Frontiers in Bioscience-Scholar (FBS) is published by IMR Press from Volume 13 Issue 1 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 School of Engineering and Science, Tecnologico de Monterrey, Campus Monterrey, Ave. Eugenio Garza Sada 2501, Monterrey, N.L., 64849, Mexico
2 Department of Biosystems and Agricultural Engineering and Robert M. Kerr Food and Agricultural Products Center, Oklahoma State University, Stillwater, OK, 74075, USA
3 Manatee Holdings Ltd, 4097 Gartelay Point Road, Courtenay, BC V9N9T2, Canada
Abstract
Harmful algal blooms in the past three decades appear to have grown in incidence, intensity and geographical distribution with negative impacts on public health and economy values. Each year the algal biotoxins are responsible for more than 60.000 intoxications with an associated mortality rate of 1.5%. The present review summarizes current knowledge and perspectives on marine and freshwater algal toxins with an emphasis on different genus of algae capable to produce toxins and their physiology. The typologies of toxins, their chemical structure and mechanisms of action, the factors that stimulate their biosynthesis and the current techniques used for algal toxins removal will be also reviewed.
Keywords
- Harmful Algal Blooms
- Toxins
- Algal Toxin Removal
- Review
In recent years, there have been alarming reports about the increase in harmful algal blooms (HABs), phenomenon associated with irrecoverable damages to socio-economic systems, human health, and the contamination of fresh and marine waters around the world (1).
Toxigenic algae are primarily classified into six classes: Cyanobacteria, Dinoflagellates, Diatoms, Raphidophytes, Pelagophytes, and Haptophytes (2), a taxonomic diversity which enables them to adapt to a wide range of environmental conditions (Figure 1). For example, the fish-killer microalga Heterosigma akashiwo, one of the widely spread documented HABs, can adapt to different temperatures (9–24.8°C), levels of salinity (4.2–34.9 psu) and irradiance (≤ 5 μmol m−2s−1) (3).
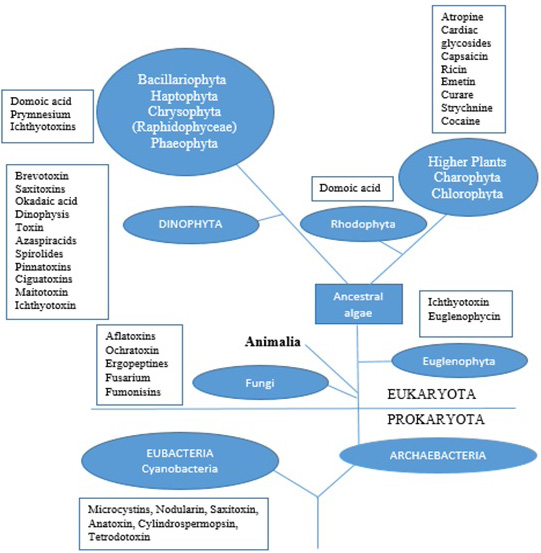 Figure 1.
Figure 1.The simplified phylogenetic tree describing the relationship between the major algal groups and higher living organisms with some of their group of toxins produced.
HABs are also classified into three main classes based on their effects on the environment and humans. The first class includes nontoxic species, which are able to discolor the water and are responsible for anoxic conditions (oxygen concentration ≤ 0.5 mg L-1) which kill invertebrates and fish. Noctiluca scintillans, Alexandrium taylori, Scrippsiella trochoidea, and Heterocapsa triquetra are a few examples of HABs belonging to this class (4, 5). HABs in the second class have the ability to produce biomolecules, such as palytoxins and maitotoxins, with hemolytic activities. Chattonella marina, Amphidinium carteri, and Gymnodinium mikimotoi belong to this group (6, 7). The third class of HABs is characterized by the production of toxins able to find their way through the food chain and as well as to cause different gastrointestinal and neurological problems in humans (8). Cyanobacteria, Dinoflagellates, Diatoms, and Raphidophytes are included in this class. Amnesic shellfish poisoning (ASP), ciguatera fish poisoning (CFP), diarrheic shellfish poisoning (DSP), azaspiracid shellfish poisoning (AZP), neurotoxic shellfish poisoning (NSP), paralytic shellfish poisoning (PSP), and cercarial dermatitis (CD) are human illnesses linked to this class of algae (9). Toxigenic algae can often cause serious health problems if concentrated in organs: gastroenteritis, liver diseases, allergies and irritations are some examples (10). Early documented evidence indicates that 114 people died in 1942 around Lake Hamana in Japan because of the consumption of clams and oysters containing the toxin venerupin from Prorocentrum minimum. Later findings highlighted a connection between secretions of the venerupin from P. minimum and certain bacteria, such as Vibrio sp., Shewanella putrefaciens, Alteromonas tetraodoni, and Pseudomonas sp. (11, 12).
Although research institutes, public health authorities, and federal agencies are significantly active against HABs, alarming data are still being recorded. The most important produced algal toxins under investigation by institutes and authorities are reported in Figure 2 in terms of their chemical structure. A recent research study demonstrates that the tetrodotoxin (TTX) (A) concentration in Greek shellfish produced by P. minimum in the area of the Vistonikos Bay has reached 222.9. μg Kg-1 (cell concentration = 1.8.9 × 103 cells L-1). These levels constitute a 12% increase in TTX concentration compared to other infected areas in which the concentration of TTX ranges from 61.0 to 194.7 μg Kg-1 (13). Statistics from health authorities reveal that algal toxins are responsible for 60.000 poisonings a year with an associated mortality rate of 1.5% (14). Reports from national centers for coastal ocean science indicate that even just the Akashiwo sanguinea, Alexandrium monilatum, Pyrodinium bahamense, Aureococcus anophagefferens, Aureoumbra lagunensis, Heterosigma akashiwo, P. minimum, Karlodinium veneficum, Cochlodinium polykrikoides, and Pseudo-nitzschia are responsible for an annual $82 million in public health costs, with repercussion on both the seafood industry and the tourism sector (15).
