1 Provincial Key Laboratory of Developmental Biology and Neuroscience, College of Life Sciences, Fujian Normal University, 350117 Fuzhou, Fujian, China
Academic Editors: José Luis Pérez-Castrillón and Ricardo Usategui Martín
Abstract
Background: The characterization of neuropathic pain is maladaptive
plasticity within the nociceptive system. Multiple alterations contribute to
complex pain phenotypes. Adrenomedullin (AM) has been documented to be a pain
mediator. However, its involvement in pathological pain is poorly understood. We
studied the contribution of AM to chronic neuropathic pain in the spinal nerve
ligation (SNL) model. Methods: Daily injection of the AM receptor
antagonist AM
Keywords
- adrenomedullin (AM)
- dorsal root ganglion (DRG)
- neuropathic pain
- spinal nerve
- spinal cord
Neuropathic pain is often caused by damage within the nervous system arising from physical injury, diabetes mellitus, infection or autoimmune disease [1]. Pronociceptive mediators are released at the lesion site in response to nerve injury [2] and increase the excitability of both the damaged and adjacent undamaged nerve fibres [3] and primary afferent neurons. As a result, peripheral sensitization is produced [4]. Ectopic spontaneous activity is generated in dorsal root ganglion (DRG) neurons by ongoing activity originating in injured nerves and increased expression of voltage-gated sodium channels [5]. Neurotransmitters, such as glutamate and substance P, are therefore released at the presynaptic sites on terminals of primary afferent nociceptors [1]. These molecules cause rapid-onset homo- and heterosynaptic facilitation in the spinal dorsal horn, resulting in hyperexcitability of secondary sensory neurons, called central sensitization [6]. However, mechanisms underlying neuropathic pain is not fully understood. As neuropathic pain is refractory to most available analgesics including opioids [7], investigating mechanisms of neuropathic pain is essential for the development of new therapies.
Adrenomedullin (AM) is a 52-amino acid peptide. It belongs to calcitonin family
with a partial sequence homology with calcitonin gene-related peptide (CGRP). AM
is broadly expressed in the peripheral and central nervous systems [8, 9],
including the spinal dorsal horn and small-diameter neurons in DRG [10], the
important tissues of pain processing. AM receptors that mediate AM activity are
the obligatory association of the calcitonin-like receptor (CLR) with the
receptor activity-modifying protein (RAMP) 2 or 3 [11]. CLR and RAMP2/3 are
distributed in superficial laminae of the spinal cord [10] and DRG neurons [12].
AM
Male Sprague-Dawley rats (Fuzhou Animal Center, Fuzhou) weighing 220–260 g at
the time of surgery were used. Animals were housed in a quiet room kept at 22
Nerve lesion in the periphery was produced by ligation of unilateral spinal nerve at L5 [19]. Rat was anesthetized with pentobarbital (50 mg/kg, i.p.). Ligation of L5 spinal nerve was carried out as described before [19]. The dorsal vertebral was exposed at lumbar level (L4 to L6). The paraspinal muscles were bluntly separated and transverse spinal processes at L5 was removed. L5 nerve at the right side was exposed and ligated tightly with 5-0 suture. The muscle layer was closed with nylon threads. Ceftiofur sodium (Shenggong, Shanghai, China) was given in the wound to prevent possible infection. Sham surgery was performed with same procedure without removing spinal process and disturbance of the spinal nerves. The same procedure was followed without ligation of the spinal nerve in the sham operated animals. The rats were allowed to fully recover.
Animals were implanted with indwelling catheters in the subarachnoid space.
Briefly, rat was injected with pentobarbital (50 mg/kg, i.p., Shenggong,
Shanghai, China). The dura mater overlying the atlanto-occipital membrane of rats
was exposed and a small slit was cut in the membrane. A PE-10 catheter
(Stoelting, Wood Dale, IL, USA) was inserted into the subarachnoid space with its
tip being positioned at the L4 segment. The catheter was flushed with saline (10
Nociceptive test responding to mechanical stimulation was used to evaluate the effect of drugs on pain threshold in rats. Mechanical pain threshold was tested in the right hindpaw (n = 7–9 each) using an electrodynamic von Frey apparatus (Dynamic Plantar Anesthesiometer, Ugo Basile, Italy). Animals were placed in plastic cages with a metal net floor. They were acclimatized to the environment for 40 min for five days and again habituated for 15 min prior to testing to minimize variability of behavioral measurements. The von Frey filament (0.5 mm diameter) was applied to the mid-plantar surface of hindpaw. Paw withdrawal threshold (PWT) was tested by application of a force. The actuator automatically lifted the filament and applied an increasing force. The force was stopped once rat moved its hindpaw. Forty g for 30 s was preset as a cut-off force in the apparatus to avoid possible injury in the tissue. PWT was measured three times at 2 min interval to generate a mean value. Investigator was blind to the tested drug conditions.
Animals were anesthetized with pentobarbital (50 mg/kg i.p.). Rats were perfused
intracardially with cold 0.01 M PBS (phosphate buffered saline) followed by 4%
paraformaldehyde in 0.1 M PB (phosphate buffer). L4/L5 spinal nerves and L4/L5
DRG (n = 5 or 6 each) were cut out. After being fixed in 4% paraformaldehyde
overnight, the tissues were transferred to sucrose (30% in PB). The nerve or DRG
was sectioned at a thickness of 10
AM and RAMP2 immunoreactivity-stained DRG neurons was
quantified using software Image-Pro Plus 6.0. A field of 870
The preparation of stained sections was consistent with the method described above by immunofluorescence. The tissue sections were incubated with rabbit AM (1:500, Bioss Biotechnology Inc., Beijing, China) or RAMP2 (1:200, Bioss Biotechnology Inc., Beijing, China) or CLR (1:500, Bioss Biotechnology Inc., Beijing, China) antibody and mouse nNOS antibody (1:100, Santa Cruz Biotechnology Inc., California, USA) together overnight at 4 °C. After being thoroughly washed in PBS, the sections were incubated with donkey anti-rabbit or anti-mouse IgG conjugated to FITC (1:1000, Abcam, Cambridge, UK) or Rhodamine (1:800, Abcam, Cambridge, UK) for 2 hours at room temperature. AM, CLR and RAMP2 immunoreactivity were labeled as green, whereas nNOS were labeled as red.
