1 LPCMIO, Materials Science Center (MSC), Ecole Normale Supérieure, Mohammed V University in Rabat, 10200 Rabat, Morocco
2 First Source Laboratory Solutions LLP (Analytical Services), 500076 Hyderabad, India
Abstract
Background: Salvia verbenaca of the Lamiaceae family is a
Mediterranean plant widely used in the Moroccan traditional folk medicine. The
aim of this work was to explore the phytochemical composition of Salvia
verbenaca extracts and its antioxidant activity. Methods: Separation
and identification of the major phytochemicals present in the two hexane and
ethyl acetate explored extracts have been achieved through ultra-high-performance
liquid chromatography separation technique coupled to photodiode array and
high-resolution time-of-flight mass spectrometry detectors. Antioxidant activity
of the obtained extracts was evaluated through DPPH
Keywords
- Salvia verbenaca
- UHPLC/PDA/ToF-ESI/MS
- phytochemicals
- pharmacological activities
Humans have relied since ancestral times on plants as a source of food, flavours, fragrances, and medicines. As far as we go back in history, man has always used the plants for healing, for feeding or for embellishing and many vegetal species have been reported to be used for the prevention and treatment of various diseases and ailments. The use of plants has been a part of all the civilizations where plant species were always the preferable source of medicines and constituted an important part of the health care system for the prevention and treatment of various pathologies. Medicinal plants have thus played a key crucial role in health and disease management for many centuries. The natural substances extracted from plants have also been subsequently used to treat a wide variety of diseases worldwide.
Nowadays and despite the appreciable substantial progress made in modern therapy and modern medicine, herbs and their derived products are still considered and used as valuable sources of medicine for many health problems and the use of plants for health care is still current. Even today a large number of people use traditional medicinal plants containing mixtures of various compounds. As claimed by the World Health Organization (WHO), thousands of plant species are still worldwide used for medicinal end [1]. The worldwide use of herbs and herb-derived products could be explained by the fact that they are less expensive and are generally locally available. Among the other cause of their use the fact that medicinal plants constitute a source of bioactive molecules with potent biological activities. Some of these bio-molecules are of complex structure and are thus difficult to obtain by chemical synthesis allowing no alternative to their extraction from their corresponding vegetal sources. Some plants are also used for the extraction of some bioactive compounds used as good starting material for the hemisynthesis and the discovery of new bioactive molecules with more enhanced biological activity and serving for drug development.
In Morocco, the climate diversity permit many plants to propagate and/or to be cultivated. Morocco is well known for its diverse vegetation in which, various medicinal plants have been reported in older Moroccan pharmacopoeia and in some ethnobotanical studies [2, 3]. Among these plants, and as part of our screening program aimed to the phytochemical and biological studies of medicinal plants from Morocco, we were interested in Salvia verbenaca from Rabat region. This vegetal specie which belongs to the Lamiaceae family is a Mediterranean plant currently distributed in Morocco and generally found at low altitude near agricultural fields and paths. The plant is extensively used in Moroccan traditional folk medicine as cholagogue, antiseptic, diuretic and astringent [2, 3, 4]. All over Morocco, the crushed or chopped fresh leaves of Salvia verbenaca are applied in poultices on wounds to facilitate their healing [2]. Phytochemically speaking, several natural products have been described in Salvia verbenaca. Previous reports on the Salvia verbenaca plant have shown the presence of phenolic acids, terpenoids, and flavonoids [5, 6, 7, 8, 9].
