1 Laboratory for Molecular Reproduction and Genetics, Department of Anatomy, All India Institute of Medical Sciences, 110029 New Delhi, India
2 Department of Psychiatry, All India Institute of Medical Sciences, 110029 New Delhi, India
Abstract
Major depressive disorder (MDD) is a mind-body disorder. Cellular aging has been implicated in the pathogenesis of MDD with the altered mind-body communication markers like stress response, immune response, nutrition sensing, and a range of other regulatory feedback systems. In this age of super specializations, one specific target and interventions (preferably a drug) on it are being rigorously sought by the health care community and industry, but have failed in it in the last fifty years in spite of advances in technology. Since, depression is a complex disorder associated with increased incidence of other complex disorders, it must be treated by an integrated holistic approach that can address the complexity of MDD. Interventions targeting accelerated biological aging to increase cellular health in whole body have potential to manage complex conditions like MDD and its overlapping symptoms and comorbidities. Yoga has the potential to be the nexus between, clinical management of MDD and other lifestyle diseases.
Keywords
- Depression
- Disorder
- Mind
- Body
- Inflammation
- Sleep
- Review
Major depressive disorder (MDD) is a chronic condition marked by distinct bouts of depression causing clear shifts of attitude, desires and satisfaction, cognitive changes, and vegetative symptoms. MDD affects about 6% of the adult population worldwide each year [1]. Of all medical disorders, as calculated by years of disability, MDD is the first significant contributor to the burden of chronic illnesses [2]. Established first-line treatments for MDD include pharmacotherapy and psychotherapy (Table 1). For the last several decades monoamine hypothesis (particularly serotonin) has dominated to provide aetiopathological mechanisms and strategies for drug discovery. In fact, current first-line pharmacotherapy (SSRIs) is based on serotonin hypothesis. The diagnostic criteria based on the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) is commonly used for diagnosis and management of MDD. But, there are several limitations to define MDD. Patients often have feelings of worthlessness, or guilt or cognitive dysfunctions like diminished ability to think or concentrate, or indecisiveness. Commonly present vegetative symptoms include: considerable changes in weight, appetite and sleep; psychomotor agitation or retardation; fatigue or loss of energy; recurrent suicidal thoughts with or without a specific plan. In addition to severity other specifiers of MDD according to DSM-5 are: anxious distress, mixed feature, melancholic features, psychotic features, peripartum onset, and seasonal pattern. Current drugs that modify mainly the monoamine homeostasis are insufficient to provide relief from these symptom complexities. Moreover, there are limitations in the current directions in exploring right treatment for MDD. Industry is still focused on refining drugs based on monoamine hypothesis. With regard to emerging treatment approaches, antidepressant and neurological interventions like rTMS (non-invasive), and deep brain stimulation (DBS) (invasive) are currently under scientific scrutiny to provide clinical improvement in treatment resistant MDD. Although promising findings of efficacy are extensively reported, approximately 40% patients don’t remit from MDD [3, 4]. Mechanism of action suggested by these interventions is by modifying neuroplasticity. While, increased neuroplasticity in the brain may provide clinical improvement acutely, neuroplasticity changes in undesired regions of the brain may lead to permanent damage to brain and clinical complications similar to substance abuse and addiction.
| Therapy | Description |
| Pharmacotherapy | Selective serotonin reuptake inhibitors (SSRIs), selective noradrenaline reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs) and other cyclic antidepressants, noradrenaline-dopamine reuptake inhibitors (NDRIs), α2-adrenergic receptor antagonists, melatonin receptor (MT1 and MT2) agonists, and monoamine oxidase (MAO) inhibitors etc. |
| Cognitive-behavioral therapy | Cognitive-behavioral therapy (CBT) shows people with major depressive disorder (MDD) how to understand and question pessimistic, skewed cognitive habits that lead to depression, as well as how to test and challenge these negative emotions and substitute them with more accurate constructive ones. |
| Behavioral activation therapy | Behavioral activation treatment helps to enhance the patient’s constructive behaviors that give him/her a feeling of achievement or superiority. Identifying and addressing evasion processes is a common goal of this therapy. |
| Psychodynamic therapy | Psychodynamic therapy aids the patient in discovering and recognizing how feelings, thoughts, and past life events have generated habits that lead to current issues. |
| Problem solving therapy | Problem management therapy gives people a standardized range of skills for coming up with innovative solutions to challenges, finding and resolving possible roadblocks to meeting goals, and making rational choices. |
| Interpersonal therapy | Interpersonal counselling helps to aid individuals with understanding and addressing difficulties in their interactions and social identities, such as interpersonal problems, role changes, and deteriorated or impoverished relationships. |
| Mindfulness-based therapy | Mindfulness has its roots in contemplative traditions, especially Buddhism, which includes daily meditative meditation in which one pays nonjudgmental attention to one’s emotions, emotions, and perceptions, learning to recognize things as they are without attempting to alter them. |
| Yoga-based lifestyle intervention | Yoga is a mixture of physical activity and cognitive concentration that seeks to encourage general healing and mental equilibrium. |
There is a need to look at the wider and complex perspective of the disorder and refine future directions for exploring treatments for MDD based on it. Monoamine hypothesis has limitations in explaining the several aspects of the pathobiology, and clinical manifestations of MDD. Based on genome-wide interaction studies, the genetic contribution to MDD is estimated to be around 35 percent (GWAS). This result indicates that the likelihood of developing MDD is closely linked to environmental conditions [5]. Lifestyle factors associated with modernity such as adverse life events (ALEs) during childhood and adult life, stress, sedentary life, and unhealthy nutrition, significantly contribute to causation of MDD. Our understanding of how environmental factors interact with genetic and epigenetic factors is far from complete. Unhealthy lifestyle is a major factor to induce accelerated biological aging associated with MDD. MDD is associated with an increased risk of developing conditions such as diabetes mellitus, heart disease and stroke, thereby further increasing its burden of disease [6]. Accelerated cellular (biological) aging is at the root of increased co-morbidities and increase in global burden of disease. Cellular aging affects neuroplasticity and contribute to smaller hippocampal volumes MDD. MDD is related to changes in neural network activity or communication, such as the emotional stimulation network [7]. Cellular aging that cause accelerated biological aging is associated with derangements in the higher function neural networks. Moreover, cellular aging leads to alterations in the main neurobiological systems including the hypothalamic-pituitary-adrenal (HPA) axis, the autonomic nervous system and the immune system that mediates the stress response which is evident in MDD. Alterations in the mechanisms that regulate nutrition sensing play a vital role in cellular aging and are an extensively researched target in the field of aging [8]. Mechanisms regulating pain and pleasure also add to the complexity of MDD. Such disruptions in mind-body communicative regulatory mechanisms have not been fully addressed in current and emerging treatments. At the cellular level, hallmarks of accelerated biological aging include OS, mitochondrial dysfunction, DNA damage, and telomere attrition.
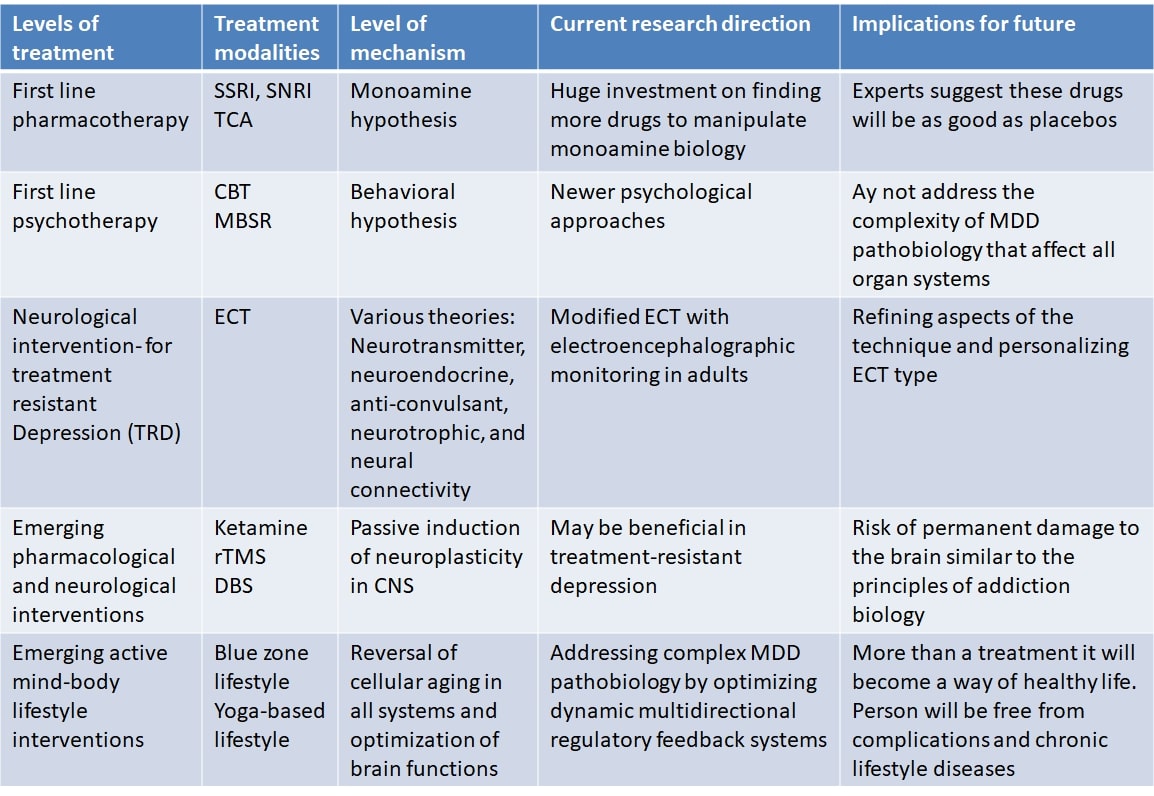
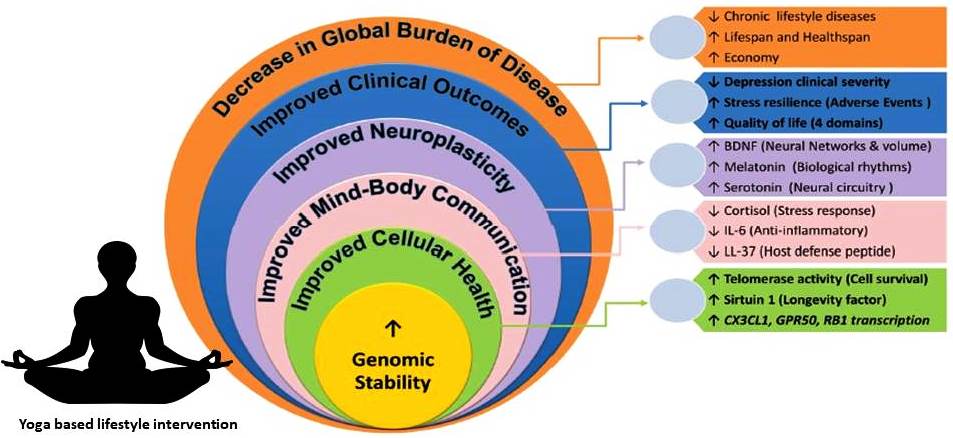
Since MDD affects both mind and body it leads to severe impairments in living of the individual, and disrupts functioning of the family, workplaces, and society as a whole. Modernity is associated with high levels of stress, increased susceptibility to stress, and decreased stress resilience among the population. Therefore, stress has a major role in MDD causation. MDD is associated with decreased quality of life in all four domains suggested by WHO, viz., physical, psychological, social, and environmental. In addition, MDD can lead to suicidal attempts. It is estimated that up to 50% of the 800,000 suicides per year globally occur within a depressive episode and patients with MDD are almost 20-times more likely to die by suicide than the general public [9]. Interventions that address the complexity of MDD and reverse accelerated biological aging have the potential to cure MDD and provide a healthy longevity. Two lifestyles are particularly prominent around the globe among people living a long healthy life and almost free chronic lifestyle diseases including depression. These include lifestyles in the blue zones of the world and yoga based lifestyle. Several recent studies have reported that YBLI can decelerate biological aging, increase neuroplasticity, and has a huge clinical utility in MDD [10, 11]. In this review, we provide an overview of the challenges for effective MDD treatment, complexities of the aetiopathology of MDD, limitations in research for future treatments, and yoga based lifestyle intervention as a simple and effective treatment for MDD. Mechanisms of treatments including yoga based lifestyle intervention are briefly explained. Fig. 1 illustrates the levels of MDD treatment during evolution of the management of MDD and the increasing complexity of the disorder that they address. We also outline the key outstanding research questions in the field that should be addressed in the coming years.
