1 Department of Biosciences and Chemistry, Biomolecular Sciences Research Centre, Sheffield Hallam University, Faculty of Health and Wellbeing, S1 1WB Sheffield, UK
Academic Editor: Corrado Angelini
Abstract
Fibromyalgia is a central sensitivity syndrome that presents with chronic pain,
fatigue, cognitive dysfunction, and disordered sleep. The pathophysiology which
due to multisensory hypersensitivity of the central nervous system involves
neuronal excitability leading to central sensitization. Treatments of the
challenges associated with the complexities of fibromyalgia involve combinations
of pharmacological and non-pharmacological therapeutic approaches which often
offer limited benefit. Potassium (K
Keywords
- fibromyalgia
- potassium channels
- pathophysiology
- pharmacology
- channelopathies
Fibromyalgia is part of the spectrum of central sensitivity syndromes characterized by persistent widespread idiopathic or “nociplastic” pain which is accompanied by fatigue, cognitive and mood disturbances, and disordered sleep [1, 2, 3]. Diffuse hyperalgesia (heightened responses to painful stimuli) and allodynia (painful responses to non-painful stimuli) with lowered pressure pain thresholds are characteristics of the pain associated with fibromyalgia. The diagnosis is often complicated by co-morbidities, e.g., chronic fatigue syndrome, with similar symptoms. Fibromyalgia, dependent on the diagnostic criteria and methods of classification, is estimated to affect 0.4–8% of the population worldwide with prevalence greater in females and increasing with age [4]. The pathophysiology of fibromyalgia involves amplified responses of the central nervous system (CNS) to peripheral stimuli associated with neuronal excitability leading to central sensitization (CS) [1]. The heightened activity of the CNS responsible for the characteristic fibromyalgia symptoms is initiated by a range of peripheral sensory generators.
Neuronal activity within the CNS and peripheral nervous system (PNS) is
suppressed and stabilized by potassium (K
Clinical features related to altered K
Evidence in patients with FM predicts responses to augmented sensory input evokes disturbed neurotransmitter functioning and neuroplasticity leading to neuronal hyperexcitability and hypersensitivity in the CNS due to enhanced excitatory and reduced inhibitory processes [1, 2, 12]. These findings are consistent with the development of central sensitization (Fig. 1) [1, 12]. Although heightened activity of the CNS can be initiated by peripheral sensory generators, such as nerve pathologies, neuroinflammation, skeletal muscle abnormalities and ischaemia, constant nociceptor activation due to a comorbid condition can also evoke secondary fibromyalgia [2, 3, 12]. Pain associated with fibromyalgia is characterized by neuropathic components in that it is chronic and intractable and unlike nociceptive pain, which serves to protect from potential or actual injury, persists after tissue healing has taken place. Even in the apparent absence of sensory stimulus symptoms of pain can present indicating that it is maladaptive and has no obvious biological purpose [2, 12].
 Fig. 1.
Fig. 1.Model of the pathophysiology of fibromyalgia. Postulated relationship between hypersensitivity due to stressors activating generators of neuronal hyperexcitability resulting in the development of central sensitization a characteristic of fibromyalgia.
In brain spectroscopy studies of patients with fibromyalgia severity of pain correlated with raised levels of neurotransmitters glutamate, glutamine, and glycine in regions of the brain associated with pain processing [1, 2, 13]. Consequently, in the spinal cord and brain the glutamate N-methyl-D-aspartate (NMDA) receptors are exposed to elevated glutamate levels. In addition, the cerebrospinal fluid (CSF) of patients with fibromyalgia has elevated levels of substance P, endogenous opioids, nerve growth factor, brain-derived neurotrophic factor and attenuated levels of 5-hydroxyindoleacetic acid, a metabolite of serotonin, and 3-methoxy-4-hydroxyphenylglycol, a metabolite of noradrenaline [1, 2, 13]. Serum serotonin and L-tryptophan are also suppressed relative to healthy subjects in people with fibromyalgia [1, 2, 13]. Consistent with these findings, the activity of descending serotonergic-noradrenergic efferent pathways in people with fibromyalgia has been observed to be reduced resulting in altered diffuse noxious inhibitory control (DNIC) of pain pathways [14]. DNIC is the descending, from the CNS to the spinal cord, control of nociception which relies on painful conditioning stimulation of one part of the body to inhibit pain in another part and inefficiency has been implicated in the development of chronic pain [14].
