1 Institute for Anatomy, Medical Faculty, TU Dresden, 01307 Dresden, Germany
2 Schönblickstraße 95, 71272 Renningen, Germany, former member of the Max Planck Institute for Intelligent Systems, Stuttgart, Germany
Abstract
This study reviews the use of magnetic and electromagnetic fields (EMF), pulsed electromagnetic fields (PEMF), and transcranial magnetic stimulation (TMS) in Parkinson’s disease, Alzheimer’s disease (AD), or Multiple Sclerosis (MS). The Introduction provides a review of EMF, PEMF, and TMS based on clinical observations. This is followed by a description of the basic principles of these treatments and a literature review on possible mechanisms describing the coupling of these treatments with biological responses. These response mechanisms include the cell membrane and its embedded receptors, channels and pumps, as well as signaling cascades within the cell and links to cell organelles. We also discuss the magnetic contribution to coupling EMF, as well as the recent finding of cryptochrome as a putative magnetosensor. Our conclusion summarizes the complex network of causal factors elicited by EMF such as those arising from the cell membrane via signaling cascades to radical oxygen species, nitric oxide, growth factors, cryptochromes and other mechanisms involving epigenetic and genetic changes.
Keywords
- Parkinson’s disease
- Alzheimer’s disease
- Multiple sclerosis
- EMF
- PEMF
- TMS
Transcranial magnetic stimulation (TMS) has been recognized as a novel neurological and psychiatric therapeutic tool useful in the treatment of several neurological diseases because it is non-invasive and painless while stimulating specific regions of the brain [1, 2]. However, the effects of electromagnetic fields (EMF) on molecular and biological systems are still not completely understood. It is known that “window-effects” are present depending on wavelength and intensity and, because of this, the effects of EMF can range from beneficial to adverse [3]. This effect describes the phenomenon in which there are specific amplitudes of frequency values, at which the response of the biological system is activated, whereas other amplitudes or frequencies can inhibit the same biological system [4].
In this review, we have studied the impact of magnetic therapy on 3 neurological diseases with a high socioeconomic impact: Parkinson’s disease (PD), Alzheimer’s disease (AD), and Multiple Sclerosis (MS). We cite only those studies that connect these diseases with magnetic therapy. We focused our efforts on those studies involved with coupling (low frequency) electromagnetic fields (EMF), pulsed electromagnetic fields (PEMF), and transcranial magnetic stimulation (TMS) to determine the pathophysiological effects on cell and molecular biology. Magnetic therapy used for stroke rehabilitation and its effects on variables such as stroke intensity and regeneration times are also included in this review [5].
In this review, we studied “magnetic field therapy” treatment with EMF, PEMF, or TMS. TMS can be applied in single pulses, multiple pulses, or repetitively (rTMS, applied in low or high frequencies). In theta-burst stimulation (TBS), there are three 50-Hz pulses applied at 5 Hz for 20–40 sec as continuous TBS (cTBS) or as intermittent TBS (iTBS) [6, 7]. A magnetic field is produced with a coil, either single or butterfly-shaped. The lines of flux pass perpendicular to the plane of the coil which is normally placed tangential to the scalp. The intensity of the magnetic field can reach up to approximately 2 Tesla, and typically lasts for approximately 100 ms. The magnetic field induces an electric field which is perpendicular to this plane. This electric field excites neurons and currents are induced leading to motor-evoked potentials [8]. Paired pulses lead to short intracortical inhibition and facilitation which reflects cortical interneuron action [9].
Positive clinical effects of TMS in Parkinson disease have been reported in several reviews [10, 11, 12, 13, 14, 15]. Treatment with TMS was superior to placebo [14] in patients with mild disease who have a greater potential for neural rehabilitation [15]. Treatment with TMS improved mobility and activities of daily living scores in the more active patient group [12]. Furthermore, weekly TMS (picotesla flux density) reduced the frequency of freezing and falling [16]. Not only can clinical symptoms of Parkinson be relieved by TMS [11], but also the concentration of dopamine and homovanillic acid in the lumbar cerebrospinal fluid also tended to return to normal values [17, 18]. The enhancement of reduced smell perception after only 7 Hz EMF is representative of EMF’s window effect, specifically, the release of dopamine and the subsequent activation of dopamine D2 receptors within the olfactory bulb [19]. In Parkinson’s disease, there are two proposed mechanisms for coupling of electromagnetic fields: radical oxygen species (ROS), and the effect of ROS on membrane potential (see Main Section) [20, 21].
