|
| Complement 3 | 5.341 | 2.060 | 6.721 | 1 | 0.012 | 208.771 | 3.681 | 11841.894 |
| 24h UP | -1.679 | 2.133 | 0.619 | 1 | 0.431 | 0.187 | 0.003 | 12.210 |
| Anti-dsDNA antibody | 2.872 | 1.498 | 3.675 | 1 | 0.055 | 17.672 | 0.938 | 333.091 |
| Anti-CL antibody | 0.165 | 0.145 | 1.288 | 1 | 0.256 | 1.179 | 0.887 | 1.569 |
| Pre-pregnancy prednisone dose | 3.127 | 2.017 | 2.403 | 1 | 0.121 | 22.794 | 0.438 | 1187.525 |
| SLEDAI | 4.263 | 1.673 | 6.491 | 1 | 0.011 | 71.045 | 2.674 | 1887.362 |
| Constant | -14.828 | 7.661 | 3.746 | 1 | 0.053 | 0.000 |
SLE: systemic lupus erythematosus; 24h UP: 24-hour urine protein; SLEDAI: systemic lupus erythematosus disease activity index.
 Figure 1.
Figure 1.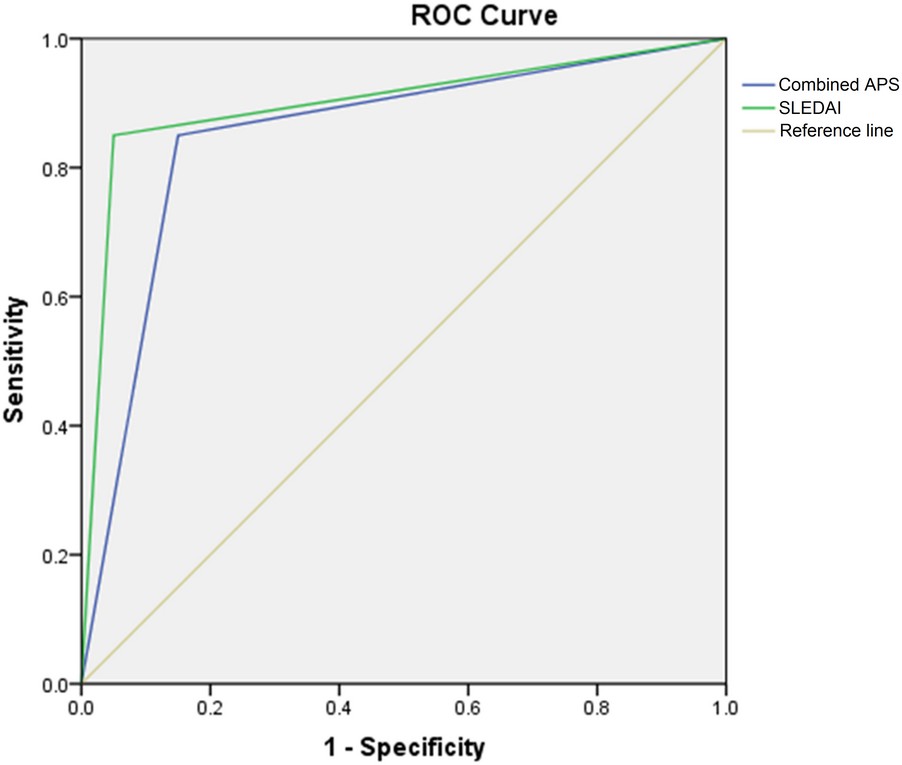 Figure 2.
Figure 2.| Index | Fetal loss | Live birth | t/χ2 | p |
|---|---|---|---|---|
| n | 27 | 55 | ||
| Age (years) | 26.32 ± 3.26 | 25.34 ± 3.18 | 1.301 | 0.197 |
| Disease course (years) | 5.61 ± 2.48 | 4.49 ± 2.72 | 1.802 | 0.075 |
| WBC (× 109 /L) | 7.19 ± 2.66 | 7.55 ± 1.87 | -0.710 | 0.480 |
| Hemoglobin (g/L) | 93.44 ± 22.67 | 87.89 ± 21.33 | 1.085 | 0.281 |
| Platelets (× 109 /L) | 161.45 ± 35.77 | 176.67 ± 39.83 | -1.679 | 0.097 |
| ESR (mm/h) | 21.78 ± 4.83 | 19.94 ± 4.79 | 1.630 | 0.107 |
| Serum albumin (g/L) | 28.47 ± 6.72 | 30.37 ± 5.83 | -1.318 | 0.191 |
| Serum globulin (g/L) | 29.97 ± 6.77 | 31.95 ± 4.84 | -1.521 | 0.132 |
| CRP (mg/L) | 3.12 ± 0.67 | 2.79 ± 0.85 | 1.764 | 0.082 |
| IgG (g/L) | 16.57 ± 3.67 | 17.57 ± 2.84 | -1.358 | 0.178 |
| IgA (g/L) | 1.92 ± 0.54 | 1.98 ± 0.52 | -0.485 | 0.629 |
| IgM (g/L) | 1.09 ± 0.32 | 1.24 ± 0.38 | -1.765 | 0.081 |
| Complement 3 (g/L) | 0.77 ± 0.22 | 0.93 ± 0.25 | -2.829 | 0.006 |
| Complement 4 (g/L) | 0.14 ± 0.06 | 0.16 ± 0.05 | -1.592 | 0.115 |
| 24h UP (g/24h) | 0.94 ± 0.31 | 0.79 ± 0.42 | 1.647 | 0.104 |
| Positive anti-dsDNA antibody [n(%)] | 8 (29.63) | 13 (23.64) | 0.341 | 0.559 |
| Positive anti-CL antibody [n(%)] | 14 (51.85) | 19 (34.55) | 2.256 | 0.133 |
| Combined APS | 7 (25.93) | 2 (3.64) | 9.208 | 0.002 |
| Pre-pregnancy prednisone dose [n(%)] | 1.898 | 0.168 | ||
| ≥ 10 mg/day | 12 (44.44) | 16 (29.09) | ||
| < 10 mg/day | 16 (55.56) | 39 (70.91) | ||
| SLEDAI [n(%)] | 7.880 | 0.005 | ||
| ≥ 5 points | 16 (59.26) | 15 (27.27) | ||
| < 5 points | 11 (40.74) | 40 (72.73) | ||
| SLE deterioration [n(%)] | 16 (59.26) | 15 (27.27) | 7.880 | 0.005 |
WBC: white blood cell; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; IgG: immunoglobulin G; IgA: immunoglobulin A; IgM: immunoglobulin M; 24h UP: 24-hour urine protein; APS: antiphospholipid syndrome; SLEDAI: systemic lupus erythematosus disease activity index.