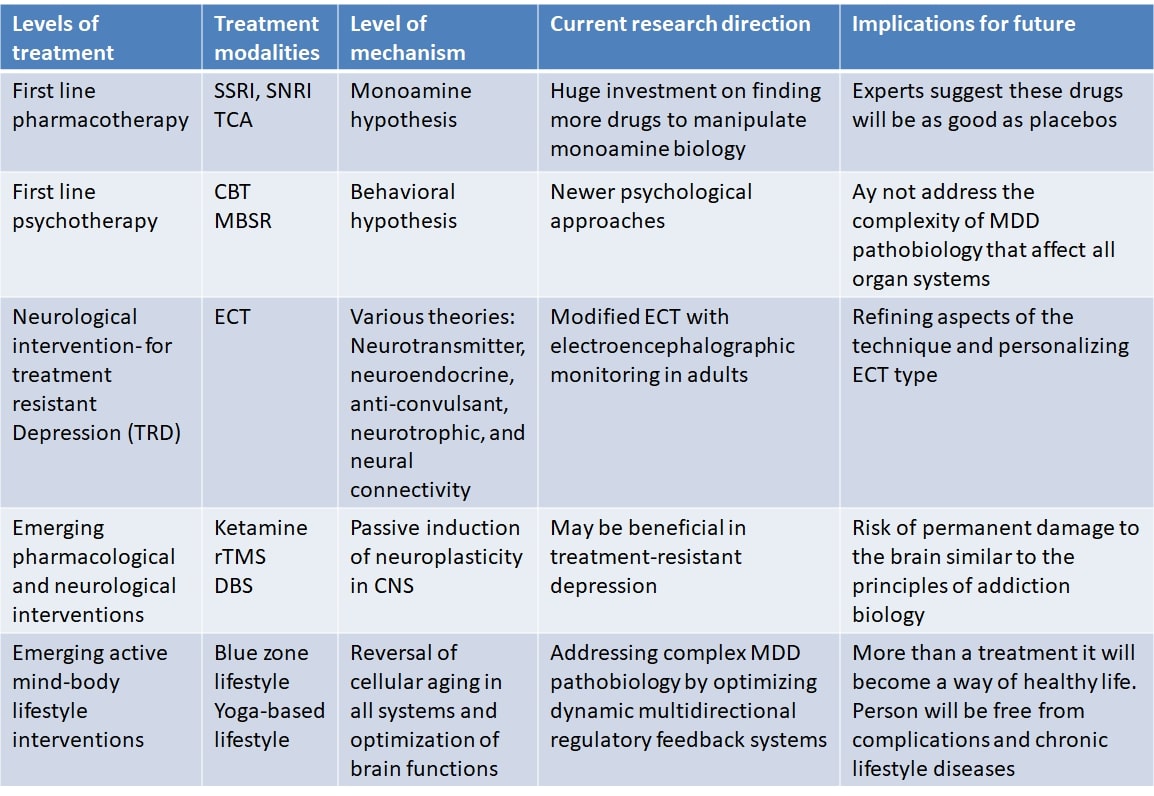 Fig. 1.
Fig. 1.Evolution of the management of MDD is illustrated where increasing complexity of the disorder is addressed as the level of MDD treatment increase.
MDD is one of the most common disorders seen in medical practice worldwide that has become the largest contributor to the global burden of disease [2, 12, 13]. Recent studies show that the prevalence of MDD in the general population is increasing, and the lifetime prevalence range from 6% to 36% [14]. Various causes of MDD are listed in Table 2. MDD occurs about twice more often in women than in men [1]. It is a new global pandemic impacting the mind as well as the body, and has increased the risk of mortality by 60–80% by causing accelerated biological aging and increasing predisposition to other lifestyle-related non-communicable diseases (NCD’s) [15]. MDD exhausts the population psychologically, socially and emotionally; furthermore, it affects productivity and economy. Therefore, MDD and other chronic lifestyle diseases have become the bane of modern society [16].
| Factors | Description |
| Demographic factors | Age, gender, and ethnicity |
| Socioeconomic factors | Socioeconomic status (for example, poverty, unemployment, income inequality and low education) |
| Healthcare affordability | |
| Lifestyle factors | Alcohol use, smoking behavior, a high fat or high sugar diet and physical inactivity |
| Adverse Life Events | Childhood adversity |
| Adulthood ALE | |
| Modern Socioenvironmental | Habitat design problems (Urban and Rural) |
| Neighborhood factors (for example, inadequate housing, overcrowding, neighborhood violence and safety) | |
| Social drift in modern habitats | |
| Healthcare policy, resources, and accessibility | |
| Modern Socioenvironmental events | Battle, conflict, displacement, inequality, job challenges, poor social care, trauma, and traumatic life events are all examples of negative life events |
| Modern Natural Environment | Environmental pollution, Environment degradation, Climate change |
| Modern Natural Environmental events | Natural disasters |
There is a rapid increase in the treatment-resistant depression (TRD) who doesn’t respond adequately to antidepressant drugs [17]. A meta-analysis found that many factors, including old age, marital status, long length of the present depressive episode, moderate to high suicidal risk, anxious comorbidity, high number of hospitalizations and comorbidity with other psychological and somatic disorders, are correlated with medication resistance [18].
In children and teenagers, depression is relatively common, but mostly unrecognized. Various genetic and environmental factors play a triggering role in the etiopathogenesis of depression in children. Children can experience some stressful events during adolescence, which are known as a significant risk factor for adult depression. These activities can contribute to physiological dysregulation, with long-term consequences of increased allostatic load until adulthood, leading to depression [19]. Clinical depression, along with diminished social functioning, is a condition with chronic mental, biochemical and psychiatric manifestations. In childhood depression, acute life events such as family dysfunction or persistent child neglect can also result. Depression leads to life-long morbidity and death, so it is especially important to identify the effects of childhood depressive symptoms because they may have long-term adverse effects on adult psychosocial transition [20]. Irritability, frustration, feelings of despair and hopelessness, social isolation, heightened vulnerability to rejection, changes in appetite, changes in sleep habits, vocal outbursts or weeping, difficulties in concentrating, exhaustion and low energy, decreased ability to cope during activities are the signs and symptoms of childhood depression [19]. Biological-hormonal shifts and environmental conditions are likely to lead to an increase in the prevalence of teenage depressive symptoms. Perhaps the greatest challenge is early diagnosis and intervention in mood disturbances. A study by S H Stewart et al. [21] has shown the association between hopelessness, depression and drug abuse and excessive drinking. An influential association between depressed symptoms and drinking to cope was being utilized, to alleviate all negative feelings of depression and obstruct pessimistic thinking typically associated with depression.
MDD is common in patients with rheumatoid arthritis, with a prevalence of
13–42%, at least double to four-times that in the general population [22]. There
is bidirectional association of depression and rheumatoid arthritis (RA) [23]. In
RA patients, the reduced quality of life, poor clinical characteristics and
functional ability are associated with subsequent depressive symptoms [22].
Patients with RA and comorbid depression experience worse health outcomes and
mortality rate. Studies have shown that complementary and alternative medicine
therapies like yoga are popularly used as an adjunct to modern medicine and help
in the reduction of depressive symptom [10, 11, 24]. Yoga involves a collection
of physical activity and mental concentration, which helps to offer complete
healing and peace of mind. Psychological components associated with RA have been
ineffectively dealt with by disease modifying anti-rheumatic drugs (DMARDs),
which leads to an exaggeration of the disease symptomatology. Yoga promotes
neurogenesis as it upregulates brain-derived neurotropic factor (BDNF), which is
a marker of neuroplasticity [25]. Also, yoga results in significant fold change
by an upregulation in levels of CX3CL1, GPR50, and RB
gene; CX3CL1 is a cytokine found in the brain, especially in neurons with
receptors in microglia with an essential role in microglial migration [26]. Its
levels are upregulated with spatial learning and facilitate neurotransmitter tone
and maintain protective plasticity of synaptic or homeostatic scaling. Also,
there was an upregulation in levels of GPR50 and expression of the RB gene, a
tumor suppressor gene [27, 28, 29]. Depression is associated with an increased risk of
cancer and also in cases like RA, which is a chronic progressive inflammatory
arthritis. A research from our laboratory showed a substantial decrease in
depressive symptoms of patients with RA who performed yoga along with regular
medicines, as indicated by their time-dependent decreased BDI-II (Beck Depression
Inventory-II) scores [24, 30, 31]. Randomized research results show that yoga
decreases symptoms of anxiety and depression, decreases inflammation and
sympathetic tone, and improves vagal function [24]. Deficiency of
neurotransmitters like dopamine, serotonin, and norepinephrine are associated
with anxiety, social phobia, and depression. Studies have shown that yoga
dramatically increases neuroplasticity-related markers such as BDNF, serotonin,
and
Currently, main initial treatment options for the management of MDD include pharmacotherapy and psychotherapy as first-line treatment, and electroconvulsive therapy (ECT) for TRD [34]. An initial strategy of ‘watchful waiting’ without treatment can be pursued in a mild depressive episode. Patient preferences and prior history of treatment are always taken into consideration during MDD management.
Pharmacologic modulation of monoamines is among the first line of treatments.
Selective serotonin reuptake inhibitors (SSRIs) are the latest generation
monoamine antidepressant drugs. Other monoamine based drugs include selective
noradrenaline reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs) and
other cyclic antidepressants, noradrenaline-dopamine reuptake inhibitors (NDRIs),
They all have fundamentally comparable moderate effectiveness, regardless of their pharmacological class, with reaction rates of about 50 percent and a characteristic sluggish response (typically more than several weeks) to medication [38]. Although SSRIs and SNRIs have a generally more favorable adverse-effect profile, they too have several tolerability issues that include acute treatment adverse effects like nausea, insomnia, headaches, dizziness, and gastrointestinal symptoms, and long-term adverse effects like weight gain, sexual dysfunction and sleep disturbances [39].
In current drug therapies only monoamine neurotransmitters are targeted, either only one in the new generation antidepressants like SSRIs or multiple in the earlier generation antidepressants like TCAs, MAOIs, and SNRIs. While monoamine theory, which is the basis for current drug therapies, has generated an abundance of fruitful research and advances in the understanding and treatment of MDD, it has not only left great gaps in our understanding of the pathophysiology of this condition, but also has failed to provide optimal remission rates [40]. Despite six decades of development of monoamine-modulating antidepressant medications, remission rates have not exceeded those seen with tricyclic antidepressants and MAO inhibitors in the 1960s [36]. Although safety and tolerability of antidepressants have indeed improved, improvement in efficacy continues to elude research efforts.
The main lacunae with monoamine theory and drug therapies based on it are due to the exclusive focus on neuronal transporters and receptors that may not be sufficient mechanism of antidepressant action. Glial cells (which represent 90 percent of the cellular population of the brain) and their uptake sites play an essential role in the pathophysiology of MDD and its treatment [36]. The majority of monoamine release is extra-synaptic and therefore out of reach of mostly pre-synaptically located neuronal transporters. Therapies that consider the complexities beyond monoamine transmission may provide optimum clinical outcomes.
