1 Biochemistry Research Laboratory, Omsk State Pedagogical University, 644099 Omsk, Russia
Abstract
Background: The features of metabolic processes associated with low human epidermal growth factor receptor 2 (HER2) expression (HER2-low) in breast cancer have not been described to date, including characteristic features of immune system reactivity, the presence and content of tumor markers, and the amino acid profile.
Methods: The case-control study involved 660 volunteers with breast cancer. Saliva was used as a biological fluid; at the time of collection, the patients were not receiving any treatment. Concentrations of 7 cytokines, 4 tumor markers, 12 amino acids, and 11 biochemical parameters were determined in the saliva.
Results: It was found that the HER2-low group was characterized by the highest pro-inflammatory activity, but the lowest metabolic activity. Three functional groups of amino acids were identified, which showed reliable differences in the HER2-low subgroup. The cancer antigen 19-9 (CA 19-9) tumor marker in the HER2-low group showed the greatest deviations from normal values compared to patients in the HER2 (–) and HER2 (+) groups.
Conclusions: Thus, patients with HER2-low breast cancer status had their own characteristic features in the course of the disease, immune response and metabolic activity. Understanding the features of metabolic processes in the HER2-low subgroup can help identify new therapeutic targets for this group of patients.
Keywords
- saliva
- breast cancer
- HER2-low
- metabolic features
- immune response
Breast cancer continues to be one of the pressing health problems worldwide today [1]. Breast cancer is highly heterogeneous [2]. The classical allocation of molecular biological subtypes of breast cancer involves taking into account only the positive and negative status of expression of the human epidermal growth factor receptor 2 (HER2). Moreover, the negative status unites cases with both true negative and low HER2 expression [3]. In 2023, the College of American Pathologists released updates to the templates for reporting biomarker testing results in primary invasive breast carcinoma, as well as in recurrent and metastatic tumors, which now include notes on low expression of the HER2 biomarker (HER2-low) [4]. Numerous studies have shown that changing the therapy regimen in HER2-low subgroups significantly improves treatment outcomes and prognosis for this group of patients [4, 5, 6]. However, the features of metabolic processes associated with low HER2 expression have not been described, including the characteristic features of immune system reactivity, the presence and content of tumor markers, and the amino acid profile. We have previously shown that a number of salivary indicators (pro-inflammatory and anti-inflammatory cytokines, amino acids, enzymes, tumor markers) significantly changed in breast cancer, but their connection with the HER2-low status has not been established to date [7].
Saliva is a unique biological fluid that combines the advantages of non-invasive collection and high information content regarding breast cancer metabolites [8, 9, 10]. If the differences in the metabolic profile of saliva with HER2 (+) status have been previously shown, then there is no information on HER2-low status in the literature [11]. At the same time, existing diagnostic methods also do not allow identifying patients with a certain HER2 status. In this regard, the purpose of this study was to analyze the metabolic features of saliva with HER2-low status of breast cancer.
The case-control study included 660 volunteers with breast cancer. Inclusion criteria were female gender, age 30–70 years, Caucasian race, histologically confirmed breast cancer, and no signs of active infection, including in the oral cavity. Patients were hospitalized for surgery or the first course of chemotherapy.
Saliva samples were collected once during hospitalization strictly before the
start of treatment as described previously [12]. Samples were analyzed for
cytokine content by enzyme-linked immunosorbent assay using Vector Best kits
(IL-1
When interpreting the immunohistochemistry (IHC) results, a staining intensity scale was used to determine the expression of HER2 receptors, which ranges from “0” to “3+” in accordance with the American Society of Clinical Oncology/the College of American Pathologists (ASCO/CAP) recommendations [13]. HER2 IHC results (1+) or 0 were interpreted as HER2-negative (HER2 (–)). If the HER2 status by IHC was indeterminate (2+), it was necessary to evaluate the presence of HER2 gene amplification by in situ hybridization (ISH). Patients with HER2 expression level in the tumor (3+) were considered HER2-positive. Patients with breast cancer by HER2 status were distributed as follows: HER2 (–)—401 (60.8%), HER2-low—137 (20.8%), HER2 (+)—99 (15.0%), no data—23 (3.4%).
Statistical analysis was performed using Statistica 10.0 software (StatSoft, Tulsa, OK, USA) by a nonparametric method. The distribution pattern and homogeneity of variances in the groups were preliminarily checked. The Mann-Whitney test was used to compare two subgroups, and the Kruskal-Wallis test was used to compare three subgroups. The statistical significance of differences between subgroups was estimated at p ˂ 0.05.