A characteristic of fibromyalgia is non-restorative sleep associated with poor sleep quality [15, 16, 17]. Superficial, fragmented and non-restorative sleep with restless leg syndrome and morning fatigue are common complaints amongst people with fibromyalgia. Diminished slow-wave sleep, which is essential to feeling refreshed upon awakening, during non-rapid eye movement sleep (NREM) is often reported [15, 16, 17]. Persistent pain related to heightened states of arousal has been linked to lower sleep efficiency and patterns of sleep discontinuity [18]. Altered production of melatonin has also been reported in patients with fibromyalgia with lower secretion in dark hours related to sleep disturbance and raised secretion in daytime contributing to pain, fatigue and mood symptoms [19].
Dysautonomia, neuroinflammation, increased pro-inflammatory with reduced anti-inflammatory cytokine profiles and reduced reactivity to stress of the hypothalamic pituitary adrenal axis have also been proposed to be involved in the genesis and enhancement of symptoms of fibromyalgia [2, 12, 13].
Investigation of sensory axonal excitability in patients with fibromyalgia
revealed enhanced superexcitability relative to healthy controls [20]. The
technique used measured membrane polarization, ion channel function and
paranodal/internodal condition of peripheral nerves. Superexcitability is
influenced by the conductance of paranodal fast K
Although findings have been inconsistent, some studies have suggested an
abnormal intrusion of alpha activity (8–13 Hz oscillations) into the delta
activity (1–4 Hz oscillations) that occurs during slow-wave sleep in people with
fibromyalgia [15, 16, 17, 23]. The pain experienced by patients with fibromyalgia may
be exacerbated by these sleep abnormalities. Depolarization of cells within the
thalamus due to alterations in conductance of K
Lower plasma levels of KCHN2 protein, which forms the Kv11.1 subtype of the hERG (Ether-a-go-go-related gene) Kv channel were observed in patients with fibromyalgia compared to healthy controls [26]. In contrast, the levels of the proteins KCHN6 and KCHN7 that form the Kv11.2 and Kv11.3 subtypes of the hERG channel were not different in patients with fibromyalgia and healthy controls. Kv11 channels are expressed in the CNS and are thought to contribute to adaptive firing of neurons [27, 28]. At the spinal cord level Kv11.1 channels have been demonstrated to be involved in nociceptive modulation [29]. Hyperexcitable states due to a lack of accommodation of nerve cells to repetitive stimuli may occur due to altered hERG expression. A relationship between the plasma levels of KCHN2 and the symptoms of fibromyalgia has not yet been established.
Autoantibodies targeting Kv channels associated with contactin-associated protein-2 (CASPR2)-IgG-positivity have been observed to contribute to hyperexcitability which could be responsible for persistent pain experienced by people with fibromyalgia [30]. CASPR2 is a member of the neurexin family that is expressed in the PNS and CNS and colocalizes with Kv1 subtype channels. In people with persistent pain conditions, such as fibromyalgia, the prevalence of symptoms correlated with a positive Kv channel-complex immunoglobulin status. Disruption of Kv localization at paranodal axons may be involved in the link between CASPR2 autoimmunity and pain. Immune modulation therapy, prednisone, methylprednisolone, intravenous immune globulin, methotrexate or hydroxychloroquine, reduced the state of pain in Kv channel-complex seropositive patients allowing the discontinuation of opioid analgesics in some cases [30].