Although some data on the composition and the pharmacologicalactivities of Salvia verbenaca were reported in previous studies [10, 11, 12], extensive investigation of phenolic compounds from the Moroccan species have not been yet reported. Even if the phytochemical composition of Salvia verbenaca has been recently reviewed [13, 14] with data on samples from Tunisia, Spain, Algeria, Jordan, Greece, Sicily, Turkey and Saudi Arabia, no information was reported on the phytochemical composition of the Moroccan species. Our work has thus the merit of having approached the phytochemical investigation of Salvia verbenaca from Morocco constituting thus the first study of the subject and making of this work an original exploration of the Moroccan plant material. This study was thus instigated in order to explore the phytochemical composition of Salvia verbenaca from the Rabat region of Morocco and to explore its antioxidant activity. The substantial significance of natural vegetal resources and their abundance in natural substances with potential bioactivities, and the absence of an in-depth and exhaustive study on the Moroccan Salvia verbenaca plant, incited us to explore the composition of this plant. New and original information on the characterization of phytochemicals will be thus given in this work in addition to the antioxidant activity of the plant. The relation between the observed activity and the phytochemical composition will be further discussed.
The used Salvia verbenaca over ground parts were harvested at Ain Aouda in the Rabat region, Morocco. Species identification was graciously done by Pr. Fennane from the Scientific Institute, Mohammed V University in Rabat. A voucher sample of the species plant was placed in the National Herbarium of this institution. The freshly collected parts were dried under shade, crushed and stored at –20 °C until use.
The used analytical grade extraction solvents and HPLC grade chromatographic solvents were bought from Sigma-Aldrich (Steinheim, Germany).
The dried Salvia verbenaca plant material was powdered. An amount of
200 g of the obtained powder was soaked under stirring for one day in a mixture
of EtOH/H
The total amounts of phenolic compounds of the instigated extracts were found using the Folin–Ciocalteu reagent with gallic acid as standard [15]. Results were given as mg of gallic acid equivalents per gram extract (mg GAE/g extract).
Flavonoids amount was determined by spectrophotometry using the aluminum chloride reagent with quercetin as standard [15]. Results were then given as mg of quercetin equivalents per gram extract (mg QE/g extract).
The phytochemical composition of the extracted samples was explored through
ultra high performance liquid chromatography coupled to mass spectrometry.
Analysis were carried out on an Agilent 1290 Infinity LC apparatus set (Agilent Technologies, Santa Clara, CA, USA) equipped
with a column chamber, an auto-sampler and a binary pump. An Inertsil C18-4
column (3
The LC separation was achieved using a binary gradient of solvent A (MeOH 20% in water containing 5 mM formate and 0.1% formic acid and solvent B (MeOH 90% in water containing 5 mM formate and 0.1% formic acid. Separation mobile phase began with 10% B and then incremented during 23 minutes to 95%. This was kept for 5 minutes and then followed by a washing and conditioning of the column. The mobile phase flow rate was 0.4 mL/min.
The used separation chromatographic system was coupled to an Agilent 6530 Quadrupole Time of Flight Mass Spectrometer using Agilent Mass Hunter Software version B.05.01 (B5125) (Agilent Technologies, Santa Clara, CA, USA). The MS detector was equipped with an ESI interface using the following conditions: capillary voltage, 3.5 kV; nebuliser, 45 psig; drying gas (nitrogen) flow rate, 8.0 L/min; sheath gas flow rate, 11.0 L/min; gas temperature, 375 °C; fragmentor, 175 V; skimmer voltage, 65 V; OCT 1 RF Vpp, 750 V. Spectra were recorded from m/z 100 to 3000.
In addition to the above analysis technique, the investigated Salvia verbenaca extracts were also explored using an UHPLC-DAD-ESI-Q-ToF/MS
platform consisting of a Waters ACQUITY LC unit coupled to both DAD (Diode-Array
Detector) and MS (Mass Spectrometry) detectors. Separations were performed on a
C18 reversed phase column (Acquity UPLC BEH 1.7
The UPLC system was connected to a quadrupole time of flight (QToF SYNAPT-G2
HDMS) mass spectrometer with an electrospray ionization (ESI) source operating in
positive and negative modes. Nitrogen was used as desolvation and cone gas at a
flow rate of 20
The antioxidant activity of S. verbenaca extracts was explored using
DPPH
The antioxidant activity of Salvia verbenaca extracts were also determined using the trolox equivalent antioxidant capacity test with the ABTS decolorization assay.