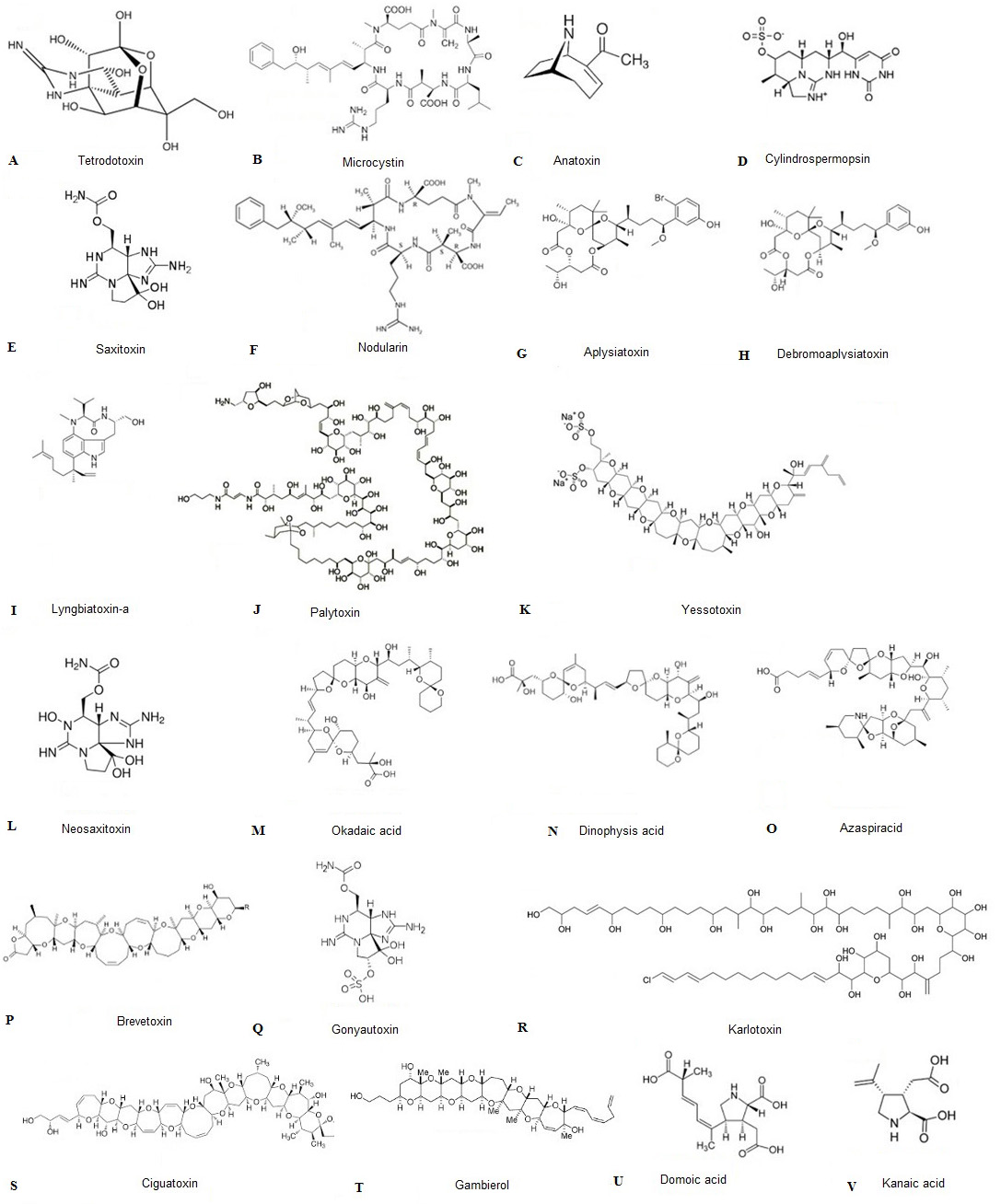 Figure 2.
Figure 2.The chemical structures of the toxins discussed in the review. The capital letter associated to each molecule (i.e A, B, C etc) corresponds to the toxin progressively cited and reported in parentheses in the text (i.e (A), (B), (C) etc.)
The objective of the present study is to give an overview of the current knowledge on marine and freshwater toxigenic algae, the chemical structure and the mechanisms of action of their toxins, the stimulatory factors that affect the production of algal toxins, and the current techniques used for algal toxins removal.
Cyanobacterial bloom can generate substantial water quality problems, since various species in this phylum potentially synthesize a variety of toxins (25% to 75% of the cyanobacterial blooms are toxigenic) (16). Cyanobacterial toxigenic species are able to grow in diverse fresh and marine water ecosystems and produce a wide range of toxins (17). Anabaena sp. (Nostocaceae), a nitrogen-fixing filamentous Cyanobacterium, has the ability to form surface blooms and vertical migration in the water and it is known to produce many biotoxins, including microcystins (MCY) (B), anatoxins (ANA) (C), cylindrospermopsin (CYN) (D) and saxitoxin (STX) (E) (18).
MCY are cyclic peptides that are synthesized non-ribosomally through the thiotemplate functions of large multifunctional enzyme complexes that contain both non-ribosomal peptide (NRPS) and polyketide synthase (PKS) domains (19). MCY have hepatotoxic activities, and almost 90 different hepatotoxins can be identified in the phylum of Cyanobacteria, although microcystin-LR (MC-LR) is the most reported one (20). The MCY accumulation occurs through the bile acid transport system and the toxin acts as an oxidative stress molecule in the liver of higher organisms. The lethal dose (LD50) for MC-RL in mice is 5 mg Kg-1 and the toxicity changes via the combination of amino acids in their molecular structure. MCY are genotoxic and they can act as tumor promoters with inhibitory effects on the mechanisms of DNA repair and protein phosphatases enzyme (21, 22). ANA, the other toxin from Anabaena sp., is a bicyclic alkaloid amine with considerable neurotoxicity. Aphanizomenon and Oscillatoria genus are also capable of producing ANA. The biosynthesis of ANA occurs after recruitment of α-amino acid proline by NRPS, chain extension and cyclization of PKS. ANA acts as an agonist for the neuronal α4β2 and α4 nicotinic acetylcholine receptors, and its lethal dosage is estimated to be less than 5 mg for an adult human male (23, 24). CYN are tricyclic alkaloids molecules that contain a guanidine group (HNC(NH2)2) linked at C7 to hydroxymethyl uracil at the hydroxyl bridge. Besides Anabaena sp., other species, such as Cylindrospermopsis raciborskii, Aphanizomenon ovalisporum, A. flosaquae, Umezakia natans, and Raphidiopsis curvata can also synthesize the CYN. Biosynthesis of CYN initiates from an amidinotransferase and completes by NRPS/PKS-type enzymes as well as tailoring enzymes (25, 26). CYN causes damage to liver, kidneys, lungs, heart, stomach, adrenal glands, vascular system, and lymphatic system. CYN has a 24-hour LD50 of 2100 μg Kg-1 and 200 μg Kg-1 at 5-6 days in mice, and its toxicity is due to the inhibition of glutathione, cytochrome P450 and protein synthesis (27). STX is a tricyclic perhydropurine alkaloid molecule, potently biosynthesized by fresh water Cyanobacteria, such as Anabaena sp., Aphanizomenon sp., Cylindrospermopsis sp., Lyngbya sp., and Planktothrix sp., and Dinoflagellates, such as Alexandrium sp., Gymnodinium sp., and Pyrodinium sp. This molecule is also a sodium channel blocker. It inhibits normal cellular functions leading to paralysis by affecting the voltage-gated sodium channels of neurons (28, 29). The synthesis of STX starts from non-terpene alkaloid pathway by charging of the acyl carrier protein with acetate from acetyl coenzyme A. STX is extremely toxic and has an intraperitoneal, intravenous and oral LD50 of 10 μg Kg-1, 3.4 μg Kg-1, and 260 μg Kg-1 respectively in mice. The 4-aminopyridine (C5H4N–NH2) molecule, as one of three isomeric amines of pyridine, can reverse the lethal effects of STX (30, 31).
The genus Oscillatoria is documented as another toxigenic cyanobacteria. These filamentous motile blue-green or brown-green Cyanobacteria carry out anoxygenic photosynthesis, and reproduce asexually via clonal fragmentation (32). In this genus, the specie Oscillatoria rubescens produces several molecules with carcinogenic, gastrointestinal, and hepatic toxic effects. Physiologically, O. rubescens needs low temperatures for growth because of its photosynthetic pigments. Thus, under high temperature and high light radiation levels, such as during the summer, it moves to the deepest parts of lakes, while under favorable conditions (low temperatures and low radiation levels) it moves to surface for toxic blooms (33). Homoanatoxin-a (HANTX) is the toxin that is isolated from O. rubescens. HANTX has a low molecular-mass, a bicyclic secondary amine structure, and it is a homologue to Anatoxin-a (ANTX). LD50 of HANTX and ANTX range from 200 to 250 mg Kg-1 in mice. The binding of HANTX or ANTX to nicotinic acetylcholine receptors (nAChRs) induces conformational changes in the postsynaptic ion-channel complex, leading to a blockage of neuromuscular depolarization (34).
