Uterine necrosis is one of the rare complications that may follow uterine arterial embolization for postpartum hemorrhage (PPH), and its incidence remains unknown. The authors report four cases of uterine necrosis in Korea. The mean time interval between uterine artery embolization (UAE) and diagnosis of uterine necrosis was 72 days. Patients’ main symptoms were abdominal pain, fever, profuse vaginal discharge, and vaginal bleeding. Decisions related to management depended on the condition of the patient and the patient’s desire regarding conservation of the uterus. Based on these cases, the authors suggest helpful decisions for the therapeutic guidelines for uterine necrosis after UAE.
Postpartum hemorrhage (PPH) is defined as estimated blood loss in excess of 500 mL following a vaginal delivery, or greater than 1,000 mL following a cesarean section (severe PPH: an estimated blood loss > 1,500 mL, peripartum fall in hemoglobin concentration > 4.0 g/dL, or acute transfusion of four or more units of blood) [1]. Uterine atony, cervical or vaginal wall lacerations, vessel injury during cesarean section (CS), remnant placenta, pathological placentation, and disseminated intravascular coagulopathy are the main causes leading to PPH. When conservative management fails to control hemorrhage, surgical treatments or uterine artery embolization (UAE) must be considered. Successful control of hemorrhage can be achieved in 83-100% of patients, allowing for preservation of the patient’s uterus and future fertility. The most frequent complications associated with these procedures include post-embolization syndrome, endometritis, leg or buttock paresthesia, and hypoesthesia [2-5].
Pelvic pain associated with fever may be involved in uterine necrosis after UAE. Pelvic CT scan or MRI is required in order for this to be confirmed. Recent publications have described cases demonstrating the occurrence of partial or total uterine necrosis after UAE in the setting of PPH [6]. Through four cases in this experience, the authors analyzed and reviewed uterine necrosis after embolization. Further study and discussion are needed in order to determine the clinical, radiological, and therapeutic aspects of uterine necrosis after UAE.
A 37-year-old woman at 38 weeks 3 days (parity 2-0-1-2) delivered a healthy female child through cesarean section, due to placenta abruption at a primary health center. She had already developed consumption coagulopathy at the time of hospitalization. Her international normalized ratio (INR) was 2.7 and D-dimer level was > 35.2 mg/L. Her abdomen was very distended and hard with tenderness. Immediate resuscitation was performed with the transfusion of blood products and a decision for embolization was taken in view of the intractable vaginal bleeding. In abdomen CT (enhanced), a pseudoaneurysm (1.5 cm) was found in the uterine incision site of the lower uterine segment, and the vagina was found to be fully filled with hematoma. Angiography showed multiple active bleeding at a branch of the right circumflex iliac artery and pseudoaneurysm formation of the left uterine artery. The authors performed embolization with microcoil, gelfoam, and Nbutyl cyanoacrylate (NBCA). At eight hours after UAE, she had continuous abdominal distension with uncheckable blood pressure despite the massive blood transfusion. The team diagnosed her with rebleeding and performed reembolization with gelfoam. In abdomen CT after ten days, endometrial perfusion was markedly decreased, and hypertrophied myometrium was replaced by a lowdensity area, suggesting ischemic change. Intramuscular and subperitoneal hematomas were found in the lower anterior abdominal wall. Her status was stable without abdominal pain or fever. Five months later, she complained of lower abdominal pain, profuse vaginal discharge, dizziness, and general weakness without fever. The authors performed aspiration biopsy of the uterus and found abscess. In ultrasonography (US), the size of uterus was 12.90×8.58 cm with anterior wall thickness of 3.8 cm, and posterior wall thickness of 3.2 cm. Bulky uterus was shown without vascularity in myometrium and visible endometrial line. The internal low-density area of the uterus was decreased, and newly developed multiple transmural infarctions and internal air bubbles were found in abdomen CT. The authors suspected pyometra and endometritis. Laparoscopic total hysterectomy was performed. The pathologic finding was acute and chronic inflammation with abscess, necrosis, and hemorrhage of endometrium and myometrium (Figure 1).