The rats were decapitated, L4–L6 dorsal root ganglia were removed and
collected. A mixture of cell lysate and protease inhibitor (10:1, Beyotime,
Shanghai, China) was added to the tissue, and then all samples were centrifuged
(15,000 r/min, 30 min, 4 °C). The extracted protein was quantified by BCA kit
(Beyotime, Shanghai, China). Proteins were denatured with SDS loading buffer (95
°C,10 min) and processed to SDS polyacrylamide gels. After transferring the
protein, nitrocellulose filter membrane was blocked with 10% skimmed milk in
TBST at room temperature for 2 h. Then the membrane was incubated with rabbit
anti-nNOS (1:500, Bioss Biotechnology Inc., Beijing, China) and
The dorsal half of the spinal cord at L4/5 was dissected. To isolate RNA,
samples were homogenized in 1 ml of Trizol reagent using RNA prep pure kit
(Transgen Biotech, Beijing, China). Deoxyribonuclease I treatment was done to
prevent DNA contamination. Concentration of RNA was measured using an
ultraviolet–visible spectrometer (NanoDrop ND-2000, Thermo Scientific, Waltham,
USA). The quality and quantity of the RNA were assessed at 260/280 A. All samples
showed absorbency ratios ranging from 1.8 to 2.0. A total of 1
Forward (5’-3’) and reverse (5’-3’) primer sequences used were:
GTTTCCATCGCCCTGATGTTATT and GTAGTTCCCTCTTCCCACGACTTAG for the gene encoding AM;