Algae belonging to the genus Cylindrospermopsis are filamentous facultative diazotrophs Cyanobacteria that inhabit almost every eutrophic waters. Cylindrospermopsis species are able to grow at temperatures as low as 14–17°C, and they can tolerate wide temperature and light regimes and different nutritional concentrations. Cylindrospermopsis produces the hepatoxin toxin CYN (35). The existence of the cyr gene cluster in toxigenic species is associated with the biosynthesis of CYN (26).
The genus Nodularia consists of filamentous nitrogen-fixing Cyanobacteria that grow in brackish or saline water. These Cyanobacteria produce molecules, such as shinorine and porphyra-334, that are mycosporine like amino acids (MAAs). These molecules act as photo-productive agents against ultraviolet radiation, and they help Nodularia to live on the surface of water (36). Nodularin (NOD) (F) is a cyclic pentapeptide carcinogenic toxin produced by Nodularia species that inhibits the synthesis of the protein phosphatase, causing protein hyperphosphorylation, cytoskeletal collapse, and massive hepatic bleeding. NOD has an almost similar molecular structure to MCY, the only difference being the absence of two core amino acids and the substitution of the MCY residue, N-methyldehydroalanine, with N-methyldehydrobutyrine in the NOD peptide structure. Evidence shows that higher salinity levels and lower ultraviolet radiation lead to a higher production of the NOD (37, 38).
Lyngbya genus are non-heterocystous autotrophic Cyanobacteria that reproduce asexually and form periodic nuisance blooms in lagoons, reefs, and estuaries that can attack a range of aquatic ecosystems. Lyngbya blooms form dense floating mats. Typically, the blooms inhibit the proliferation of other microorganisms that are competing for light and nutrition (39). A range of toxins, including STX, aplysiatoxin (ATX) (G), debromoaplysiatoxin (DAT) (H) and lyngbyatoxin-a (LTA) (I), are identified in the Lyngbya species (40). LTA and DAT toxins induce ornithine de-carboxylase activity and they are responsible for causing asthma-like symptoms and severe dermatitis in humans. LTA is a nitrogenous toxin from a non-dermatitis-producing variety of L. majuscula. The LD50 is about 0.3 mg Kg-1 in mice. LTA is closely related to teleocidin-b, a toxic substance associated with different strains of Streptomyces. The LD50 of ATX and DAT is estimated to be 0.3 mg Kg-1 in mice. Skin exposure can cause redness, blisters, and pus on contact and has shown tumor promoter activity. At sub lethal concentrations, DAT exhibits fair activity against P-388 lymphocytic mouse leukemia (41, 42). Studies reveal that alternations in phosphate, nitrate, calcium, irradiance, and temperature causes changes in toxin production (43).
Trichodesmium genus consists of diazotroph filamentous Cyanobacteria with unstacked thylakoids structures and very high nitrogen fixation ability (the N2 fixation by Trichodesmium is estimated to about 60–80 Tg N annually). Trichodesmium species grow in oligotrophic waters, with heavy blooms typically occurring during periods of low wind stress and warm temperatures. The blooms can reach densities of 3.000 to more than 10.000 trichomes L-1, with a concentration of chlorophyll ranging from 1 to 5 mg L-1. The optimum temperature for growth and nitrogen fixation inTrichodesmium is 24–30°C (44). The colonies host a wide diversity of microorganisms, including specific epibionts, viruses, bacteria, eukaryotic microorganisms, and metazoans. The presence of gas vesicles in Trichodesmium enable them to regulate their buoyancy and to move vertically (45). This Cyanobacterium can produce toxins, such as lipophilic chlorinated trichotoxin, CTXs, 42-OH-palytoxin, b-N-methylamino-L-alanine, and palytoxin (PLTX) (J), which cause clupeotoxism in humans through consumption of contaminated fish (46). With the exception of polypeptides and protein toxins, PLTX is one of the most toxic substances among these toxins, with a LD50 of 0.15 mg Kg-1 in mice. PLTX turn the Na+/K+ pump into a shape that allows the passive transport of Na+ and K+ ions (47).
Dinoflagellates are diverse photosynthetic mixotrophic or heterotrophic groups of flagellate eukaryotic organisms with haplontic lifecycles in both fresh and marine waters. Various Dinoflagellates (i.e. Gonyaulax polyedra, Pyrodinium bahamense, and Pyrocystis lunula) are bioluminescents. Dinoflagellates blooms can sometimes reach more than one million cells ml-1 and cause the red tide phenomena. Notably, the vast majority (75%) of the approximately 300 identified HABs are Dinoflagellates, which produce a wide range of biotoxins as reported in Figure 1 (48).
In recent years, human illnesses linked with Ostreopsis have been paid increasing attention because some of the benthic toxigenic Dinoflagellates belonging to this genus have been found to produce toxins such as PLTX, palytoxin-like compounds (i.e. ovatoxins) and ostreocin-D, mascarenotoxins (49). Ostreopsis blooms have been connected to human poisoning (after consumption of clupeid fish, crabs, or sea urchins) and respiratory difficulties in swimmers and people near affected areas. Some evidence indicates that the production by Ostreopsis of toxins like PLTX is a natural occurrence and that climate does not play a major role in either bloom formation or toxin production. However, other reports have been providing evidence of the important role played by climatic changes and salinity levels (50).
Gymnodinium is another genus of toxigenic Dinoflagellates. Typically, the species that belong to this genus have mixotrophic characteristics with vertical migration toward the light source. The genus can tolerate wide ranges of salinity (5 to 40 psu) and temperature (10 to 30°C), and uses sexual resting cysts as a mechanism for distribution (51). A recent study suggests that 70 kilodalton heat shock proteins (AsHsp70) are responsible for the adaptation of Gymnodinium sanguineum (also known as Akashiwo sanguinea) to different temperatures and has a role in the encystment of Dinoflagellates (52). Microarray-based technology (GeoChip) tests indicate that the genes involved in carbon degradation, along with nitrogen and phosphorus limitations, are involved in the bloom of G. sanguineum (53). Recent studies show that the water soluble MAAs synthesized by G. sanguineum have the potential to reach humans through the food web and that light, salt stress, and nitrogen levels have a substantial influence on the synthesis of MAAs from G. sanguineum (54). Recent advances in technology have allowed the classification of Dinoflagellates genus like Coolia, previously thought to be nontoxigenic, as toxic genus. Coolia species are euryhaline and they have the ability to survive in wide salinity ranges (20-50%). Coolia monotis is able to produce a monosulphated polyether toxin with a LD50 of 1 mg Kg-1 in mice called cooliatoxin, which causes similar symptoms to those of yessotoxins (YTXs) (K) infection (55, 56).
