1 Food Science Department, Agriculture College, Basrah University, 61001 Basrah, Iraq
Abstract
Flavonoids are among the most important compounds found in plants, since laboratory studies have shown them to be a daily requirement in human diets due to their various health benefits. Therefore, this study focused on extracting, purifying, and measuring the antioxidant activity of the flavonoid quercetin, which is widely found in plants and possesses a variety of biological activities, from different plant sources.
The extraction of quercetin was performed using several methods (chemical, physical, and enzymatic) and several extraction solutions (water, ethanol, and chloroform) from several plants (spinach, dill, Onion Skin, Pistacia eurycarpa, sumac, digalkhasab chemri, and leelwi chemri). The alcoholic extract extracted by chemical method was purified and the content of total flavonoids based on quercetin in all plant extracts was determined using adsorption chromatography on a silica gel column (100–200 mesh), followed by thin layer chromatography (TLC). TLC and high performance liquid chromatography (HPLC) were used to assess the purity of quercetin. The ability of quercetin to capture free radicals using 2,2-diphenyl-1-picrylhydrazyl (DPPH) was compared to that of butylated hydroxytoluene (BHT). Statistical analyses were performed using completely randomized designs (CRD) for factorial experiments, and the least significant difference (LSD) test was used to calculate the significant differences between the averages of the coefficients at the 0.05 probability level.
The alcoholic Pistacia extract extracted by chemical method yielded the highest concentration of quercetin (84.037 mg/g). Furthermore, it was found that quercetin purified from Pistacia possessed strong antioxidant activity, and its antioxidant activity increased with increased concentration.
Pistacia eurycarpa showed the highest quercetin content among the assessed plants. Moreover, solvents played a major role in extracting plant components due to the high polarity of flavonoids. Quercetin purified using a silica gel column demonstrated antioxidant activity.
Keywords
- quercetin
- extraction
- purification
- antioxidant activity
- Pistacia eurycarpa
Flavonoids are among the most important compounds found in plants due to their various health benefits. Indeed, laboratory studies have shown them to be a daily requirement in human diets due to their antioxidant, anticancer, and cardiovascular-protective properties, as well as antibacterial, antifungal, and antiviral activity; this has, in turn, highlighted the importance of using flavonoids in nutritional processes as well as therapeutically [1]. Flavonoids are compounds that result from the secondary metabolism of plants, and cannot be produced by animals. They are responsible for the color and flavor characteristics of food plants and have many functions related to growth physiology. Based on the composition of these compounds, they can be classified into six main categories: flavanols, flavones, flavanones, flavanols, isoflavones, and anthocyanidins [2].
The flavanols comprise one of the most prominent classes of flavonoids and are found in fruits and vegetables. Among the flavanols is quercetin (3, 5, 7, 3′, 4′-pentahydroxyflavone). The name “quercetin” is derived from the Latin word “Quercetum”, which means “oak forest”. Chemically, quercetin is composed of three benzene rings and five hydroxyl groups. It is a yellow powder that is insoluble in cold water but soluble in hot water and alcohol. Quercetin is widely found in plants, including in foods such as apples, berries, grapes, onions, green tea, and tomatoes, as well as in many nuts, flowers, seeds, bark, and leaves. It is also present in some medicinal plants, such as Ginkgo biloba [3, 4]. As a biologically active substance, quercetin is used as a dietary supplement that may be beneficial against a variety of ailments due to its cardiovascular-protective, anticancer, antineoplastic, antiulcer, neurological, antiallergic, antiviral, antiinflammatory, antidiabetic, and hypotensive properties [5, 6]. The compound has received much attention recently due to its natural antioxidant activity and the high availability of the raw material from which it is extracted [7]. Several methods have been used to extract quercetin, including soaking, filtration, thermal digestion, Soxhlet, ultrasonic extraction, microwave extractions, and extraction using enzymes [8, 9].
The genus Pistacia, which belongs to the order Sapindales and the Anacardiaceae family, contains 11 species. This native tropical and subtropical Asian genus has nutritional and medicinal importance because it contains phenolic compounds, terpenoids, monoterpenes, flavonoids, alkaloids, saponins, fatty acids, and sterols, for which it has recently gained scientific attention [10]. Pistacia eurycarpa is a perennial tree with a height of 4 to 10 meters. It grows naturally and, at maturity, produces small fruits the size of a lentil or slightly larger, with a blue or pinkish-red color. The fruit is considered an edible nut and is also used in the production of oil and soap. This species is among the major tree species that grow in the mountainous regions of Iraq [11, 12].
This study aimed to extract and estimate the quantity of quercetin from a selection of plants. It also investigated more than one method of extracting quercetin and more than one extraction solution to obtain the best method and the best extraction solution in terms of extracted quercetin content. The study indicated that Pistacia eurycarpa had the highest quercetin content among the studied plants. This is the first study in the world to show the importance of this plant, which is affordable and available in abundance in northern Iraq and in other regions of the world, with regard to exploiting it to obtain quercetin. The present study presents the first part of a master’s thesis project, which involved the extraction of quercetin. The second part of the thesis, which will be published in another study, will demonstrate the importance of quercetin in the field of food science due to its antioxidant and antimicrobial properties, as it demonstrably increased the shelf-life of food.
Many devices and materials were used to complete the requirements of this study, including Power Sonic-405 (DAIHAN LABTECH(LTD), Namyangju-si, South Korea), Soxhlet apparatus (Daihan scientific, Wonju, South Korea), High performance liquid chromatography (HPLC) (Sykam, Eresing, Germany), and Spectrophotometer (Jenway, Germany). We also used many Materials including Quercetin (manufactured by the American company Microingredients), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (manufactured by Sigma-aldrich, Steinheim, Germany), Snailase enzyme (manufactured by Solarbio, Beijing, China).
Table 1 (Ref. [12, 13, 14, 15, 16, 17, 18]) shows all the plants used in the study, their cultivation areas, and harvest periods.
| Common name | Scientific name | Region | Harvest period | Reference |
|---|---|---|---|---|
| Spinach | Spinacea oleracea | It is found throughout Iraq, Iran, Kazakhstan, Turkey, Turkmenistan and Uzbekistan. | It is an annual plant that is grown from mid-August to mid-November, and the harvest is usually 8–10 weeks after planting. | [13, 14] |
| Onion Skin | Allium cepa | The United States, China, India, Egypt, Brazil, and in Iraq, the onion crop is grown in the central and northern regions of Iraq, especially in the Nineveh and Anbar governorates. | The plants mature after about 1–1.5 months and are available in the market all year round. | [15] |
| Dill | graveolens Anethum | Its original homeland is southwest Asia and Europe. | The first mowing takes place about two months after planting, and the next mowing takes place monthly, and the harvest stops until the plants begin to flower. | [16] |
| Pistacia eurycarpa | Pistacia eurycarpa | It is spread in Central Asia and is distributed across southern Europe (the Mediterranean region), western and northern Africa, Central Asia, Central America and the Middle East, as its distribution is concentrated in the Middle East, especially in the Kurdistan region of Iraq, where it is located between northeastern Iraq, southern Turkey, northeastern Syria, and western Iran. | Harvesting the fruits of Pistacia eurycarpa trees located in northern Iraq begins in the fall. | [12] |
| Sumac | Rhus coriaria | It is distributed in North America and temperate regions of Asia, the Mediterranean basin and Africa. | A perennial tree whose fruits, which are lentil-like berries, are harvested at the end of August. | [17] |
| Digalkhasab Chemri, and Leelwi Chemri | Phoenix dactylifera L | It originated in Mesopotamia (Iraq) and from there it spread widely in the Arabian Peninsula, North Africa and the Middle East. | Date palm fruits change from the basil stage to the chemri stage during the month of June. | [18] |
Fresh plants were obtained at the time of their harvest and availability in the markets, such as spinach, dill, and chemri (digalkhasab chemri, and leelwi chemri). They were then cleaned and washed, and the pits and funnels were removed from the chemri. After that, they were dried in the sun. As for the dry plant matter, such as onion peels, sumac, and Pistacia eurycarpa, which are available throughout the year in markets, these were purchased and then individually ground into a fine powder using an electric grinder. The powder was then stored in sterile glass containers with airtight seals and kept in a freezer until used in the experiments.