In the occurrence of an antioxidant, the transformation of the ABTS radical to
the free form is accompanied with a diminution of the absorbance at 734 nm. A
volume (2.5 mL) of the ABTS solution was blended with 200
A discussion on the relation between the chemical composition of the hexane and ethyl acetate extracts and their observed activities will be presented. In addition to the antioxidant activity, the discussion will concern also the cytotoxicity [11], the acute and subchronic oral and dermal toxicity [12] in addition to the effects of the extracts for healing of second-degree burns wounds [10]. We have previously performed and reported these activities [10, 11, 12] and the relation of the observed results with the phytochemical composition herein presented will be discussed in this work.
The obtained results were given as means
The Salvia verbenaca samples were instigated by UHPLC coupled to both diode array and high-resolution mass spectrometry detectors. The latter was used with an electrospray ionization source operating in both negative and positive ion modes. UHPLC-ESI/MS analysis was performed using parameters providing helpful additional cleavage information.
The HPLC chromatographic profiles showed evidence of various chromatographic compounds in the instigated extracts. The structures of the main detected compounds are detailed below. Among the detected compounds, eighteen phytochemicals pertaining to phenolic acids, phenolic diterpenes and flavonoids have been identified in the present study using the obtained spectral characteristics and literature results [18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29]. Fig. 1 showed the 18 identified phytochemicals in the explored Salvia verbenaca samples, while their spectral characteristics are gathered in Table 1. Relative quantification of the phytochemicals in the instigated extracts (hexane, AcOEt) is also given. This research presents thus an exhaustive investigation of the chemical composition of the Moroccan Salvia verbenaca.
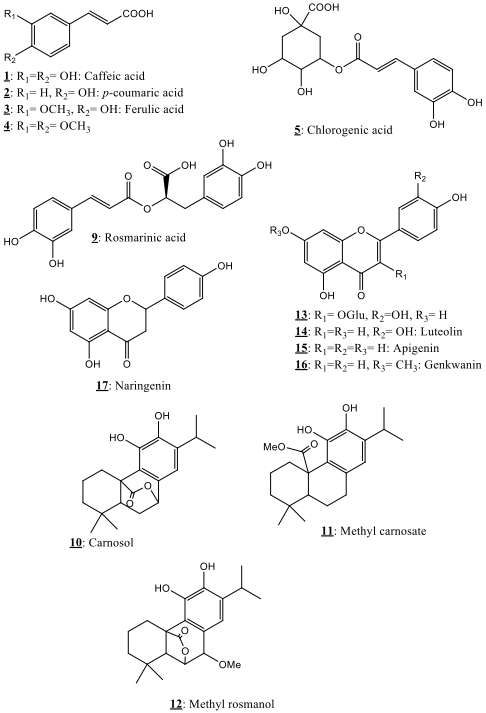 Fig. 1.
Fig. 1.Phytochemicals identified in Salvia verbenaca extracts.