Alexandrium genus is recognized as autotrophic, mixotrophic and heterotrophic armored Dinoflagellates (covered by thecal plates), that can reproduce through asexual or sexual cycles. This genus is an invader with the ability to form HAB in different ecosystems. It is characterized by various life cycles phases, including motile vegetative cells, haploid gametes, diploid zygotes, resting cysts, and cysts. The species, including A. andersoni, A. taylori, A. lusitanicum, and A. minutum, are able to produce different toxic molecules, including PSP toxin, and their cysts are much more toxic than motile vegetative cells (57). A recent study demonstrates that the genome size of Alexandrium can reach 56.48 ± 4.14 Gb almost nineteen times that of a human genome (58). Alexandrium genus produces the alkaloid neurotoxin neosaxitoxin (neoSTX) (L), a biotoxin that has a high affinity for voltage-dependent sodium channels. This affinity makes them potentially lethal, as they can prevent the transmission of nerve impulses and lead to muscle paralysis. The LD50 is about 33.5 μg Kg1 (57). The 13-desmethylspirolide is another cyclic amine toxin isolated from Alexandrium, which has been associated with PSP. The molecule is a fast acting agent (LD50 = 0.028 mg Kg-1 in mice) that produces a response within minutes of exposure (in mice) by blocking the sodium conductance of the squid axon membrane with no effect on the potassium conductance (59).
Dinophysis genus is documented as phototrophic and heterotrophic armored Dinoflagellates with asexual and sexual reproduction cycles. They are found worldwide from tropical, to moderate, to boreal aqua systems. Statistical modeling indicates that Dinophysis growth is linked to seasonal changes, thermal stratification, and changes in nutrient concentrations. Some of the species like D. caudata bloom in tropical and subtropical waters and others like D. acuta bloom in late summer–autumn in thermal stratified temperate waters (60). The genus includes more than 200 species, such as D. acuminate, D. norvegica, D. caudate, D. rotunda, D. fortii, D. sacculus, D. acuta, and D. miles. This genus produces two types of lipophilic toxins: acid polyethers, such as okadaic acid (OA) (M) with its derivatives and dinophysistoxins (DTX), and polyether-lactones, such as pectenotoxins (PTXs) (N), YTXs, and azaspiracids (AZAs) (O) (61). PTXs are macrocyclic lactones that cause damage to the liver cells. They are said to be in the neutral class of DSP toxins, although this is still debated as they do not provoke diarrhea, a symptom characteristic of DSP toxins. LD50 values for PTX vary between 219 and 411 μg Kg-1 (62). Furthermore, AZAs are open-state blockers of the human ether-a-go-go-related gene (hERG) potassium channels characterized by a high cytotoxicity, teratogenic and probably carcinogenic activities.
AZAs are firstly identified in the dinoflagellate Azadinium spinosum, and currently twenty analogues of AZAs have been identified. A minimal LD50 of 150 μg Kg-1 has been reported in mice. In humans, AZAs cause DSP like symptoms with a LD50 of 15 AZA mg (63).
DTX are effective at cell densities as low as 1-2 × 102 cell L-1. DTX1, DTX2, DTX3, DTX4, and DTX5 are known to be the analogues of OA and are considered the main cause of DSP. They cause symptoms such as diarrhea, vomiting, and abdominal pain, starting 30 minutes after exposure. OA and the DTXs inhibit protein phosphatases and are hepatotoxic in mice (61).
The Amphidinium genus are known as tropical marine water Dinoflagellates, which lack cellulosic material. Some of the species, like A. carterae, can reach a density of 1.8. x 108 cells L-1 and form HAB in shallow, sandy, open coastal lagoons, causing water discoloration. A range of toxic molecules, such as short and long chain polyketides, macrolides, amphidinolides, amphidinols, and luteophanol are identified in the Amphidinium genus (64).
Gymnodinium genus is another documented toxigenic dinoflagellate. The species of this genus are responsible for the production of SXT, brevetoxins (PbTx) (P), gonyautoxin (GTX) (Q), neostx, gymnocin A and B, and karlotoxins (KmTxs) (R) (65).
PbTx is a lipophilic polyether and a neurotoxin molecule that attaches to the DIS6 unit and causes a negative shift in the voltage-dependency of activation. PbTx has multiple active centers, including A-ring lactone, and C-42 of R side chain, which affect the sodium channel-binding site (66). Studies reveal that PbTx are released into the water by excretion. During a bloom, cells rupture and cause the amount of extra-cellular toxins to increase. The extra-cellular toxins then undergo bubblemediated transport to the sea surface where they are ejected into the air as jet drops from the bursting bubbles. Subsequently, with on-shore winds and breaking surf, the toxins combine into marine aerosol causing severe respiratory irritation to humans and other mammals along the shore (67). GTX is a neurotoxin and causes PSP with a LD50 from 1 to 4 mg in humans. GTX as a neurotoxin that can bind with high affinity to the α-subunit of voltage-dependent sodium channels in the postsynaptic membrane of the cells (68). KmTxs are lytic compounds that have negative influences on blood cells, and they cause cell lysis. Two classes of karlotoxins were reported: KmTx 1-type (UV max ∼225 nm) and KmTx 2-type (UV max ∼235 nm). Evidence shows that the spectral shift is due to chlorination of the terminal diene of the lipophilic arm. KmTxs are extremely active against erythrocytes (complete cell lysis = ∼0.5. μg ml-1): the toxin targets the cell membrane affecting membrane permeability, similarly to how amphidinols, a new class of polyhydroxyl polyene compounds, behave (69).
Gambierdiscus genus consist of photosynthetic, epiphytic, and epibenthic toxigenic Dinoflagellates that grow in tropical coral reef ecosystems at water temperatures from 24 to 29°C. The cells have an anterior-posteriorly compressed shape and attach to macroalgae or seagrass. This genus produces ciguatoxins (CTXs) (S). The global number of people affected by diseases caused by G. toxicus species, which are responsible for CFP via production of a series of toxins including CTXs, maitotoxin, gambierol (GAM) (T), scaritoxin, and PLTX, is estimated at 20.000 to 50.000 people annually (70). The toxin GAM is a polycyclic ether toxin with a trans-fused octacyclic polyether core and 18 stereogenic centers. GAM starts showing toxicity signs in mice at 50 μg Kg-1 and, with a LD50 of 80 μg Kg-1, death occurs after one hour. The most affected organs are the lungs and the heart. The symptoms are similar to those of CTXs. GAM can act as a low-efficacy partial agonist at voltage-gated sodium channels (VGSC) or as a high affinity Kv channel inhibitor (71).
CTXs are a class of toxigenic polycyclic polyethers that decrease the threshold for opening VGSC in synapses of the nervous system. CTXs are lipophilic molecules that are able to cross the blood brain barrier, and can cause both central and peripheral neurologic symptoms. Opening sodium channel causes depolarization, which could consequently cause paralysis, heart contraction, and a change in sensitivity to heat and cold. This condition is known as ciguatera. Evidence shows that CTXs and PbTx share a common receptor site on the neuronal voltage dependent Na+ channel. The major symptoms caused by CTXs develop within 1-3 hours from toxin ingestion and include vomiting, diarrhea, numbness of extremities, mouth, and lips, reversal of hot and cold sensation, and muscle and joint pain. The symptoms may last from a few days to several weeks or even months depending on each individual (72, 73).
The Lingulodinium genus is represented by armored photoautotrophic, motile Dinoflagellates belonging to the Gonyaulacaceae family with various life cycles and the ability to display magnificent bioluminescence in warm coastal waters. The cells can have an angular, roughly pentagonal, and polyhedral-shape and can have peridinin molecules stored inside their chloroplasts. Reduction of irradiance, temperature increase, and turbulence are known to increase the size of the cells. Lingulodinium machaerophorum is the cyst of this alga, and its size varies from 31 to 41 μm. Disulfated polyether toxins, such as YTXs, have been discovered in this genus. YTXs have been traditionally associated with DSP, although new evidence indicates that YTXs should be excluded from the DSP toxins class as they are dissimilar to OA and DTX; diarrhea and inhibition of protein phosphatases are not among the symptoms caused by YTXs (74, 75).