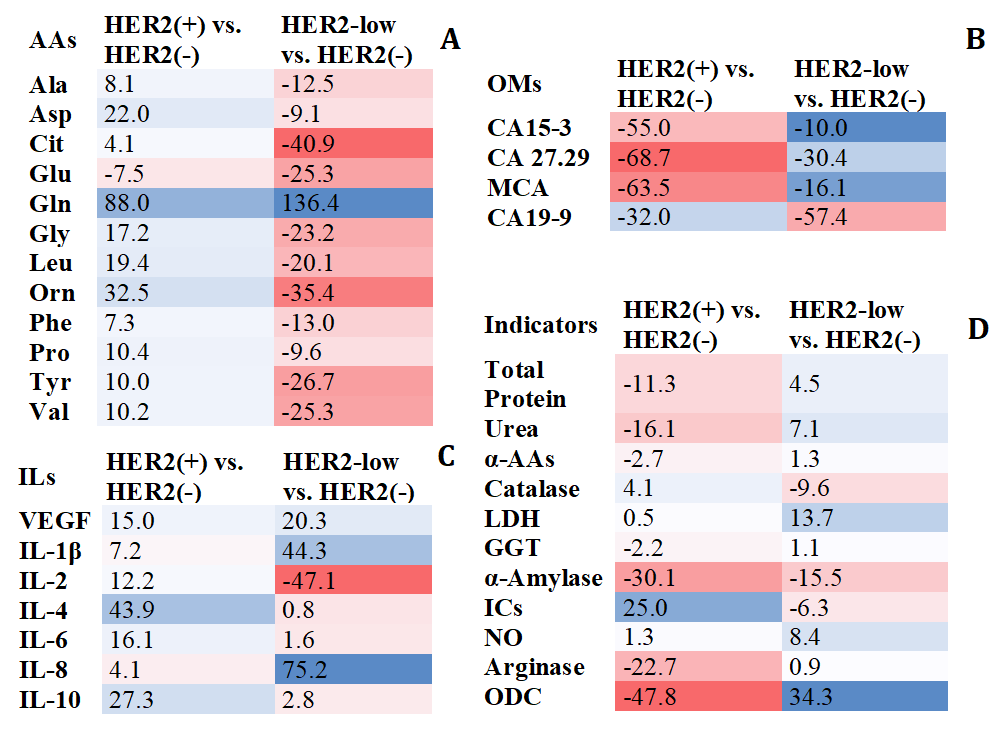
In HER2 (+) status, an increase in amino acid content was observed, whereas in HER2-low status, the amino acid content decreased compared to the HER2 (–) subgroup. The exception was glutaminic acid (Glu) for the HER2 (+) subgroup and glutamine (Gln) for the HER2-low subgroup (Fig. 1A). In the subgroup with low HER2 levels, the concentrations of citrulline (Cit) (p = 0.0251), ornithine (Orn) (p = 0.0375), Glu (p = 0.0430), Gln (p = 0.0137), and glycine (Gly) (p = 0.0402) statistically differed significantly from the group with HER2 (–) (Fig. 1A). No significant differences were found for the HER2 (+) subgroup compared to HER2 (–). The HER2 (+) and HER2-low subgroups differed the most in the concentrations of alanine (Ala) (p = 0.0157), asparaginic acid (Asp) (p = 0.0283), Cit (p = 0.0415), Glu (p = 0.0190), Gln (p = 0.0203), Gly (p = 0.0059), Orn (p = 0.0022), phenylalanine (Phe) (p = 0.0130), tyrosine (Tyr) (p = 0.0156) and valine (Val) (p = 0.0410).
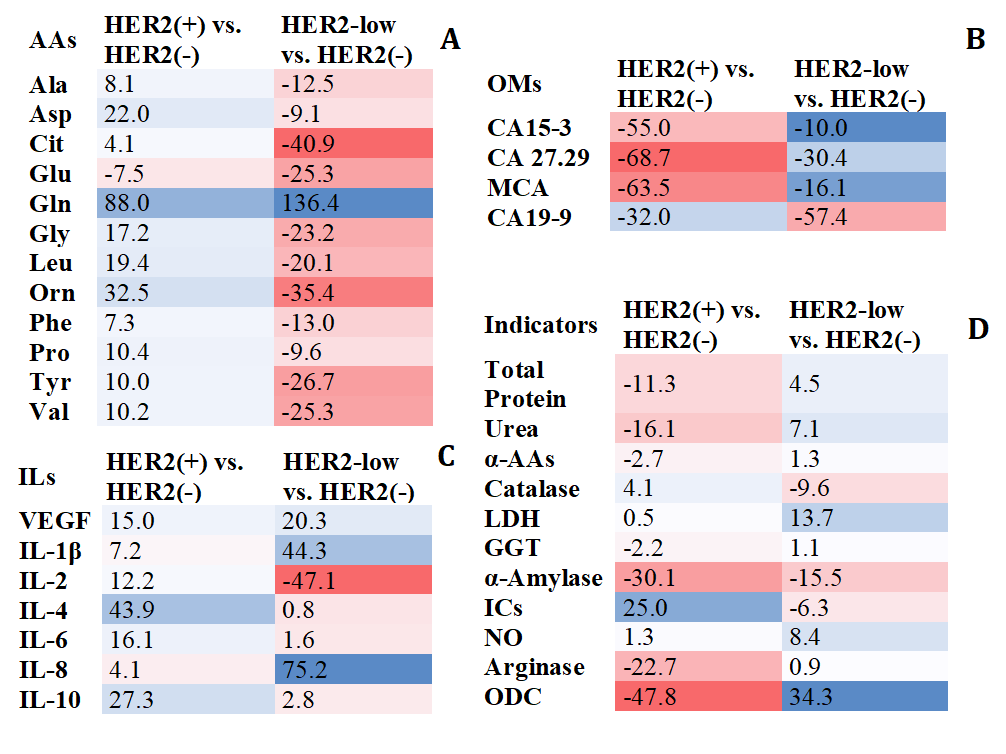 Fig. 1.