AACCTTAGAAAGCAGCCCAGGCATG and GTGGGCACAAAGTTGTCCTTCACCA for the gene encoding
CGRP; GTTTGTGATGGGTGTGAAC and TCTTCTGAGTGGCAGTGA for the gene encoding the gene
encoding GAPDH. Twenty ng of cDNA from the same cDNA batch was subjected to
real-time PCR to amplify all genes in triplicate in a total reaction volume of 20
All data were presented as the mean
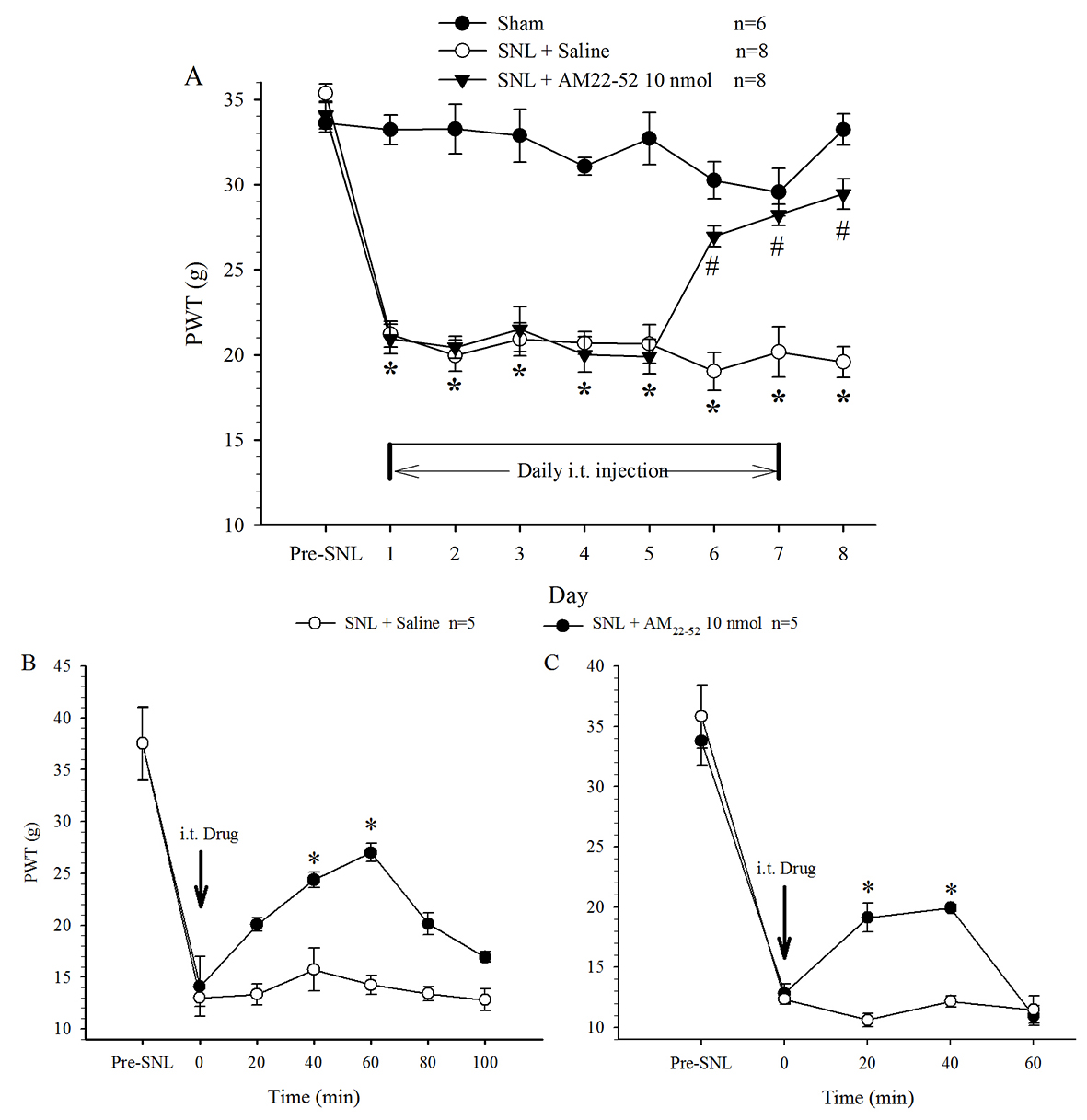
PWT in the hindpaw on the surgical side significantly shortened one day
following nerve ligation operation. Behavioral examination showed that basic
mechanical threshold (the value measured before i.t. administration) was
remarkably decreased by SNL, but not by sham, surgery, indicating mechanical pain
hypersensitivity or allodynia. This state was maintained thereafter throughout
the experiment (15 days) and was not changed by i.t. administration of saline
(once per day). Daily administration of AM
 Fig. 1.
Fig. 1.Effect of i.t. administration of AM
To characterize the association of AM receptor activity with neuropathic pain,
nerve ligation was performed on day 0. L4/5 spinal nerves, DRG and the dorsal
half of the lumbar spinal cord were taken on naive rats (day 0 or D0) and 1, 3, 7
and 15 days post-SNL. The samples were proceeded by qRT-PCR assay or
immunofluorescence staining to examine the changes of AM mRNA or protein with
respect to the development of neuropathic pain. The experiment in each assay was