Quercetin was extracted from the plants under study using the Soxhlet apparatus according to the method of Sharifi et al. [9], with some modifications. Extraction was performed in a ratio of 1:75 w/mL (the individual plant matter under study:the solvent, which was ethanol, chloroform, and water separately). Two grams of plant powder was placed in the filter paper, then it was wrapped and placed in the designated place for the sample in the Soxhlet device. 150 mL of solvent was added to the beaker of the device and left for 5 hours. After that, the extracts were concentrated under low pressure using a rotary evaporator at a temperature of 40 °C and stored at 4 °C.
Quercetin was extracted from the plant samples under study according to the method of Sharifi et al. [9], with some modifications. The extraction was performed in a ratio of 1:20 w/mL (the individual plant matter under study:the solvent, which was ethanol, chloroform, and water separately). Two grams of the plant sample was weighed and added to 40 mL of solvent, then placed in an ultrasonic device at 40 KHz (Daihan Labtech - Korea) for 10 minutes at 25 °C, after which the extracts were filtered using a piece of cloth followed by filter paper. The extracts were then concentrated using an evaporator at 40 °C and stored at 4 °C.
Quercetin was extracted using the enzyme snailase according to the method of Wang et al. [8], with some modification. Five grams of the plant sample was mixed with 20 mL of water (pH 5 using 0.02 M citric acid), then 1.5 g of the enzyme was added, after which the mixture was left for 12 hours at 50 °C, then placed in a water bath at 80 °C for 20 minutes to stop the activity of the enzyme. Finally, the mixture was dried at 60 °C to remove the water.
The content of total flavonoids was estimated using the aluminum chloride method with quercetin as a standard according to the method followed by Dewanto et al. [19]. First, 0.1 mL of the extracts was mixed with 0.9 mL of distilled water in test tubes, after which 75 µL of NaNO2 (5%) was added. After 6 minutes, 150 µL of aqueous aluminum chloride (AlCl3·5H2O 10%) was added and left for 5 minutes, then 0.5 mL of NaOH (1 M) was added. Next, 2.5 mL of distilled water was added and shaken well, immediately after which the absorbance was measured at a wavelength of 510 nm using a spectrophotometer.
A standard solution of quercetin was prepared according to the content of flavonoids in plant extracts at different concentrations (20–140 mg/mL). The absorbance was measured at a wavelength of 510 nm, and the content of flavonoids was calculated based on the graphical relationship between the concentration of quercetin and the absorbance.
Quercetin was purified using adsorption chromatography. For the adsorbent column
preparation, a separation column with dimensions of 50 cm
The thin layer chromatography technique was used to identify the tubes
containing quercetin obtained from the adsorption column, according to a previous
method [20]. Ready-made silica gel aluminum sheets (20
The purity and concentration of quercetin extracted from the Pistacia eurycarpa were diagnosed and confirmed in comparison with standard quercetin using a HPLC device at the Ministry of Science and Technology - University of Baghdad/Environment and Water Department laboratories, according to a previously described method [21].
The antioxidant activity of both standard and purified quercetin was estimated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical capture method [22], with some modifications. The method included preparing several concentrations (5, 10, 15 mg/mL) using water once and ethanol again by mixing 2 mL of different concentrations with 2 mL of (DPPH) solution concentration of 0.1 mM prepared in methanol, while the control sample was prepared by mixing 2 mL of distilled water instead of the sample. After that, the industrial antioxidant butylated hydroxytoluene (BHT) was used for comparison. This was prepared at a concentration of 0.01 mg/mL in methanol and kept in the dark at a temperature of 25 °C for 30 minutes, after which the absorbance was measured at a wavelength of 517 nm according to the following equation:
where Ac is the control reaction absorbance and As is the testing specimen absorbance.
Statistical analysis of the data was conducted using completely randomized designs (CRD) for factorial experiments, and the least significant difference (LSD) test was used to calculate the significant differences between the averages of the coefficients at the 0.05 probability level using SPSS version 26 (IBM SPSS statistics, Chicago, IL, USA).
Plant extracts obtained from a wide range of plants are of great importance as they contain many biologically active compounds. These resources have received much scientific interest due to their positive effects on health, demonstrating nutritional, economic, and medicinal importance. These extracts can be sourced from fruits, vegetables, medicinal herbs, spices, and so forth, and their consumption is closely linked to improving the health of the digestive system and reducing the risk of many diseases, as there is an inverse relationship between the consumption of fruits and vegetables and many diseases. Therefore, choosing an effective extraction method and the appropriate extraction solution to ensure the optimal concentration and high performance are obtained is the most important step in the extraction of these products [23, 24]. In the present study, quercetin was extracted and quantified from the plants under study based on the standard curve shown in Fig. 1.
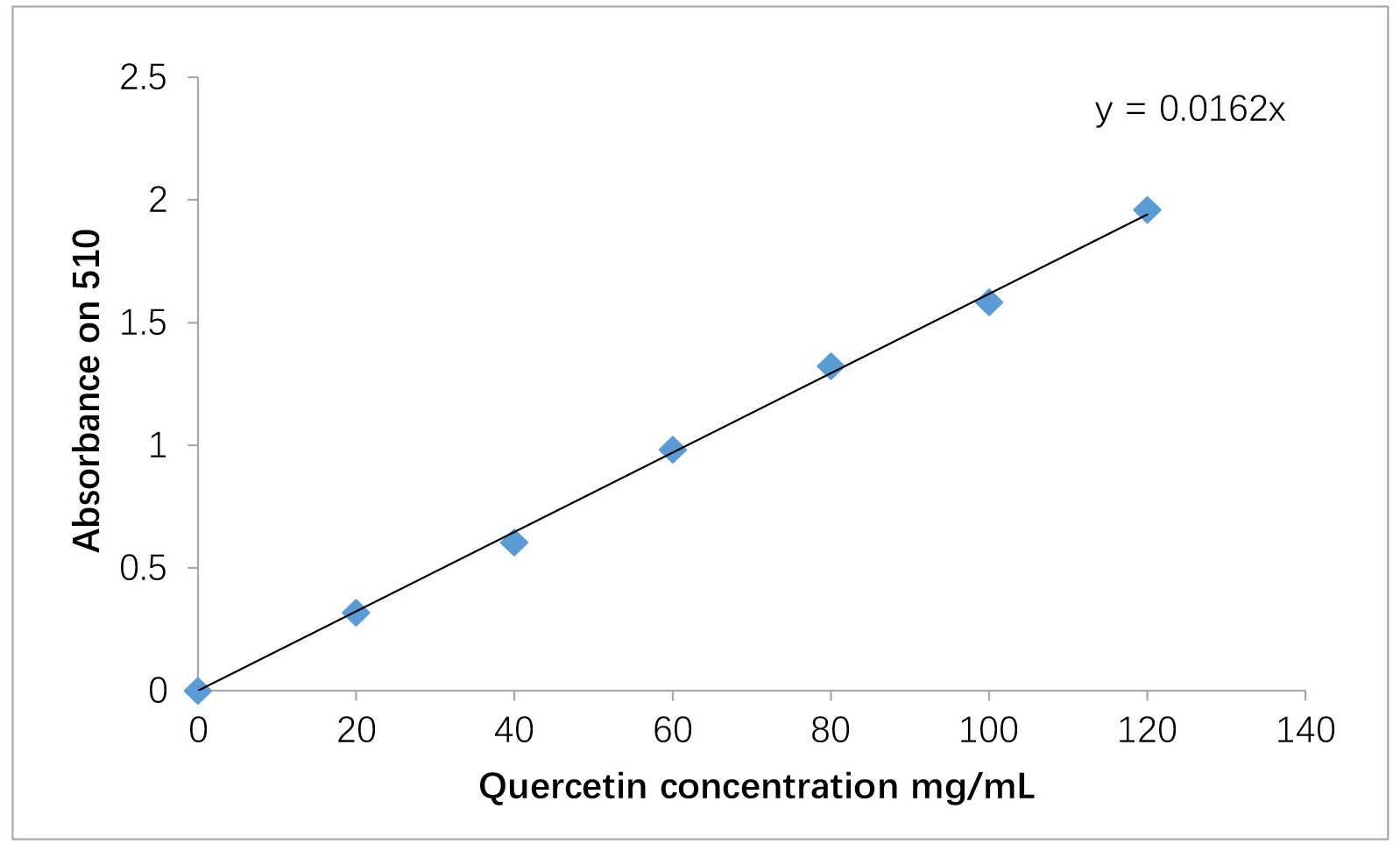 Fig. 1.