Substance P (SP) levels are elevated in the CSF of people with fibromyalgia [1, 2]. SP is a mediator of pain involved in the generation and transmission of pain
signalling which can also exhibit antinociceptive properties [31]. CS results due
to SP activating excitatory post-synaptic potential in the CNS, whilst peripheral
neurogenic inflammation is caused by the release of SP from nociceptive nerve
fibres [32, 33]. SP decreases the activity of Kir channels in locus coeruleus and
nucleus basalis neurons, and IK
The gabapentinoid pregabalin has demonstrated clinically significant
improvements of core symptoms in patients with fibromyalgia and is a recommended
treatment of the condition [40]. The benefits reported have been dose-related
pain alleviation with improvement in quality of life and sleep [40]. The efficacy
exhibited by pregabalin as a treatment of pain is a consequence of preventing
sensory propagation with reduction of neuronal excitability. Alpha-2-delta (
Intravenous lidocaine has been demonstrated to reduce hyperalgesia, pain
intensity and FIQ scores in people with fibromyalgia [46]. Although inhibition of
sodium channels is considered the primary mechanism of action, lidocaine
suppresses neuronal activity within the dorsal horn due to hyperpolarization
following blockade of Kv channels [47, 48]. Hyperpolarization-activated cyclic
nucleotide-gated (HCN) channels, which are related to Kv channels and generate
inward K
Patients with fibromyalgia reported an improvement in their symptoms,
particularly pain and physical activity, following flupirtine treatment [51].
Flupirtine evokes hyperpolarization and stabilization of neuronal membranes due
to activation of Kv7 subtype channels, with subsequent NMDA receptor activity
reduction [52]. Kv7 channels are present in central terminals of primary
afferents and spinal cord dorsal horn neurons and involved in the regulation of
peripheral and central nociceptive pathway activity [52, 53]. Activation of
neuronal Kv7 channels evokes stabilization of membrane excitability producing
inhibition of action potential initiation and thereby these channels are a
therapeutic target for the treatment of diseases involving neuronal
hyperexcitability [54, 55]. The location of Kv7 channels in other neuronal areas
responsible for the pharmacological properties of flupirtine are relevant to the
management of the spectrum of symptoms characteristic of fibromyalgia [52, 53].
Thus, K
Melatonin regulates the sleep-awake cycle due to synchronization of circadian
rhythm which impacts on fatigue and mood levels and enhances pain inhibition [56, 57]. Some studies in patients with fibromyalgia have reported altered levels of
melatonin with secretion during dark hours being lowered contributing to
disordered sleep leading to daytime pain and fatigue and raised daytime secretion
enhancing the pain, fatigue, and mood symptoms further disturbing sleep quality
[19]. Although such findings could suggest abnormal secretion of melatonin being
involved in the pathophysiology of fibromyalgia, inconsistencies have been
reported where studies have observed no difference in the melatonin levels in
patients with fibromyalgia and healthy controls [19]. Administration of melatonin
as a treatment of fibromyalgia reduced pain and fatigue, and improved sleep
quality and quality of life measurements [19]. The anti-nociceptive effects of
melatonin following stimulation of melatonergic receptors have been associated
with the activation of SK
In rat reserpine-induced myalgia and vagotomy-induced myalgia models that mimic
pain features of fibromyalgia, such as hyperalgesic symptoms, the activator of
IK
Palmitoylethanolamide (PEA) is a peroxisome proliferator-activated receptor
(PPAR
In patients with fibromyalgia treatment with the orexin receptor antagonist suvorexant, which would allow recovery of Kv7 channel activity, resulted in an improved sleep time and subsequent reduced pain sensitivity [70]. The hypocretin/orexin neurons within the lateral hypothalamus are involved in sleep-wake control where stimulation of neuronal activity leads to sleep-to-wake transition, whilst suppression induces NREM sleep [71]. Hyperexcitability in hypocretin/orexin neurons in a mouse model leading to destabilized sleep involved a decreased density and impaired functioning of Kv7 channels [72]. When the Kv7 channel activity was augmented with flupirtine the resulting hyperpolarization suppressed the activity of the hypocretin/orexin neurons, increased NREM sleep and restoration of sleep stability. Interestingly, the flupirtine enhanced sleep quality was related to an improvement in cognitive function in the mice, a characteristic of fibromyalgia that was not assessed during the clinical trial of suvorexant.