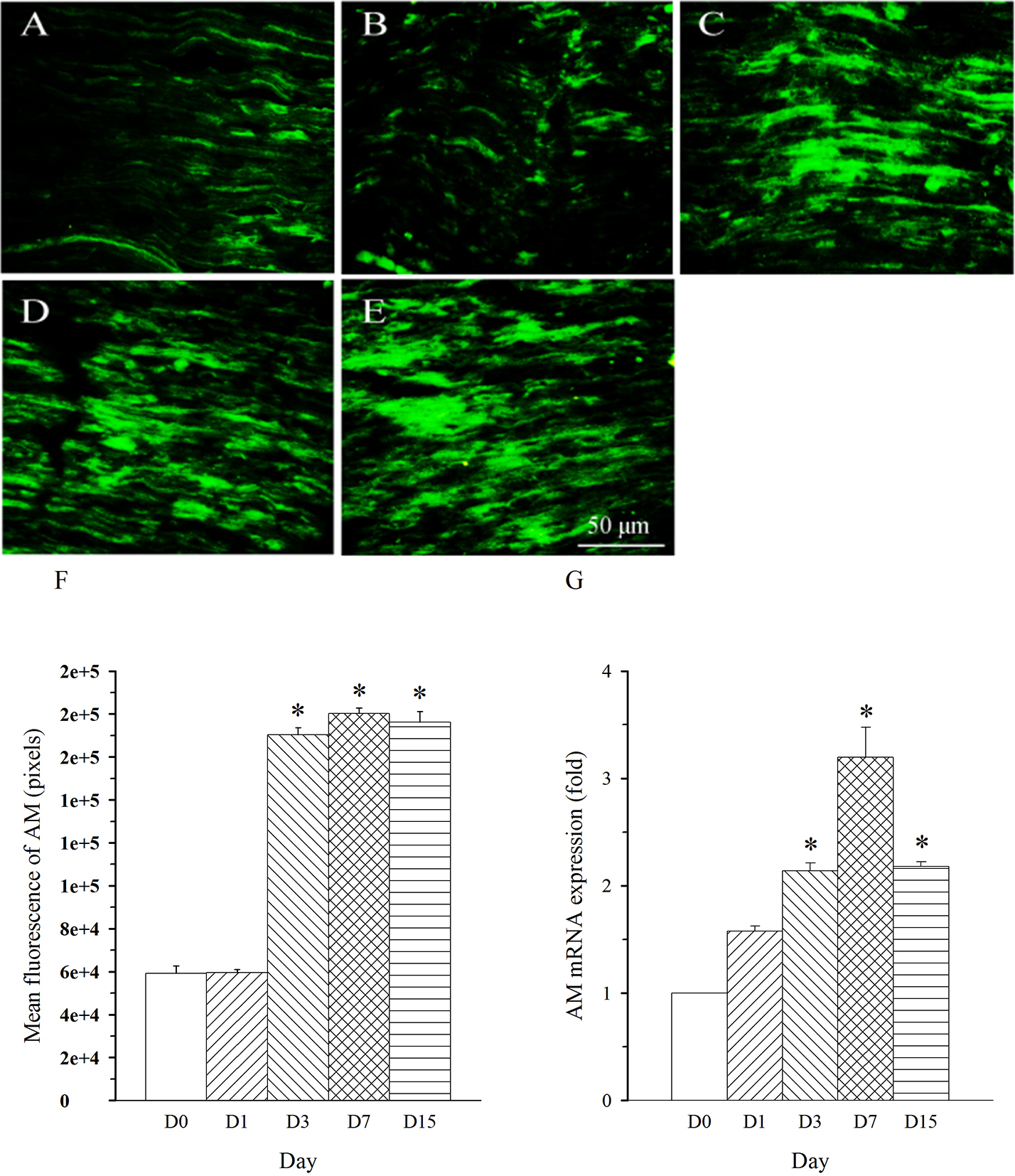
repeated five times. AM immunoreactivity was shown in nerve fibers. The density
of AM-immunoreactive fibers was remarkably increased in L5 nerve (damaged nerve)
on day 3 and remained at a high level throughout the experiment (up to 15 days,
Fig. 2A–F, p
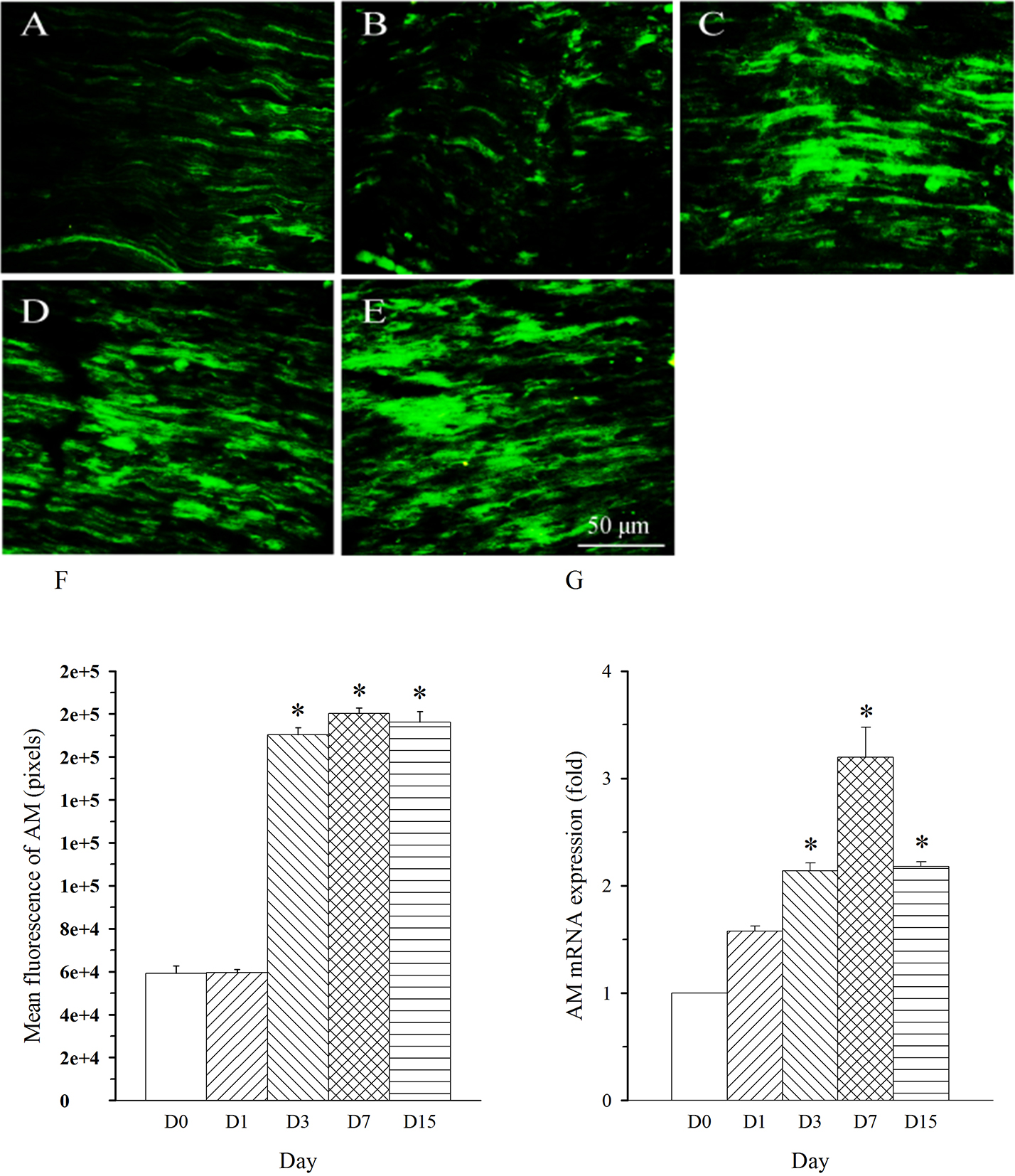 Fig. 2.
Fig. 2.Expression pattern of AM protein and mRNA in L5 spinal nerve in
SNL rats. Spinal nerve ligation (L5) or sham surgery was performed on day
0. The L5 spinal nerve on the operate side was harvested at various times. The
samples were assayed using immunofluorescence staining and RT-PCR techniques.
Representative immunofluorescence images are presented as following: A-day 0,
B-day 1, C-day 3, D-day 7, E-day 15. The AM staining intensity was quantified as
pixels in F. AM mRNA levels at various times were shown in G. *p
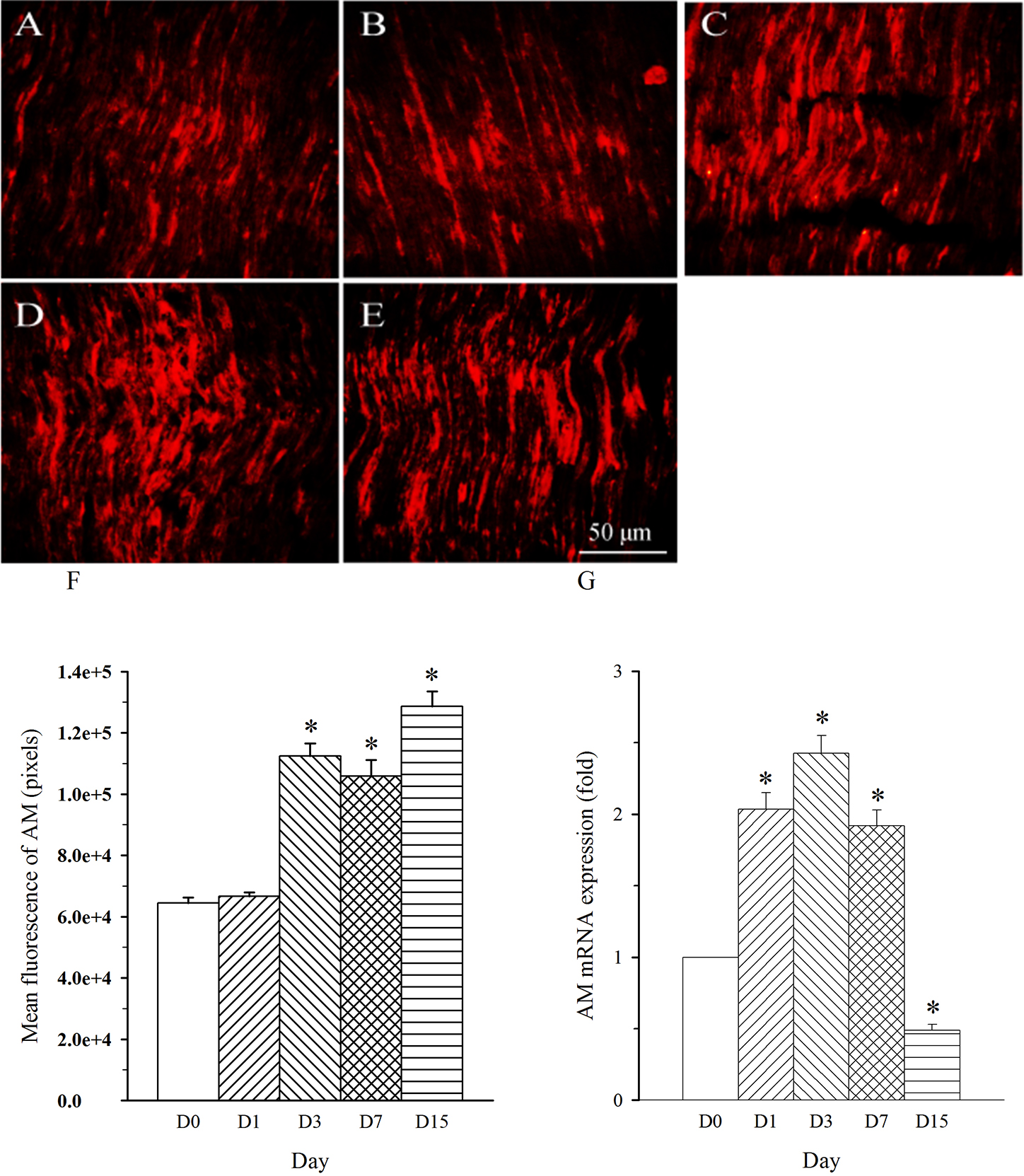
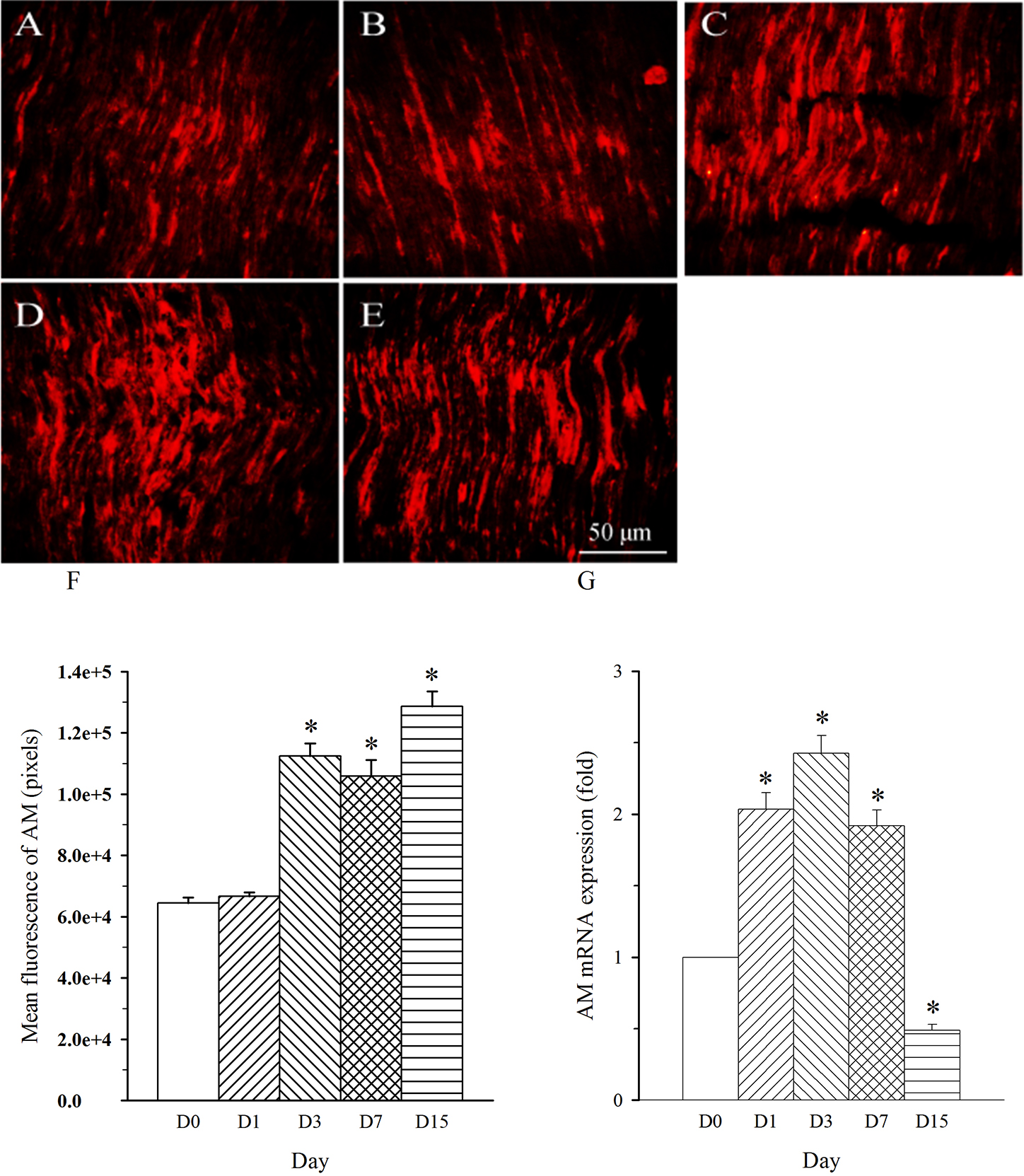 Fig. 3.
Fig. 3.Expression pattern of AM protein and mRNA in L4 spinal nerve in
SNL rats. Spinal nerve ligation (L5) or sham surgery was performed on day
0. The L4 spinal nerve on the operate side was harvested at various times. The
samples were assayed using immunofluorescence staining and RT-PCR techniques.
Representative immunofluorescence images are presented as following: A-day 0,