The genus of Noctiluca is a heterotrophic bioluminescent free-living un-armored Dinoflagellate that feeds on phytoplankton. Noctiluca reproduces asexually (via binary fission) and sexually (via formation of isogametes). The toxic blooms of Noctiluca have been associated with considerable fish and marine invertebrates deaths. This Dinoflagellate accumulates and secretes ammonia into the water, process which can lead to skin irritations among swimmers (76).
The Phalacroma genus is identified as heterotrophic Dinoflagellate. The strains of this species are able to synthesize the hydrophilic and lipophilic neurotoxins, such as OA, dinophysistoxin-2, and pectenotoxin-2. The species typically existed in a concentration < 500 cells L-1, although the population can reach to 8.7 x 105 cells L-1 during the bloom, which occur mainly in late winter to early spring (77).
Diatoms are in the division of Chrysophyta, with orders Biddulphiales and Bacillariales. They are a part of heterokonts that include autotrophs and heterotrophs, diatoms that have a siliceous skeleton and range from 2 to 200 μm in size. Reproduction among these organisms is primarily asexual by binary fission. They are responsible for 40% of the marine primary production and almost one-quarter of the global primary production (78).
The diatom bloom typically occurs in upwelling areas and coastal oceans where nutrient concentrations are considerably high. Silicate depletion is the first factor that controls the reduction of diatom blooms (79).
Pseudo-nitzschia (Bacillariaceae) is known as a genus of diatoms with some toxin producer species, including P. australis, P. calliantha, P. cuspidate, P. fraudulenta, P. multiseries, P. multistriata, P. pungens, and P. subpacifica. The biotoxin of Pseudo-nitzschia genus produces neurotoxins domoic acid (DA) (U) (80). This toxin is a cyclic amino acid, and its structure is composed by three carboxylic groups responsible for its polarity and hydrophilicity. Pseudo-nitzschia species accumulate DA and its isomers in concentrations of 1.3. pg DA cell-1, and they are associated with ASP. The toxicity from DA occurs at a cell density of > 5 x 104 cells L-1 and human intoxication can occur with doses between 1 and 5 mg Kg-1 (Table 1). DA functions similarly to kanaic acid (KA) (V), another neurotoxin (81).
| Toxin | Action level 1 | Associated human syndrome | Vector for human | Other affected resources | Method for toxin analysis |
|---|---|---|---|---|---|
| PbTx | 5000 cell L-1 (close shellfish beds); <20 MU/100 g | NSP, ARDS2, and eye/skin irritation | Bivalves, gastropods aerosol exposure | Marine mammals, seabirds, sea turtles, fish, and invertebrates | Mouse Bioassay, ELISA3, LC-MS4, receptor binding assay |
| DA | 20 ppm | ASP | Bivalves | Seabirds and marine mammals | HPLC-UV5, LC-MS, ELISA |
| OA, DTX | 0.1.6 ppm | DSP, tumor promotors | Bivalves | Fish disease, potential tumor promoter in turtles | Mouse bioassay, LC-MS, ELISA, PP2A6 |
| STX | 80 μg STX eq./100 g | PSP | Bivalves puffer fish | Marine mammals and other organisms | Mouse bioassay, HPLC-FD7, ELISA, LC-MS, receptor binding assay |
| MCY, CYN | 1.6.-3.0. μg L-1 in water | Hepatotoxic activities | Drinking water | Marine mammals and other organisms | Mouse bioassay, HPLC-UV, LC-MS, ELISA |
| 1: refers to the levels recommended by U.S. Environmental Protection Agency (EPA) for enforcement by food and drug administration and by U.S. Department of Agriculture when microlgal toxins occur in food or feed commodities; 2: ARDS = Acute respiratory distress syndrome; 3: ELISA = Enzyme Linked Immunosorbent Assay; 4: LC-MS = Liquid chromatography–mass spectrometry; 5: HPLC-UV = High-Pressure Liquid Chromatography with UV Detector; 6: PP2A = Protein serine/threonine phosphatase; 7: HPLC-FD = High-Performance Liquid Chromatography with Fluorescence Detection. | |||||
This class of HABs is composed of motile unicellular eukaryotic fresh and marine algae that are responsible for red tides. Raphidophytes are autotrophic, although the latest reports demonstrate that they are able to feed on heterotrophic and autotrophic bacteria (82).
Chattonella is a genus of marine Raphidophytes that contains ichthyotoxic species like C. marina that is able to produce neurotoxic molecules including CaTx-I, CaTx-II, and CaTxIII. Chattonella grows in brackish waters and the blooms occur at cell densities of up to 66.000 cells L-1 (83). The genus Heterosigma contains relatively small mixotrophic alga (18 to 34 μm) that inhabit shallow waters. The species have the ability to reproduce asexually. The Heterosigma toxins (HaTx) are HaTx-i, HaTx-iia, HaTx-nb and HaTx-iii and they occur at slow growth phase (temperature = 20°C, light intensity = 200 μmol m−2s-1) (84).
Fibrocapsa is a new genus in Chloromonadophyceae that inhabits temperate and tropical coastal waters worldwide. Some species of this genus, like F. japonica, are toxin producers. Elliptical shape and the presence of mucocysts are typical identifiers of the genus, and Fibrocapsa are commonly existed at low concentrations (500 cells L-1). Optimal growth conditions for this microalga include temperatures of 10°C and 26°C with a salinity of 11 up to 20 PSU. F. japonica produces a neurotoxin called fibrocapsine. Research has linked Fibrocapsa mechanism of action to the synthesis of mucous, the production of reactive oxygen species (ROS), and the production of haemolytic compounds (85).
Physiologically the biosynthesis of algal toxins is associated with the algal genetic characteristics. However, due to the importance of the toxin for aquatic ecosystems and human health it is important to understand the factors that induce and stimulate toxins.
Fertilizers, industrial or agricultural wastewaters, costal aquaculture, and atmospheric inputs are all associated with increased levels of nutrients in aquatic ecosystems. Reports demonstrate that a rise in the accumulation of nitrogen (N) in coastal ecosystems and alterations of relative and absolute discharges of phosphorus (P) and silicon (Si) levels are not in a balance with the requirements of algae but are linked to the production of different HABs and toxins (i.e. AZA2 by Azadinium poporum) (86). There is a considerable difference between algal genus, species, and strains and their toxin content response to different nutrient conditions. The N stresses predominantly affect the somatic growth and size of the cells, while P stresses have the potential to influence their metabolic regulation, although P stresses are those which are mainly associated with the production of toxins. For example, P deficiency in Alexandrium tamarense is associated with the upregulation of PSP toxin (4 fold). Further, reports on A. fundyense show that severe P-limitation leads to an increase in toxin content only when N is a co-limiting factor. This result suggests a synergistic effect of N and P availability on toxin synthesis and turnover (87). Toxin content and the intracellular concentration of arginine also have a positive relationship, though this connection is altered by the nutrient concentration of the cells (in particular P-status). In A. minutum, cell toxicity and total toxicity reach maximum levels at the post-stationary growth phase and decrease quickly, while toxins GTX 2 and 3 increase when the cells are cultured in high salinity medium (88).