Fig. 1.
Standard curve for determination of flavonoids.
Figs. 2,3 show the extraction of quercetin from plants (spinach, dill, Onion Skin, sumac, Pistacia eurycarpa, digalkhasab chemri, and leelwi chemri)
by the chemical method (Soxhlet) and the physical method (ultrasound),
respectively, using three extraction solutions (water, ethanol, and chloroform).
The results of the statistical analysis (p
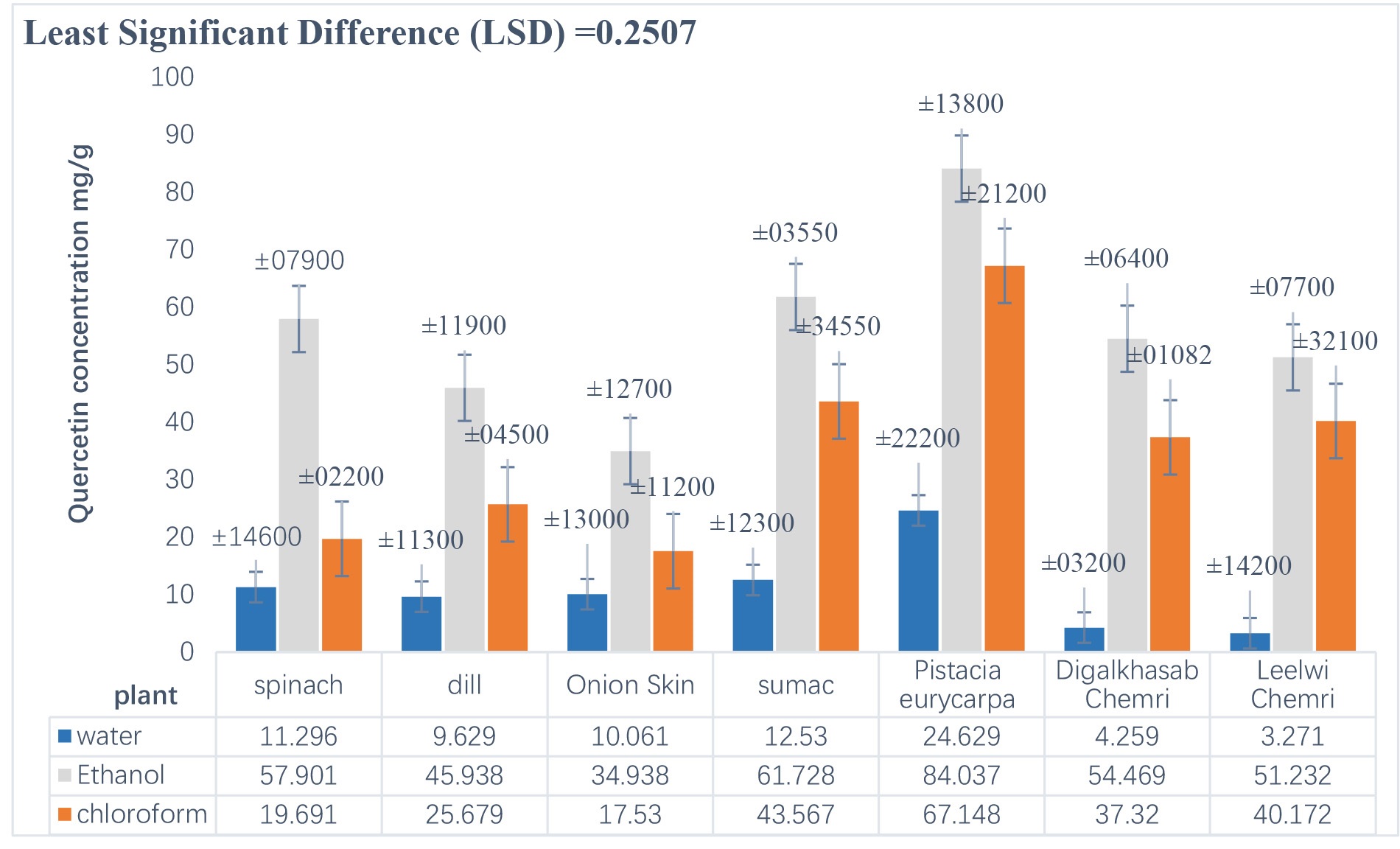 Fig. 2.
Fig. 2.
Concentration of quercetin extracted from plants using the chemical method (Soxhlet).