| N° | UV |
[M-H] |
Fragment signals | Experimental MW & Formula | Theoretical MW & Formula | MW difference | Identification | |
|---|---|---|---|---|---|---|---|---|
| Phenolic Acids | 1 | 320 | 179 | 135 | 179.1815, C |
179.1495, C |
0.0320 | Caffeic acid |
| 2 | 310 | 163 | 145, 119 | 163.1887, C |
163.1501, C |
0.0386 | p-coumaric acid | |
| 3 | 331, 289 | 193 | 179, 149 | 193.0664, C |
193.1761, C |
–0.1097 | Ferulic acid | |
| 4 | 323, 294 | 207 | 135 | 207.0749, C |
207.2026, C |
–0.1277 | 3,4-Dimethyl-caffeic acid | |
| 5 | 326 | 353 | 191 | 353.4881, C |
353.3008, C |
0.1873 | Chlorogenic acid | |
| 6 | 317 | - | 181 | - | - | - | Caffeic acid derivative | |
| 7 | 314 | - | 305, 135 | - | - | - | Phenolic acid derivative | |
| 8 | 317 | - | - | - | - | - | Phenolic acid derivative | |
| 9 | 325, 287 | 359 | 197, 179, 161 | 359.37645, C |
359.3069, C |
0.0695 | Rosmarinic acid | |
| Phenolic diterpenes | 10 | 294, 224 | 329 | 285, 269, 201 | 329.483, C |
329.4101, C |
0.0729 | Carnosol |
| 11 | 319 | 345 | 301, 286 | 345.5204, C |
345.4526, C |
0.0678 | Methyl carnosate | |
| 12 | 319, 225 | 359 | 315, 301, 249 | 359.7237, C |
359.4361, C |
0.2876 | 7-methyl rosmanol | |
| Flavonoids | 13 | 348, 251 | 463 | 301, 257, 179, 151 | 463.3971, C |
463.3684, C |
0.0287 | Quercetin 3-O-glucoside |
| 14 | 344, 268 | 285 | 241, 253, 151, 133 | 285.0693, C |
285.2284, C |
–0.1591 | Luteolin | |
| 15 | 337, 265 | 269 | 117, 107 | 269.0768, C |
269.2290, C |
–0.1522 | Apigenin | |
| 16 | 333, 267 | 283 | 271, 185, 165 | 283.2925, C |
283.2555, C |
0.0370 | Genkwanin | |
| 17 | 330, 282 | 271 | 165, 151, 119 | 271.2609, C |
271.2448, C |
0.0161 | Naringenin | |
| 18 | 338, 294 | - | 287 | - | - | - | Eriodictyol derivative | |
| MW, Molecular Weight. | ||||||||
Salvia verbenaca extracts (hexane, AcOEt) were analyzed by UHPLC coupled to both DAD and MS detectors. Structural characterization of products for which standards were accessible was performed by injecting the latter in the same conditions and comparison with the obtained chromatographic and spectroscopic UV and MS data. Structural elucidation of the others was initiated using the obtained UV which permit to determine the phytochemical family since each one exhibits a characteristic UV–vis spectrum [30]. This was followed by the interpretation of the obtained MS spectra and the further observed fragmentations giving useful information on each compound molecular weight and also on the presence and the nature of substituents. The compound molecular mass was assigned from MS spectra obtained through low collision energy, since in such conditions the protonated/deprotonated molecules are easily obtained. Mass spectra with several fragment signals were recorded through high collision energy experiments allowing the occurrence of fragmentations and providing useful structural information related to the aglycon and glycosides moieties. The compounds evidenced in Salvia verbenaca extracts were split into phenolic acids, phenolic diterpenes and flavonoids which structural elucidation is discussed below.
Nine polyphenols in the category of phenolic acids were detected in the explored Salvia verbenaca samples presenting UV spectral properties of hydroxycinnamic acid adducts. Six of them, namely, caffeic acid 1, p-coumaric acid 2, ferulic acid 3, 3,4-dimethyl-caffeic 4, chlorogenic acid 5 and rosmarinic acid 9 were identified by comparing their characteristic UV and MS characteristic data with those of previously reported data [27, 31, 32, 33].
Through UHPLC-QToF-MS in the negative ionization mode, compound 1 gave
a signal at m/z 179 (C
In a comparable manner, the mass characteristics of compound 2
exhibited the deprotonated molecule [M-H]
Ferulic acid 3 and 3,4-dimethoxycaffeic acid 4 have also been
detected. Their characterizations were identified by matching their spectral
results with those of literature data [31]. Compound 3 molecular ion
signal was located at m/z 193 corresponding to the ion [M-H]
In addition to the phenolic acid indicated above, chlorogenic acid 5
was also detected giving deprotonated molecule [M-H]
Besides these five phenolic acids, three additional phytochemicals 6–8 presenting spectral characteristics of phenolic acid derivatives were also evidenced in the explored extracts. This was deduced from their ultraviolet spectra which were similar of hydroxycinnamic acid derivatives [30]. Compound 6 which MS spectrum showed a fragment ion signal at m/z 181 was concluded to be caffeic acid derivative [31, 36, 37].