In another case, the relationship between temperature, phosphate limitation, and production of GTX-3, STX and NEO toxins from A. tamarense was analyzed and revealed that low temperatures had a negative impact on the growth of Dinoflagellates (A. catenella, A. cohorticula and Gymnodinium catenatum), while having a positive impact on the production of toxins (89). The production of six cyclic, heptapeptide toxins from cyanobacterium Microcystis aeruginosa was associated with seasonal changes. The six toxins were either not detectable or in very low concentrations during the winter (May-August), reaching maximum concentrations during the summer. Combined concentrations of four of the toxins ranged from 5 to 415 wg (g of dry scum) and were strongly correlated to primary production factors, including chlorophyll a, solar radiation, surface water temperature, pH, and percent oxygen saturation (90). In another study, UV-B irradiation has shown significant influence on the expression level of the sxtU gene responsible for STX production from C. raciborskii. The response to UV-B radiation suggested that it was a part of the survival mechanism and fitness of the species in the environment (91).
Allelopathic interaction factors also have the potential to trigger the production of toxins from algal species. For example, the GTX1 toxin in A. minutum can be transformed into GTX 2 and 3 through the marine bacterium Pseudomonas sp. (92). The DA synthesis increases when the alga has grown with a mixture of gluconic acid/gluconolactone produced by the bacterium Alteromonas sp. (93). In addition, the toxins from M. aeruginosa increase through interaction with herbivorous zooplankton, such as Moina macrocopa, Daphnia magna or D. pulex, supporting the hypothesis that this response is an induced defense mechanism mediated by the release of signaling molecules from zooplankton (94).
Toxins production in algae subjected to different treatment technologies is based on the nature of the toxin and on whether they are completely water soluble or not. Indeed, toxins are produced and retained within healthy cells or released into the surrounding water when cells die and break open. Suitable water treatment methods thus depend upon whether toxins are dissolved compounds in the raw water or are present within cells. On one hand, if toxin-containing cells are not removed at the early stages of treatment, great care must be taken to avoid cell lysis in order to prevent the shift of toxins into the water phase reducing the benefit of the treatment. On the other hand, in some cases, it is desirable to release toxins like MCY into the liquid phase where they can be removed through treatment processes. Methods of water treatment for toxins removal can be roughly divided into physicals and chemicals. They have been reviewed in the last fifteen years by several authors (95-98). A standard framework and steps for public response to toxigenic HAB events is represented in Figure 3.
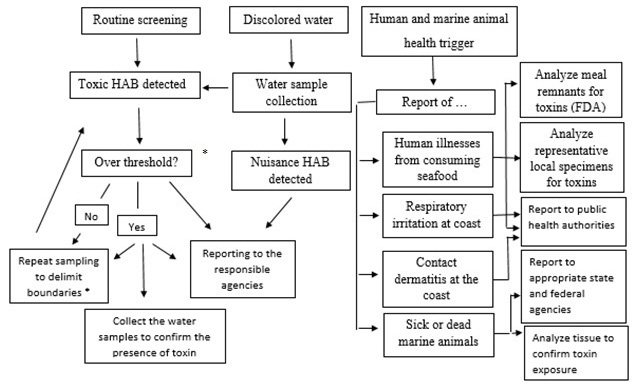 Figure 3.
Figure 3.The suggested framework and steps for a state response to toxic HAB events. Note: *After the first report on suspected HAB event and the subsequent investigation, adopt appropriate cautionary plan of action. The thresholds for cell density and toxicity in seafood for Pbtx, STX, OA/DAX and DA are reported as follow: for Pbtx 5000 cells L-1; 20 MU 100g-1; for STX 10000 cells L-1; 80 μg 100g-1; for OA/DAX 1000 cells L-1; 0.1.6 ppb; and for DA 1000000 cells L-1; 20 ppb.
There are two general types of well-established physical methods for toxin removal: absorption technologies, such as activated carbon, and reverse osmosis (RO). In the water industry, granular activated carbon (GAC) and powdered activated carbon (PAC) are commonly used for the removal of trace organics. The former is intermittently added to water contained in flow-through column reactors in a powder or slurry form, whereas the latter can be continuously added directly to the water prior to coagulation or filtration. Early studies carried out by Hoffman (97) demonstrate that common methods, such as flocculation/sedimentation, sand filtration, and chlorination, all failed to eliminate the toxicity associated with the peptide toxins released by cyanobacteria. However, the author demonstrated that filtration through activated carbon, in particular the addition of PAC, was successful in removing the toxins, although this was dependent on dosing levels. Subsequent studies confirmed that activated carbon could be effective to varying degrees and that for most carbon types high doses of PAC are required. A number of investigations have assessed the suitability of a range of carbons, both PAC and GAC, produced from a variety of starting materials (99). In the case of PAC, dosing is an important parameter to consider (10 pg L-1 toxin: > 200 mg PAC-1 L-1), whereas a key point when using GAC is the choice of the carbon source (coal, wood > peat, coconut), possibly due to the different pore sizes relative to the size of the MCY molecule (100). However, it was concluded that surface characteristics are more important in determining sorption performance rather than the parent carbon source (99). A drawback to be taken into consideration when employing activated carbon for water treatment is the formation of a biofilm, which can significantly impair the ability of the filter to adsorb toxins (101).
RO is a technology pioneered in the 1960s which takes salt out of seawater. Although operating in different ways, RO is similar to the filtration method in its separation of a liquid from a mixture of suspended or dissolved solids. The semipermeable membranes commonly used in RO exclude dissolved salts although they exclude organic compounds with a molecular weight above 100 dalton, whereas filtration excludes components from the filtrate due to their size.
Since the molecular weight of MCY is around 1000 dalton, it can be assumed that RO membranes can retain them, and actually, RO is currently in use in a number of countries to provide drinking water. Neumann and coworkers (102) compared the retention rates of MC-LR, MC–RR, and RO and found out that the RO rates were higher by 95%. Seubert et al. (103) carried out a 5-year monitoring (2005-2009) of the intake and desalinated water at the pilot RO desalination plant of El Segundo (California) to explore the potential of extracellular algal toxin contaminating the RO products. DA, STX, PbTx and OA were the toxins investigated. DA and STX were detected sporadically in the intake waters but never in the desalinated water, while PbTx and OA were not detected in the intake nor the desalinated water. The results of this study demonstrate the ability of typical RO operations to remove these toxins. Nevertheless, although RO appears to be a suitable method of producing safe drinking water, this process retains toxin-enriched water, which has to be disposed safely since the toxins are not destroyed by this treatment.
The use of chemicals in toxin treatments has been well documented since the mid 70’s and has mainly involved oxidising agents commonly used for the treatment of potable water. Amongst them hydroxyl radical, ozone, hydrogen peroxide, perhydroxyl radical, permanganate, hypochlorous acid (HClO), chlorine (Cl), and chlorine dioxide (ClO2) to name a few. Broadly speaking, the studies reporting the use of these chemicals are characterized by lack of information on the fate of decomposed products, mainly focusing on how quickly the parent toxins disappear. Most of these oxidizing reagents tend to react readily with unsaturated bonds such as those included in most of the toxins. Inside toxins, these bonds act as chromophores and absorb the UV at 238 nm when analyzed with a spectrophotometer. This means that when modifying the chemical composition of the parent toxin it will seem to disappear upon UV analysis, even if this does not provide information on the nature, fate, and potential hazards of the by-products that are generated.