The influence of electromagnetic fields on membrane potential and cortical excitability is also mentioned in clinical studies of AD by Lopez et al. [22]. TMS therapy has been associated with “cortical rewiring” or “synaptic plasticity”. These phenomena are also reviewed in this manuscript in combination with treatment of the aging brain [23, 24, 25]. Clinically, it was found in AD patients that the application of repetitive TMS can transiently restore or compensate damaged cognitive functions [26].
It has also been reported that in AD patients, application of three dimensional (3D)-pulsed magnetic fields reduces inflammation and produces vasodilatory effects which, in turn, improve blood circulation most likely due to the release of nitric oxide (NO) [27]. In the peripheral blood mononuclear cells of AD patients, Capelli et al. [28] tested the ability of low frequency-PEMF to modulate gene expression in cell functions that are dysregulated in AD (i.e., beta-site amyloid precursor protein cleaving enzyme 1 or BACE1). These investigators observed that LF-PEMF can stimulate epigenetic regulation mediated by miRNAs, which may lead to a rebalancing of dysregulated pathways. The expression of typical AD proteins, such as tau, showed the positive effects of rTMS with low and higher frequencies in studies in AD mouse models [24].
MS is not a typical neurodegenerative disease because of the involvement of the immune system which attacks the myelin sheath of nerve fibers [29]. It is reported that in MS, EMF exerts therapeutic effects through modulation of immune-relevant cells [30]. Another characteristic found in MS patients is reduced blood oxygen, reduced blood circulation, and impaired cell metabolism. Sakamoto and co-workers found that application of magnetic fields with low frequency and intensity improves these parameters and reduces symptoms of MS [31].
Low levels of NO were also found in the brains of MS patients [32, 33]. Following application of magnetic fields, this parameter normalized in cell models [34, 35]. The dual role of NO is discussed for pain transmission [35] as NO inhibits nociception in the peripheral and in the central nervous system, as well as mediating the analgesic effect of opioids and other analgesic substances. Hochsprung et al. [36] found that treatment with PEMF may be effective in reducing pain in patients with MS, using monopolar dielectric transmission of pulsed electromagnetic fields.
In summary, a number of clinical parameters are positively altered after electromagnetic therapy in patients with neurodegenerative diseases.
Time-varying magnetic fields produce forces on charges and are more effective
than static magnetic fields [37]. Time-dependence of the magnetic field (B(t))
induces an electric field (E) according to Faraday’s law: CurlE =
–1/c dB/dt. In this equation, the vector E stands for the electric field, the
vector B represents the magnetic induction, and B = H + 4
Time-oscillatory magnetic fields induce intracellular eddy currents, which, according to the Lentz rule, counteract the change of the external magnetic field. Eddy currents appear in materials which are electrically conducting, especially on cell membranes. Static magnetic fields produce forces on charged particles in motion. Because cellular plasma membranes are constantly moving, even static magnetic fields produce time-varying forces on the charged particles in the brain [38, 39].
Charged ions such as sodium, potassium, calcium, and magnesium are present in
all tissues of the body. Most of the biomolecules possess charges and therefore
they can be directly influenced by electric fields [40, 41]. In general, there
are multiple methods for coupling electrical fields, for example, by voltage
gated calcium channels, nonspecific charged moieties like Ca
The cell membrane and its embedded molecules are the most relevant candidates for EMF-coupling because of the very high gradient of electric field at this location [40]. The cell membrane generates a resting potential which comes from the segregation of charged ion concentrations by molecular machines such as pumps, transporters, and ion channels largely situated within the plasma membrane [41]. Levin and coworkers showed that artificial depolarization holds the cells in an undifferentiated and proliferative state, while artificial hyperpolarization accelerates differentiation [42]. A switch between pathological (e.g., inflamed) and normal states can be elicited by external changes of the membrane potential [43, 44]. EMF, PEMF and TMS [45] can each influence this resting potential.