MDD psychotherapy appears in many distinct ways. These various paradigms depend on various philosophical models and recommend strategies that differ in their emphasis and methodology to some degree. A large number of randomized controlled trials and meta-analyses consistently show that psychotherapy is effective in treating MDD; no consistent or clinically meaningful differences are evident between different types of psychotherapy [41, 42]. This conclusion has led to two large theories to understand the success of psychotherapies: the explanation of unspecific or general variables and the explanation of specific factors [43]. The former claims that primarily those common to all psychotherapies are the key agents for improvement in psychotherapy, such as the therapeutic alliance and therapist influences. The above suggest that treatment-specific interventions induce improvement through multiple mechanisms, such as cognitive restructuring, activation of actions or enhanced interpersonal functioning.
The persistence of the beneficial effects of psychotherapy is only for a short duration [44]. Many persons have barriers to entry, including time restrictions, the lack of resources and costs available.
Strategies commonly used in treating TRD include high-dose drug therapy, combination therapy, the strategy of switching, augmentation with non-antidepressant drugs (such as lithium, l-triiodothyronine (T3), or atypical antipsychotic drugs, and combination treatment (more than one antidepressant simultaneously). Several studies like the STARD study have shown that such strategies provide only a limited success in remission (10–25%) [3]. Often psychotherapy (most commonly cognitive-behavioral therapy) is used as either augmentation or substitute therapy in TRD with mixed results.
ECT is one of the most established strategies for treating TRD. In ECT, once the patient has given informed consent, a seizure is elicited during brief anesthesia. The most commonly used and effective non-pharmacological biological therapy for TRD is known to be ECT [45]. It is widely used, for example, in extremely deeply depressed and/or highly suicidal patients where a rapid antidepressant reaction is needed.
Its adverse effects on memory, in particular anterograde and retrograde amnesia, are the key tolerability concerns with ECT. Several refinements are being tested to increase tolerability like right unilateral ECT [37] and ultra-brief pulse-width stimulation [46].
Newer treatments for MDD include numerous pharmacological and non-pharmacological approaches. Pharmacological approaches include non-monoamine based drugs and parenteral or intranasal ketamine and esketamine [47]. Non-pharmacological approaches include non-invasive neurological interventions like rTMS, deep TMS, magnetic seizure therapy (MST), transcranial direct current stimulation (tDCS), low-field magnetic stimulation (LFMS), and vagus nerve stimulation (VNS), as well as invasive neurological interventions like DBS [48].
Most of these emerging approaches are developed based on the recent proposition that disruption of neuroplasticity and, accordingly, neurogenesis is the primary aetiopathological mechanism in MDD. These approaches increase neuroplasticity through several mechanisms [49]. Various MDD-associated pathways eventually cause depressive symptoms by influencing neuronal brain activity, mainly by undermining neuroplasticity and, subsequently, neurogenesis, the process by which new neurons are produced from pluripotent stem cells in the adult brain. By regulating neurogenesis, BDNF and other neuroplasticity regulators may influence behavior [50]. BDNF mRNA levels are also lowered and pharmacological and non-pharmacological antidepressant treatments have been shown to normalize BDNF levels [51].
In the last two decades, attempts have been made to create non-monoamine-based
antidepressant medications that are devoid of any of the untoward effects of
these drugs and can cause therapeutic improvements even quicker [47]. Various
compounds like neurokinin 1 antagonists, glutamatergic system modulators,
anti-inflammatory agents, opioid tone modulators and opioid-
Standard rTMS uses an eight-shaped coil to modulate neuronal activity to a maximum depth of 1.5–2.5 cm from the scalp. A latest overview of 18 TRD research of rTMS concluded that, for sufferers with MDD with at the least antidepressant remedy failures, rTMS is a reasonable, powerful consideration [52]. However, a meta-amalysis has proven that rTMS is not as good as ECT with reference to efficacy in TRD [41]. Newer methods under research are deep TMS, MST that combines elements of rTMS and ECT, tDCS, and LFMS that refers to a form of brain stimulation delivered in a magnetic field waveform inducing a low, pulsed electric field in the brain. VNS involves the surgical implantation of a pacemaker-like pulse generator in the chest, which is connected to a stimulating electrode attached to the vagus nerve. VNS outcomes result in activation of various subcortical mind systems and the stimulation of hippocampal neurogenesis [53]. Clinical utility of these is unknown.
DBS includes the implantation of a pulse generator related to 2 stimulating electrode wires, surgically positioned in precise mind regions. DBS is normally reserved for sufferers with the most intense sorts of TRD and calls for in addition assessment of management methods and its function in MDD therapy [54].
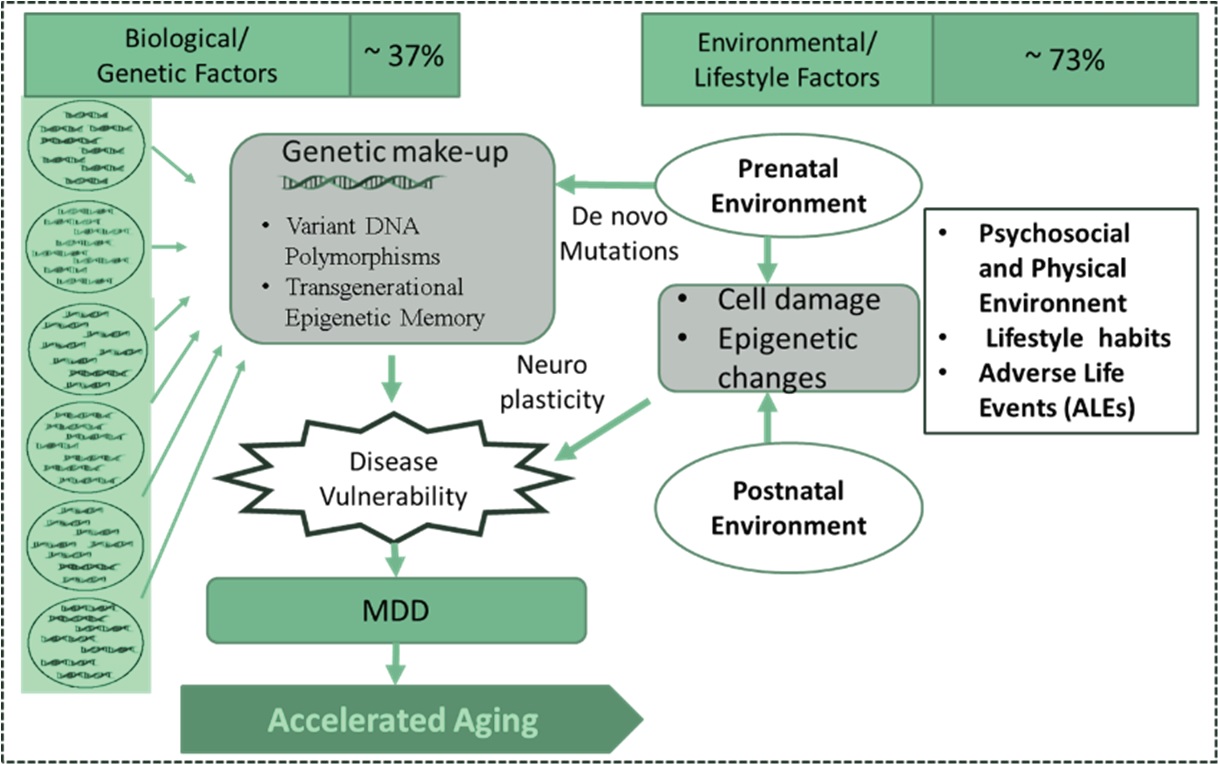
MDD often persists and lead to devastating problems [55]. Narrow perspectives on depression are common, but to understand the complex aetiopathogenesis of MDD, it is essential to consider multifactorial genetic environmental and stochastic factors (Fig. 2).
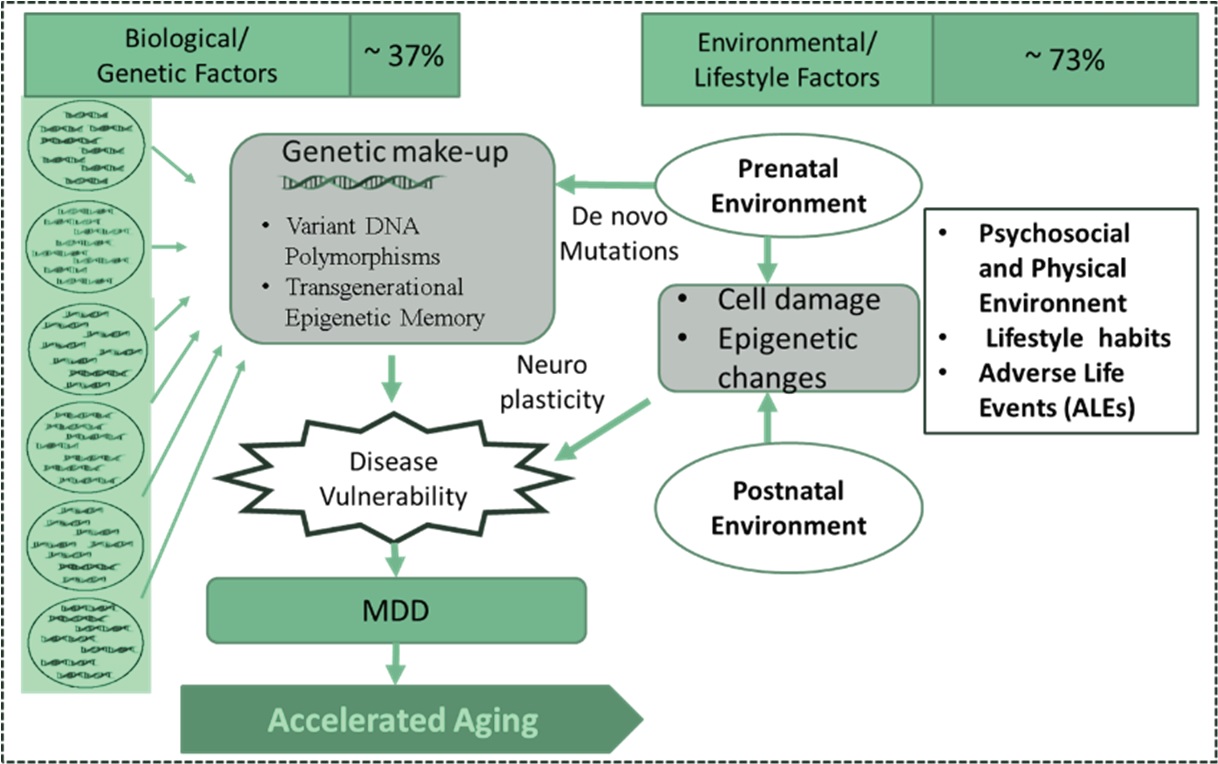 Fig. 2.
Fig. 2.Complex aetiology of MDD: Role of genetic, environmental, and stochastic factors.
Heritability of MDD is approximately 35% [56, 57]. The specific genes that are causal or increase the risk for the development of depression remain unknown. Numerous susceptibility genes have also been implicated, but none has yet been definitively established as a bona fide depression gene. Most of the known genes interact extensively with the gene pathways associated with aging, stress, inflammation, and nutrition sensing pathways. Risk of MDD is highly polygenic and multifactorial, and it is possible that virtually all genetic risk factors have individually small effects [58].
The biological and environmental factors together determine the events that happen in one’s life, and the impact varies depending on the life stage of the person. The peak prevalence of MDD is seen in adulthood. Environmental and social conditions are increasingly stressful, and susceptibility to stress varies across the population. Events during early life and childhood also contribute to the events in adulthood. Stress, in its various forms, is a major determinant in the causation of MDD.