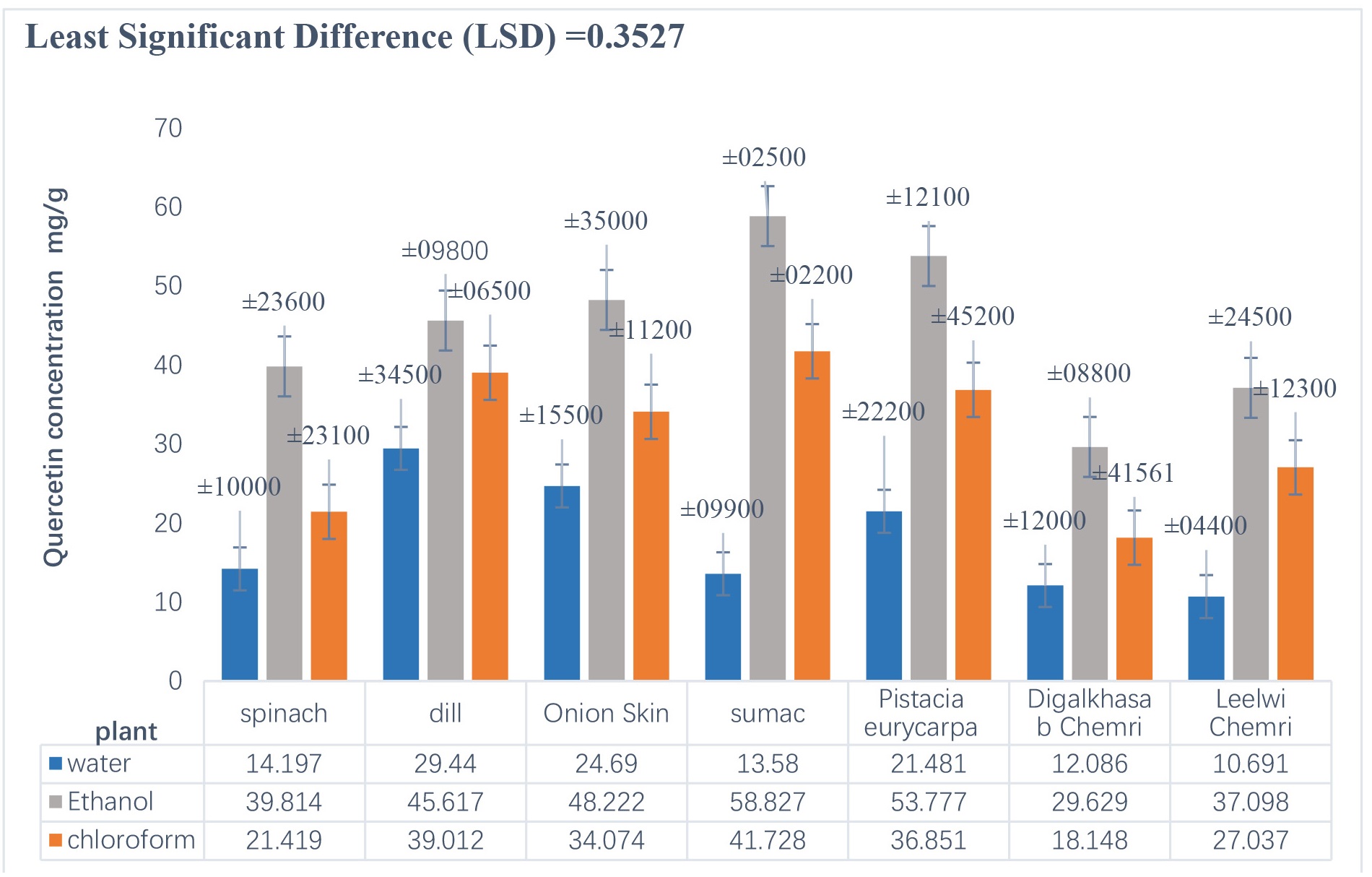 Fig. 3.
Fig. 3.
Concentration of quercetin extracted from plants using the physical method (ultrasound).
These results agree with those of Awang et al. [26], who extracted quercetin from the leaves of the Melastoma malabathricum plant with Soxhlet using three extraction solutions (ethanol, ethyl acetate, and hexane), finding that ethanol was the best extraction solution. The present study’s results also agree with the findings of Sharifi et al. [9], who extracted quercetin from the leaves of radish (Raphanus sativus) using a variety of extraction solutions and methods, reporting that ethanol was better than chloroform and than water in extracting quercetin. In addition, our results agreed with those of Sambandam et al. [20], who extracted quercetin from the leaves of the fenugreek plant (Trigonella foenum-graecum) by soaking for 48 hours using ethanol, hexane, and ethyl acetate, also finding that ethanol was the best solution for extraction.
It is clear from the results in Fig. 2 that the concentration of quercetin extracted from the plants using the chemical method ranged from 3.271–84.037 mg/g, and that Pistacia eurycarpa yielded the highest concentration of quercetin, which was 84.037 mg/g. On the other hand, Fig. 3 shows that the concentration of quercetin extracted from plants using the physical method ranged from 10.691–58.827 mg/g, and that sumac yielded the highest concentration of quercetin (58.827 mg/g), which was close to the concentration found by Khanam and Anis [27] when they extracted quercetin from the golden trumpet plant, Allamanda cathartica, using ultrasound (51.39 mg/g). The present study’s concentration was also higher than that obtained by Jin et al. [28] when they extracted quercetin from onion peels using ultrasound under different conditions of ethanol concentration, wave intensity, and time period, which ranged between 1.74–4.34 mg/g.
Also more than what Jang et al. [29] obtained when extracting onion peels using ultrasound technology using aqueous ethanol and under specific conditions such as 59% ethanol and the extraction temperature 49 °C, which led to obtaining a total quercetin content of 11.08 mg/g of dry weight of onion solid waste. And less than what Wei et al. [30] found when they carried out the extraction process of flavonoids, especially quercetin, from the leaves and bark of the Abies nephrolepis plant, with the help of ultrasound technology and by determining the optimal conditions for the extraction process in terms of temperature, frequency, and time. The total yield of quercetin reached 69.59 mg/g.
Fig. 4 shows the extraction of quercetin from plants under study using the enzymatic method. Enzymes are biological catalysts that accelerate chemical reactions in living organisms [31] and are considered one of the modern methods increasingly used to extract active ingredients from natural products such as plants. The plant cell wall is a dense structure consisting of polysaccharides and proteins that can prevent the release of active ingredients from the plant. Therefore, enzymes can be used to facilitate the release of these ingredients, as they work to break down the structure of the cell wall and thus accelerate the disintegration of the active ingredients in plant cells. This contributes to the dissolution of the active ingredients, which improves the extraction efficiency [32], as has been demonstrated for the enzyme snailase.