Evidence is presented demonstrating involvement of K
| Target channel | Model | Outcomes | Comments | References | |
| Pathophysiology | |||||
| Peripheral axonal function | Fast K |
Patients with fibromyalgia. | Testing nerve excitability and nerve conduction indicated axonal superexcitability increased in fibromyalgia relative to healthy controls. | Indicating dysfunction of paranodal channels. | [20] |
| Thalamic neuron activity | Potassium leak (KL) channel | Imaging studies in fibromyalgia patients. | Biophysically realistic mathematical model indicating increased I |
[24] | |
| KCHN2 protein | Kv11.1 | Patients with fibromyalgia. | Plasma levels of KCHN2 protein lower in patients than healthy controls. | KCHN2 protein widely expressed in the CNS and forms hERG voltage-gated potassium channel. | [26] |
| VGKC-complex-immunoglobulin G (IgG)–seropositive patients | Kv1 | Patients with persistent pain, including fibromyalgia. | Autoimmunity, autoantibodies to channel complex. | [30] | |
| Substance P | Kv4, IK |
Dorsal root ganglion neurons, locus coeruleus neurons, nucleus basalis neurons (rat); Stellate ganglion neurons (guinea pig). | Decreased channel activity led to increased afferent nerve excitability, sensitized nociceptors. | [34, 35, 36, 37] | |
| Kv7 (M-type), K |