 Figure 1.
Figure 1.— Case 1. (A) Laparoscopic images of the uterus with both normal ovaries. (B) Total uterus measures 12×10×5 cm and weighs 280.0 grams with a tan and brown smooth perimetrial surface. The exocervix is grayish white, smooth, and firm. The endocervical canal containing the mucoid material is brown and patent throughout. The endometrium, 2.5 cm in thickness, is pale pink and doughy. The myometrium, 1.0 cm in thickness, is pale brown and shows a well-demarcated round mass, and measures up to 1.3×1.0 cm, with a whitish gray solid, compact, rubbery, and whoring cut surface.
A 41-year-old primiparous woman was transferred to the hospital due to uncontrolled vaginal bleeding after normal spontaneous vaginal delivery. Her vital signs were: BP of 110/60 mmHg, pulse rate of 84/minute, and drowsy mental state. Abdomen CT (Figure 2A) showed active bleeding into the uterine cavity at the lower anterior uterine wall. UAE was performed at both uterine arteries with gelform particles and polyvinyl alcohol (PVA) (Figure 2B). She was discharged from the hospital after three days without any complications. Three weeks later, she showed gait disturbance, persistent abdominal pain, and vaginal spotting. The authors found that she had leukocytosis and intractable fever (over 38℃). They rechecked her with abdomen CT and pelvis MRI (Figures 2C, D) which showed the absence of endometrial and myometrial enhancement except for in the peripheral areas of the uterine corpus. She strongly desired to preserve her uterus, so the authors began intravenous antibiotics and vaginal dressing daily for two weeks with fever control. US and abdomen CT revealed a