Unhealthy lifestyles are associated with MDD. The key factors associated with unhealthy lifestyle are often referred to as “anthropogens” (or “…man-made environments, their by-products and/or lifestyles encouraged by these, some of which may be detrimental to human health”) and include high-calorie diet, lack of exercise, abnormal sleep patterns and sexual behavior, unhealthy habits like smoking, alcohol consumption, and abuse of drugs, medication, and modern technologies [59]. These factors predispose to stressful events.
Early epidemiological research centered on traumatic events such as such as loss of employment, financial insecurity, chronic or life-threatening health problems, and exposure to violence, separation which might be temporally associated with MDD. More recent research, however, has centred on childhood exposure as a precedent for MDD later in life [60]. These incidents include physical and sexual assault, psychological deprivation, and exposure as a result of death or separation to domestic violence or early separation from parents, with strong evidence of a dose-response interaction between the intensity of adverse life events and the risk, severity, and chronicity of MDD [5].
Individuals with MDD die at an earlier average age [50]. They are also at elevated risk of contracting somatic disorders, such as cardiovascular diseases, metabolic syndrome, immune dysregulation and dementia that are usually associated with old age [61]. The “accelerated biological aging” is an intrinsic factor in MDD pathogenesis. MDD can no longer be described as either a “mental illness” or even a brain condition, but rather as a multi-system disease of the entire body. This idea of accelerated biological ageing in MDD could broaden the likelihood of prevention and rehabilitation in affected people to improve physical and mental health.
DNA damage may represent a common pathophysiological mechanism in depression that increases the vulnerability to accelerated aging in MDD. Accumulation of genetic damage throughout life is one the common denominators of aging [62, 63]. Genetic lesions resulting from extrinsic or intrinsic damage are extremely complex and include point mutations, translocations, chromosomal gains and losses, shortening of telomeres, and gene destruction induced by virus or transposon integration. These lesions are associated with errors in the complex network of DNA repair mechanisms [69]. The specific mechanisms for maintaining the appropriate length and functionality of telomeres, and those for ensuring the integrity of mitochondrial DNA (mtDNA) are found to be disrupted [65, 66]. The literature provides evidence for accelerated biological aging in major depressive disorder, as indicated by shorter telomere length [67, 68].
Several papers have documented connections between depression and oxidative stress (OS). Increases in oxidative damage and modifications in the ETC complex I in the prefrontal cortex of depressed patients were documented by Ben-Shachar and Karry [69]. Other researchers noted decreased levels of antioxidants and antioxidant enzymes in MDD [70]. The primary source of ROS is mitochondria, which play important roles in cell signaling and homeostasis under normal conditions. In the oxidative phosphorylation pathway, ROS is produced; however, mitochondria generate protective factors in normal physiological conditions that can neutralize harmful free radicals [71, 72]. Endogenous and exogenous causes such as smoking, excess alcohol intake, electromagnetic radiation exposure, cancer, xenobiotic exposure and psychological stress are attributable to supraphysiological ROS levels [73]. Even ROS levels below physiological limits are detrimental to normal cellular activity, and it is necessary for cell survival to preserve OS at physiological levels. Macromolecular damage, including harm to DNA and telomeres, is caused by increased OS. Signal transduction and gene transcription are both impaired by genome-wide hypomethylation and gene-specific hypermethylation, inducing epigenome-specific changes [74].
Oxidative stress-induced mitochondrial dysfunction changes intracellular metabolism and can damage both nuclear and mtDNA. The level of resistance to stress or “physiological reserve” of mitochondria may explain the difference in the clinical appearance and seriousness of the disease. Mitochondria reproduce the energy requirements of particular intracellular microenvironments and react to them [75].
One major characteristic of MDD is the uncoupling of circadian rhythms [76]. This leads to disruptions in the incorporation of melatonin levels that control the circadian rhythms in relation to Zeitgeber, a normal rhythmic mechanism that serves as a guideline in the modulation of circadian rhythms of the body such as light/dark cycle, seasons in a year, and social experiences. Circadian rhythms are related to the increase in endogenous melatonin secretion at night. Melatonin modulates circadian rhythms through signalling pathways linked to the MT1 and MT2 melatonin receptors [61, 77]. Melatonin has recently been reported to increase the expression of the clock genes Per1 and Per2, which play a crucial role in resetting the circadian clock [78]. There is a decrease in plasma levels of melatonin in MDD patients [70]. Depressed patients show disruptions in the rhythm of melatonin secretion, and melatonin may increase the quality of sleep in these patients. The suprachiasmatic nucleus (SCN) regulates melatonin secretion, and elevated melatonin receptor concentrations exist in the SCN. Its suitability as a medication is limited because of the low half-life of melatonin [79]. Pharmacological treatments with agomelatine, a prolonged-release melatonin, have shown antidepressant benefits with a distinct 5-HT2C receptor and melatonin receptor agonist (MT1/MT2) selective antagonist profile [80]. In addition, in brain regions such as the hippocampus and prefrontal cortex, agomelatine is able to strengthen neuroplasticity processes and adult neurogenesis [80]. Mind-Body Interventions (MBIs) have also shown improvement in melatonin and circadian rhythms in clinical settings [81]. These findings highlight the importance of circadian rhythms in neuroplasticity and depression therapy.
Many studies have found that stress-related improvements in inflammatory and glucocorticoid signaling are connected to sufficient functional changes in several brain networks [82]. Indeed, MDD neuroimaging experiments have reported anomalies within the affective-salience circuit, the medial prefrontal-medial parietal default mode network and the fronto-parietal cognitive function system in either stimulation or communication.
The HPA axis is among the most researched biological systems in MDD [83].
Several researches concluded that cortisol levels were raised, with a moderate
effect size in patients with MDD [84]. In these patients, HPA variations are
related to diminished cognitive performance [85]. In addition, multiple
experiments have prospectively demonstrated that elevated cortisol levels are a
contributing factor in at-risk populations for subsequent MDD [86]. Meta-analyses
in the past have confirmed that cortisol levels have risen in patients with MDD
[87]. Higher levels of cortisol are correlated with reduction in the size of the
hippocampus [88]. Finally, a review using evidence from a survey in primary care
involving
Same authors have also reported the occurrence of severe neuropsychiatric outcomes including depression following discontinuation of long-term glucocorticoid therapy [89]. Moreover, the central hormones regulating cortisol levels are also shown to be dysregulated in MDD. For example, increased levels of corticotropin-releasing hormone in the cerebrospinal fluid (CSF) have been shown in patients with MDD.
The immune system is an essential part of the mechanisms of physiological stress-sensing and interacts directly with the major integrative systems of the body [90]. A large body of evidence from animal studies has supported the role of peripheral immune dysfunction and neuro-immunological mechanisms in MDD. These models have also provided intriguing insights into how peripheral cytokines can affect brain circuits, behaviour, and mood, directly and indirectly. Through the blood-brain barrier, peripheral cytokines can be transported to act directly on CNS-resident cells, including astrocytes, microglia, and neurons [90]. In addition, inflammatory signals can be transmitted to the CNS through cellular mechanisms (peripheral immune cell infiltration of the CNS) or via vagus nerve (‘inflammatory reflex’) signalling. Animal models have shown that these pathways converge to modify molecular programmes (such as receptor expression), neurogenesis and plasticity in the CNS [90]. Clinical observations indicate that similar inflammation mechanisms may also be relevant to patient development of MDD. A population-based study, has shown that both previous serious infections and autoimmune diseases increase the risk of subsequent MDD development [91]. Finally, MDD patients display elevated serum cytokine levels, such as TNF-alpha and IL-6, as confirmed by meta-analysis [92]. There was an increased gene expression of IL-6 signalling in MDD patients relative to healthy controls [93]. A few prospective studies have also shown that increased IL-6 levels in childhood dramatically increase the likelihood of developing MDD in adulthood [94]. Neuro-inflammation and microglial activity in the CNS of patients with MDD have been documented in recent research using PET imaging as well as post-mortem brain tissue analysis [95]. Finally, a potential role for inflammation in MDD is also supported by clinical trials of non-steroidal anti-inflammatory drugs (NSAIDs), reviewed in a meta-analysis [96].
The effects of food consumption and metabolism on depression were studied. Previous research has shown that appetite and satiety can reward exposure to gauze, and diet plays a role in controlling actions [97]. The melanin-concentrating hormone (MCH) nerve cells spread from the lateral hypothalamus to the limb system, including the NAcc. They predominantly carry out signal transduction that encourages appetite [98]. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity [99]. Antidepressant effects in mice demonstrated a complete decline in MCH-induced signal propagation and MCH antagonism in the NAc [100].
Ghrelin can produce antidepressant effects in depressed patients with poor appetite, in contrast to the depression-inducing effects of MCH [101]. In animals, metabolic status significantly influences mood and motivation. Therefore, a new perspective on depression may be suggested by understanding the correlation between peripheral metabolic signal transmission and the regulators secreted from the central nervous system, which affect food intake and wakefulness.
Lower levels of leptin and higher levels of ghrelin may be linked to a higher prevalence of depression [102]. Leptin, a hormone for satiety, is secreted from white fat cells and is involved in regulating food intake. Considering that poor appetite and decreased food intake are common symptoms of depression, studies have been carried out on the role of leptin in depression. Depression was associated with low levels of leptin, and patients who attempted suicide demonstrated significantly low levels of leptin in their cerebrospinal fluid [103]. Other studies, however, reported that depressed patients showed high levels of leptin [104]. It is possible to explain these high levels of leptin as leptin resistance. In type 2 diabetic patients, this could be close to insulin resistance. It has been documented that obese people suffer depression more frequently than normal-weight subjects [105]. In obesity, elevated leptin levels and tolerance to leptin are frequently observed.
The lack of a partner, a family and close social networks, and the experience of recent negative life experiences, such as sickness or loss of close relatives or associates, financial or social issues and unemployment, was correlated with regularly recorded environmental determinants of MDD in both men and women [106]. In addition, the risk of MDD in men and women is substantially elevated by a number of social determinants (including childhood adversity, socio-economic status, and low social support) as well as low educational attainment. The cause-effect relationship between lower educational performance and MDD, however, is uncertain, and a major study of 25,000 participants recently reported that it may be partially due to shared genetics [107]. Patients with MDD and a history of childhood trauma have a greater degree of symptoms, a worse course of treatment and a greater lack of reaction than patients with MDD without childhood trauma [108]. Most of the stress of MDD-related illness is due to its drastic effects on one’s ability to function and the strain on family life.
Healthy life and longevity in humans are modulated by genetic and non-genetic factors. Since genetic factors contribute only around 25% of the variation in human longevity, lifestyle and socioenvironmental factors contributes significantly to healthy life and healthy longevity. Meikirch model that provides one of the most comprehensive look at the nature of health states: “Health is a dynamic state of wellbeing emergent from conducive interactions between individuals’ potentials, life’s demands, and social and environmental determinants [109]”.
In addition to DSM-5, the Research Domain Criteria (RDoC) were developed by the US National Institute of Mental Health (NIMH) and are not intended to be a diagnostic system but a research coordinating mechanism. The RDoC method consists of a matrix in which the rows reflect given functional structures characterised by genes, molecules, cells, circuits, physiology, self-report and paradigms used to quantify them [110]. Lifestyle interventions can modify the variables defined by RDoc and optimize PAP.
The characteristics shared amongst the people having healthy life and healthy longevity, like those in “blue zones” of the world and those practicing yoga and Ayurveda, include the following: engaging in moderate physical activity, eating a healthy diet, lowering levels of stress, sticking to the circadian rhythms, having a social and network and family life, having moderation in everything [111]. These factors may contribute to the optimum dynamic interactions of the determinants of health at the individual, social, and environmental levels.