B-day 1, C-day 3, D-day 7, E-day 15. The AM staining intensity was quantified as
pixels in F. AM mRNA levels at various times were shown in G. *p
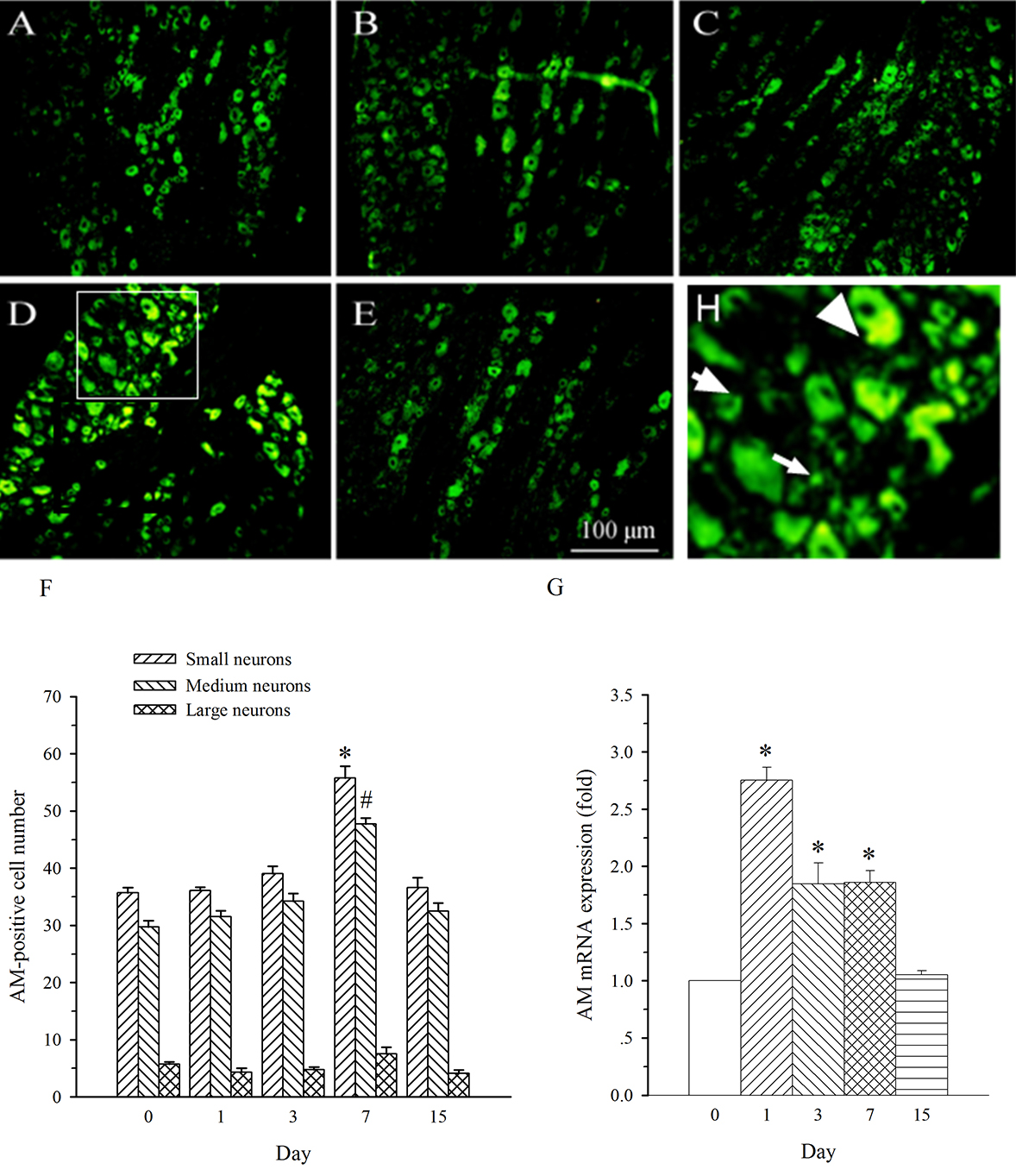
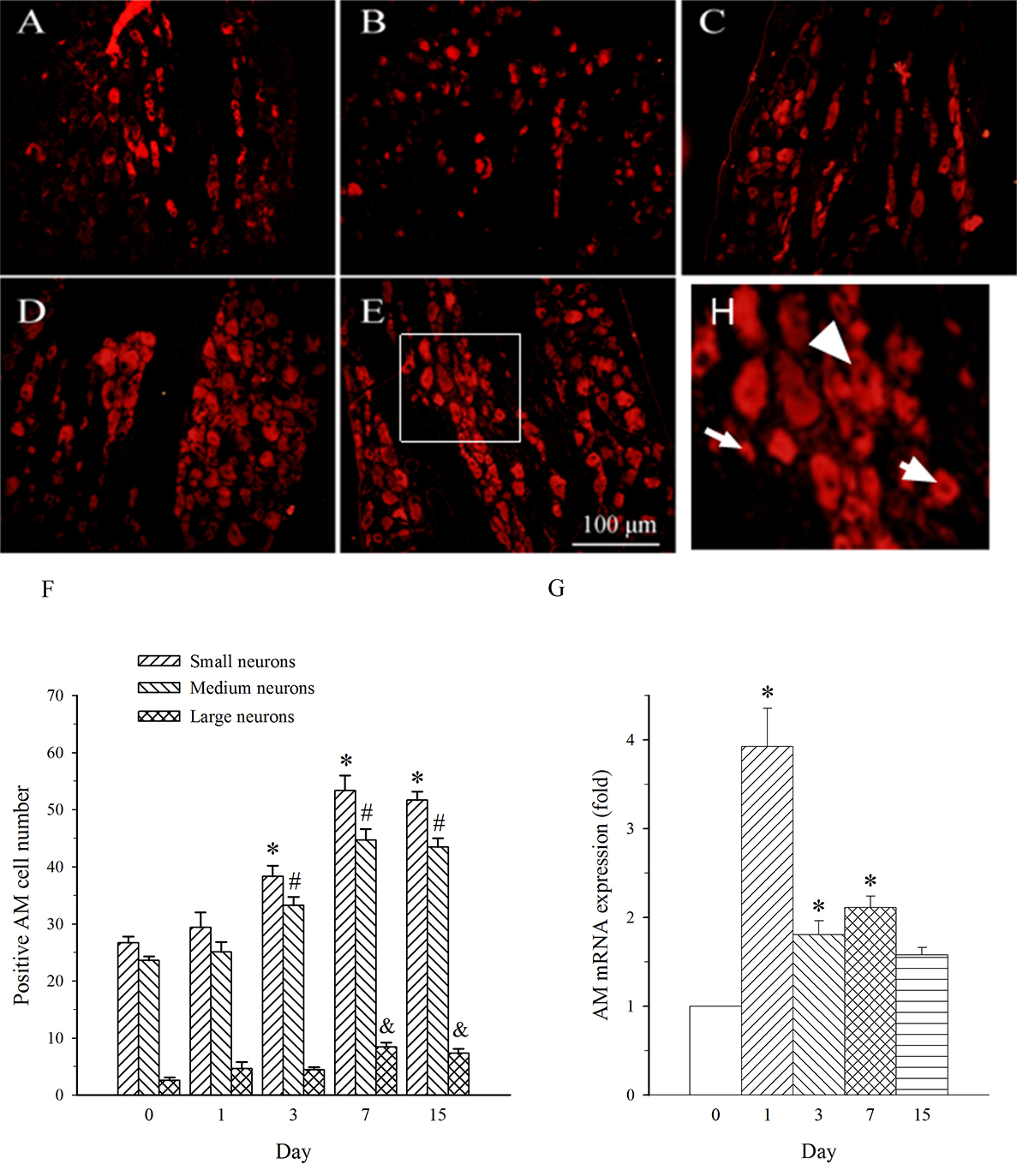
AM neurons were mainly observed in medium- and small-diameter DRG neurons. In
DRG at L5 (containing damaged neurons), SNL induced an increase in the expression
of AM in both medium- and small-diameter neurons only on day 7 (Fig. 4A–F). The
expression of AM mRNA in L5 DRG exhibited a different profile. It was increased
on days 1–7 post-SNL (Fig. 4G, p
 Fig. 4.
Fig. 4.Expression of AM protein and mRNA in DRG at L5 in SNL
rats. Spinal nerve ligation (L5) or sham surgery was performed on day 0.
The L5 DRG on the operate side was harvested at various times. The samples were
assayed using immunofluorescence staining and RT-PCR techniques. Representative
immunofluorescence images are presented as following: A-day 0, B-day 1, C-day 3,
D-day 7, E-day 15. Arrows of different sizes indicate small-, medium- or
large-sized cells (H). Quantification of AM expression is represented as a number
of AM-positive cells in small- or medium-sized subpopulation (F). AM mRNA levels
at various times were shown in G. *p
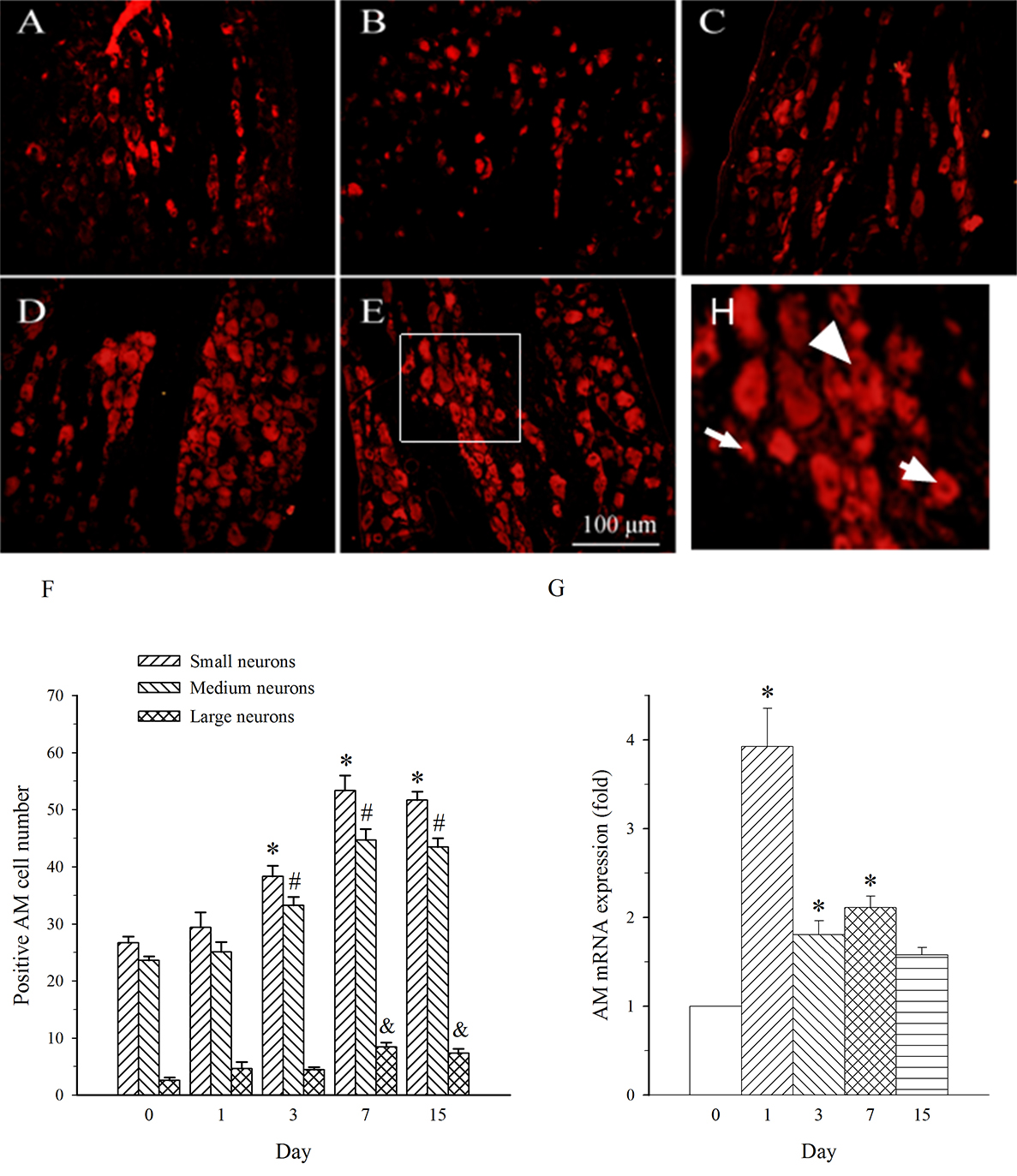 Fig. 5.
Fig. 5.Expression of AM protein and mRNA in DRG at L4 in SNL
rats. Spinal nerve ligation (L5) or sham surgery was performed on day 0.