large uterus without identifiable endometrium and multiple air foci in the subendometrial region, suggestive of uterine necrosis. She experienced persistent spiking fever (over 38℃) and purulent vaginal discharge without vaginal bleeding. Her blood, urine, and vaginal cultures were normal. After 3 weeks of fever, she expelled a globoid, fleshy, and necrotic mass (18.0×7.0 cm, Figure 2E) through the vagina, which was infarcted uterine muscle tissue and thrombosed blood vessels. Follow-up CT (Figure 2F) showed near shedding of the necrotic endometrium and myometrium with viable outer myometrium. She became afebrile but secondarily amenorrhea afterwards. The authors summarize the US finding in Figure 3 as well as the change of uterus after UAE.
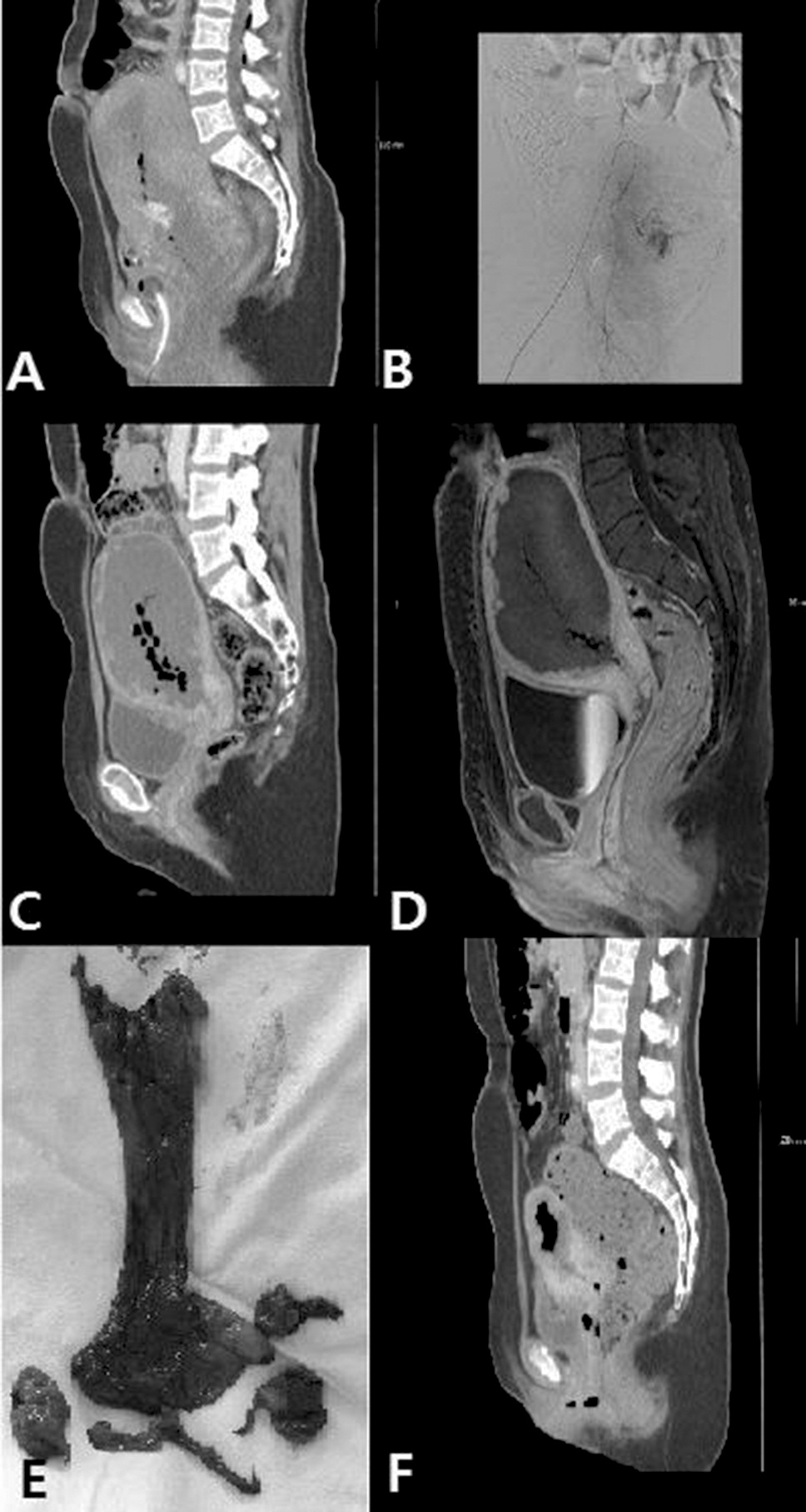 Figure 2.
Figure 2.— Pelvic imaging in 40-year-old woman (Case 2). (A) Abdomen CT scan finding before UAE: Active bleeding in the lower anterior uterine wall. (B) Selective angiography of the pelvic arteries are demonstrated. (C) Abdominal CT scans findings 21 days after UAE: enlarged uterus contain low attenuation tissue with air bubbles, consistent with uterine necrosis or uterine infarction. (D) Sagittal dynamic T1-weighted images after intravenous injection of gadolinium (day 21): only peripheral enhancement of the myometrium reflecting devascularization of the endometrium and the interior third of the myometrium, air bubbles within the uterus, and signs of necrosis. (E) Expelled necrotic mass (day 55). (F) Abdominal CT scan finding (day 56) shows near shedding of the necrotic endometrium and myometrium with visible outer myometrium (about 1 cm in thickness).