A culture of health adopting healthy lifestyle like those in ‘blue zones’ or as guided by Patanjali’s Ashtanga (eight limb) yoga may markedly improve health in the society. Health, when viewed as a complex adaptive system, offers new possibilities for addressing new challenges posed by lifestyle related chronic non-communicable diseases including depression. Study of the prevailing health services shows that much should be done to improve the health of patients in many ways and to reduce the quality of health care than is done currently. Adopting a lifestyle that incorporates the Meikirch model of health may be the most promising approach for individual and public health in both low and high income countries. It is essential to emphasize health instead of disease, like in the Meikirch model.
Studies on centenarians, who were “immune” to MDD and who have lived a long healthy life have suggested that optimizing several pathways like stress response, inflammation, and nutrition sensing, can modulate lifespan by promoting an efficient maintenance of the cell and of the organism. Recently, epigenetic experiments have demonstrated that epigenetic changes are very susceptible to the ageing process and affect the rate and efficiency of ageing, modulated by both genetic history and lifestyle. Overall, current studies indicate that it is important to modulate the relationship between genetic history and climate in order to assess the human opportunity to achieve longevity. APOE and FOXO3A are the major genes that have been reliably linked with improved survival in human candidate gene studies [112, 113]. Gene set study from GWAS research has shown that survival is associated with both the insulin/IGF-I signaling pathway and the telomere repair pathway [114]. Healthy lifestyle may be associated with optimum epigenetic regulation of longevity genes and cellular pathways.
Treaties like International Covenant on Economic, Social and Cultural Rights (ICESCR) of the United Nations, provide directions to the nations to support towards people having healthy life. The Sustainable Development Goals (SDGs) that define 17 common priorities, 169 objectives and 230 metrics leading up to 2030 have even been set by the UN General Assembly. Now is the time for the global health sector to explicitly express the well-established returns on health, reaffirmed in ‘Investing in Health 2035’ by the Lancet Commission [115]. Given the prohibitive costs of curative and chronic treatments of NCD’s, risk for which is almost doubled in depression, we may have no choice but to push for better management of depression and its prevention.
This will entail a profound rethink about how we treat the market determinants of depression, one of the influential “profit-driven diseases”. Therefore, driving society towards a healthy lifestyle is the key to realize the treaties that promise economic, social and cultural rights, decrease risk factors to health and prevalence of diseases, efficiently manage diseases, and reach the aspired developmental goals. R&D would need radically different models which are not strictly profit-oriented, it has been pointed out. There is an urgent need for change in our approach to the health of our society, where depression has become a major challenge. We need to retool our health workforce and bring it closer to communities if health-as-a-way-of-life is to be achieved [116].
Principles of yoga for better life and in clinical practice
Practitioners of yoga that contain various components of Patanjali’s Ashtanga (eight limbs), such as yoga asana, meditation, pranayama breathing, have shown benefits for optimization of health, protection from injury, rejuvenation, and increased life expectancy. Recently, imaging and molecular studies have provided evidence for the relatively healthier biological parameters compared to the general population. The aging seen in them is physiological, and the chronic morbidity related to modern lifestyle diseases that are seen in the current general population is significantly less in regular practitioners of yoga.
Previous evidence indicates that in particular cognitive domains and in brain structure, yoga can decrease the decline. Gard et al., have shown that fluid intelligence, i.e., the set of abilities involved in coping with novel environments and abstract reasoning, and resting state brain functional network architecture in middle-aged yoga and meditation practitioners, declined slower than in controls. Resting state functional networks of yoga practitioners and meditators combined were more integrated and more resilient to damage than those of controls. In addition, mindfulness was favourably associated with fluid intelligence, durability, and productivity of global networks. These results demonstrate the likelihood of growing durability and halting the decrease in fluid intellect and functional architecture of the brain and indicate that cognition plays a mechanistic role in this preservation [117]. Villemure et al. have shown that yogis did not display the well documented age-related global brain gray matter decline compared to controls, suggesting that yoga contributes to protecting the brain against age-related decline [118]. Years of yoga practise were primarily associated with variations in the amount of grey matter in the left hemisphere (insula, frontal operculum, and orbitofrontal cortex), indicating that yoga tunes the brain to a mode and supportive states that are parasympathetically guided. In the main somatosensory cortex/superior parietal lobule, precuneus/posterior cingulate cortex, hippocampus, and primary visual cortex, the amount of hours of weekly practise was associated with grey matter thickness. Yoga’s potential neuroprotective effects may provide a neural basis for some of its beneficial effects.
Cahan et al., found increased BDNF and decreased inflammatory mediators after yoga based intervention for three months and have suggested their findings reflect mind-body integration and well-being [25]. In a recent systematic review by Buric et al. exploring the molecular signature of MBIs has shown that the downregulation of the nuclear factor kappa B pathway is correlated with these practices; this is the opposite of the effects of chronic stress on gene expression and indicates that MBI practises can contribute to a decreased risk of inflammation-related diseases [119]. Krishna et al., have shown that in individuals who perform yoga frequently with lower systemic oxidative stress, the duration of the leucocyte telomere is well maintained compared to others who have a more sedentary lifestyle despite the absence of any medical conditions. The normal practice of yoga appears to prevent replicative cell senescence [120].
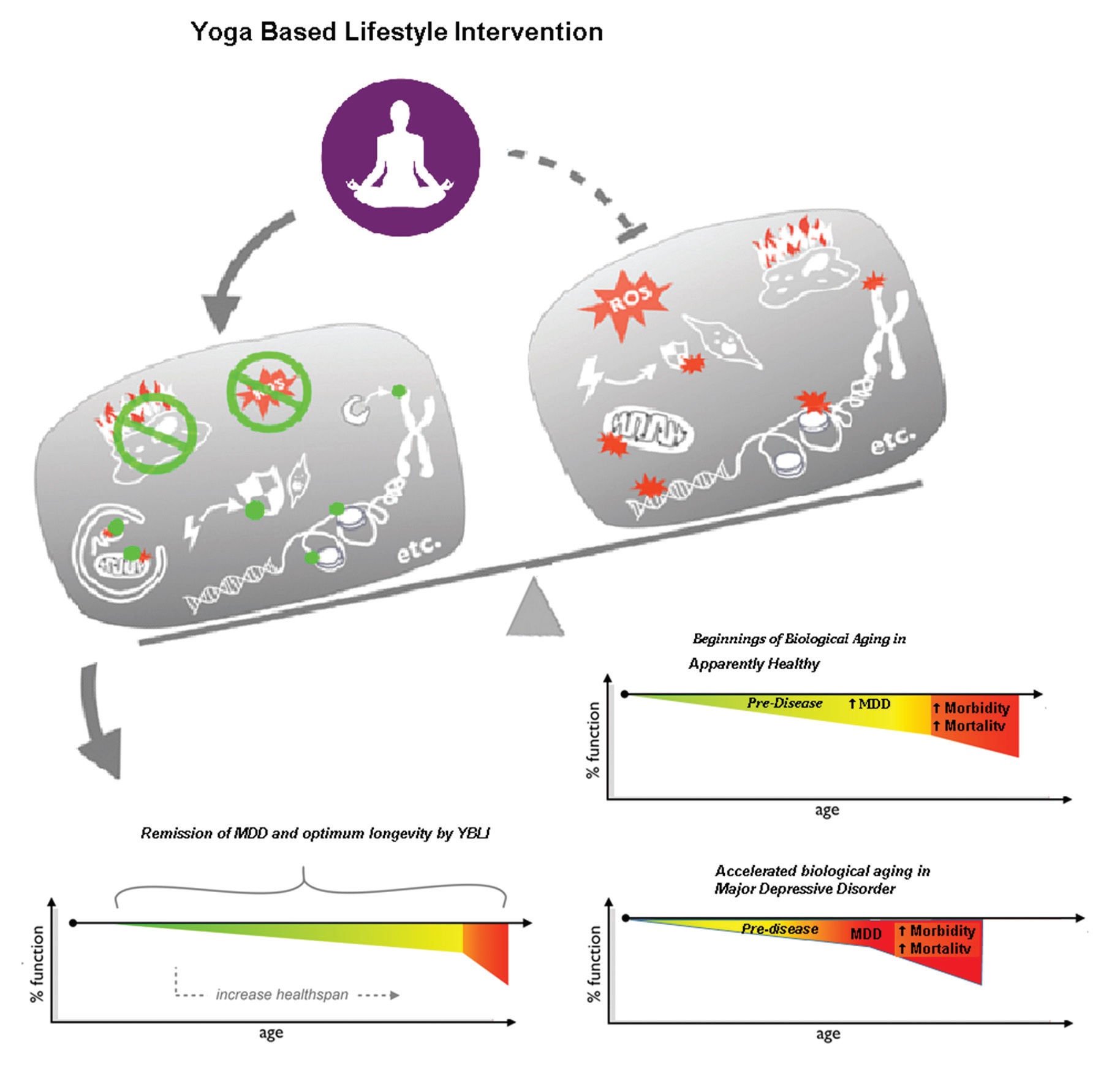
These evidences using advances in technology are supportive of yoga and the lifestyle based on it for living healthy life and has “optimum longevity”.
Yoga considers disease as a disturbance that perturbs the equilibrium. Yoga identifies different interventions that are preventive, promotional, medicinal, and rehabilitative for many disorders. Recent systematic reviews and meta-analyses have shown the usefulness of yoga in various medical conditions including metabolic syndrome, type II diabetes mellitus, chronic heart failure, and low back pain [121, 122, 123, 124]. Reviews have also suggested that yoga is beneficial for pain-associated disability and mental health [125]. Yoga may be useful in mitigating some medical problems as a supporting adjunct, and yoga may have the ability to be incorporated as a more cost-effective supportive/adjunct therapy that is helpful. In addition, it offers a life-long behavioral potential, increases self-efficacy and self-confidence, and is also related to additional beneficial health benefits. In a recent systematic review on yoga in MDD, in contrast to science-based treatments, experts have found some evidence of beneficial outcomes above placebo and comparable effects. It is possible to incorporate yoga intervention with any medical system effectively. Yoga can play a vital role in future integrative medicine [126].
The anatomical concepts of Yoga are ‘metaphysical’. The Atman, or soul, is considered to be covered by five layers of consciousness, or sheaths, known as Kosha (physical body structure): form outer to inner Annamaya Kosha (for nourishment), Pranamaya Kosha (related to the vital energy required for body functions), Manomaya Kosha (knowledge and sense of self-existence), Vidnyanamaya Kosha (emotional and intellectual aspects and the five sense organs), Anandmaya Kosha (sheath of bliss). Removing this layer liberates Atman giving the experience of Samadhi and Turya, which are the highest states of meditation. The practice of Yoga helps expose these layers of the Atman and encourages its emancipation to experience Brahman’s peaceful existence.
A recent study for our center show that increased MDD remission and response
with YBLI is independent of the genotypes the highly associated gene
polymorphisms like 5-HTTLPR and MTHFR 677C
Several epigenetic and transcriptomic studies have shown optimization of gene expressions and epigenetic modifications after yoga.
In order to promote optimal neuroplasticity, cellular health of all CNS and peripheral organ systems and their long-term preservation over the life cycle is necessary, which will guarantee continued remission of MDD, avoid complications and maintain somatic health. Achieving and maintaining optimum cellular health depend on genomic stability, oxidative eustress, and telomere length maintenance.
MDD is associated with oxidative DNA damage and consequent genomic instability [133]. This predisposes to mutations and aberrant methylations. The results may be attributed to increased oxidative stress. Psychological stress, sedentary lifestyle, intake of processed and nutritionally depleted food, unhealthy social habits (smoking, excess alcohol intake, etc.), and exposure to environmental pollutants have taken a toll on human health with the onset of MDD at a much younger age [16, 129]. These environmental and lifestyle factors are responsible for genomic instability [128]. DNA damage to mitochondrial and nuclear genome results in the accumulation of genetic and epigenetic aberrations [27, 131].