The mass spectrum of compound 9 in the negative ion mode (Fig. 2),
showed a signal at m/z 359 corresponding to the deprotonated molecular
ion [M-H]
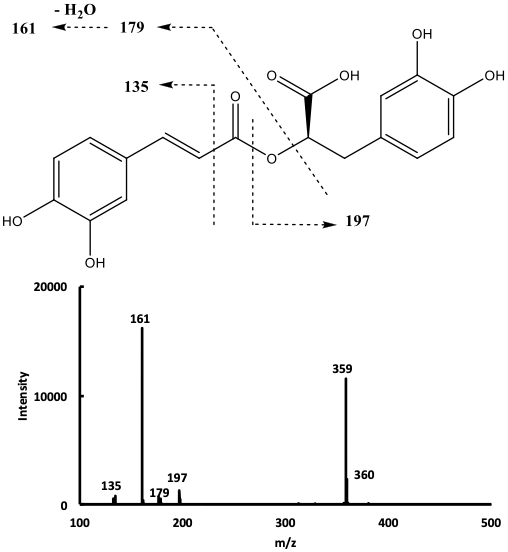 Fig. 2.
Fig. 2.Mass spectrum of compound 9 with the proposed corresponding cleavages.
The evidence of phenolic acids in Salvia genus has been formerly described in the literature. Caffeic acid, p-coumaric acid, ferulic acid and rosmarinic acid in addition to several derivatives of phenolic acids have been previously reported [18, 19, 20, 21, 22, 23, 24]. Our results corroborate the presence of such adducts in Salvia verbenaca. Rosmarinic acid has also been previously reported in extracts of Salvia verbenaca [9]. Its detection in the present work confirm thus its occurrence in the Moroccan sample. The present work confirmed also the occurrence of rosmarinic acid in vascular plants particularly in the Lamiaceae family [38, 39]. Plant extracts containing rosmarinic acid have been reported to possess dietary, cosmetic, pharmaceutical and beverage production applications [40]. This phytochemical phenolic acid derivative has also been reported to have various biological properties such as antioxidant antiviral, anti-inflammatory, antimutagenic, antibacterial and above all antioxidant [38, 39, 41]. The antioxidant activity of rosmarinic acid is due to the presence of four hydroxyl groups in its molecule [42]. An anti-HIV property of rosmarinic acid has also been reported in the literature [43]. Its presence in the studied Salvia verbenaca could justify the observed biological activities of the investigated plant extracts [11].
Three compounds 10–12 were detected in the explored Salvia verbenaca ethyl acetate extract. These compounds showed MS spectral data characteristics of carnosol 10, methyl carnosate 11 and 7-methyl rosmanol 12 as previously described in the literature [44, 45, 46].
The mass spectrum of compound 10 recorded in the negative ion mode
showed a signal located at m/z 329 corresponding to the deprotonated
molecular ion [M-H]
Compound 11 was concluded to be methyl carnosate which molecular ion
and fragmentations pattern agrees with the suggested structure and the published
information [44]. The deprotonated molecular ion [M-H]
In the same way compound 12 was identified as 7-methyl rosmanol which MS characteristics agree with published data concerning this compound [46]. The obtained MS spectrum presents in particular signals at m/z 359 and 315 matching respectively to the deprotonated molecular ion and the further elimination of a carbon dioxide molecule.
The presence of compounds 10–12 in Salvia genus has been previously reported in the literature [18, 19, 20, 21, 22, 23, 24]. Their presence in Salvia verbenaca chemical composition has also been reported [19, 20] and was confirmed by the present results.
Besides the phytochemical products discussed above, other phenolic compounds were detected in the studied extracts. Their UV spectral data were characteristics of flavonoids in agreement with previously reported data with two very characteristic bands corresponding to the cinnamoyl and the benzoyl groups [30]. Flavonoids structural elucidation was further achieved from interpretation of the obtained mass spectra and using literature data related to diagnostic fragmentation of flavonoids whether as aglycon or as O- or C- glycosylated adducts [43, 44, 45, 47, 48, 49, 50].