The L4 DRG on the operate side was harvested at various times. The samples were
assayed using immunofluorescence staining and RT-PCR techniques. Representative
immunofluorescence images are presented as following: A-day 0, B-day 1, C-day 3,
D-day 7, E-day 15. Arrows of different sizes indicate small-, medium- or
large-sized cells (H). Quantification of AM expression is represented as a number
of AM-positive cells in small- or medium-sized subpopulation (F). AM mRNA levels
at various times were shown in G. *p
Expression of AM mRNA in the spinal dorsal horn in SNL
rats. Spinal nerve ligation (L5) or sham surgery was performed on day 0.
The dorsal part of the lumbar spinal cord on the operate side was harvested at
various times. The samples were assayed using RT-PCR techniques. Histograms
indicate the mean
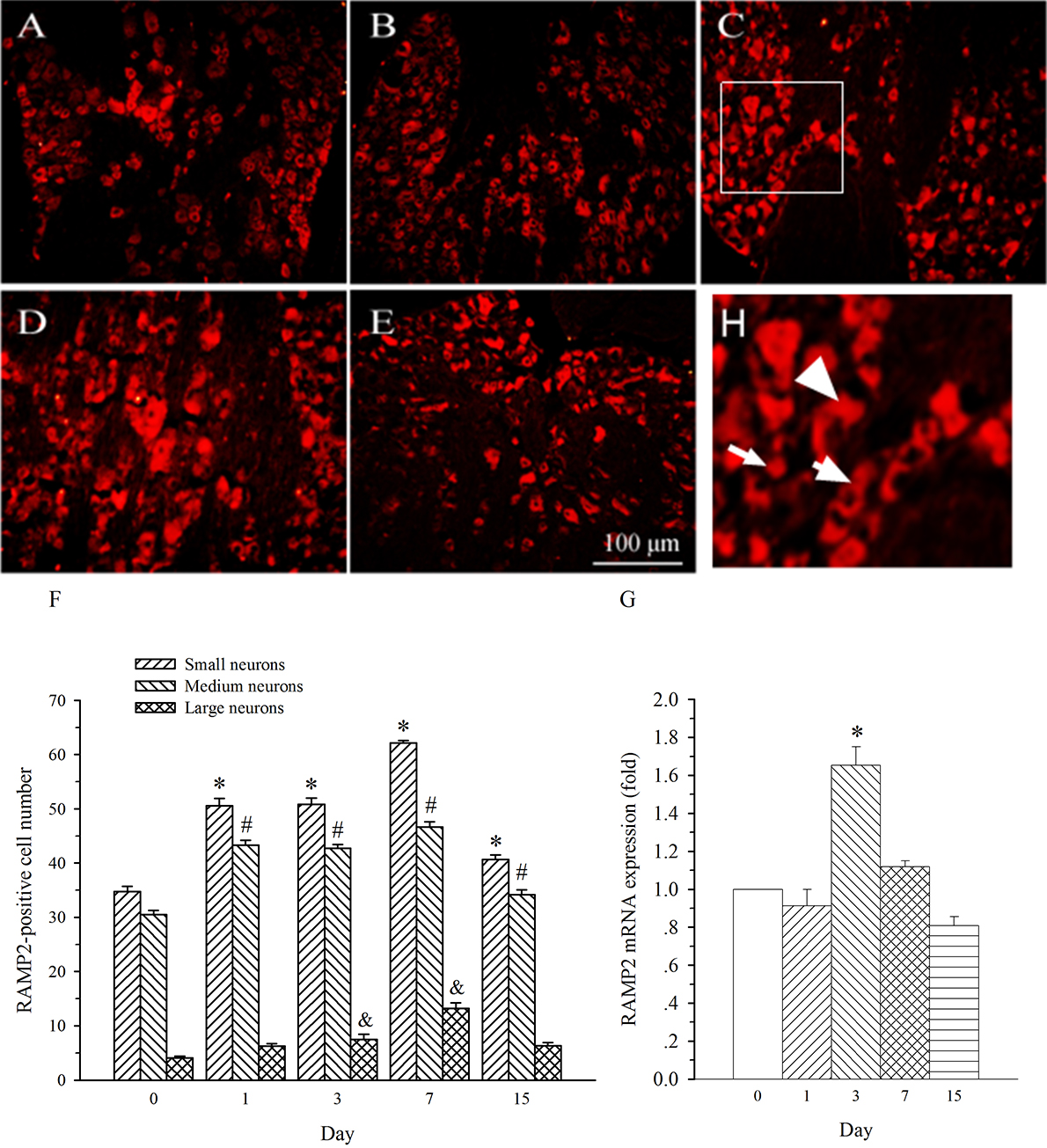
AM mainly activates AM1 receptor which is a complex of CLR with RAMP2 [13]. This
study examined characteristics of the temporal expression of RAMP2 in DRG. In
consistence with previous report [12], RAMP2 immunofluorescence staining was seen
in medium- and small-diameter DRG neurons (Fig. 7). The expression of RAMP2 in
both sized neurons was remarkably increased on day 1 post-SNL in L5 DRG. This
increase maintained throughout the experiment (Fig. 7A–F, p
 Fig. 7.
Fig. 7.Expression of RAMP2 protein and mRNA in DRG at L5 in SNL
rats. Spinal nerve ligation (L5) or sham surgery was performed on day 0.