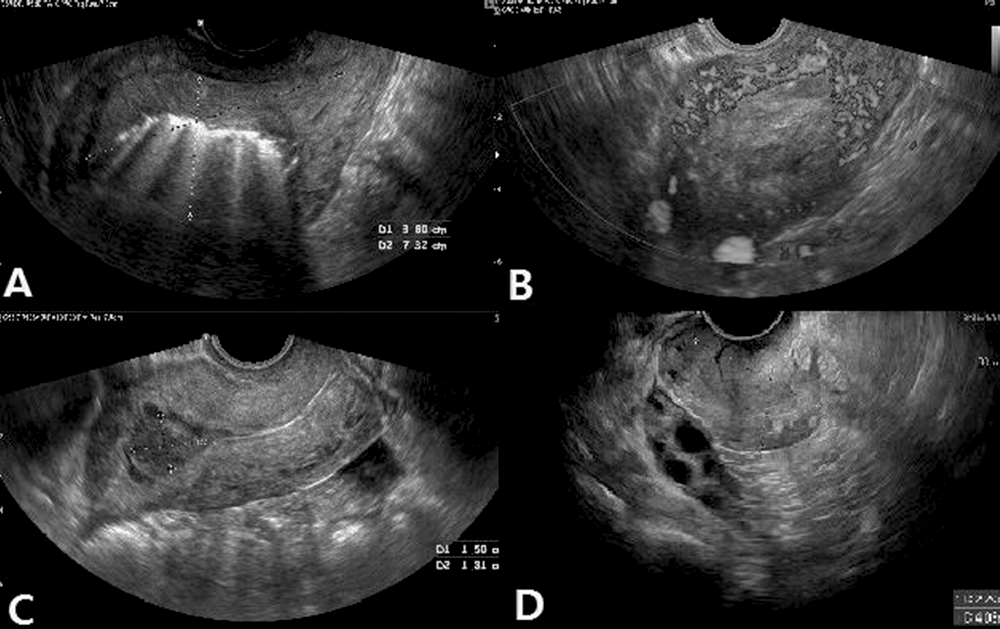 Figure 3.
Figure 3.— The ultrasonographic change of uterine necrosis after UAE in case 2. (A) Two-dimensional sonography reveals a hyperechoic heterogeneous myometrium without endometrium identification. The size of the suspected necrotic mass is 3.8×7.32 cm (day 21). (B) Power Doppler sonogram. Some irregular vessels are visible in the peripheral area, suggesting a vascularized myometrium (day 21). (C) The uterus size decreased with heterogeneous endometrial debris (1.5×1.8 cm) (day 76). (D) The uterus size is 2.2×4.05 cm with invisible endometrium (day 300).
A 29-year-old woman (parity 2-0-0-2) was transferred to the hospital due to postpartum bleeding. Repeat cesarean section was performed at 39 weeks and uterine atony was found, and despite transfusion of PRC, vaginal bleeding continued and dyspnea developed. Her past history showed laparoscopic myomectomy due to leiomyoma and previous postpartum bleeding due to placenta accreta. Her vital signs showed a BP of 80/50 mmHg. In abdomen CT (enhanced), large amounts of hematoma were found in the endometrial cavity and uterine atony was suspected and the authors performed UAE with gelfoam. On day 57, the patient was reexamined for pelvic pain and abnormal vaginal spotting with nausea. The uterus was bulky and showed a homogenous pattern in US. Small amounts of air bubbles were observed in the endometrial cavity and except for in the peripheral areas of the uterine corpus, contrast enhancement was not found in the endometrium or the myometrium of the uterus on abdomen CT. The authors suspected uterine necrosis, and broad-spectrum antibiotics were supplied, leading to the improvement of her symptoms ten days later. She was discharged without clinically significant events and followed up with for ten months. In the last US, the size of the uterus showed dimensions of 11.7×9.12 cm with anterior wall thickness of 2.06 cm and posterior wall thickness of 5.89 cm. Bulky uterus was seen with hypoechoic endometrial line without vascularity in myometrium.
