Introduction The increase in the placental thickness (mm) in the second trimester of pregnancy is associated with placenta-mediated pregnancy complications. The authors aimed to evaluate the effect of maternal BMI (kg/cm ²) on the placental thickness in the second trimester. Materials and Methods The second trimester is a prospective study in which 832 pregnant women (gestational age between weeks 18 and 24) were examined. The measurements of BMI and placental thickness of the participants were statistically evaluated by simple linear regression analysis. Results The average chronological age of the 832 participating pregnant women was 28.64. The average placental thickness was 25.30 mm and the average BMI value was 26.46 kg/cm ². Simple linear regression analysis was performed for evaluating the effect of BMI (independent variable) on the placental thickness (dependent variable). A regression equation in the form of [F (1.830) = 5.833; p= 0.016 < 0.05] and R ² = 0.007 was obtained in the analysis. The placental thickness was 23.063 + 0.085 mm for the second trimester.Conclusion A positively significant linear relationship was observed between the placental thickness and BMI. According to this, a one-unit increase in the BMI affects the placental thickness by 0.085 mm. The importance of these findings will be understood in a future result-oriented study. Using modifications according to the maternal BMI in calculating the placental thickness will contribute to the sensitive medicine approach as well.
The placenta is an organ that is affected by and affects both the fetus and the mother. The placental thickness in the second trimester of pregnancy is associated with placenta-mediated pregnancy complications [1]. Placentomegaly or placental insufficiency was associated with some maternal metabolic conditions and feto-maternal pathologies in many studies. However, not all interactions of the placenta are known yet. For example, why does the placental thickness vary from one pregnant woman to another? The placenta is evaluated by US imaging, and the thickness measurement and maturation grading are used; however, conditions of change in the thickness that cannot be explained or early maturation are observed as well. As the present authors reviewed the literature, they observed that the fetal effects of placental thickness were more commonly discussed and some studies that affected the placental thickness were mentioned. However, they did not see any studies with large samples related to association with the maternal BMI. In this era in which personalised and sensitive medical approaches are discussed, the authors considered whether the thickness and development of placenta should be evaluated specific to the pregnant woman. In this study in which 832 pregnant women were examined, they demonstrated that there was a positive significant linear relationship between the maternal BMI and placental thickness and that a one-unit increase in the BMI affected the placental thickness by 0.085 mm. Another multi-scale study of the authors in which some maternal biomarkers and fetal sonographic-Doppler data are added as well is ongoing. They aim to also share the results of this large-scale study, which will be completed and in which the participants are followed up until the date of delivery for the pregnancy results.
The approval was obtained from the local Ethics Committee of Recep Tayyip Erdoğan University Faculty of Medicine and study permission was obtained from the management of the affiliated training and research hospital. One thousand pregnant women, who were followed up in the gynaecology polyclinic of this hospital and who presented for US imaging for the second trimester screening, were prospectively evaluated in 2017. Of these, 832 healthy pregnant women were included in the study, and 178 pregnant women who had unknown last menstrual period (LMP), wrong registration information, high-risk or multiple pregnancies, and diseases that would affect the placental thickness such as Rh incompatibility, diabetes mellitus, thyroid function disorder, and cardiovascular disease, were excluded from the study. The gestational age was determined according to the last menstrual period. The measurements of age (years), weight (kg), and height (cm) registered in the cards of the pregnant women were confirmed by the patients, and the BMI (kg/cm²) was calculated. All US imaging examinations were performed in a transabdominal manner with an ultrasound device and a 3.5-MHz convex probe. Each evaluation was performed by an experienced radiology specialist. The placental thickness was measured in the central or paracentral section of the placenta by vertically implanting the ultrasound probe on the placenta plane (Figure 1). In the measurements, special attention was paid to the fact that an average value was calculated by repeating 2-3 times in a manner such that the uterine in the central section of the placenta did not include contraction or large lakes. SPSS 22.0 was used to calculate the data. P values under 0.05 were considered to be significant. Simple linear regression analysis was used to statistically determine the effect of maternal BMI on the placental thickness.