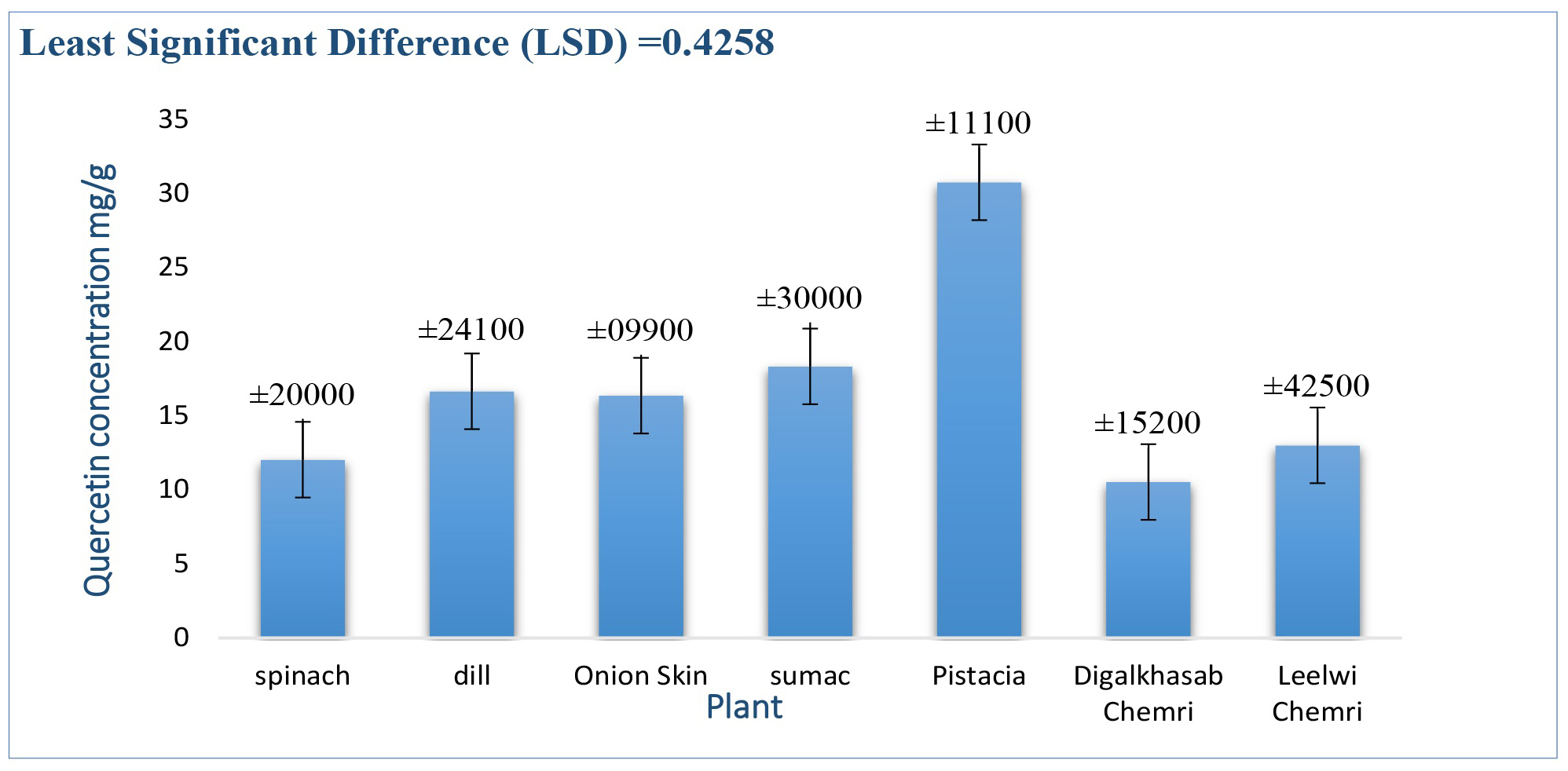 Fig. 4.
Fig. 4.
Concentration of quercetin extracted from plants using the enzymatic method.
The results of the statistical analysis (p
We conclude that the best of the three methods used in extracting quercetin is the chemical method. This method has many major advantages, including that it is possible to manipulate temperature to increase the extraction of medium to low-soluble compounds using this technology, and that it does not require filtration after completing the extraction process [33, 34].
These results agreed with Zhang et al. [35] who found that the Soxhlet method was the best method for extracting quercetin from the stems of the Euonymus alatus plant, as it produced the highest concentration of quercetin compared to ultrasound and microwave methods. The results in Table 2 also showed that the best-quercetin-yielding plant was Pistacia eurycarpa, so it was chosen to complete the subsequent purification steps to obtain pure quercetin.
| Plants | Extraction solution | ||||||
| Soxhlet method | Ultrasound method | Enzymatic method | |||||
| Water | Ethanol | Chloroform | Water | Ethanol | Chloroform | ||
| Spinach | 11.296 |
57.901 |
19.691 |
14.197 |
39.814 |
21.419 |
12.2330 |
| Dill | 9.629 |
45.938 |
25.679 |
39.44 |
45.617 |
39.012 |
16.8890 |
| Onion Skin | 10.061 |
34.938 |
17.53 |
24.69 |
48.222 |
34.074 |
16.4550 |
| Sumac | 12.53 |
61.728 |
43.567 |
13.58 |
58.827 |
41.728 |
18.6280 |
| Pistacia eurycarpa | 24.629 |
84.037 |
67.148 |
21.481 |
53.777 |
36.851 |
30.8630 |
| Digalkhasad chemri | 4.259 |
54.469 |
37.32 |
12.086 |
29.629 |
18.148 |
10.6790 |
| Leelwi chemri | 3.271 |
51.232 |
40.172 |
10.691 |
37.098 |
27.037 |
13.4250 |
The date presented are mean
The process of separation and purification of quercetin from the alcoholic extract of Pistacia eurycarpa was carried out using an adsorption column containing silica gel (100–200 mesh). Thirty tubes were collected from this process, each tube containing 3 mL of purified quercetin, then the tubes containing quercetin were identified using TLC.
Pure quercetin was determined in the tubes obtained from the adsorption column using TLC by comparing the Rf of the material spot in each tube with the Rf of the standard quercetin spot. Random tubes were chosen and the process was repeated until the tubes containing pure quercetin were identified, which were tube Nos. 10–15 (6 tubes), and compared to standard quercetin, which was shown using an ultraviolet lamp. The Rf value of the separated spots from tubes containing pure quercetin ranged between 0.63–0.64 and the materials was a luminous pale yellow in comparison with the standard Rf of quercetin, which was 0.64 and also a luminous yellow color.
Fig. 5 shows the chromatogram of quercetin purified from Pistacia eurycarpa using HPLC. The figure illustrates that the retention time was 7.95, while the concentration reached 272 parts per million (ppm). On the other hand, Fig. 6 is the chromatogram of the alcoholic extract of Pistacia eurycarpa using HPLC, for which the retention time was 7.93 and the concentration was 370 ppm. Fig. 7 is the standard quercetin chromatogram, which had a retention time of 7.90.
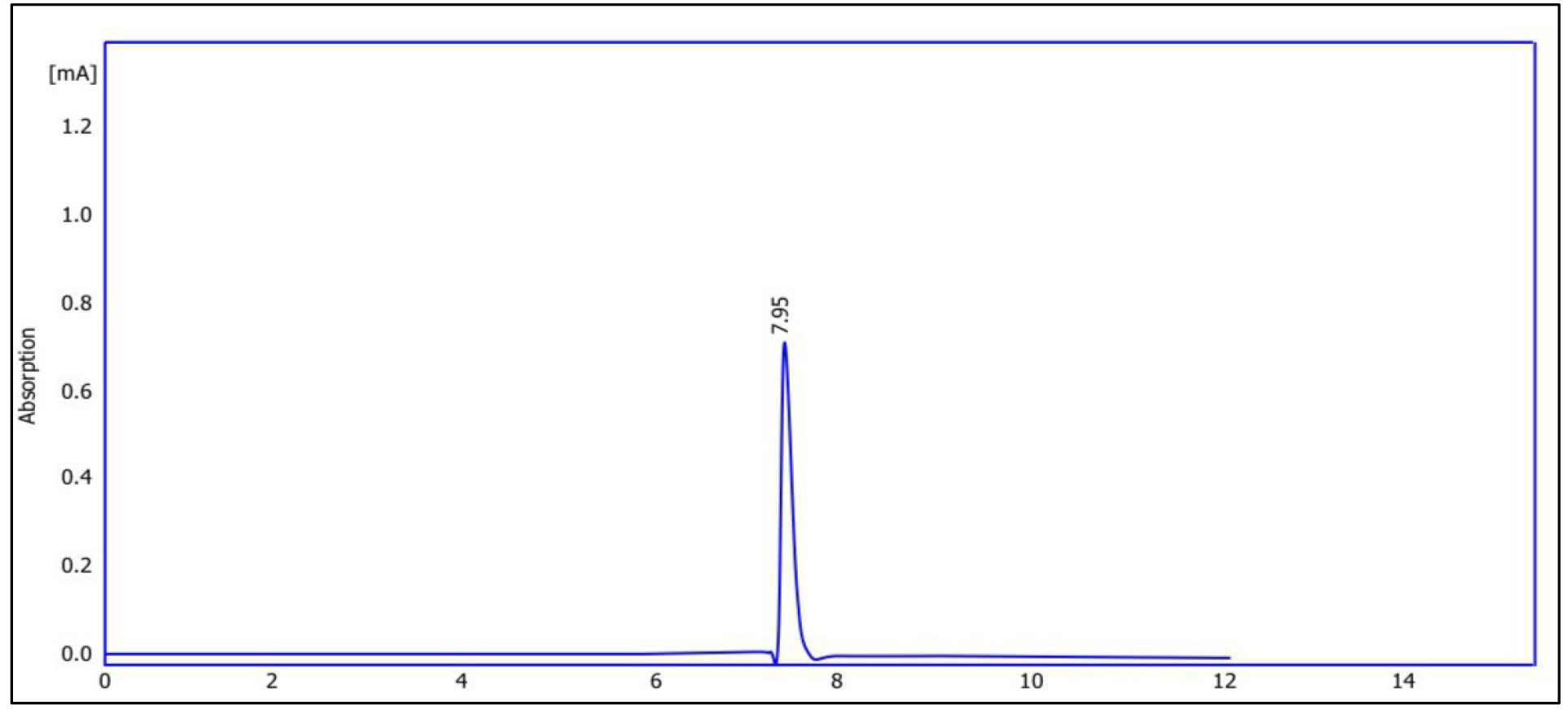 Fig. 5.