Genomic stability is essential to cellular survival, youthful cancer free, healthy life, and persistent health issues such as MDD prevention and care. Recent studies suggest the reduction of genomic instability (decreased levels of 8-OHdG) after YBLI in MDD. Previous studies have shown that active interventions like psychotherapy reversed DNA strand break accumulation originating from traumatic stress [132]. Commonly used drugs for MDD including SSRIs have shown adverse effects on genomic stability with increased complications like male infertility by causing sperm DNA damage [133].
DNA damage is mostly due to the aberrant pathway of the DNA damage response, which is important for DNA repair and genomic integrity monitoring. In order to facilitate irreversible ageing, inadequate DNA repair causes structural consequences [130]. DNA damage reduction by YBLI suggests that yoga has the capacity to optimize the DDR pathway to repair genomic damage and improve genomic stability. Improved oxidative stress and telomere stability along with changes in mind-body communicative and neural markers by YBLI contribute to stabilizing the genetic-make-up [11].
Several research and meta-analyses have documented a systematic decrease in telomere duration in both depressed and MDD individuals [134]. Recent studies have also documented dysregulations in telomere metabolism within the brain of rat model of depression and post mortem brain of MDD patients [135]. OS is a crucial player in the maintenance of telomere length, and there is an inverse relation between OS and telomere length. Studies have shown that OS can decrease telomere length by causing telomerase deficiency. ODD is significant among the factors which adversely affects telomere length [136]. Telomere attrition, due to DNA damage at the ends of chromosomes, has been traditionally associated with senescence and related disease conditions [137]. Obviously, enhanced oxidative stress may result in impairment in telomere metabolism and accelerated cellular aging and decrease cellular longevity in patients with depressive disorder.
It has recently been shown that optimum oxidative stress may increase induction of the expression of several genes involved in genomic stability and telomere maintenance, leading to improved cellular processes like autophagy, intercellular communication, stem cell renewal, and neuroplasticity. Study data shows that frequent yoga participants have increased telomere length relative to controls, and yoga exercise may enhance telomere metabolism by seemingly stable individuals [10]. The potential molecular pathways for increasing telomere protection after exercise were also established in recent studies [138]. The function of telomerase is understood to mediate cell survival through the promotion of BDNF actions [139]. Recent studies from our lab have shown significant increase in telomerase activity after yoga in MDD and that telomere length was maintained in them [11]. Although the impact of SSRI treatment on telomere metabolism is not known, a recent study suggests that short leucocyte telomere length may serve as a vulnerability index of poorer response to SSRI treatment in MDD [139, 140]. More research is needed to investigate the processes of how telomere metabolism can be positively changed by yoga and other therapies.
Increased ROS, and decreased TAC and cytochrome c oxidase (COX2) activity in MDD patients is suggested by several studies that have reported mitochondrial dysfunction and unregulated oxidative stress in the pathogenesis of MDD [10, 11]. At the cellular level, environmental influences such as perinatal insults, childhood maltreatment, and other adverse pathophysiological or psychosocial life events may activate oxidative stress pathways, thereby altering neuronal plasticity and function [141]. Mitochondria are the main energy metabolism organelles that are highly essential for adaptation to OS. Interestingly, OS process has also been observed in the frontal cortex of patients with recurrent depressive disorder [142].
Oxidative eustress variance is associated with macromolecular injury, turnover of telomeres, epigenetic changes and altered gene expressions. It is strongly associated altered neuroprotection, neurogenesis, and neuroplasticity in the brain [143]. HPA hyperactivity predisposes to increased cellular oxidative stress [144]. Szebeni et al., have even documented the elevated expression of DNA oxidation and DNA repair enzymes in brain white matter in MDD [135].
Achieving maximum oxidative eustress is a highly responsive activity, even under extremes of stress related to lifestyle and environmental challenges. A number of methods are followed to minimize or prevent the OS [145]. A meta-analysis by Liu et al. confirms the fact that antioxidant levels are raised and that after antidepressant treatment, the levels of oxidative damage products are reduced [61]. Passive interventions such as drugs and anti-oxidants can change the status of OS. In chronic lifestyle conditions, successful strategies such as daily physical activity are also helpful in minimising OS [148]. Asanas and pranayama contribute to voluntary exercise may improve mitochondrial health [149]. Findings from our recent study have shown that MDD therapy with YBLI contributes significantly to achieve oxidative eustress (homeostatic balance) and decrease DNA damage (Fig. 3). Another study of YBLI in apparently healthy also reports that yoga and meditation could re-establish oxidative eustress [10]. COX2 is an important component of cytochrome c oxidase (complex IV), and increased COX2 levels by YBLI stabilize the cytochrome c oxidase super complex organization. Cytochrome c oxidase regulates the functions of cytochrome c that maintains mitochondrial transmembrane potential and ATP generation. Its activity is an indicator of the oxidative capacity of the cells. Moreover, the activation of apoptotic mechanisms are suggested for the mediation of psychosocial stress in inducing depression-like behavior, and ceramides that mediate apoptosis have been suggested as targets for therapies in depression [148]. YBLI mediated increase in COX2 activity may further improve mitochondrial function by decreasing the levels of the pro-apoptotic C16 : 0 ceramide mediated by inhibition of ceramide synthase 6. The modulation of cellular OS within physiological limits after YBLI suggests the ability of this intervention to defend cells from DNA disruption and telomere attrition triggered by oxidative stress and to reverse epigenetic modifications accumulated by unhealthy behaviours and unfavourable environmental conditions. Previous studies have reported conflicting findings for the impact of MDD drug treatment on oxidative stress and mitochondrial dysfunction, some of the adverse effects may be due to disruptions in these mechanisms [61]. A recent study in our laboratory has documented that Yoga improves mitochondrial integrity and functionality. This is seen by increased expression of genes which maintain mitochondrial integrity and increase in mitochondrial copy number. There is also increase in mitochondrial membrane potential and COXII activity. Thus improvement in mitochondrial health is critical as it results in higher ATP and less free radical production and thus is able to meet the metabolic demand of the brain and also improves neuronal health along with various neurotrophins and factors which promote neuroplasticity like BDNF and DHEA. This improvement in mitochondrial DNA integrity has implications in various mitochondrial diseases like Leber hereditary optic neuropathy (LHON) and various skeletal, cardiac and liver diseases.
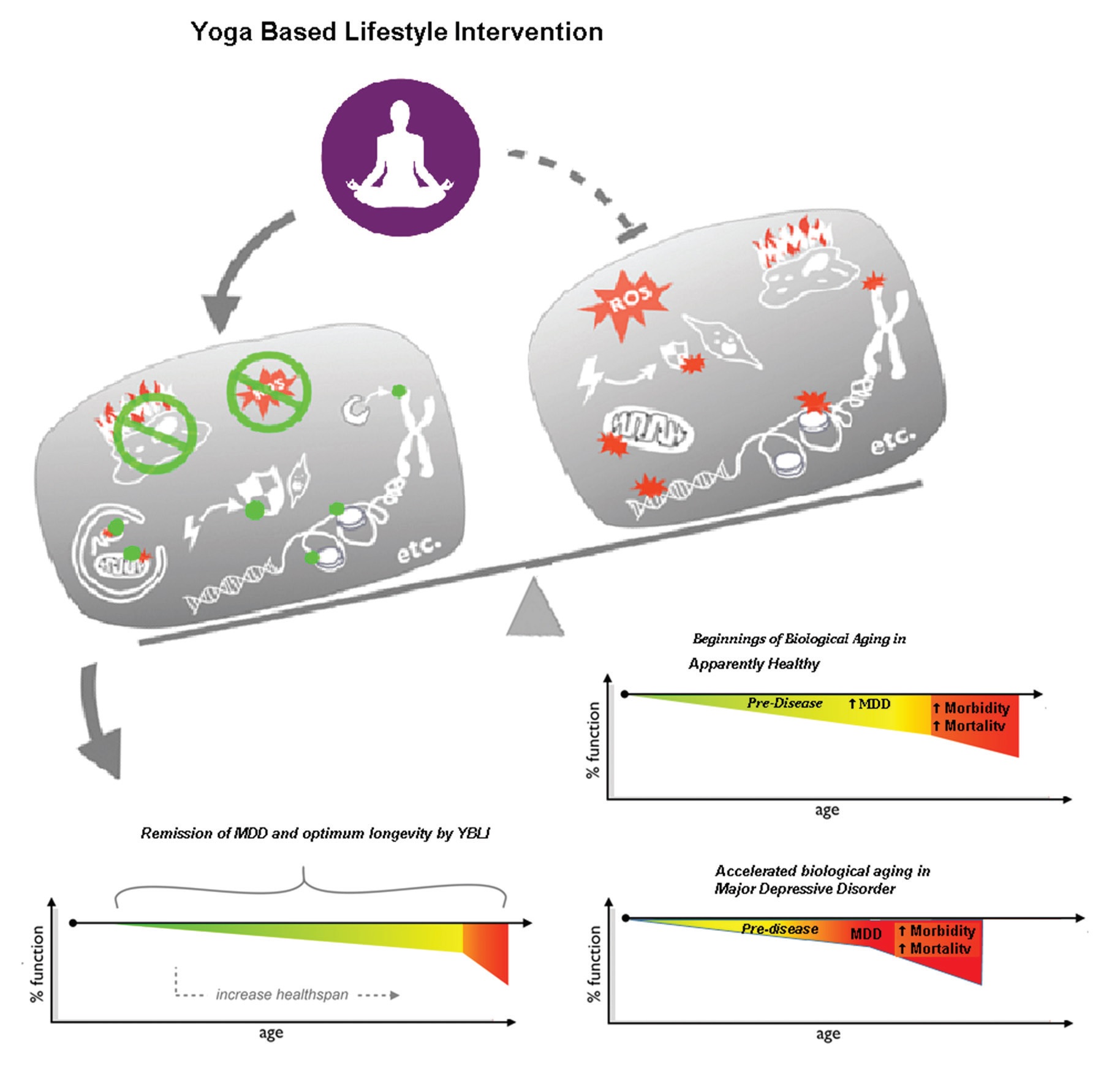 Fig. 3.
Fig. 3.Yoga based lifestyle intervention is designed to activate signaling pathways of cellular longevity.
Yoga, a premier MBI, has been increasingly accepted as a cost-effective strategy
for promoting health, including brain health. This is evident from the reduced
age associated decline in gray matter of yogis and regular meditators [149].
