Bilateral pulmonary agenesis (BPA) is a rare congenital lung malformation incompatible with extra-uterine life, diagnosed by various prenatal imaging modalities in the second and third trimesters. Essentials for diagnosis are complete absence of lungs and bronchi and no pulmonary vascular supply. In the present case, both convex and vaginal ultrasound scan performed at 13th GW revealed an anechoic content in the entire fetal thorax with no evidence of pericardial effusion, fetal heart diseases, or any other accompanying congenital malformations. On three-vessel view (3VV), bifurcation of pulmonary artery could not be identified, which confirmed the diagnosis of BPA. In conclusion, clinical importance of combining the up-to-date technological tools and clinical expertise in establishing the diagnosis as early as in the first trimester allows the clinicians to terminate the pregnancy with the lowest incidence rate of eventual complications related to the procedure. According to the literature data, this is the first and therefore unique case of BPA diagnosed in the 13th week of gestation.
Congenital lung malformations can be identified in the immediate birth period or in early childhood; however, they can be diagnosed incidentally on routine imaging or autopsy [1]. Bilateral pulmonary agenesis (BPA) is a very rare congenital lung malformation incompatible with extra-uterine life [2]. BPA is usually suspected during a prenatal US scan and diagnosed by fetal MRI, in the second and third trimesters [3-5].
The aim of this report is to present the case of BPA diagnosed as early as in the 13th week of gestation, thus representing the unique case report diagnosed at this gestational age.
A 32-year-old patient, G 0, P 0, spontaneously conceived and checked in another tertiary institution at 11th GW, two weeks prior to referral to the present authors. Although Double test was normal, anechoic content was present in the entire thorax (Figure 1a). This finding was interpreted as pericardial effusion and accompanied by a precisely undefined - suspected congenital heart disease. Due to this sonographic finding, the patient was referred to this Clinic for an expert exam.
On ultrasound scan, performed by two expert Consultants, using convex probe C 9-2 MHz and endovaginal probe C 10-3v Mhz, with CD and CPA, CRL had a diameter of 6 cm, which corresponded to the pregnancy of 12 W 4 days, and exactly matched a period of amenorrhea.
Sonomorphologic and Doppler markers for chromosomal abnormalities were found to be negative (NT 1.4 mm, both nasal bones, Vomer present, pallatum without interruption, and PI of ductus venosus adequate). “Soft markers” for chromosomal anomalies were also negative: both plexus chorioideus, intestinal convolutes with normal echogenicity, excluding estimation of pulmonary echogenicity (as a part of soft markers) which was not possible due to bilateral hydrothorax (Figure 1b).
Negative ultrasound marker for neural tube diseases (the relationship between pons and posterior cerebral fossa diameter adequate) was also clearly visualized. Lateral brain ventricles were with adequate diameter, relating to the plexus chorioideus, diverticulation of CNS was adequate. In this gestational age, ossifications of vertebral nuclei could be visualized.
Scan of abdominal structures detected an anechoic ventricular/gastric content, and situs solitus of visceral organs, with both kidneys without pielon dilatation. There was an anechoic content in the bladder, along with intestinal convolutes with normal echogenicity and position, and intact anterior abdominal wall. There were no signs for diaphragmatic hernia. Bone structures of extremities were clearly differentiated, both hands with five fingers, and feet without abnormalities.
Visualization of heart confirmed adequate cardial axis, including ventricle axis, and atrio-ventricle unisonity/harmony (Figure 1c). Positions of both atrio-ventricle valves and ventricle-arterial connection were adequate, i.e. aorta in normal site, and normal relationship between aorta and pulmonary artery (sign “X” and “B”). Retrograde flow on AV valves was not present, while IVS was intact, with clearly identified ostium primum.
 Figure 1.
Figure 1.— a) CRL. First examination. Fetal hydrops is absent. b) Bilateral hydrothorax and/or BPA. c) M-MOD excludes the presence of pericardial effusion (exudate) (endovaginal probe view). Parenchimatous abdominal organs in normal position, diaphragmatic hernia is absent. There are no elements of thorax hypoplasia.