Compounds 13–18 (Fig. 1) which UV and MS characteristics are reported in Table 1 were the main flavonoids detected in the explored Salvia verbenaca extracts. The structural elucidation of these compounds was achieved through combination of UV visible spectrophotometry and HRMS in addition to the further observed fragmentations which agree with the suggested structures and the published results concerning the same compounds [18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29].
The ultraviolet spectrum of compound 13 showed two absorbance maxima
located at 251 nm (bnzoyl BII band) and 348 nm (cinnamoyl BI band) suggesting a
flavonol derivative [30]. The molecular ion signal observed in the mass spectrum
recorded in the negative ion mode was located at m/z: 463 suggesting a
molecular weight of 464 Da. Among the signals observed in the ESI-MS analysis a
fragment signal was observed at m/z: 301 corresponding to the subsequent
loss of hexose moiety [M-H-162]
In addition to the flavonol discussed above, flavonoids 14–16
of the flavone’s family have also been detected. Compound 14 was deduced
to be luteolin from the obtained spectral data which agree with published data
[49]. Compound 14 mass spectrum exhibited the molecular ion [M+H]
Besides luteolin 14, one more free flavone was evidenced in the studied samples showing a deprotonated molecular ion signal at m/z 269 suggesting apigenin molecule 15 which molecular ion signal and the subsequent observed fragmentation signals agree with the advanced structure [55]. This was also committed by high resolution mass spectrometry giving an elemental composition compatible with the suggested structure. This free flavone has been formerly outlined in Salvia genus [18, 19, 20].
The third detected flavone derivative 16 was concluded to be genkwanin as confirmed through its observed UV and MS spectral data. Its mass spectrum exhibited signals at m/z 285 and 271 for the protonated molecular ion and the fragment corresponding to the further loss of a methyl group.
Compound 17 showed UV spectral data corresponding to flavanone family.
Its mass spectrum recorded in the negative ion mode showed signals at
m/z 273, 151 and 119. These corresponded respectively to the
deprotonated molecular ion [M-H]
Compound 18 representing the last detected flavanone in the explored extracts was concluded to be a derivative of eriodictyol. In addition to the characteristic UV spectral data, the mass spectrum of compound 18 showed a signal at m/z 287 suggesting the presence of the eriodictyol aglycon and indicating that compound 18 was an eriodictyol derivative [26].
The occurrence of the above eighteen phytochemicals in Salvia verbenaca will be undeniably and assuredly involved to increase and enhance the pharmacological properties of this plant. By the way, these compounds constitute evidently a scientific basis to the use of Salvia verbenaca in folk medicine against various pathologies.
Following the identification of the phytochemicals occurring in the instigated Salvia verbenaca samples, the relative percentages of these compounds have been determined using UHPLC chromatography connected to diode array and ESI-ToF-MS detectors. An example of the obtained profile is presented in Fig. 3 and the obtained results for the studied extracts are gathered in Table 2. The relative quantitative analysis of the detected phytochemicals has been made from the area % calculation procedure which gives the area of each chromatographic peak as a percentage of the total peaks area. This method assumes that the detected phytochemicals answer in a similar manner to the detector. Even if these criteria are may be not guaranteed, this method gives at least a useful approximation of the relative amounts of the evidenced phytochemicals.
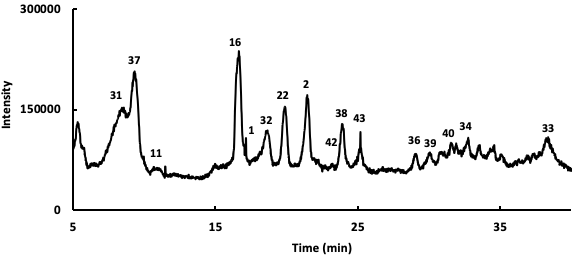 Fig. 3.