When dissolved in water, Cl tends to form HClO. For pH values above 5, the latter starts to dissociate forming hypochlorite ions, and dissociates completely with values above pH 10. The undissociated HClO molecule represents the most effective disinfecting agent. Early studies on the use of Cl for the treatment of toxins indicated that the process was ineffective, probably due to the use of a pH range that reduced the presence of free Cl concentrations (104, 105). The positive correlation between high pH values and the effectiveness of Cl for the destruction of toxins was proved by Hoffman (97), who performed his investigation at pH 8.5, and by Nicholson et al. (106), who investigated the use of Cl and chloramine for the destruction of MCY and NOD. They found that the treatment was pH dependent with the use of NaOCl and Ca(OCl)2, and, in particular, it was less effective for pH values above 8, with 79% and 0.4.% destruction at pH 7 and pH 10, respectively. They speculated that the reason was probably associated to the decreasing concentrations of HClO, which is a more effective oxidant than the hypochlorite ion. Zamyadi et al. (107) investigated the chlorination as a method for the treatment of STXs produced by Anabaena circinalis. They found that with a Cl exposure value of 7.0 mg-min L-1 all cells lost viability causing toxin release. Further, they found that all STXs and more than 95% of other STXs analogues were subsequently oxidized. The use of ClO2 as method of toxin removal has been less investigated in the literature so far, though Kull et al. (108) studied the effectiveness of ClO2 in oxidative elimination of cyanotoxin MC-LR. They studied MCY and ClO2 at natural concentrations of 10 μg L-1 and 1 mg L-1, respectively, in the presence and absence of natural organic matter and found that fulvic and humic acids rapidly consumed ClO2, leaving less residual ClO2 to oxidize MC-LR. Rodriguez et al. (109) also investigated the removal of toxins such as MC-LR, CYN, and ANTX, produced by the cyanobacteria Cylindrospermopsis, Anabaena, Aphanizomenon, Oscillatoria, and Nostoc, and found out that ClO2 was not an appropriate oxidant for the oxidative removal of these toxins. On the other hand, they concluded that chlorination was a suitable option for oxidation of MC-LR and CYN, whereas the oxidation of ANTX by Cl was too slow. Recently, a work on the use of ClO2 for the treatment of toxins produced by the cyanobacteria M. aeruginosa demonstrated that ClO2 could not only inhibit the photosynthetic capacity of cyanobcaterial cells, but also rapidly degrade the toxins increasing ClO2 dosages from 0.1 mg L-1 to 1.0 mg L-1. The only drawback associated with this treatment is the formation of trihalomethanes and halocetic acids as disinfection by-products, process that can limit the use of this treatment (110).
Ozonation relies on the use of the instable gas ozone (O3) characterized by a relatively high oxidation potential. It is able to rapidly lyse cells and is effective against toxins at doses of 5 ppm or less and at very low concentration-time (CT) values (in mg-min L-1), with an elimination of MC-LR that is virtually instantaneous. Its use for water treatment, through the dispersion of the gas in aqueous media, is widespread even if it is an expensive and sometimes unpredictable reagent. Ozonation is highly reactive towards double bonds; thus, MCY would be susceptible to such an attack (111). Studies carried out by Rositano et al. (112) proved that 99% of MCY was removed in 15 seconds when treated with 0.05 mg L-1 of O3, whereas a 100% removal was achieved by Keijola et al. (105) treating 60 mg L-1 of MCY with 1 mg L-1 of O3. Both authors also found that the efficiency of O3 treatment was greatly enhanced when it was combined with hydrogen peroxide. Bernazeau (113) also reported a 99% destruction of 500 μg L-1 MC-LR after 4 minutes of treatment with a 0.2 mg L-1 of O3 dose. Onstad et al. (114) further evaluated the reactivity of O3 with the double bonds of MC-LR and CYN, demonstrating that with the former it is not pH dependent while with the latter it is. The effect of O3 as pre-oxidant for algae-containing water, and the mechanisms responsible for algal cells rupture and intracellular cytoplasm release was investigated by Miao and Tao (115). Their results showed that ozonation was an effective method for the removal of MCY produced by M. aeruginosa and that the removal efficiency of MC-LR was higher than that of –RR.
The permanganate method is based on the use of potassium permanganate (KMnO4) as a strong oxidizing agent capable of destroying organic compounds and microorganisms. This method was very effective in lysing cells and eliminating toxins with a CT value of about 25 mg-min L-1. Studies undertaken at the water research center in Buckinghamshire, UK, on the removal of algal toxins during water treatment showed that MC-LR and ANTX were successfully removed by ozonation and treatment with KMnO4 (116). Similar conclusions were reached by Hall et al. (95), which evaluated the effectiveness of some toxins treatment methods on a laboratory scale. A 95% removal of MC-LR was achieved in 30 minutes, treating 200 mg L-1 of this toxin with 1 mg L-1 of a KMnO4 solution. The kinetics involved in the removal of MCY toxins by KMnO4 were investigated by Rodriguez et al. (117), who took into consideration a pH range of 6.8-8.2. They found that the influence of pH on the oxidation process was not appreciable and that a KMnO4 dose of 1-1.25 mg L-1 was enough to reduce MCY concentration below the guideline value of 1 μg L-1. An algaecide made of KMnO4 combined with zeolite was developed to investigate its effect on the removal of MCY toxins produced by M. aeruginosa (118). A removal rate of 97% with a 0.22 g L-1 dosage of zeolite carrying potassium permanganate was achieved. A study carried out by Gad et al. (119) in a full scale plant for the removal of MCY from water (where the water surface was pre-chlorinated) demonstrated that, when KMnO4 was used as a pre-oxidant instead of Cl, the integrity of algae cells was preserved allowing the removal of intact cells with their toxins and providing better control over harmful disinfection byproducts. Pre-chlorination produced cell lysis and the release of cell-bound toxins. Ding et al. (120) compared the efficiency of Cl, KMnO4, ClO2, O3 and monochloramine for the removal of MCY from M. aeruginosa. The results suggested that KMnO4 was the most effective disinfectant for achieving both disinfection and removal of the released cyanotoxins with typical disinfectant dosages.
The use of hydrogen peroxide (H2O2) in water treatment is limited due to the fact that the kinetics for many water treatment applications are unfavorable, even if its effectiveness can be enhanced by irradiating water with UV light. There are some reports dealing with the ineffectiveness of H2O2 in degrading MCY when used alone. A recent study to control the bloom of M. aeruginosa and Anabaenopsis sp. in a brackish storm-water pond and mitigate their toxins content was carried out using three chemical algaecides, where H2O2 was the main component (121). The results showed that the addition of algaecides to the pond did not reduce overall MCY levels, and a release of toxin from the particulate to the dissolved phase was observed in most treatments. Only a 17% removal was reported after 60 minutes with a 20 mg L-1 solution of H2O2, while no destruction at all was observed after 10 minutes when treating 1 mg L-1 solution of MCY with 2 mg L-1 of H2O2 (112, 122). In this last case, all the toxins were completely removed within 30 seconds when H2O2 was coupled with O3. Barrington et al. (123) investigated the use of H2O2 for the removal of MCY from waste stabilization ponds. Evidence showed a reduction of Cyanobacteria and MCY concentration in the upper layer of the pond. As H2O2 significantly decreased the MCY concentrations within days of application, it was suggested that H2O2 can be regarded as an efficient algicide treatment in waste stabilization ponds. Qiao et al. (124) successfully demonstrated the use of UV/H2O2 for the treatment of MCY-containing water. He et al. (125) used low-pressure mercury vapor germicidal lamps as source of UV, providing a UV dose of 80 mJ cm-2 (considered as the total incident radiation per unit area), and an initial H2O2 concentration of 882 μM. Under these conditions, a 94% removal rate was obtained with an initial MC-LR concentration of 1 μM. Recently, the use of H2O2 was coupled with ultrasounds to test their efficiency in reducing M. aeruginosa release of cyanotoxins. Laboratory assays with H2O2 dosed at 4 or 8 mg L-1 lowered total MCY concentrations by 23%, yet led to a significant release of the toxin into the water, with dissolved MCY concentrations that were 9 and 12-times higher than in the control. Ultrasound treatment with commercial transducers was ineffective since it caused some release of toxins into the water (126).