Microdomains of ion channels and transporters are distributed in patterns across the entire two-dimensional surface of the cell membrane [41, 42]. Within the membrane, PEMF can activate voltage-gated calcium channels (VGCC) [46] (Fig. 1). From these channels, specific signal amplification processes carry membrane-mediated effects into the interior of the cell [47, 48]. During TMS stimulation of the cortex, neurons are most excitable when their membrane potential is just below threshold but not discharging [45]. It has been shown that TMS directly acts more on the surface layers of the cortex, where an electric field will induce a change in the resting transmembrane potential by superimposing an electrically induced transmembrane potential [25].
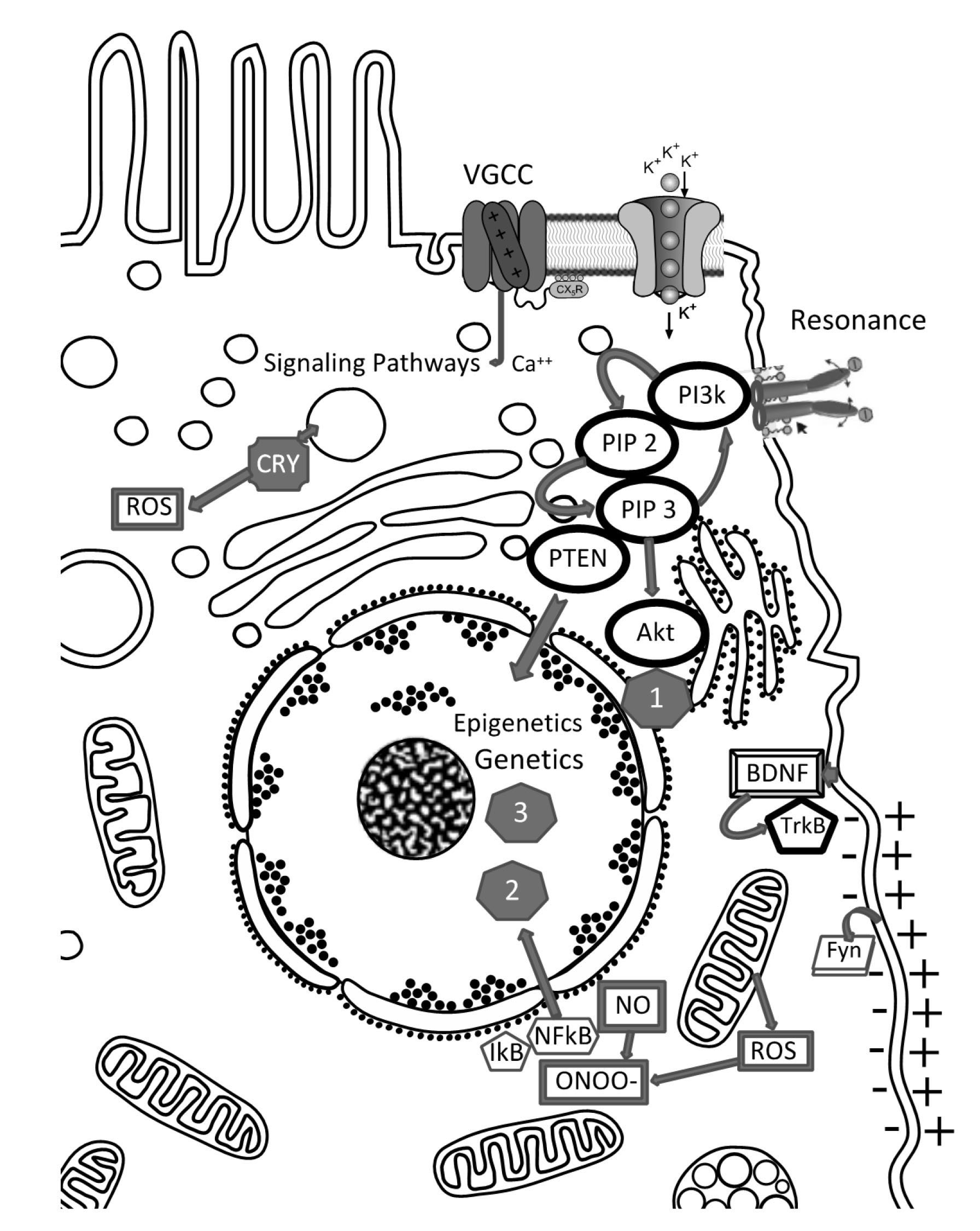 Fig. 1.