Fig. 5.
Chromatogram of purified quercetin from Pistacia eurycarpa using high performance liquid chromatography (HPLC).
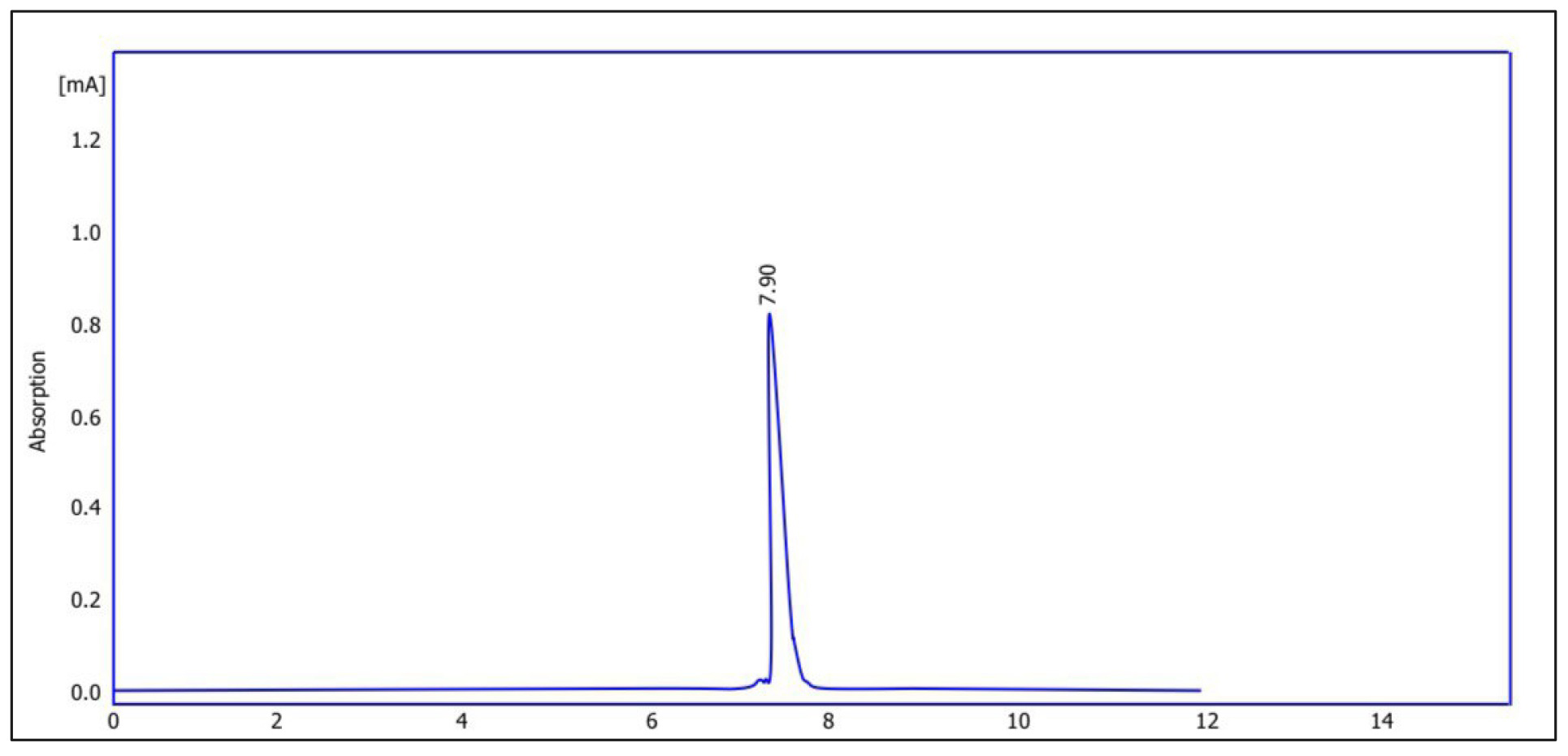 Fig. 6.
Fig. 6.
Chromatogram of alcoholic extract of Pistacia eurycarpa using high performance liquid chromatography (HPLC).
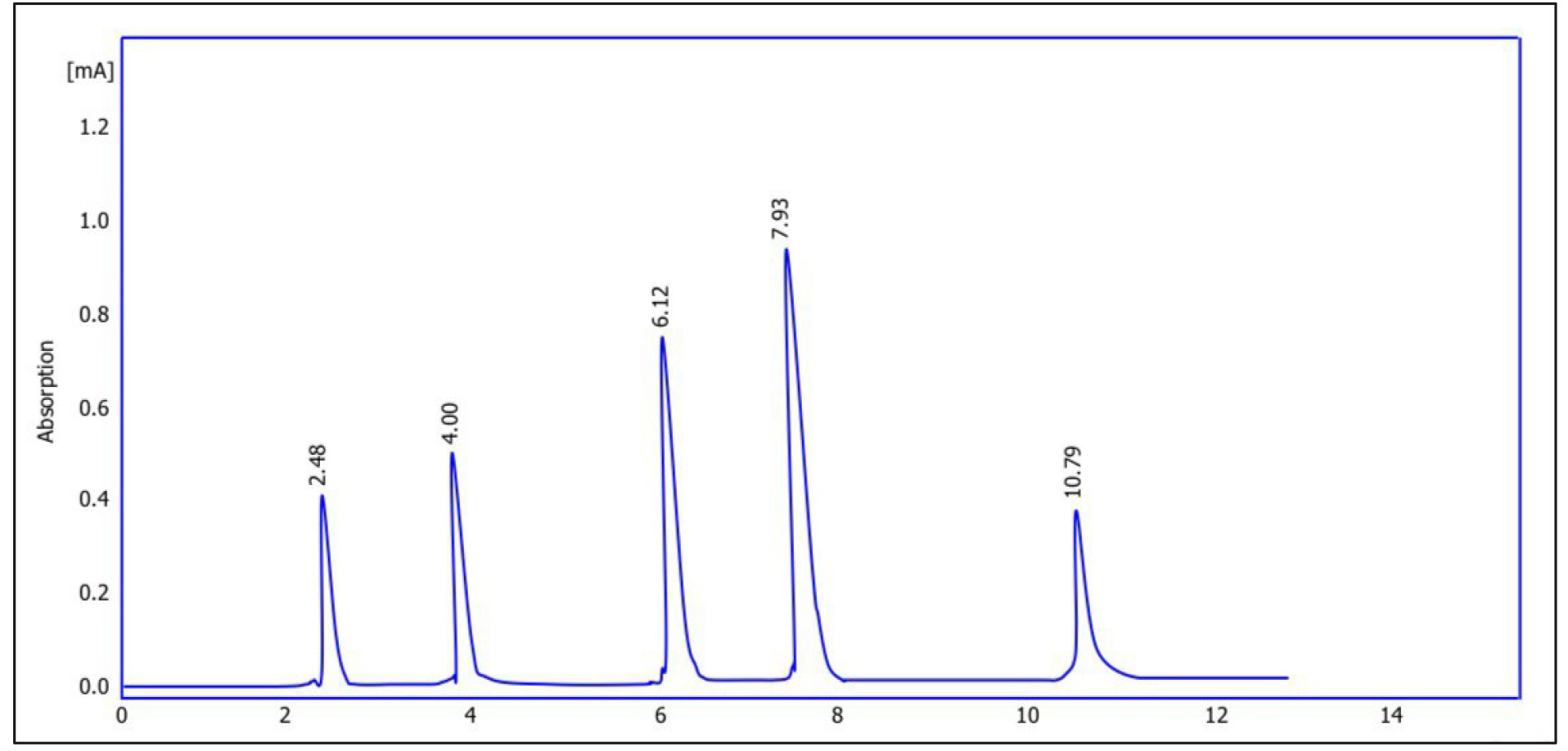 Fig. 7.