Particular attention was devoted to the vascular pattern evaluation (Figure 2). There was adequate relationship between pulmonary artery, aorta, and vena cava superior on 3VV. Bifurcation of pulmonary artery could not be visualized, which confirmed BPA. CPA could identify both left and right ventricle outflow (Ao arch having laminary flow, with three branches, ductal arch without interruption). Venous drainage of the right atrium below diaphragm was adequate; flow through ductus venosus was found to be normal too. Vena cava superior was visualized on 3VV. M mode excluded pericardial effusion; heart frequency was 167/minute.
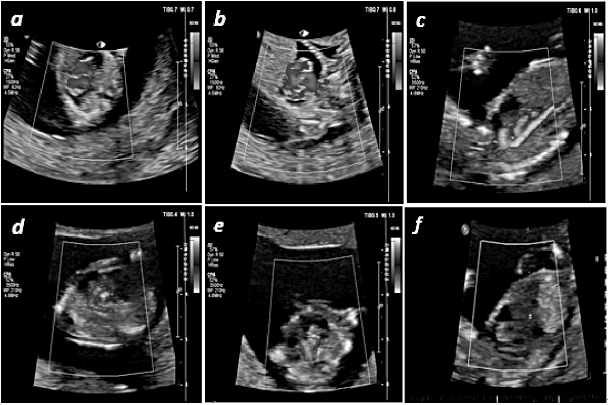 Figure 2.
Figure 2.— a) 4CV ColorPower angio mode (CPA) of fetal heart. Intraventricular septum (IVS) intact, ventricle situs adequate, without retrograde flow at the level of AV valves (endovaginal probe examination). b) ColorPower angio mode (CPA) of the left and the right ventricle outflow tract (LVOT and RVOT). Obtained sign “B”, “X”. Venous drainage of the left atrium cannot be seen. c) Aortic arch with three branches. Transabdominal view with CPA mode. d) Transabdominal view with CPA mode - Ductal arch without interruption. e) Branching of pulmonary artery is not present on the vascular pattern view. Pulmonary artery without its branches on 3VV. f) Transabdominal CPA mod of ductus venosus. Flow velocity PSV = 47 cm/second, and pulsatility index PI = 1.04. The obtained values indicate normal venous drainage and filling of the left ventricle. Normal echogenity of intestinal convolutes. Anterior abdominal wall intact.
There were no sonomorphologic markers for congenital heart diseases (major and minor). Due to detected BPA, pregnancy termination was done by D/C procedure, a few days after ultrasound scan and expert opinion’s advice.
Lung agenesis is a rare congenital anomaly, that can be unilateral and bilateral [6]. Unilateral lung agenesis has an incidence of 1.22 to 9.66 per 100,000 live births [6-8]. BPA is a very rare anomaly with the complete absence of lung tissue/parenchyma, bronchi, and pulmonary vasculature [9] In the complete absence of lung tissue, the diaphragm and abdominal organs are usually displaced cephalad, mimicking congenital diaphragmatic hernia (CDH) on prenatal ultrasound scan [10]. Suspicion on BPA is usually done by ultrasound examination and diagnosis made by MRI in the second and the third trimester of pregnancy [11]. Unfortunately, it is not rare that BPA can be missed on routine prenatal screening [2]. As BPA is incompatible with extrauterine life, there is a need to make a reliable diagnosis in pregnancy at the earliest. In the present case, bilateral hydrothorax identified on transabdominal and endovaginal ultrasound scan in the 13th week of gestation was considered as a pathologic finding. This finding could be either interpreted as the first sign of fetal hydrops, or be a consequence of the complete congenital pulmonary agenesia. Throughout detailed ultrasound scan, after exclusion of CDH, fetal hydrops and congenital fetal heart diseases, pulmonary artery was found to be without its branches on 3VV. This sonographic finding, along with the systematic ultrasonographic evaluation of all other organs and systems, enabled the authors to establish the diagnosis of BPA in the 13th week of gestation. Sonographic absence of pulmonary artery branches was confirmed to be a sign of BPA [5, 12, 13]. This case confirms that a systematic and meticulous approach is critical to achieve a prenatal diagnosis of BPA.
In conclusion, this report of BPA detected by US scan in 13th GW, the first one in the literature at this gestational age, supports the need to combine expertise and up-to-date diagnostic tools. Early detection of this extra-uterine life incompatible anomaly enables the physician to terminate pregnancy with the lowest incidence rate of eventual complications related to the procedure.