Fig. 1.Primary entities for direct coupling EMF to cell: cell membrane (- +, de-, hyperpolarization) with embedded or coupled molecules: Fyn (Fyn kinase - see main text); BDNF - TrKB pathway; VGCC channels (voltage gated channels), other ion channels (like potassium channels). Resonance: ligands with polar moieties can go into resonance with EMF-frequencies.
When the electric current penetrates the membrane, a neuronal membrane may be depolarized and/or hyperpolarized from its resting value, which causes excitation or inhibition of the cell. This can lead to secondary training effects of the neurons evoking new synaptic wiring also via long term potentiation and activating a family of tyrosine kinases (e.g., Fyn) [49, 50] (Fig. 1). Training effects are especially important for the aging brain. TMS enhanced synaptic markers activate the brain-derived neurotrophic factor (BDNF)-tropomyosin receptor kinase B (TrkB) pathway (Fig. 1) as well as the downstream kinase Fyn, enhancing glutamatergic synaptic transmission and increasing phosphorylation of the subunits of N-methyl-D-aspartate (NMDA) receptors in the hippocampus [23]. This suggests that these events lead to changes in structural plasticity in the aged hippocampus and improve cognitive function.
In the cortex of the rat brain, TMS fields stimulate other neurons that inhibit
the activity of dendrites from neurons within the deeper cortex layers [51]. This
inhibition process depends on a type of receptor protein in the dendrites termed
GABA
The topographical pattern of the cell membrane can encode additional information
[42, 45]. For example, time varying patterns of molecular fluctuation and
specific rhythms can enhance such information [52] and, accordingly, the
signal-noise ratio can be lowered significantly. For coupling EMF, a
discontinuous cell geometry with clustered receptors favors EF detection [53].
Specifically, if macrophage-operated Ca
In mitochondria, a very high membrane potential is normally present as the outer membrane potential measures 180–220 mV compared to maximal 70–90 mV resting potential of the cell’s plasma membrane [57]. Using “nano-pebble” sensors, Tyner et al. [58] and Lee and Kopelman [59] found that the membrane potential of mitochondria spreads to a wider distance than was predicted using the parameters for shielding and damping by stochastic Brownian movement of random water molecules. Thus, magnetic therapy can also affect mitochondrial function, and this can lead to changes in ROS and NO production (see below).
In the cell membrane, receptors or channel proteins can also function as levers or antenna, activated by resonance phenomena. This is because charged molecule elements can be addressed “non-specifically” by appropriate resonance frequencies of EMF (Fig. 1). Following this step of a signaling cascade, secondary messengers are elicited and this initiates “classical” pathways [38, 41, 60].
Secondary, downstream events are elicited, e.g., via receptor tyrosine kinases,
PIP2 (Phosphatidylinositol 4,5-biphosphate), PIP3 (Phosphatidylinositol
3,4,5-triphosphate) and lipid Phosphatase PTEN (Phosphatase and Tensin homolog).
PIP3 can signal further via Akt and Akt itself is the center of many other
signaling pathways (1): for protein synthesis acting on growth, differentiation,
migration etc. The Ca
Tertiary reactions arise within the nucleus via epigenetic modification of gene expression or direct gene regulation, leading to (2) Redox homeostasis, cell survival and growth or (3) altered gene expression or, e.g., changes in the cell cycle.