Fang et al. (127) reported effectively detoxifying MCY in water using an inexpensive, environmentally friendly and biocompatible mineral. In this study, the iron-oxide mineral maghemite was investigated as a visible light catalyst for decomposing MC-LR in water using H2O2 as the initial oxidant. Visible light appears to play a new role in this unique photocatalytic oxidation system producing the opening of the cyclic peptide structure and, therefore, detoxifying MCY. The photolytic oxidation of toxins is based on the absorbance of ultraviolet (UV) energy that produces one or more breaks in molecule bonds without the addition of chemicals. To do this, it is necessary that the compound absorbs light at a wavelength, which is the same as that emitted by the light source. Normally, this process involves the use of low- or medium UV lamps characterized by a wavelength range between 200-300 nm (128). To quantify the total exposure of a substance or organism to UV radiation, a UV index is used and can change depending on the microorganisms or substance. The proposed mechanisms involved in the reduction of toxicity in MCY with UV are severals. Photo isomerization may take place if an electron in one of the unsaturated bonds of Adda group is promoted to a higher orbital, causing a rotation around the newly formed single bond. In many cases the molecule undergoing degradation is subjected to an electron transfer from excited state to ground state oxygen, generating the superoxide radical anion and the radical cation that may then be hydrolysed (98, 129). These radicals may also react with other species in water, causing further oxidation of the toxin. A number of research groups has reported the photolysis of MCY, although the mechanistic explanation for the process has not been always provided (126, 127). It should be noted that most of this research was carried out on a laboratory scale where the UV dosages used are limited, controlled, and sometimes not appropriate for practical treatments. UV dosages can effectively degrade both MC-LR, ANTX, and CYN ranges from 1530 mJ cm-2 to 20.000 mJ cm-2 (122, 126). The UV dosage has several orders of magnitude higher than that needed for disinfection and usually applied in a water treatment plant, as 643 mJ cm-2 produced by low-pressure narrow-band mercury vapor lamps at 254 nm and required to degrade CYN (130). Photo inactivation of coupled with other catalysts in Photo-Fenton processes and TiO2/UV toxins has been also investigated (131, 132). Fotiou et al. (133) compared the removal of CYN from water through photocatalysis by using UV-A, solar, and visible light. Two commercial TiO2 were used as catalysts. Complete degradation of toxin with photocatalysts was achieved in 15 and 40 minutes under UV-A and in 40 and 120 minutes under solar light irradiation; photolysis in the absence of photocatalysts was negligible. Choi et al. (134) also investigated a mesoporous nitrogendoped TiO2 photocatalyst and its ability to inactivate MC-LR under UV and visible light. Advanced oxidation processes technique can be considered as a relatively nonselective oxidant, derived by both photochemical and non-photochemical reactions. OH- has been reported to be reactive with MCY, CYN, and ANTX. Inactivation of MCY was also investigated with new emerging technologies. Zhang et al. (135) used a boron-doped diamond electrode, varying the supporting electrolyte and the applied current density, in order to determine its effect on the inactivation of MC-RR. They determined that a pH value of 3 and 20 mM sodium chloride were optimum conditions for the process, although the low pH value can represent a drawback for the feasibility of this technology, as pH 3 is not practical for drinking water treatment. In another study by Song et al. (136), ultrasonic irradiation at 40 kHz was demonstrated to be feasible for the inactivation of the MC-LR. Overall, these emerging technologies were proven to be effective for the inactivation of MCY and it should be worth to extend the investigations to CYN, ANTX, and STXs.
The threats from HABs and algal biotoxins are rising globally and they are representing a serious concern to public health and wildlife. Some of the toxins like ANA have a lethal dosage of less than 5 mg for an adult human male, and can target liver, kidneys, lungs, heart, stomach, and vascular and lymphatic systems. Further, the costs of containing these threats keep rising worldwide. Broadly, for public health costs (45%), market loss costs (37%), tourism costs (13%), and management costs (4%), the United States spends an estimated 100 million dollars per year and the European Union an estimated 1 billion dollars per year, respectively.
During the past decade, different strategies have been introduced for the prediction of incidence, distribution, and toxicity of HABs. Also, various countries have limited sewage, agricultural, and industrial discharges to coastal zones and decreased or removed nitrogen and phosphorus chemicals in order to cut down the potential nutrients for HABs growth.
Nevertheless, these strategies are pointless if are not well understood the physiology and the growth strategy of HABs, considering that HABs are characterized by a complex life cycle, including both spores and cysts. To this end, the structure and the mode of action of algal toxins should be thoroughly investigated, and easy and rapid methods for analysis of the toxins must be introduced for the protection of human health and the stability of ecosystems. Further, it is essential to develop multi-lateral international programs to allow cooperation among scientists from different regions and countries. In addition, public awareness of the threats that algal toxins pose to human health must be increased. Lastly, risk assessment of algal toxins must be refined in order to obtain the most possible accurate scenario and undertake all the due enforcement actions.
We appreciate the School of Engineering and Science, Tecnologico de Monterrey, Campus Monterrey, México for supporting this study.
Abbreviations: ANA, anatoxins: ANTX, anatoxin-a; ASP, amnesic shellfish poisoning; ATX, aplysiatoxin; AZAs, azaspiracids; AZP, azaspiracid shellfish poisoning; CD, cercrical dermatitis; CFP, ciguatera fish poisoning; CYN, cylindrospermopsin; DA, domoic acid; DAT, debroaplysiatoxin; DSP, diarrheic shellfish poisoning; DTX, dinophysistotoxins; GAC, granular activated carbon; GAM, gambierol; GTX, gonyautoxin; HABs, harmful algal blooms; HaTx, Heterosigma toxins; HANTX, homoanatoxin-a; KA, kanaic acid; KmTxs, karlotoxins; LTA, lyngbyatoxin-a; MAAs, mycosporine like amino acids; MC-LR, microcystin-LR; MCY, microcystins; neoSTX, neosaxitoxin; NOD, nodularin; NRPS, non-ribosomal peptide; NSP, neurotoxic shellfish poisoning; OA, okadaic acid; PAC, powdered activated carbon; PbTx, brevotoxins; PKS, polyketide synthase; PLTX, palytoxin; PSP, paralytic shellfish poisoning; PTXs, pectonotoxins; RO, reverse osmosis; STX, saxitoxin; TTX, tetrodoxin; YTXs, yessootoxins; VGSC, voltage-gated sodium channels