Fig. 7.
Standard quercetin chromatogram using high performance liquid chromatography (HPLC).
Natural antioxidants have gained increasing attention recently due to their essential roles in maintaining human health and preventing and treating various diseases by preventing oxidation chain reactions [36].
Figs. 8,9 show the results of the antioxidant activity assay of quercetin to capture free radicals using DPPH compared to BHT, which involved using different concentrations (5, 10, 15 mg/mL) of purified and standard quercetin dissolved in hot water and ethanol, respectively. For purified and standard quercetin dissolved in hot water, this reached 70%, 79%, and 86% and 80%, 86%, and 92%, respectively. As for purified and standard quercetin dissolved in ethanol, it reached 66%, 75.1%, and 82.3% and 71.3%, 79.1%, and 86.9%, respectively, compared to BHT (98%). The antioxidant activity of quercetin is due to its structure, which contains hydroxyl groups capable of giving an electron to the free radical and converting it into stable products—the higher the hydroxyl number, the greater the antioxidant activity—and the double bond C2 = C3 in the C ring, which contains a hydroxyl group attached to carbon 3, preventing oxidation and resulting in strong antioxidant activity by quercetin [37, 38].
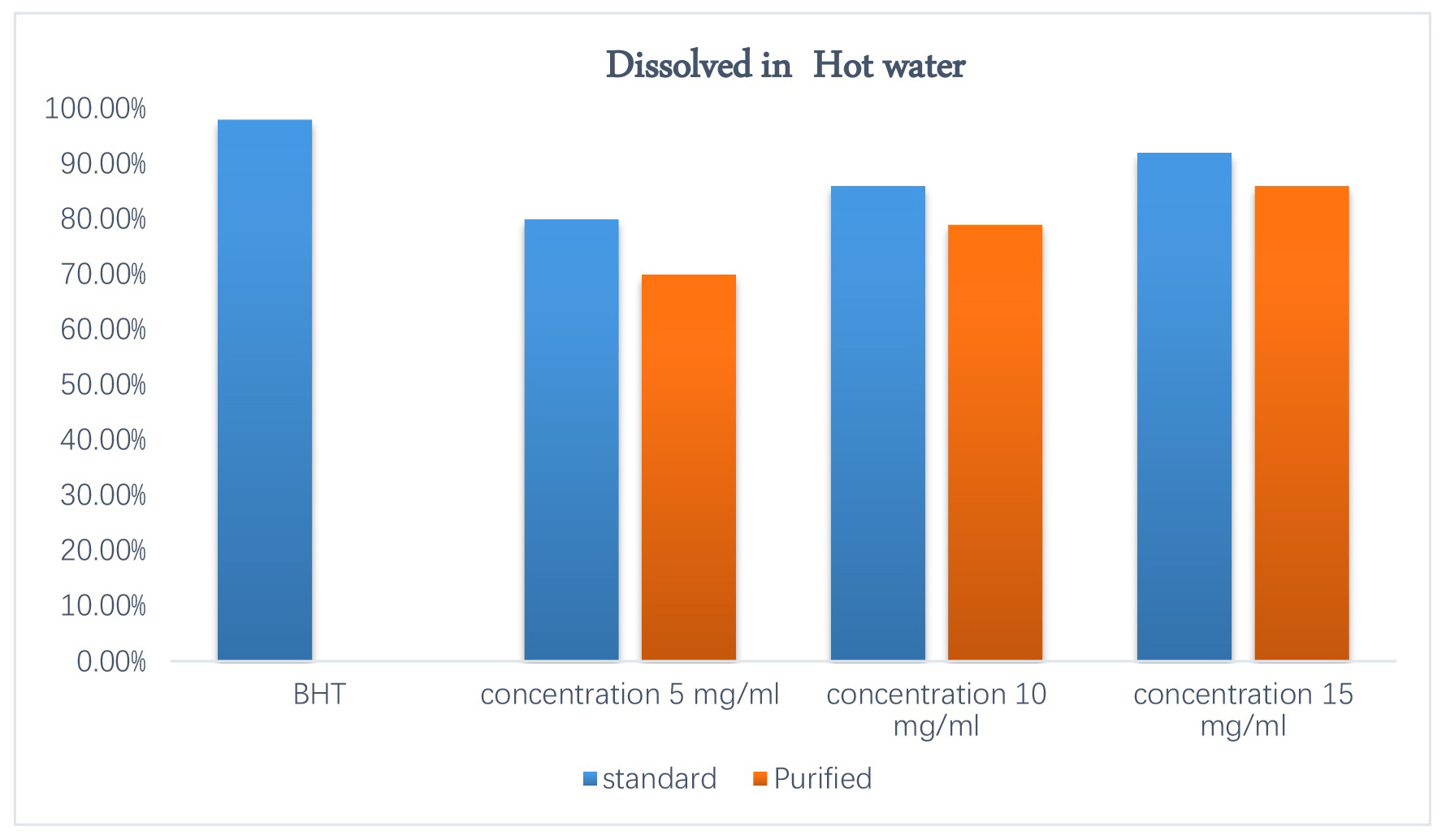 Fig. 8.
Fig. 8.
Antioxidant activity 2,2-diphenyl-1-picrylhydrazyl (DPPH) of quercetin dissolved in hot water.