The L5 DRG on the operate side was harvested at various times. The samples were
assayed using immunofluorescence staining and RT-PCR techniques. Representative
immunofluorescence images are presented as following: A-day 0, B-day 1, C-day 3,
D-day 7, E-day 15. Arrows of different sizes indicate small-, medium- or
large-sized cells (H). Quantification of AM expression is represented as a number
of AM-positive cells in small- or medium-sized subpopulation (F). AM mRNA levels
at various times were shown in G. *p
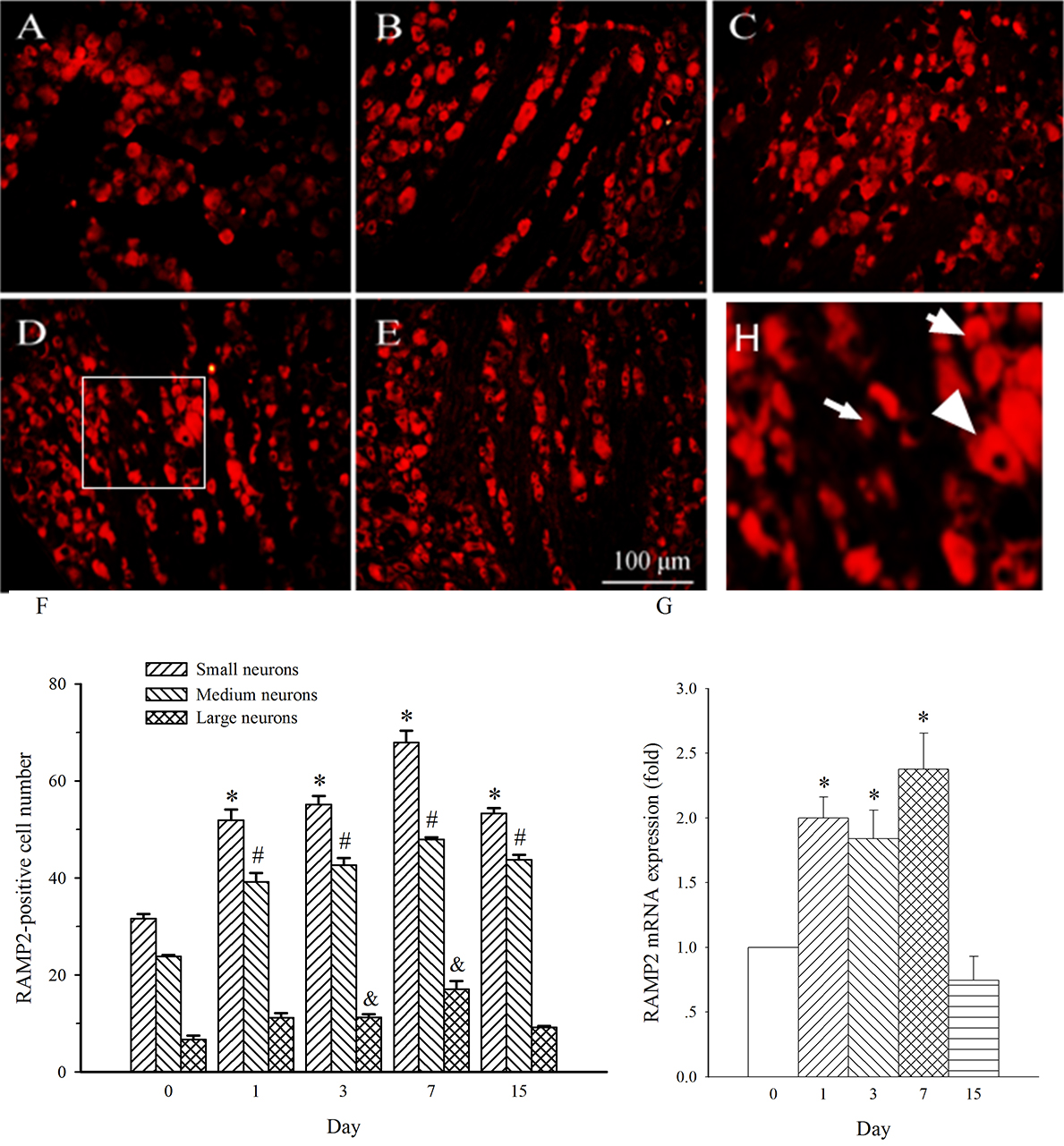
RAMP2 expression in medium- and small-diameter neurons in L4 DRG was also
increased on day 1 post-SNL (Fig. 8A,B, p
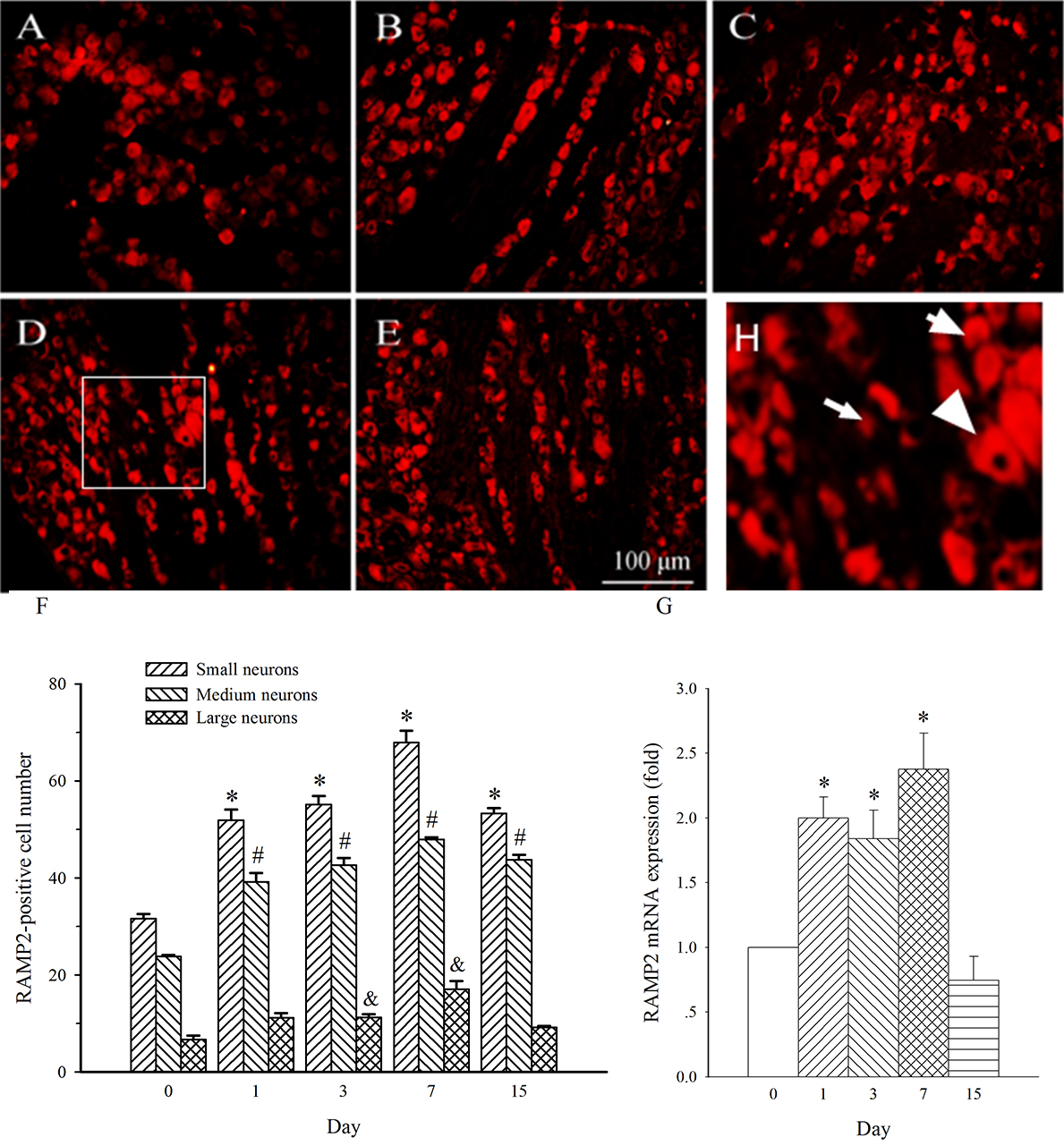 Fig. 8.
Fig. 8.Expression of RAMP2 protein and mRNA in DRG at L4 in SNL
rats. Spinal nerve ligation (L5) or sham surgery was performed on day 0.
The L4 DRG on the operate side was harvested at various times. The samples were
assayed using immunofluorescence staining and RT-PCR techniques. Representative
immunofluorescence images are presented as following: A-day 0, B-day 1, C-day 3,
D-day 7, E-day 15. Arrows of different sizes indicate small-, medium- or
large-sized cells (H). Subpopulation Quantification of AM expression is
represented as a number of AM-positive cells in small- or medium-sized
subpopulation (F). AM mRNA levels at various times were shown in G. *p
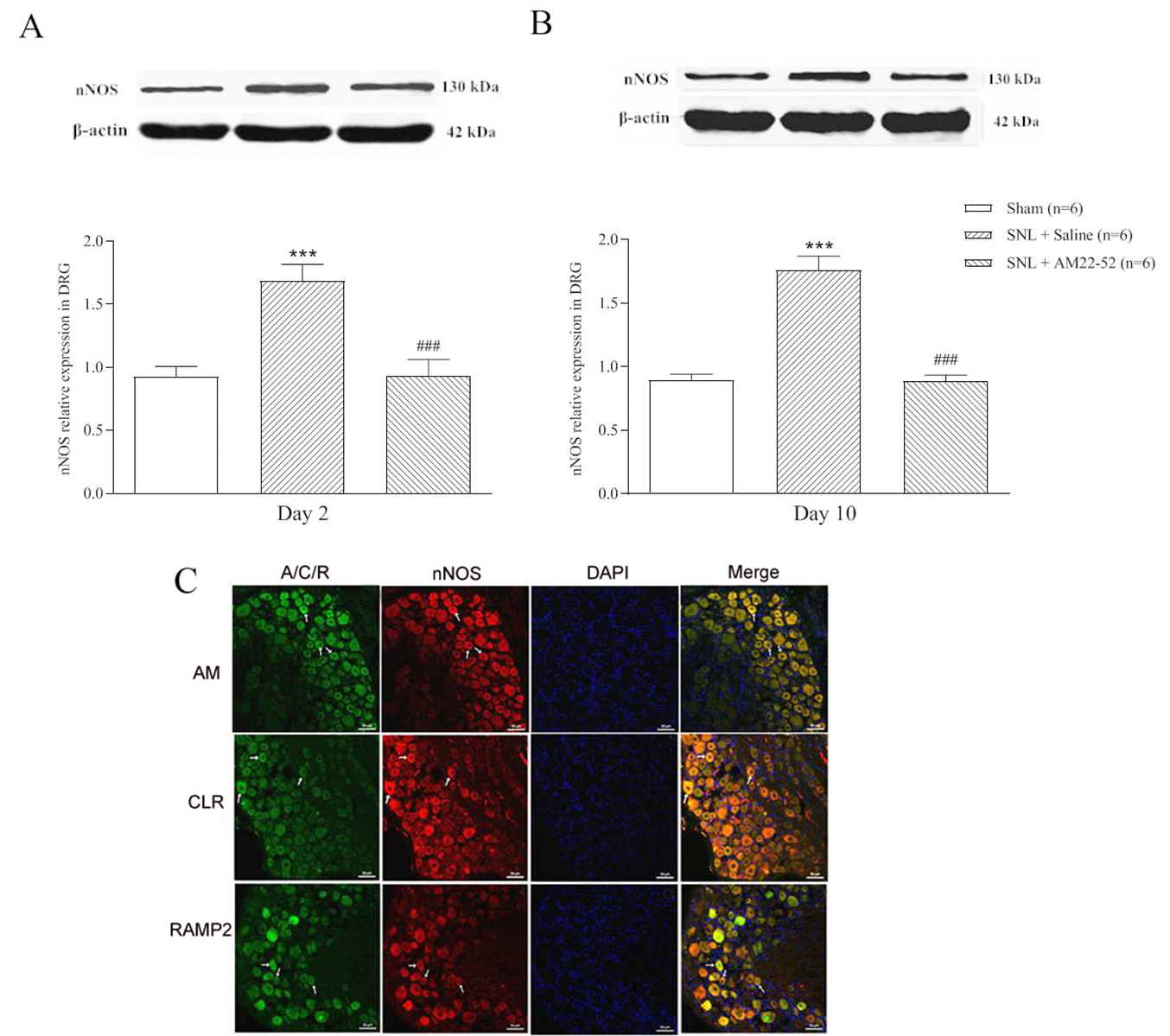
To explore cellular mechanism of AM-induced activity, nNOS protein in DRG was
assayed. AM
 Fig. 9.