Dorsal root ganglion neurons (rat and mouse). Trigeminal nociceptors (rat). Vagal sensory (PNS) neurons (ferret). | Enhanced channel activity leading to hyperpolarization and suppression of excitability. | Substance P levels raised in patients with fibromyalgia. | [38, 39, 73] | |
| Treatment | |||||
| Pregabalin | K |
Patients with fibromyalgia. | Reduction of pain, and improvement in quality of life and sleep. | Activation of K |
[42, 43, 44, 45] |
| Lidocaine | Kv, HCN | Patients with fibromyalgia. | Hyperalgesia, pain and FIQ score reduced. | Neuronal activity suppressed in dorsal horn due to Kv blockade. | [46, 47, 48] |
| Flupirtine | Kv7 (M-type) | Patients with fibromyalgia. | Reduced pain and improved physical activity. | Flupirtine activates Kv7 (M-type) channels. | [51] |
| Melatonin | SK |
Patients with fibromyalgia. | Reduced pain and fatigue, and improved sleep quality and quality of life. | Anti-nociceptive effects due to stimulation of melatonergic receptors involve activation of K |
[58, 59, 60] |
| ASP0819 | IK |
Rat myalgia models of fibromyalgia. Patients with fibromyalgia. | Reduced excitation in dorsal root peripheral nerve fibres. Improved FIQ score, sleep and pain. | ASP0819 is an activator of K |
[61, 64] |
| Palmitoylethanolamide (PEA) | IK |