Fig. 3.UHPLC chromatographic profile of Salvia verbenaca (ethyl acetate extract).
| Type of extract | Compound identification | Retention time (min) | Percentage (%) |
|---|---|---|---|
| Hexane | Not identified | 28.83 | 5.94 |
| Phenolic acid derivative | 29.90 | 5.23 | |
| Not identified | 30.71 | 3.28 | |
| Phenolic acid derivative | 31.49 | 22.13 | |
| Caffeic acid derivative | 32.685 | 16.41 | |
| Genkwanin | 36.36 | 39.25 | |
| Ethyl acetate | Quercetin 3-O-glucoside | 5.37 | 2.57 |
| Ferulic acid | 8.46 | 0.42 | |
| Rosmarinic acid | 9.35 | 8.28 | |
| Luteolin | 16.83 | 23.71 | |
| 3,4-dimethyl caffeic acid | 18.69 | 23.71 | |
| Apigenin | 19.85 | 5.42 | |
| p-coumaric acid | 21.51 | 8.28 | |
| Naringenin | 21.78 | 8.28 | |
| Carnosol | 23.97 | 3.34 | |
| Caffeic acid | 16.32 | 3.34 | |
| Eriodictyol derivative | 25.20 | 2.57 | |
| Phenolic acid derivative | 29.189 | 1.42 | |
| Methyl carnosate | 30.08 | 0.71 | |
| 7-methyl rosmanol | 31.64 | 1.42 | |
| Caffeic acid derivative | 33.10 | 3.42 | |
| Chlorogenic acid | 38.29 | 1.85 |
The obtained results showed that the Salvia verbenaca hexane extract contained six compounds representing a percentage of 92.24% of the total peaks area. This extract was dominated by genkwanin as major compound (39.25%), followed by phenolic acid derivatives (38.54%).
The quantitative analysis of the Salvia verbenaca ethyl acetate extract showed the presence of 16 phytochemicals representing a percentage of 98.74% of the total peaks area (Table 2). The major compounds were luteolin (23.71%) and 3,4-dimethyl caffeic acid (23.71%). These were followed by p-coumaric acid, rosmarinic acid and naringenin with similar percentage (8.28%).
The antioxidant properties of the Salvia verbenaca samples were assessed using DPPH
The obtained results presented in Table 3 revealed higher antioxidant activity
on DPPH
| Extracts | IC |
IC |
Polyphenols contents |
Flavonoids contents |
|---|---|---|---|---|
| DPPH |
ABTS Assay | |||
| Hexane | 0.0925 |
0.1867 |
15.4 |
10.5 |
| Ethyl acetate | 0.0744 |
0.1338 |
23.3 |
17.2 |
| Trolox | 0.0621 |
0.0604 |
- | - |
In term ABTS model assay, the ethyl acetate sample exhibited the highest potent
activity with an IC
The difference obtained between the two tests (DPPH
The study of the relation between the observed antioxidant activities of the explored extracts and the polyphenols and flavonoids contents showed that the importance of such metabolites in enhancing the antioxidant activities. Thus, the ethyl acetate extract which was the richer extracts either in total polyphenols contents and in total flavonoid contents showed the higher antioxidant activity. This indicated the importance of such phytochemicals in the antioxidant activity observed with the two used methods.
The medicinal properties of plant are obviously related to their composition. The biological activities of plant extracts are evidently linked and dependent of the extract’s chemical content.