| Variable | B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. | for Lower EXP(B) |
|---|---|---|---|---|---|---|---|---|
| Complement 3 | 2.183 | 1.560 | 1.958 | 1 | 0.162 | 8.869 | 0.417 | 188.549 |
| Combined APS | 3.269 | 1.523 | 4.610 | 1 | 0.032 | 26.296 | 1.330 | 519.958 |
| SLEDAI | 4.377 | 1.562 | 7.854 | 1 | 0.005 | 79.612 | 3.728 | 1699.958 |
| SLE deterioration | 0.086 | 0.135 | 0.409 | 1 | 0.523 | 1.090 | 0.837 | 1.420 |
| Constant | -8.178 | 5.950 | 1.889 | 1 | 0.169 | 0.000 |
SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome; SLEDAI: systemic lupus erythematosus disease activity index.
It is found that SLE disease itself may interact with pregnancy in pregnant women with SLE. Previous study had shown that the SLE activity did not deteriorate significantly during pregnancy, and the pregnancy was not an aggravating factor for SLE [9]. However, in recent years, more and more studies have confirmed that pregnancy can affect the activity of SLE. A majority of pregnant women have SLE activity in varying degrees, mostly mild and moderate SLE activities [10, 11]. The main manifestations of SLE include arthritis, skin lesions, blood system involvement, etc. In some patients, the SLE activity can involve the kidney, heart, lung and brain, and even leads to death [12]. The recurrence or deterioration of SLE during pregnancy is common in the middle and third term of pregnancy. It is also necessary to pay attention to the recurrence or deterioration of SLE after delivery [13]. The causes of recurrence or deterioration of SLE during pregnancy are related to the dysfunction of autoreactive B cells caused by changes in estrogen and prolactin levels in the body [14].
In this study, LAI-P was used to evaluate the SLE activity during pregnancy. The results showed that, there were 31 (37.80%) cases of SLE with deterioration and 51 (62.20%) cases of SLE without deterioration during pregnancy. The single-factor analysis showed that, there was significant difference in complement 3, 24h UP, anti-dsDNA antibody, anti-CL antibody, pre-pregnancy prednisone dose, and SLEDAI between SLE with deterioration group and SLE without deterioration group, respectively. The multi-factor regression analysis found that, the complement 3 and SLEDAI were the main risk factors of SLE with deterioration during pregnancy. This is similar to the results of previous studies [14, 15].
Effects of SLE on pregnancy are mainly related to the vascular lesions and autoantibodies of SLE itself. The vasculitis caused by maternal SLE activity can lead to poor placental blood supply, thereby affecting the fetal blood circulation. This leads to fetal growth retardation and even death [16]. In addition, the immune complex attack can cause placental dysplasia, villus growth damage, thus affecting the growth and development of the fetus [17]. Therefore, the condition of maternal SLE activity will directly affect the state of the fetus. Pregnant women with SLE are prone to miscarriage, premature delivery, intrauterine growth retardation, stillbirth, and various congenital abnormalities [18]. The live birth rate of SLE patients with good disease control before pregnancy is higher than that of SLE patients with active disease before pregnancy [19]. Clowse et al. [20] find that SLE activity before pregnancy and in early pregnancy is the most important factor leading to fetal loss. Tian et al. [21] report that proteinuria, thrombocytopenia, and hypertension in early pregnancy are the independent risk factors for fetal loss. In addition, Mankee et al. [22] report that, the risk factors of fetal loss are associated with APS and SLE activity before pregnancy.
In this study, there were 27 (32.93%) cases of fetal loss and 55 (67.07%) cases of live birth. The single-factor analysis of factors for fetal outcome showed that, there was significant difference in complement 3, combined APS, SLEDAI and SLE deterioration between fetal loss group and live birth group. The binary logistic regression analysis showed that combined APS and SLEDAI were the main risk factors of fetal loss. This is basically the same with the results of the above studies.
In conclusion, pregnancy is at a high risk in women with SLE. The complement 3 and SLEDAI are the main risk factors of SLE with deterioration during pregnancy, and the combined APS and SLEDAI are the main risk factors of fetal loss. Therefore, the reasonable and effective strategies should be formulated based on these factors, so as to reduce the risk of adverse pregnancy outcomes.