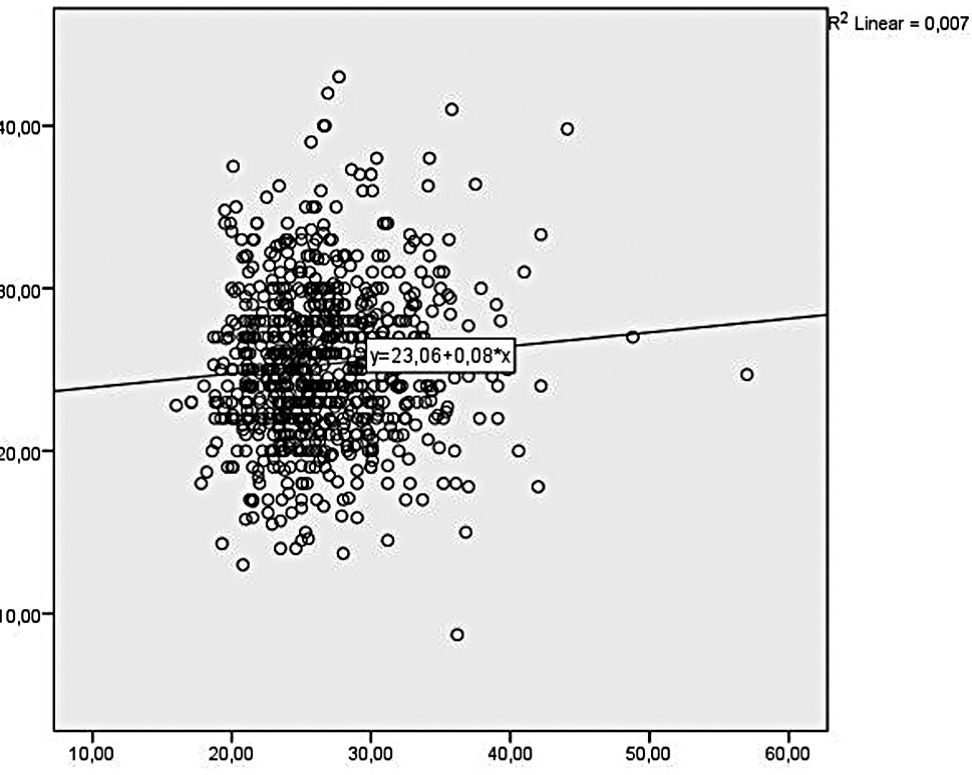 Figure 1.
Figure 1.— Relationship between maternal BMI and placental thickness.
The chronological age of 832 pregnant women included in the study was between 16 and 49 years, and the average age of the pregnant women was 28.64 years (Table 1). The standard deviation of the ages was 5.502 years. The gestational age of the pregnant women, who presented for the US imaging in the second trimester, was between 18 weeks and 2 days and 23 weeks and 6 days. The average placental thickness was 25.30 mm and the average BMI value was 26.46 kg/cm² (Table 2). Simple linear regression analysis was performed to be able to determine the effect of BMI (independent variable) on the placental thickness (dependent variable). A regression equation in the form of [F (1.830) = 5.833; p = 0.016 < 0.05] and R² = 0.007 was obtained. Maternal BMI was evaluated in kg/m² and placental thickness was in mm; a statistical model in the form of placental thickness = 23.063 + 0.085 mm was obtained for the second trimester. According to the statistical model obtained, there is a positively significant linear relationship between the placental thickness and maternal BMI, and a one-unit increase in the BMI affecting the placental thickness by 0.085 mm. (Table 3) (Figure 1). Some biochemical maternal and ultrasonographic fetal markers and Doppler data of the pregnant women included in the study were collected and the deliveries are awaited. After formation of all data, a multiple regression analysis will be performed to evaluate the maternal and fetal effects on the placental thickness.