Patients with fibromyalgia. | Improved pain and FIQ scores. | PEA evokes channel activation. | [66, 67, 68] |
| Suvorexant | Kv7 (M-type) | Patients with fibromyalgia. | Improved sleep and reduced pain sensitivity. | Suvorexant, an orexin receptor antagonist, reversed the decreased channel function due to action of hypocretin/orexin neuron hyperexcitability. | [70] |
| FIQ, Fibromyalgia Impact Questionnaire; HCN, hyperpolarization-activated cyclic
nucleotide-gated channel; hERG, human ether-a-go-go-related gene; K | |||||
Modulation of K
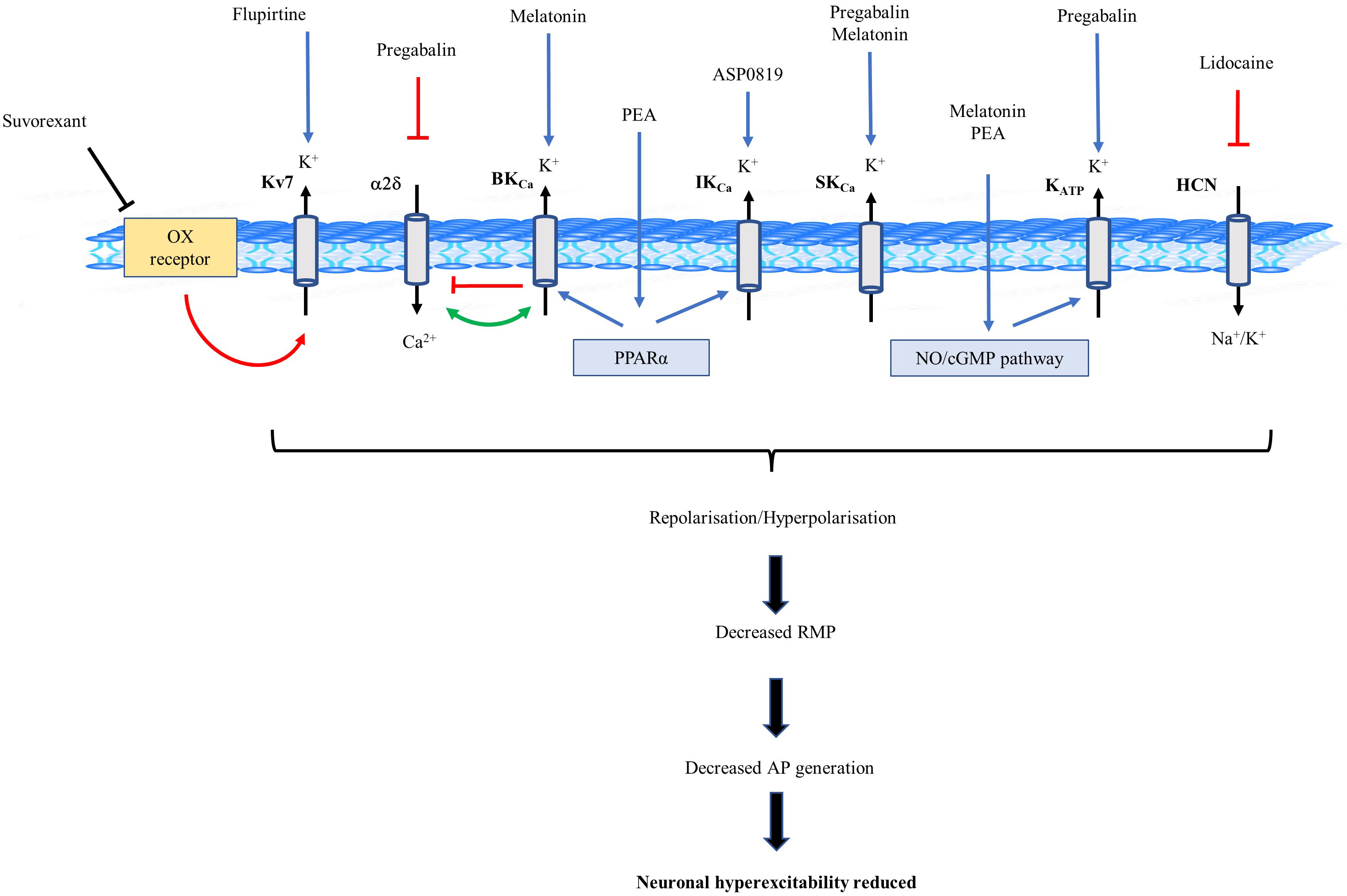
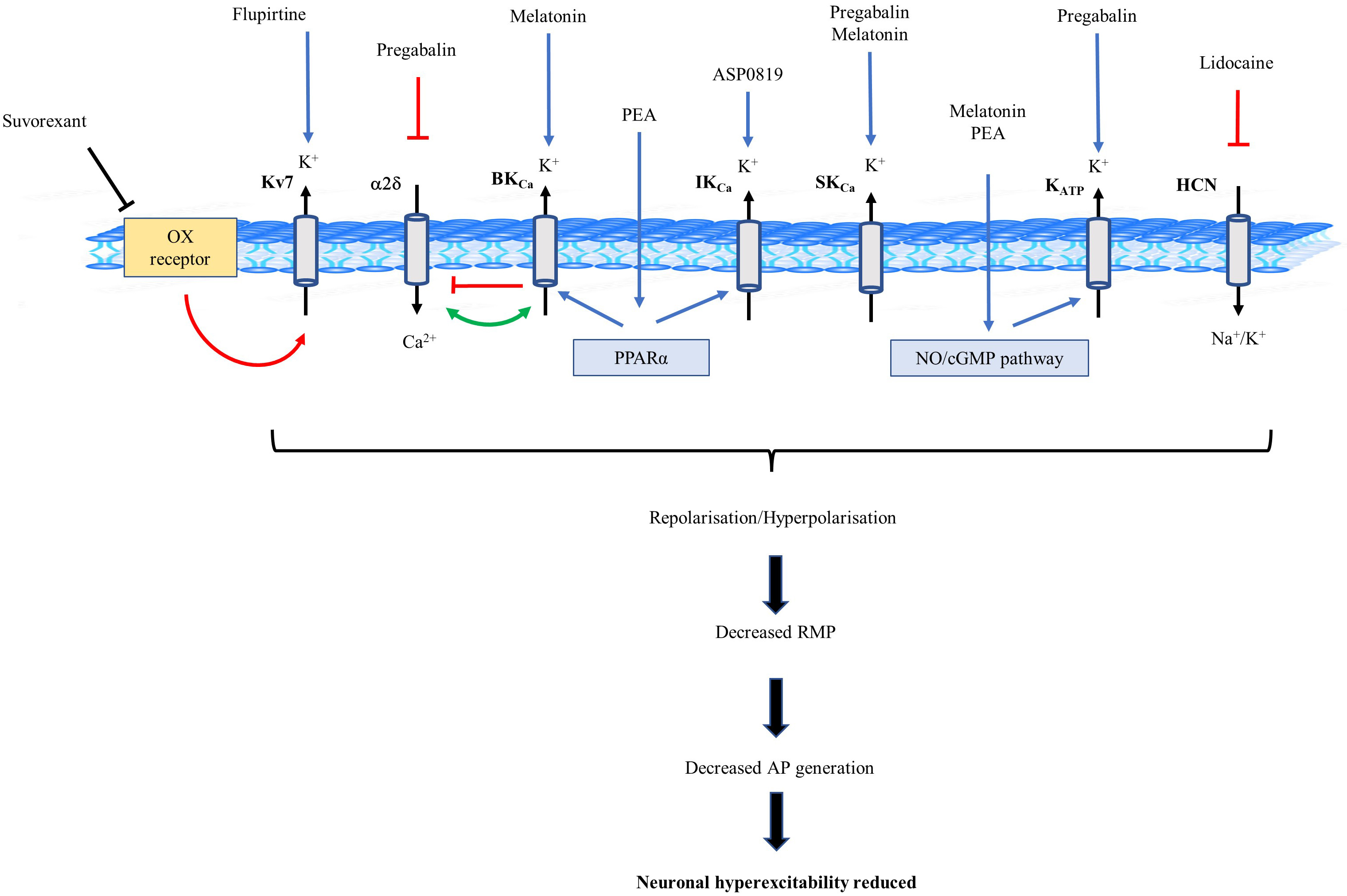 Fig. 2.
Fig. 2.Schematic diagram of potassium (K
Identification of the role K
CASPR2, contactin-associated protein-2; CNS, central nervous system; CS, central
sensitization; CSF, cerebrospinal fluid; DNIC, diffuse noxious inhibitory
control; FIQ, Fibromyalgia Impact Questionnaire; HCN, hyperpolarization-activated
cyclic nucleotide-gated channel; hERG, human ether-a-go-go-related gene;
K
KL is solely responsible for all the work leading to and resulting in this paper, including researching, writing and editing the paper.
Not applicable.
Not applicable.
This research received no external funding.
The author declares no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.