Fig. 1.
Salivary metabolic composition in subgroups with HER2-positive
and HER2-low status compared to HER2-negative status (%). (A) Amino acid
composition. (B) Tumor markers. (C) Cytokines. (D) Biochemical composition.
Relative content is calculated as the content in the HER2 (+) or HER2-low
subgroup minus the content in the HER2 (–) subgroup, divided by the content in
the HER2 (–) subgroup, %. The color scale shows the relative change in the
content of the indicators from the minimum (red) to the maximum (blue). The color
intensity is directly proportional to the change in concentration compared to
HER2 (–) breast cancer. HER2, human epidermal growth factor receptor 2; AAs, total concentration of alpha-amino acids; Ala,
alanine; Asp, asparaginic acid; Cit, citrulline; Glu, glutaminic acid; Gln,
glutamine; Gly, glycine; Leu, leucine; Orn, ornithine; Phe, phenylalanine; Pro,
proline; Tyr, tyrosine; Val, valine; ILs, interleukines; VEGF, vascular
endothelial growth factor A; OMs, oncomarkers; CA 15-3, cancer antigen 15-3; CA
27.29, cancer antigen 27.29; MCA, mucin-like carcinoma antigen; CA 19-9, cancer
antigen 19-9;
According to the content of tumor markers with different HER2 expression, differences were shown for antigens of the MUC1 family (CA 15-3, CA 27.29, MCA, CA 19-9) (Fig. 1B). In the HER2 (+) group, the concentrations of tumor markers CA 15-3 (p = 0.0185), CA 27.29 (p = 0.0006) and MCA (p = 0.0034) decreased consistently, only for CA 19-9 a more pronounced decrease in concentration was shown in the HER2-low subgroup (p = 0.0013).
For six cytokines, differences in content were found at different HER2
expression levels (Fig. 1C). The HER2-low subgroup differed from the HER2 (–)
subgroup by higher content of IL-1
When assessing the biochemical composition of saliva, it was found that changes
in the content of indicators for the HER2 (+) and HER2-low subgroups were not
unidirectional (Fig. 1D). The HER2-low subgroup was characterized by a higher
level of NO (p = 0.0318), increased activity of LDH (p =
0.0444), GGT (p
The HER2-low group showed the highest pro-inflammatory activity, as evidenced by
the high level of IL-1
Thus, the group with HER2-low status of breast cancer has its own characteristic features in the course of the disease, immune response and metabolic activity. Understanding the features of metabolic processes in the HER2-low subgroup can help identify new therapeutic targets. In particular, the effectiveness of anti-HER2 targeted drugs has already been recognized [5, 6]. However, the pronounced metabolic differences between HER2-low and HER2 (+) breast cancer subtypes suggest that some new type of therapy will be effective in the case of HER2-low status, which may be a promising direction for future research. This once again emphasizes the importance of identifying the HER2-low group in patients with breast cancer.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
LB designed the research study and performed the research. LB performed the experiment and analyzed the data. The author contributed to editorial changes in the manuscript. The author read and approved the final manuscript. The author has participated sufficiently in the work.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Omsk Regional Clinical Oncological Dispensary (21 July 2016, protocol code 15) and by the Ethics Committee of Omsk State Pedagogical University (20 March 2024, protocol code 46-04/2). A written consent was signed by the patients or their families/legal guardians.
Thanks to all the peer reviewers for their opinions and suggestions.
This research was funded by Russian Science Foundation, grant number 23-15-00188, https://rscf.ru/project/23-15-00188/.
The author declares no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.