Recent studies and meta-analysis of neuroimaging in meditation practitioners,
found consistent alterations and activations of brain areas involved in
processing of higher mental functions [150]. BDNF plays an important role in
neuroplasticity and neurogenesis, and is a cardinal biomarker, which positively
correlate with the improved neuroplasticity seen at morphometric, neural network,
and molecular levels [151, 152]. There is even growing interest to translate BDNF
biology into therapies for depression. Passive interventions, although modify
neuroplasticity and related processes in the brain, they fail to provide long
term remissions, and are associated with complications [153]. In a recent study
exercise increased BDNF levels in MDD patients [154]. In another study,
Taekwondo, a form sport and exercise, improved neuroplasticity-related growth
factors, including BDNF, in healthy children [155]. Yoga has also been shown to
improve levels of BDNF in MDD [186]. Our recent study further confirmed that in
MDD subjects 12 weeks of YBLI could significantly increase systemic BDNF level,
in association with improvement in biomarkers of mind-body communications and
cellular health (Fig. 4). We observed that levels of BDNF were 42% higher
(P
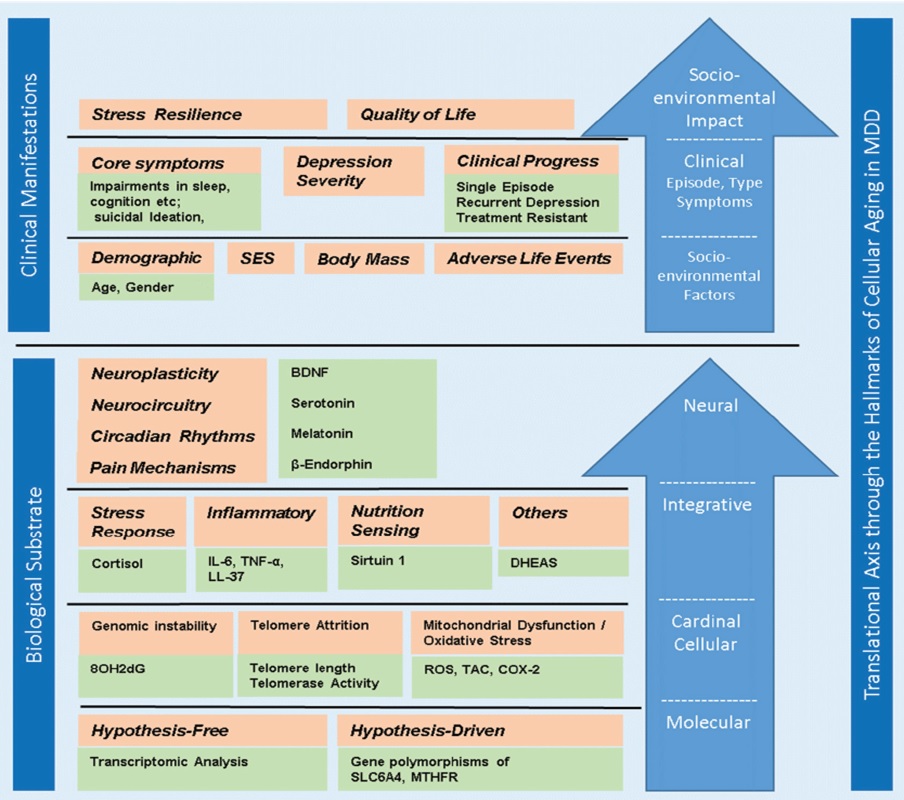
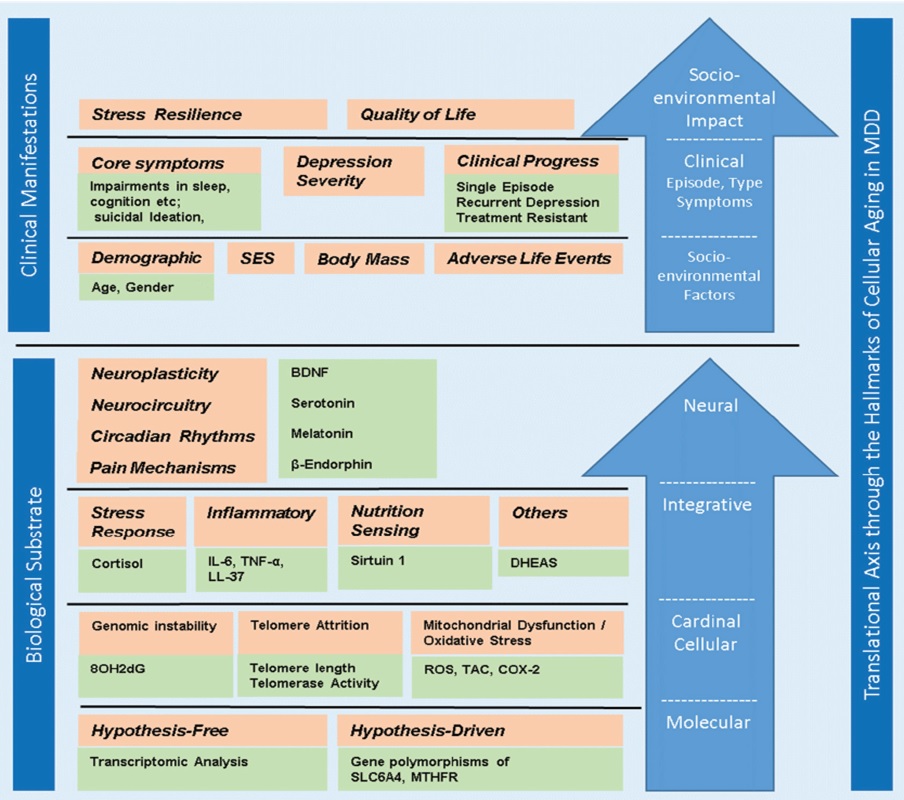 Fig. 4.
Fig. 4.Hallmarks of cellular aging in major depressive disorder.
A recent review has analyzed how peripheral somatic stimulation by acupuncture may increase neurotrophic factors like BDNF, and improves neuroplasticity [156]. Asanas (postures) and pranayama (breathing exercise) in YBLI may provide similar peripheral stimulation to CNS. In this regard, increased BDNF levels and improved clinical outcomes by YBLI, suggest that it may be an intervention to improve neuroplasticity, reverse pathobiology of MDD, and provide long-term remission [11].
Although drug treatment can improve neuroplasticity, they do so through the “GPCR-cAMP” pathway that is commonly seen in other organs or tissues, but is not the major pathway regulating the function of cAMP response element binding protein in the brain that is activated by BDNF [157]. BDNF also promotes neuroplasticity and neurogenesis in the amygdala, ventral tegmental area and NAc, which is believed to cause depressive-like behaviors or exacerbate depressive symptoms [158, 159]. Therefore, the effectiveness of the antidepressant is not completely contradictory to the site-specific neurophysiological and neurochemical efficacy of tension in various areas of the brain, which prevents neuroplasticity, causes atrophy in the hippocampus and prefrontal cortex, encourages maladaptive neuroplasticity and induces amygdala hypertrophy. The hypertrophy and increased activation of amygdala may underline the heightened risk of relapse in chronic MDD.
Adult neurogenesis facilitates resistance to stress at a cellular level by strengthening glucocorticoid-mediated negative feedback on the HPA axis [50]. Lower levels of BDNF have been reported in depression patients [152]. Decreased BDNF in association with altered regulatory systems of mind-body communication like stress and immune response, along with accelerated cellular aging support the neuroplasticity theory of MDD [61, 160]. In particular, during chronic stress response, elevation of endogenous cortisol will exert a neurotoxic impact on hippocampal neurons through the glucocorticoid receptor and its downstream effects, resulting in reduced neurogenesis, synaptogenesis and dendritic spines and increased neuronal apoptosis [161]. In addition, stress will decrease cell proliferation and facilitate glial cell apoptosis, which is the primary cell responsible for glutamate clearance in the brain and may be responsible for hippocampal atrophy in MDD [162]. Recent studies suggest that disruption of microglia’s normal structure and function, triggered either by extreme inflammatory activation or by the decline and senescence of these cells, may lead to depression and related impairment of neuroplasticity and neurogenesis [163].
Improved homeostasis of other neurotransmitters, including serotonin, dopamine, norepinephrine, acetylcholine, GABA, and glutamate, may be associated with increased neuroplasticity in after YBLI in MDD [164]. Crosstalk between improvements in other hallmarks of cellular aging mediates this neurotransmitter homeostasis. Lower concentrations of serotonin in MDD patients have been reported previously. Serotonin is thought to be implicated in multiple MDD-dysregulated physiological and behavioural mechanisms, including mood, appetite, sleep, exercise, suicide, sexual behaviour and cognition. In tandem with the mood-lowering effects of tryptophan depletion and the effectiveness of serotonin-modulating antidepressants, reduced serotonin metabolite levels in CSF have supported the hypothesis that serotonin system dysfunctions are implicated in the pathogenesis of MDD [165].
Recent studies show that YBLI-optimized serotonin homeostasis in MDD patients and is associated with significantly improved crosstalk with others hallmarks of cellular aging, which may contribute to increased remission and response. YBLI-optimized neurotransmitter homeostasis, including serotonin, in MDD patients is associated with improved neuroplasticity by the optimization of the dynamic multi-directional regulatory feedback systems that are discussed in relevant places for other biomarkers of cellular aging in this thesis. Crosstalk between these biomarkers provides the necessary explanations for the combined mechanisms because of the changes in the biomarkers assessed. Further discussion on the mechanism of YBLI is provided in a separate section below.
Melatonin is decreased in MDD patients that highlight the importance of the biological clock in the pathogenesis of MDD [166]. Biological clocks at the molecular level have been observed in almost every body organ and tissue so far examined, and these clocks are synchronized by the suprachiasmatic nucleus directly (via neural connections) or indirectly via hormonal inputs (e.g., cortisol, melatonin) or behavioral outputs (e.g., feeding). The clock also monitors the timing of sleep and, as such, communicates with sleep and circadian processes to monitor periods of rest-activity and maintain an individual in harmony with the natural world. Disruption of certain main homeostatic mechanisms could clearly lead to allostatic overload and depression [166]. This is partially due to a sustained inflammatory brain response mediated by immune cell penetration across the blood brain barrier and elevated levels of pro-inflammatory soluble proteins [70]. Recently studies have identified several circadian rhythm genes that are dysregulated in depression [167]. Several transcriptomics analysis show the association of MDD patients with pathways related to circadian rhythms.
Diverse strategies aimed at counteracting circadian misalignment, and thereby decrease allostatic load, have been proved to be beneficial for the clinical management of MDD. Sleep constraints have been shown to raise blood pressure, decrease parasympathetic tone, increase levels of cortisol, pro-inflammatory cytokines and insulin, and encourage increased appetite, likely by raising the pro-appetitive hormone ghrelin, along with decreased levels of leptin [168]. Therefore, improved sleep and melatonin levels by YBLI may optimize biomarkers of cellular aging including cortisol and inflammatory markers.
It is hypothesised that melatonin’s anti-stress and antidepressant-like activities interfere with many neurotransmitter processes, including GABAergic, serotoninergic, glutamatergic and nitrergic, as well as hypothalamic-pituitary-adrenal axis regulation [169]. Moreover, melatonin is also shown to protect cells from oxidative stress and improve mitochondrial function [170]. A recent study suggests that antidepressant effects of melatonin are by inhibition of acid-sphingomyelinase/ceramide system [171]. YBLI induced antidepressant action may be due to improved melatonin and mitochondrial function, thereby inhibiting acid-sphingomyelinase/ceramide system.
Improved melatonin homeostasis and circadian rhythms optimize neuroplasticity, and evidence in rodents has shown that it stimulates all stages of neuroplasticity [172]. Preclinical experiments have found that improvements in neuroplasticity in the adult brain follow a circadian pattern and are often caused by the light/dark cycle, with neuroplasticity during the dark period achieving its highest levels. Electrophysiological experiments have shown that long-term hippocampal potentiation was greater in magnitude and had improved flexibility during the dark period, which is indicative of synaptic strength. Drug therapies with SSRIs have been associated with decreased melatonin levels and disruption of sleep [140]. Therefore, while drug therapy is associated with relapses and complications, YBLI may optimize the circadian rhythms of neuroplasticity in MDD, which may contribute to long-term remission.
The most significant dysregulation of mind-body communications in MDD includes exaggerated stress response, immune hyperactivity abnormalities in nutrition sensing and other regulatory feedback systems [173]. These dysregulations in mind-body communications have a detrimental impact on central nervous system, and damage neuropil structure and function leading to deficits of neuroplasticity and higher mental functions.
Previous findings confirm the correlation of elevated cortisol with reduction in BDNF and increased incidence of depression. Through repressing glucocorticoid receptors, increased cortisol is known to inhibit BDNF secretion throughout the brain, and decreasing activity-dependent expression of BDNF mRNA in hippocampal neurons, and thereby contribute to neurodegeneration [174].
Decreasing cortisol levels has been shown to reverse the reduced hippocampus volume [175]. A research by Taren et al. reveals that instruction in mindfulness meditation facilitates physiological neuroplastic improvements and includes decoupling of the amygdala and subgenital anterior cingulate cortex as a possible neurobiological process influencing the intervention results of mindfulness training [176]. Yoga-related cortisol reduction in MDD was associated with a rise in blood BDNF levels. Our research confirms that YBLI reduces the amount of cortisol and enhances contact between the mind and body that controls MDD neuroplasticity [11].