A 36-year-old woman with parity 2-0-0-2 was transferred to the hospital due to postpartum bleeding after normal vaginal delivery at 38 gestational weeks. Her vital signs were: BP of 110/70 mmHg and pulse rate of 110/minute. Her vaginal bleeding continued, and so the authors performed abdomen CT (enhanced) and found active bleeding in the lower uterine segment. UAE was then performed with gelfoam. She was discharged from the hospital three days later without complication. After 46 days, she was readmitted to the hospital due to acute abdominal pain and vaginal bleeding with expulsion of necrotic tissue (10.0×4.5×4.2 cm, 72 grams) through the vagina. Her uterine size was 8.2×5.1 cm in transvaginal US. The pathologic finding was degenerative and necrotic chorionic villi and decidua, with neutrophils and focal calcification. After 14 months, she complained of amenorrhea without breastfeeding. The uterine size was 6.69×3.16 cm in US without visible endometrial line. In hormone assays, FSH of 0.43 mIu/ml and estradiol-E2 of 87.33 pg/ml were found. The authors diagnosed her with secondary amenorrhea. The authors summarize all four of the above cases in Table 1. Case 1 was diagnosed as total uterine necrosis and subsequent total laparoscopic hysterectomy was performed. Cases 2 and 4 were considered as partial uterine necrosis with expelled necrotic mass within two months after uterine artery embolization (Table 1). The authors analyzed all four cases for changes in uterine size (sagittal view in abdomen CT) after UAE. The maximum longitudinal length was measured in the sagittal view imaged by abdominal CT. Length reductions were 46.1% in case 1 (19.3 cm to 10.4 cm after 163 days), 51.1% in case 2 (18.8 cm to 9.2 cm after 55 days), 29.7% in case 3 (22.2 cm to 15.6 cm after 57 days), and 51.2% in case 4 (16.8 cm to 8.2 cm after 46 days).
| case 1 | case 2 | case3 | case 4 | |
|---|---|---|---|---|
| Age(years) | 37 | 41 | 29 | 36 |
| Gestational weeks | 38 | 40 | 39 | 40 |
| Parity | 2-0-1-2 | 1-0-0-1 | 2-0-0-2 | 2-0-0-2 |
| Mode of delivery | C/Sec | NSD | C/Sec | NSD |
| Cause of C/Sec | Placenta abruptio | repeat c/sec | ||
| Cause of PPH | Pseudoaneurysm | Active bleeding in the lower anterior uterine wall | Uterine atony | Active bleeding in the anterior myometrium of the lower uterine segment with hematocolpometra |
| Initial Hgb/Hct(g/dL) | 7.3/21.1 | 8.5/25.5 | 12.0/36.6 | 9.3/27.9 |
| Initial platelet(/ul) | 112,000 | 151,000 | 165,000 | 254,000 |
| Blood transfusion (pint) | PRC 26 + FFP 14 + PC 20 | PRC 5 | PRC 5 + FFP1 | PRC 3 |
| Embolic material | Gelatin sponge particles, microcoil, NBCA (1st) | Gelatin sponge particles plus PVA | Gelatin sponge particles | Gelatin sponge particles |
| gelatin sponge particles (2nd) | ||||
| Size of the embolic material | 350~560 um | 560~710um | 350~560um, 560~710um, 710~1000um, 1000~1400um | 560~710um, 710~1000um |
| Target artery of embolization | Rt. superficial circumflex iliac artery, Rt. internal iliac artery, Lt. uterine a | Both uterine artery | Both uterine artery | Both uterine artery |
| Both inf. epigastric a, Rt. internal iliac a | ||||
| Time of interval (days) | 163 | 23 | 57 | 46 |
| Major clinical symptoms | General weakness, profuse vaginal discharge | High fever, Lower Abdominal pain | Lower Abdominal pain, nausea | Vaginal bleeding |
| Time of procedure (days) | 163 | 55 | 57 | 46 |
| Type of surgery | TLH | Necrotic tissue expulsion | Conservative treatment | Necrotic tissue expulsion |
| Pathology | Acute and chronic inflammation with abscess, necrosis, and hemorrhage, endometrium and myometrium | Infarcted smooth muscle tissue and thrombosed blood vessels | No | Degenerative and necrotic chorionic villi and decidua, with neutrophils and focal calcification |
C/SEC: cesarean section, NSD: normal spontaneous delivery, TLH: total laparoscopic hysterectomy, PRC: pack red cell, FFP: fresh frozen plasma, PC: platelet concentrates.
This study was approved by the Ethics Committee of the Catholic University of Korea (approval number: HC18ZEI0028).
UAE is currently the most effective treatment for the management of PPH that fails to respond to conservative treatment, but the long-term outcomes of UAE have been unknown. The minor complication of UAE is postembolization syndrome (low grade fever, pelvic pain, nausea, and general malaise), and the major complications are technical or traumatic complications (vessel injury, dissection puncture site aneurysm, or thrombosis), ischemic complications (uterine or vaginal necrosis related to injection of embolization particles in the systemic circulation), and infectious complications (endometritis, puncture site infection, or sepsis) [5, 7].