As messengers, NO and also ROS may induce activation of the Nrf2 antioxidant pathway and exert protective effects [61, 62] with a reduction of cell and oxidative damage biomarkers. Regarding NO production, Chinon et al. [63] observed that increased NO levels in stroke patients after TMS are associated with neural nitric oxide synthetase (nNOS) and/or endothelial NOS (eNOS) activities, but not with inducible NOS (iNOS) expression. Cho et al. [5], showed that ELF-EMF (60 Hz, 2 mT) increased the expression and activation of nNOS in rat brain [63]. In contrast, activation of nNOS and eNOS are dependent upon calcium ions and there are many reports that the biological effects of ELF-EMF are related to the control of calcium channels [64]. Therefore, the observed mechanism of increased NO generation and metabolism may be associated with calcium-ion flux.
Amplification via calcium flux may also provide a means by which the
membrane-mediated effects of EMFs could be carried into the cell [41, 57]. The
cellular site of F-actin-based Ca
Other cellular events are elicited via receptor tyrosine kinases (RTK), Phosphatidylinositol 4,5-biphosphate (PIP2), Phosphatidylinositol 3,4,5-triphosphate (PIP3), and lipid Phosphatase and Tensin homolog (PTEN). PIP3 can activate pathways via the serine/threonine kinase Akt, and Akt itself is the center of diverse signaling pathways. Hence, these signaling cascades may be functionally accessed by various mechanisms [38] (Fig. 1). Yao et al. [65] have also shown that the PEMF effects can also effect gene expression as they found, in vitro, that PEMF promote differentiation of oligodendrocyte precursor cells.
Epigenetic changes have also been reported as repetitive TMS applied over the frontal cortex of awaken mice induce dopamine D2 receptor dependent persistent changes of CDK5 (cyclin dependent kinase 5) and PSD-95 (postsynaptic density protein 95—a member of the membrane-associated guanylate kinase) protein levels specifically within the stimulated brain area [66]. These modifications were associated with changes of histone acetylation within their gene promoter region and this event was prevented by administration of a histone deacetylase inhibitor. Consales et al. [67] presented a critical overview of the epigenetic changes triggered by deep brain stimulation and TMS in both Parkinson patients and neurons from different experimental animal models. In peripheral blood mononuclear cells of AD patients, Capelli et al. [28] tested the ability of Low Frequency-PEMF to modulate gene expression in cell functions that are dysregulated in AD (i.e., BACE1). They observed that LF-PEMF can stimulate epigenetic regulation mediated by miRNAs, which would lead to a rebalancing of the pathways deregulated in the pathological state. However, further studies at the molecular level are necessary regarding the complex network of epigenetic signals and the possibility of potential adverse effects.
AD mice showed a long-term impairment of cognition and memory after PEMF exposure and this resulted in AD symptoms in these mice [68]. The authors of this study argue that EMF can enhance oxidative stress, and this might be related to the autophagy dysfunction seen in these animals. Higher MHz frequency and a longer duration of autophagy can lead to demyelinization in mouse brains [69]. In contrast, in keeping with the phenomena of EMF windows and intensities, Marcesi et al. [70] found that autophagy is positively modulated in human neuroblastoma cells through direct exposure to low frequency electromagnetic fields. As a proposed mechanism, the authors cite in vitro the expression of a microRNA sequence that affects autophagy via Beclin1, an ortholog of autophagy-related gene 6 and BEC-1, expression. The authors of this study discuss the positive cytoprotective effect of autophagy in the clearance of protein aggregates within the cells in diseases such as AD.
The significantly enhanced expression of plasticity genes 24 h after intermittent Theta Burst Stimulation (iTBS) as compared to sham TBS was found in a human neuron-like cell model [71]. This specific effect provides support for the widely assumed plasticity mechanisms underlying iTBS effects on human cortex excitability.
ROS production is another molecular link regarding magnetic stimulation. Changes in cellular ROS levels, induced by PEMF devices, may explain their beneficial and healing effects. Interestingly, concentrations of ROS induced by such devices are much lower than those induced by oxidative stress [72, 73]. Paradoxically, ROS plays a beneficial role by stimulating antioxidant defense and repair pathways, and the therapeutic effects of PEMF have been documented in several pathologies involving defined cellular mechanisms [74].