YBLI increased stress resilience in association with reduced depression severity and optimized stress response in MDD patients. Previous findings have demonstrated that cortisol concentrations were not impaired by a particular type of antidepressant medication in patients with MDD, so stress physiology was unlikely to be a contributing factor in MDD drug therapy results [177].
Several studies suggested the role of various inflammatory processes and
mediators in the pathobiology of MDD [178]. The inflammatory biomarkers that were
significantly altered in MDD patients in comparison to healthy population
included increase in the levels of IL-6, and LL-37, and decrease in the levels of
CX3CL1 gene expression. In a previous study, patients with MDD displayed
elevated levels of IL-1
Crosstalk between different hallmarks of cellular aging is important for the
improved clinical outcomes mediated by optimized inflammatory responses in MDD.
Emerging research has indicated that in patients with MDD, many inflammatory
cytokines such as IL-6 and TNF-alpha are associated with OS [178]. Latest
research indicates that microglial SIRT1 deficiency contributes to a cognitive
impairment in ageing and neurodegeneration by epigenetic modulation of immune
molecules such as IL-1
Preclinical study has demonstrated that chemokines are associated with peripheral-central crosstalk and may be essential to mediate suicidal behaviors. In both physiological and pathological settings, crosstalk between CX3CR1 and CX3CL1 tends to be an essential means of coordination between microglia and neurons [185]. Future studies should investigate the putative processes driving this relationship, strive to reproduce current outcomes in broader communities and seek to establish novel diagnostic and therapeutic strategies.
A recent trial investigating differential antidepressant treatment response by
proteomic approach, has reported that several cytokines (IL-5, IFN-
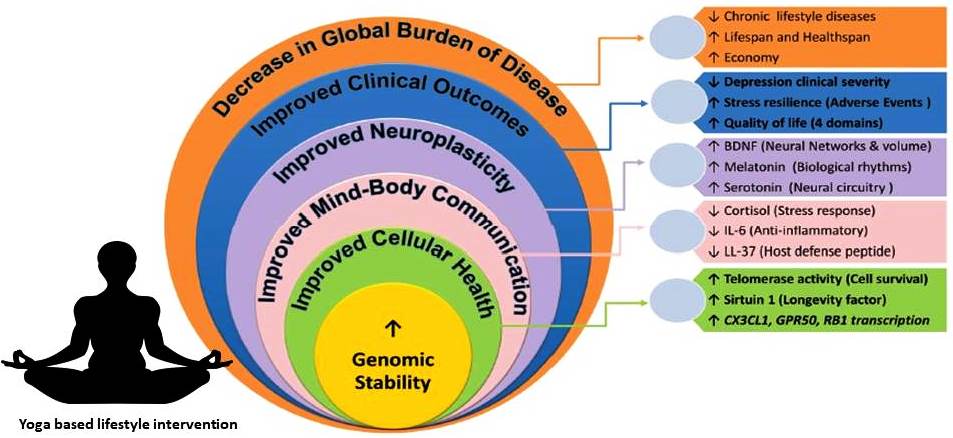 Fig. 5.
Fig. 5.Schematic representation of improved cellular health by yoga and meditation and its association with molecular and clinical parameters.
Nutrition and energy sensing pathways are important among the factors that
promote longevity, and Sirtuin 1 plays a prominent role. Literature available
with respect to blood sirtuin-1 levels in patients with MDD is limited, but most
have shown decreased levels of sirtuin 1 in MDD [188]. In support of this
observation, lower sirtuin-1 concentration was associated with MDD in a recent
study from our lab. Latest research indicates that microglial SIRT1 deficiency
contributes to a cognitive impairment in ageing and neurodegeneration by
epigenetic modulation of immune molecules such as IL-1
SIRT1, a mammalian class III member of histone deacetylases dependent on nicotinamide adenine dinucleotide (NAD+) and a nutrient and energy sensor, has been a target for longevity and aging-related disease interventions [187]. Recent evidence suggests that caloric limitation and resveratrol may increase the levels of circulating sirtuin 1 [190]. Our recent study is the first to document an increase in levels of sirtuin-1 independent of caloric restriction after yoga practice [10, 11].
These improved processes may result in delaying onset and slowing down progression of diseases associated with accelerated cellular aging. Sirtuin 1 strongly contributes to the correlation between BDNF and BDI-II scores. A research in rats indicated that therapy exercise and resveratrol increased the regeneration of neurological and motor activity via the SIRT1 signalling pathway following cerebral ischemic injury [191]. Potential underlying molecular mechanisms for decrease in MDD severity by increased sirtuin 1 include: (1) increasing cell survival and neurogenesis through mTOR signaling, (2) promoting synaptic plasticity by increasing BDNF transcription, mediated by inhibition of miR-134 expression that inhibit binding of cAMP response element-binding protein to several BDNF promoters, (3) regulating circadian rhythm mediated by inhibiting the central circadian timer protein CLOCK, a histone acetyltransferase, (4) by powerful antioxidant mechanisms [192]. SIRT1 provides cells with tolerance against OS. By modulating forkhead transcription factors, SIRT1 may offer protection against oxidative stress in some cells [193]. In addition, SIRT1 defends cells against OS by increasing catalase activity [194]. Sirtuin-1 provides resistance to OS, anti-inflammation and immunosuppression [195]. SIRT1 over-expression enhances tolerance to free radical toxicity in neuronal cells and through microRNA-regulated DNA repair and genomic stability pathways. A previous research revealed that SIRT1 modulates synaptic plasticity and memory development through a pathway mediated by microRNA [196]. SIRT1 stimulation promotes synaptic plasticity, although its lack of action impairs. Appropriately, these effects were mediated by a brain-specific microRNA, miR-1344 miR, through post-transcriptional regulation of cAMP reaction binding protein expression. SIRT1 usually acts to restrict miR-134 expression through a transcription factor YY1 repressor complex. It also unchecks miR-134 expression following SIRT1 deficiency which results in downregulated cAMP reaction element-binding protein and BDNF expression, thereby impairing synaptic plasticity. The results of our research indicate a role for the increase in SIRT1 caused by YBLI in correcting MDD pathology via a microRNA-based mechanism.
Previous studies and meta-analyses showed that mind-body interventions improve quality of life in patients with NCD’s [196]. In our study we found a significant increase of WHOQOL-BREF scores in YBLI group for overall quality of life, overall satisfaction with health, and all four domains of QOL-physical, psychological, social, and environmental. The improvement was more marked for physical and psychological in comparison to social and environmental domains. Comprehensive improvement in QOL in YBLI group could be due to physical, mental, and spiritual benefits from yoga and meditation [125]. YBLI induced reduction in biological aging could be associated with improved functions in the brain, organ systems, and the regulatory feedback pathways. Cells and tissue environments related to these derive unique differential benefits from the YBLI-induced improvement in cellular health. Several recent studies suggest MBIs improve expression patterns of genes that regulate cellular health [119]. Yoga is shown to decrease age associated telomere attrition and age associated decline in gray matter, and improve neuroplasticity, adiposity, and vascular health [120]. Asana and pranayama contribute to physical fitness, in addition to creating necessary foundations for meditation [197]. In-depth psychological benefits are derived from the four steps followed for meditation- pratyahara (detachment from senses), Dharana (concentration), dhyana (meditation), and samadhi (mindfulness/self-awareness). Physical and mental fitness in combination with adopting moral principles (Yama) and discipline (niyama) in YBLI contribute to improved perceptions in social and environmental facets of QOL. In addition, contributions to improved QOL could be due to YBLI induced moving away from sedentary life, improved dietary habits and decreased unhealthy habits like alcohol, smoking and digital technology abuse [198].
Provided that MDD has reached epidemic levels globally, introducing successful care in low-income countries where fewer than 10 percent of MDD patients receive appropriate treatment should be one of the top priorities in the region. The new WHO Mental Health Gap Action Programme seeks to ramp up programmes for mental illnesses in low- and lower-middle-income countries.
Many concerns about the aetiology and pathophysiology of MDD remain unanswered. Like, how is the immune system dysregulated in MDD? Furthermore, there is a lack of replicated findings in GWAS studies. As a consequence, it’s also uncertain how environmental conditions interfere with the genome to cause MDD. Furthermore, how stable are genomic read-out epigenetic changes, and are they reversible with a good successful therapy? The persistent sex differences in MDD prevalence rates have been identified as an epidemiological anomaly, and it would be important to investigate the mechanisms that are responsible for the increased MDD prevalence in women. Finally, since MDD is a strong risk factor for developing metabolic and cardiovascular disorders, as well as a worse outcome in these diseases, more research into the pathways of interaction between MDD and other medical diseases, such as diabetes mellitus or coronary heart disease, is required. Future studies should look at whether treating comorbid MDD in psychiatric patients decreases morbidity and mortality.
Yoga should be viewed alongside other main lifestyle medicine components including physical activity or exercise, dietary change, appropriate relaxation/sleep and social contact, and the elimination of addictive behaviours such as smoking, narcotics, and alcohol. Other behavioural variables that have a major potential to impact health, such as environmental problems (e.g., urbanisation and pollution) and the increasing human interaction with technology, should also be addressed. While evidence shows that yoga is a modifier that promotes general mental and physical wellbeing, further research is needed into the long-term use of yoga for depression prevention and management, as well as health promotion. Importantly, research exploring lifestyle changes involving several lifestyle components, such as yoga, is required. Owing to the complexities of human depression and an understanding that daily yoga and dietary change can be a normal aspect of care and mitigation efforts, the judicious application of medicine and therapeutic methods is also promoted.
In the age of super-specialization health care, medical care is too specific. MDD is a mind-body disorder and should be managed accordingly by a mind-body intervention. Yoga is a profound science and technology of wellbeing which improve physical, mental, and behavioral health. MDD has considerable effects on the human condition and its etiology, pathophysiology, and management remain a complex puzzle. Consistent with Winston Churchill’s famous quote “Success is not final, failure is not fatal: it is the courage to continue that counts”, it will be worth every effort to relieve the enormous burden of MDD.
All authors contributed equally to the conception, design and writing of this manuscript and approved the submitted version.
The study was initiated after ethical clearance (ESC/T-370/22-07-2015) from the Institute Ethics Committee. All participants provided signed informed consent.
The authors are thankful to all the patients who participated in the yoga intervention and the yoga therapists, Ms. Shalini Jha and Ms. Richa Mishra, for providing yoga instructions and counseling to the patients.
Financial assistance by the Department of Science and Technology (DST), India [SR/SATYAM/55/2016], the Ministry of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy (AYUSH), India [Z.28015/66/2016-HPC (EMR)-AYUSH-B], and Indian Council of Medical Research (ICMR), New Delhi, India, in the form of a Senior Research Fellowship [45/2/2019-ANA/BMS] to Ms. Surabhi Gautam is thankfully acknowledged.
The author declares no conflict of interest.
8-OHdG, 8-hydroxy-2’ -deoxyguanosine; ALEs, adverse life events; BDI-II, Beck
depression inventory-II; BDNF, brain-derived neurotropic factor; BGP,
biologically given potential; CNS, central nervous system; COX2, cytochrome c
oxidase; CRP, C-reactive protein; CSF, cerebrospinal fluid; DAS28-ESR, disease
activity score erythrocyte sedimentation rate; DBS, deep brain stimulation; DHEA,
dehydroepiandrosterone; DMARDs, disease modifying anti-rheumatic drugs; DSM-5,
diagnostic and statistical manual of mental disorders 5th edition; ECT,
electroconvulsive therapy; ESR, erythrocyte sedimentation rate; GBD, global
burden of disease; GWAS, genome-wide association studies; HAQ, health assessment
questionnaire; HPA, hypothalamic-pituitary-adrenal; ICESCR, International
Covenant on Economic, Social and Cultural Rights; IFN