| N | Valid | 832 |
|---|---|---|
| Missing | 0 | |
| Mean | 28, 64 | |
| Median | 28, 00 | |
| Std. Deviation | 5, 502 | |
| Range | 33 | |
| Minimum | 16 | |
| Maximum | 49 | |
| Percentiles | 25 | 25, 00 |
| 50 | 28, 00 | |
| 75 | 32, 00 | |
| Mean | Std. Deviation | N | |
|---|---|---|---|
| Plasental thickness(mm) | 25, 3018 | 4, 69255 | 832 |
| BMI | 26, 4679 | 4, 63350 | 832 |
| N | R | R Square | F | Sig. | t | β0 | β1 | ε |
|---|---|---|---|---|---|---|---|---|
| 832 | 0.084 | 0.007 | 5.833 | 0.016 | 2.415 | 23.063 | 0.085 | 1.805 |
The placenta provides the interaction between maternal and fetal circulation and it plays an important role in the development of the fetus [2]. The placenta is a complex organ that provides numerous hormones and enzymes for maternal circulation. Sufficient growth of the fetus and following this, the normal birth weight, depend on the transmission of effective nutritional substances from the mother to the fetus through the uteroplacental route [3]. By placental examination, an idea can be obtained regarding the specific diagnosis, risk of recurrence and chronic statuses of the complications such as preeclampsia or IUGR [4]. IUGR is highly associated with perinatal morbidity and mortality [5, 6]. Maternal obesity affects the hormonal and lipid profile of the new-born. This leads to consideration that it is fetal metabolism that affects the placental lipoidosis. Therefore, an affected fetal metabolism can increase the predisposition of the fetus for IUGR by causing a high predisposition for lipoidosis [7]. As a matter of fact, asymmetrical late-onset IUGR is due to uteroplacental insufficiency and it is commonly observed after the mid-trimester [8]. It is well known that fetuses with asymmetrical IUGR have higher rates of morbidity and mortality than fetuses with symmetrical IUGR. It can be difficult to determine these pregnant women who are at risk [1]. The investigators, until now, evaluated the placental size, volume, and blood flow in order to be able to better estimate the abnormal fetal growth [9]. On the other hand, the correlation between the placental thickness and SGA is still controversial. However, two new studies mentioned that the placental thickness decreased in the fetuses with SGA [10]. A correlation between the placental weight and fetal birth weight during the delivery has been known for a long time. The placental thickness is usually below the 10th percentile according to the gestational week in the pregnant women affected by IUGR and preeclampsia [4]. Another study demonstrated that there was no difference between the placental thickness and the groups with SGA and without SGA. This study mentioned that since the ratio of placental thickness and estimated fetal weight is a useful ultrasonographic marker that can be easily obtained for foreseeing the labour with SGA; it can be helpful for pregnant women in obstetrics consultancy for the pregnancies with a high risk associated with SGA [8]. As a result, the placental thickness is closely related to fetal development and not all factors that affect the placental thickness are known yet. Early determination of any pathology in the placental bed and villi helps provide careful antenatal care [3]. In order to evaluate the increase in the placental thickness in pregnancy, different descriptions were made due to the effects of gestational age, measurement technique, and maternal status of the fetus [3]. Some studies accepted that an increase greater than 35 mm was an increase in the placental thickness in the second trimester [11] and some other studies considered a measurement of 40 mm as a cut-off value [6]. In this study, the authors evaluated the effect of maternal BMI on the placental thickness during the US screening period in the second trimester. As a result of analysis of the data, a statistical model in the form of “placental thickness = 23.063+ 0.085×(BMI)” was obtained for the second trimester. It was demonstrated that there was a positive correlation at the 0.84 level between the maternal BMI and placental thickness; in other words, a one-unit increase of BMI affected the placental thickness by 0.085 units (mm).
As a result, it was demonstrated in this study that the maternal BMI had an effect on the placental thickness. Modification of the placental thickness, which is followed up with percentile according to the maternal BMI, can be used in following up the pregnancy. Additionally, the doctor’s use of modification according to maternal BMI in calculating the placental thickness will contribute to the sensitive medicine approach as well.
The authors thank their family and Meryem Demirtaş.