In our previous research investigation on Salvia verbenaca, some biological activities of its extracts have been explored. Thus, the cytotoxicity and the ability of the plant to accelerate second degree burn healing in addition to its acute and subchronic oral and dermal toxicity of the hexane and ethyl acetate extracts have been investigated [10, 11, 12]. Among the obtained results, and in relation with burn healing, the ethyl acetate extract was as efficient as silver sulfadiazine which constitutes the basic principle of the most widely used topical treatment against injury [10]. The phytochemical investigation conducted in the present work showed that the ethyl extract contained various phenolic compounds such as flavonoids and phenolic acids which are recognized and reported to have different biological activities. Our results indicated that the flavone luteolin was the major compound of the ethyl acetate extract and the total percentage of the identified flavonoids was 42.55%. This demonstrated the role played by flavonoids as wound healing dressings and justify the use of flavonoids in different formulation for the treatment against burns. It is obviously evident that the multiple identified flavonoids in the extract could act in a synergistic effect or combined effect and the observed effect could not be obviously due to this or that compound even if it constitutes the major one. The positive role played by flavonoids in wound healing could be explained by the fact that they possess all the activity involved against burn such inflammation, proliferation and re-epithelialization. In addition to flavonoids, the ethyl acetate was also rich in phenolic acids which are also recognized for their properties in wound management [56]. The global observed action of the extracts involved obviously the total phytochemicals which could act in synergetic manner.
In addition to the ability of Salvia verbenaca extracts to accelerate the healing process, its cytotoxic effect was also explored against two cancer cell lines, Vero and RD, using the colorimetric MTT assay [11]. The obtained results showed that the effect observed for the ethyl acetate extract was higher than that of the hexane. This is also attributed to richness of this extract in phenolic acids (caffeic acid, p-coumaric acid, ferulic acid, chlorogenic acid and rosmarinic acid) and flavonoids (quercetin 3-O-glucoside, luteolin, apigenin and naringenin) constituting more than 90% of the total phytochemical composition. This confirmed the fact that polyphenols in general and flavonoids in particular have a range of biological activities including antioxidant, anti-inflammatory, and anti-tumorigenic properties. Flavonoids have been reported to be active at different stages of cancer development inducing cellular cytotoxicity [57]. Reported data suggest that flavonols may provide protection against oxidatively induced cellular damage in vivo [57]. Flavones such as luteolin and apigenin have also been reported to have strong anticancer [57]. Our findings are in agreement with these statements as these flavonoids were detected as both apigenin and luteolin were detected in the ethyl acetate extract.
Concerning the antioxidant activity, examination of the obtained results gathered in Table 3, showed a relatively higher effect of the ethyl acetate extract compared to the hexane one. As indicated above, this was quite obvious given the relatively higher total polyphenols and total flavonoids contents of the two extracts. The obtained results agreed also with the qualitative phytochemical composition of the two extracts. Indeed, Table 2 showed the presence of flavonoids pertaining to flavones and flavonols such as apigenin, luteolin and quercetin derivatives which are recognized as potent antioxidant agents. The structures of these compounds contained a double bond between the carbons 2 and 3, conjugated with a 4 oxo group in the ring C and the presence of additional hydroxyl groups at the C-3, C-3’, C-4’ positions. These structural characteristics are known to improve the radical scavenging capacity of flavonoids and to increase their antioxidant activity [58]. The presence of other compounds such as rosmarinic acid which was detected in the ethyl acetate extract could also enhance its antioxidant activity.
In the current research work, a simple and rapid prcedure involving the use of UHPLC, PDA and Q-ToF-HRMS techniques was used for the phytochemical characterization of Salvia verbenaca, plant widely used in the Moroccan traditional medicine. Eighteen phenolic derivatives have thus been characterized and their relative percentages determined. The current research work showed thus that the used analysis procedure can serve as a potent tool for the instigation of natural products metabolic profiles. The use of such procedure allowed us in this study to have useful data about the characterization of Salvia verbenaca phytochemicals and gave original results on the chemical composition of this Moroccan plant.
Our findings gave thus a scientific basis and support to the traditional use of this plant in the remediation of tumor diseases and for wound healing dressings. This work gave thus convenient information for supplemental pharmacological investigations and the design of new drugs based on this plant and will facilitate the use of Salvia verbenaca in the clinic and its safety studies. It is also hoped that the information presented here might motivate other studies that will possibly conduct to development of therapeutic agents from this species.
Data generated or analyzed during this study are included in this published article.
FG, NE conceived and designed this study; FG and SY performed the experiments; FG, NE, SY analyzed the data; FG, NE, SY interpreted the results of the experiments; FG, NE wrote and revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