PEMF can stimulate a rapid accumulation of ROS in mammalian cells [72]. Following exposure to PEMF, cell growth is slowed, and ROS-responsive genes are induced [72]. These effects require the presence of cryptochrome, a putative magnetosensor, which synthesizes ROS. Cryptochromes are ubiquitously expressed flavoproteins that undergo conformational change and generate a radical pair in the presence of either light or magnetic fields [75, 76]. Conversely, a positive effect of magnetic field exposure was reported during seizure recovery in Drosophila larvae [77]. Similarly, this effect is dependent on cryptochrome suggesting a magnetically sensitive, photochemical radical pair reaction in cryptochrome that alters levels of neuronal excitation. Finally, repetitive TMS at low intensity induces axon outgrowth and synaptogenesis which can repair a neural circuit in in vivo and ex vivo situations such as postlesion axonal outgrowth and olivocerebellar reinnervation in the mouse. This repair depends on complex biomimetic patterns being particularly effective, and the presence of cryptochrome [78].
These contradicting results regarding ROS concentration can be resolved by a single exposure to ELF-PEMF induced ROS production in human osteoblasts without reducing intracellular glutathione [79]. Repetitive exposure to PEMF, however, reduced ROS levels suggesting alterations in antioxidative stress response. Scavenging of radical species diminished the PEMF effect on osteoblast function [73]. Thus, it is concluded that PEMF elicited non-toxic amounts of ROS and that reactions to ROS generated by PEMF may also result in preconditioning for these cells [81].
This compilation of reports regarding magnetic and EMF stimulation in neurological diseases paints a complex picture, due to the many variations in duration, intensities, resonance effects, as well as window effects.
In this manuscript, we have tried to determine important molecular and cell biological links for coupling low frequency electromagnetic fields derived from animal and clinical studies. Among other factors, the resting potential of stressed, inflamed, or compromised cells may initiate this switch and result in improved outcomes for these patients with neurologic disorders [81]. Charge-sensitive receptors and channels embedded in the cell membrane can activate a variety of signaling cascades leading to different secondary cellular and tissue reactions such as protein synthesis, growth, migration, and differentiation. We also stress the importance of ROS generation, especially from mitochondria with their very high outer membrane potential. This organelle has to handle the electron transfer chain which comes with the risk of escaping electrons leading to ROS and NO production. Both messengers, as well as associated signaling cascades, have the capability of inducing epigenetic and genetic alterations that can ultimately lead to changes in gene expression which can affect cell survival, redox homeostasis, and many other cellular reactions.
Compared to the electrical coupling, the role of “magnetic interactions” remain controversial. The newly found putative magnetosensor, cryptochrome, has the potential to shift the focus on EMF, PEMF and TMS effects onto their magnetic component. Therefore, it is important that biophysics, and related disciplines, investigate the quantum radical pair mechanism and the role of the cryptochromes [82, 83].
With numerous publications emerging in this field in recent years, we are now beginning to better understand the causal principles of coupling EMF to biological phenomena. Hallet [8] noted that TMS is a powerful instrument for the clinical neurophysiologist especially in the diagnosis of neurological disorders. Since most of these effects are mild and often transient, further investigation is necessary to understand the underlying principles of these EMF-induced effects.
A more thorough understanding is necessary regarding the electric nature of the inner components of the cell, such as organelles and biomolecules of mitochondria using nano-pebble sensors to determine the mechanism for a wider spreading of internal, cellular electric fields. By developing precise EMF measurements within the cell´s interior, these limitations of EMF-magnetic and TMS studies can be better understood.
MF provided the basic concept and the principles in physics and of magnetic therapy for the relevant diseases. RHWF provided the description of biological principles of magnetic and electromagnetic effects and of clinical effects. RHWF performed the final editing of the manuscript.
Not applicable.
The work mentioned in this review was partly funded by the Saxonian Ministry of Science and Education, GWT, HZDR, TUD (project NeuroMaX).
This research received no external funding.
The authors declare no conflict of interest.