In order to prevent ischemic complications, embolization should be aimed with great care at the bleeding vessel. Gelatin particles are preferred, as they are temporary occlusive embolizing agents that can achieve blood flow reduction for sufficient time to stop bleeding while also lowering the risk of long-term ischemia. These particles are non-harmful, economical, easily available, and can be expected to dissolve in almost three to six weeks of time, leading to recanalization of the embolized artery.
Uterine necrosis has been reported in the literature with a mean time interval of 21 (range, 9-730) days between pelvic embolization and the diagnosis of uterine necrosis [8]. The main symptoms of uterine necrosis are fever, abdominal pain, vaginal bleeding, and profuse vaginal discharge. Surgical management includes total hysterectomy or subtotal hysterectomy [9].
US is the first-line diagnostic tool, and a CT scan or MRI is needed to confirm uterine necrosis by checking for air bubbles in the uterus. The best predictive finding for necrosis is localized hyperechogenicity in the uterine myometrium without vascularization, whereas the peripheral uterine area is normally vascularized [10]. Findings based on imaging of uterine necrosis include a bulky uterus, necrosis of the central uterine myometrium with a strong peripheral enhancement, and an air bubble in the necrotic area [11, 12].
In the present cases, patients complained of fever and lower abdominal pain following UAE. In US, bulky uterus, overall myometrial avascularity, and cervical or peripheral vascularity were found. It is difficult to make a definite diagnosis in uterine necrosis using only US.
The definition of partial and total uterine necrosis has not been clearly classified yet. The destruction of the margin of the inner half of the myometrial wall is a key to distinguishing partial or total uterine necrosis. Partial uterine necrosis is a potential mechanism supporting amenorrhea due to the loss of endometrium.
A complete embolization may occur after the use of gelatin particles that are too small or too emulsified, thus traveling too far distally and reaching collateral vessels [6, 11]. The risk of using combined particles smaller than 300- 500 um to occlude microcatheters used for vascular embolization has been proven previously [6, 13]. Current guidelines recommend the use of relatively large absorbable particles, such as gelfoam particles, in order to prevent prolonged obliteration of the distal vascular bed downstream from the uterine arteries [11].
Older age, radiation therapy history, absence of antibiotic prophylaxis, sepsis, massive bleeding, and hypovolemic shock are the risk factors of uterine necrosis after UAE [6, 14, 15]. In the present cases, all patients were over 35-years-old and received blood transfusion with the use of gelatin sponge particles. In case 1, UAE was performed twice due to rebleeding.
Necrotic tissue expulsion was found 40 days after UAE. Change of myometrium after UAE was found serially in case 2. After UAE, the patient experienced fever and lower abdominal pain. The authors could not confirm the diagnosis of uterine necrosis at an early stage by US.
To date, there is no definitive treatment guideline for uterine necrosis. Total hysterectomy is recommended in total uterine necrosis. When the uterus size was reduced by more than 50% within 60 days of embolization, uterine conservation was possible in the present cases. In contrast, when the uterus size was reduced by less than 50% after UAE, a total hysterectomy was required. With partial uterine necrosis, the size of the uterus was decreased after expulsion of necrotic tissue (< 5 cm), they experienced amenorrhea after UAE.
It is very difficult to choose between surgery and conservative treatment options when deciding how to manage uterine necrosis. It is important to fully explain the possibility of amenorrhea and other complications to patients when conservative treatment is being considered in the management of uterine necrosis after UAE. There is no evidence supporting uterine shrinkage prognosis, therefore continuous follow-up is warranted if a conservative course of therapy is used.
PPH-associated delivery is a very serious and emergency problem. Following UAE in PPH, the medical team should aggressively evaluate and manage symptoms of fever, lower abdominal pain, profuse vaginal discharge, and bulky uterus without visible endometrium in US. Moreover, the embolizing materials and procedure used during UAE should be carefully selected.