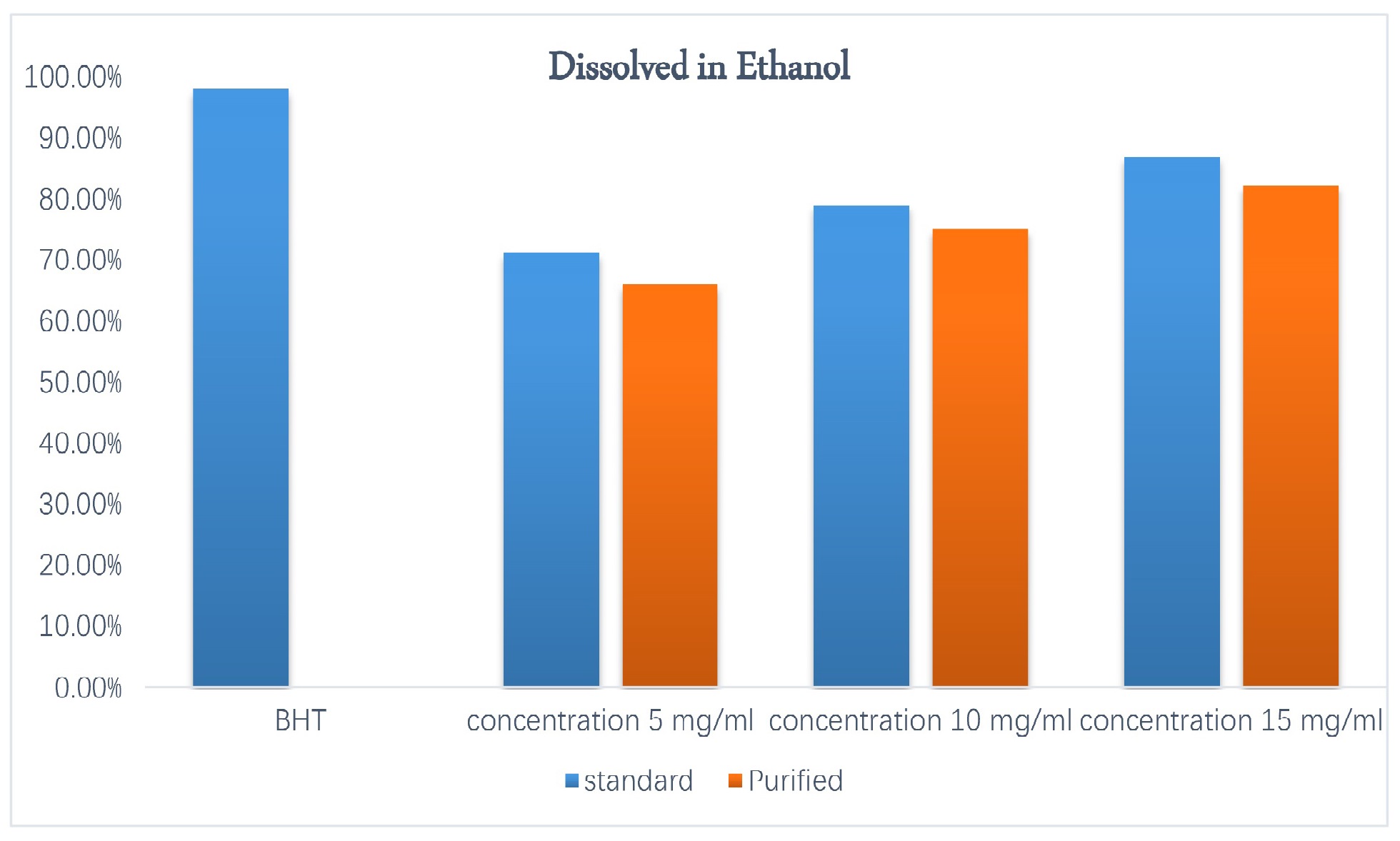 Fig. 9.
Fig. 9.
Antioxidant activity 2,2-diphenyl-1-picrylhydrazyl (DPPH) of quercetin dissolved in ethanol.
We also noted from Figs. 8,9 that the antioxidant activity of standard quercetin was higher than that of purified quercetin, and that antioxidant activity increased with increasing quercetin concentration. This agreed with previous estimations [39] of the antioxidant activity DPPH for different concentrations of quercetin.
The results also agreed with Tian et al. [40] who demonstrated the
antioxidant activity of a group of flavonoids, namely quercetin, apigenin,
luteolin, and kaempferol, against DPPH radicals, showing that these compounds
exerted stronger antioxidant activity than BHT, with the order of DPPH radical
scavenging activity as follows: quercetin
These results were similar to what Geetha et al. [39] found when estimating the antioxidant activity of both quercetin and ascorbic acid. The results showed that quercetin has a significant effect in scavenging free radicals, reaching 71.94%. It also showed that the effectiveness of quercetin is two times higher than ascorbic acid.
The results of the present study also came close to the findings of Diao et al. [41], where the antioxidant activity of quercetin reached 93% when estimating the activity of DPPH radical capture, indicating the high activity that quercetin possesses against free radicals. Furthermore, the results show that the antioxidant activity of quercetin dissolved in hot water was higher than that dissolved in ethanol for all concentrations and for both extraction types (purified and standard). The solubility of quercetin in water increases with increasing temperature. This is because the rise in temperature leads to an increase in the kinetic energy of the quercetin molecules and their release from the attractive forces that keep them together, and this in turn leads to their dissolution in water [42].
In this work, quercetin was extracted from different plants using different solvents and different methods. Pistacia eurycarpa, was the best plant in terms of its high quercetin content. It was also found that solvents play a major role in extracting plant components, and because of the high polarity of quercetin, which is due to its structure that contains hydroxyl groups, ethanol gave the highest concentration of quercetin extracted from plants, which ranged between 34.938–84.037 mg/g in the chemical method (Soxhlet) and between 29.629–58.827 mg/g in the physical method (ultrasound), then chloroform, which ranged between 17.53–67.148 mg/g in the chemical method (Soxhlet) and between 18.148–41.728 mg/gm in the physical method (ultrasonic waves) and was poorly soluble in water, as it ranged from 4.259–24.629 mg/gm in the chemical method (Soxhlet) and 10.691–29.44 mg/gm in the physical method (ultrasonic waves). As for the Enzymatic method, the concentration of quercetin reached 10.679–30.864 mg/gm, and the presence of ethanol and temperatures in the chemical method (Soxhlet) led to an increase in the concentration of quercetin extracted from plants. Pistacia eurycarpa plant gave the highest concentration, which was to 84.037 mg/gm. Quercetin purified using a silica gel column achieved antioxidant activity, and this antioxidant activity increased with increasing quercetin concentration.
TLC, Thin Layer Chromatography; HPLC, High-performance liquid chromatography; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; BHT, butylated hydroxytoluene.
The raw date used and/or analyzed during the current study are available from the corresponding author on reasonable request.
SHB and ZKAY designed the research study. SHB performed the research. SGAS provided help and advice on all experiments. ZKAY analyzed the data. SHB, ZKAI and SGAS wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Spinach (Spina Oleracea) leaves were obtained from the local markets of Basrah Governorate in Iraq. Onions (Allium cepa) orange peels were obtained from the local markets of Basrah Governorate in Iraq. Dill (Anethum graveolens) leaves were obtained from the local markets of Basrah Governorate in Iraq. Sumac (Rhus coriaria) fruits were obtained from the local markets of Dohuk Governorate in northern Iraq in dried form, as the sumac tree is widely cultivated in the northern region of Iraq. Pistacia (Pistacia eurycarpa) were obtained from the local markets of Dohuk Governorate in northern Iraq. Chemri (Phoenix dactylifera L.) the date palm, were obtained from the local markets of Basrah Governorate in Iraq.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.