Fig. 9.Effect of i.t. AM
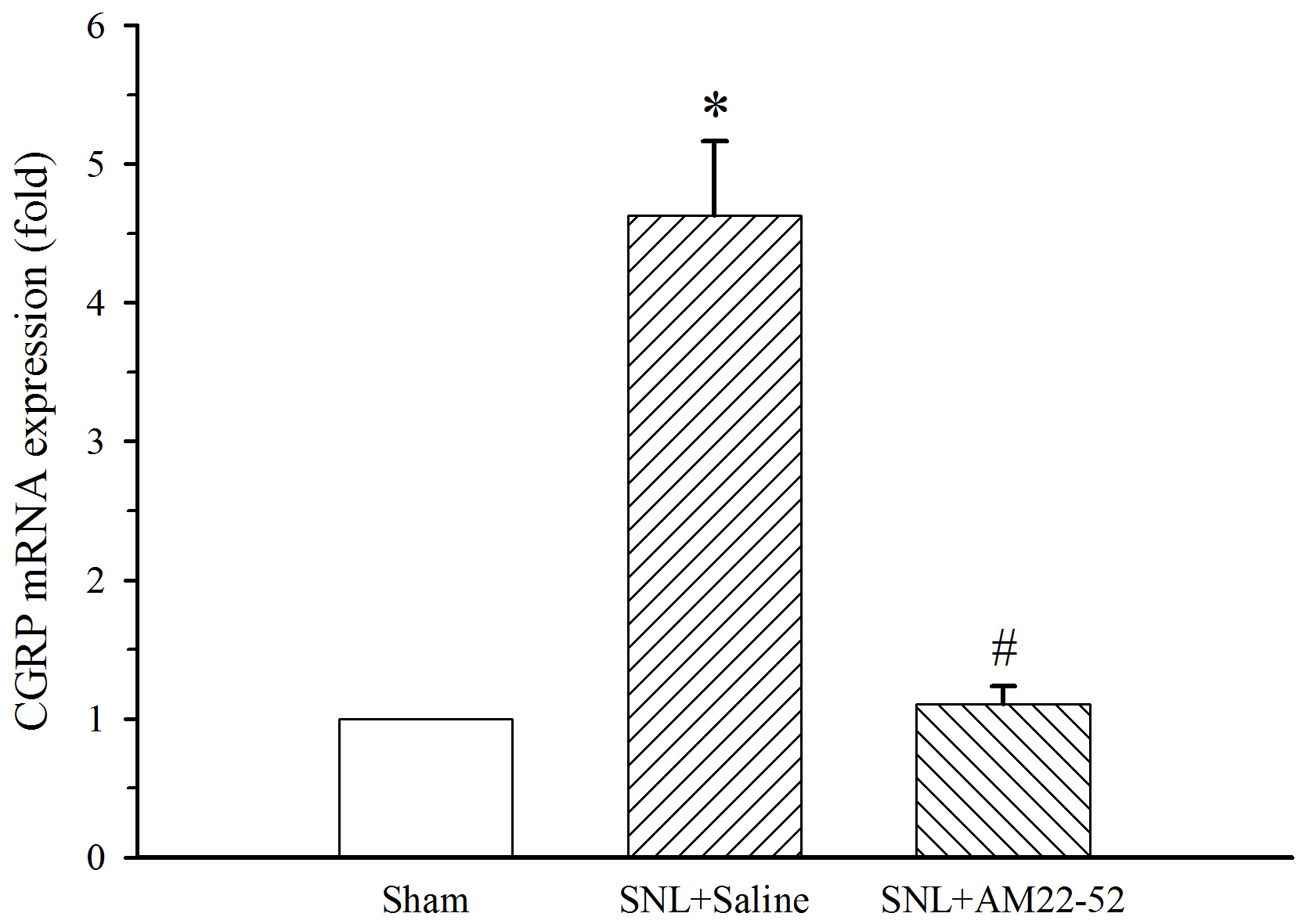
To further explore cellular mechanism of AM-induced activity, CGRP mRNA in the
spinal dorsal horn was assayed. AM
 Fig. 10.
Fig. 10.Effect of i.t. AM
The current study investigated the role of AM receptor signaling in neuropathic
pain. Our results showed that blockade of AM receptor inhibited nerve
injury-induced mechanical allodynia. AM mRNA expression was increased in the
damaged (L5) and adjacent undamaged (L4) peripheral nerves following SNL surgery.
The levels of AM mRNA were also elevated in DRG (L5) containing damaged neurons
and adjacent DRG (L4) containing complete neurons as well as the spinal dorsal
horn. AM protein was increased in L4/L5 peripheral nerves and L4 DRG. SNL also
increased the protein level of RAMP2 in both L5 and L4 DRG but enhanced mRNA
level mainly in L4 DRG. Furthermore, chronic injection of AM
Bolus injection of AM
The spatio-temporal expression profile was examined to explore functional
characterizations of AM in neuropathic pain. It has been documented that sensory
signals transported in adjacent uninjured afferents, but not injured afferents,
contribute to the sensitization of primary and the secondary sensory neurons,
producing hypersensitivity response [25, 26, 27]. Moreover, some molecules, such as
CGRP [28], Nav1.3 sodium channel [29], MrgC [30] and GABA
Physiological relevance of AM bioactivity depends on AM receptor. AM receptor is the association of CLR with RAMP [11]. CLR actually forms the basis of the receptors for CGRP and AM. CGRP and AM bind to the same receptor CLR with receptor specificity being determined by RAMP. Three different RAMP that have been found in human tissue are RAMP1, RAMP2 and RAMP3. Coexpression of RAMP1 and CLR reveals CGRP receptor whereas coexpression of RAMP2 or RAMP3 and CLR forms AM1 or AM2 receptor [33]. As RAMP2 is a component of AM1 receptor and is not expressed in AM2 or CGRP receptor, we examined the expression of RAMP2. RAMP2 has been found to be expressed in DRG [12]. The current study demonstrated that RAMP2 expression was increased in both medium- and small-diameter neurons on days 1–15 in L5 DRG. Interestingly, RAMP2 was also increased even in large-sized neurons on days 3 and 7. However, RAMP2 mRNA was increased only on day 3. For RAMP2, different expression patterns from AM was happened in L5 DRG which is possible that maybe no matter increases in AM or its receptors, it will produce enhanced cytological effect. These alternating changes act together to cause neuropathic pain. In L4 DRG, RAMP2 protein was increased in medium- and small-diameter neurons on days 1–15 and in large-sized neurons on days 3 and 7. RAMP2 mRNA was increased on days 1–7 which was longer than that in L5 DRG. These results suggest that SNL promoted ongoing post-translational changes of RAMP2 in DRG containing injured and uninjured neurons but transcriptional activation of the RAMP2 gene mainly in the adjacent DRG containing complete neurons. The changes of RAMP2 exhibited a similar profile with AM. Based on the notion that the increase impulses are ascribed to neurons in the adjacent uninjured DRG [26], studies to determine the involvement of the increased AM/RAMP2 expressions in adjacent uninjured DRG in hyperexcitability of neurons are warranted.
Peripheral nerve injury increases the excitability of DRG neurons, which AM must
be involved in this process. Our results showed that nNOS protein was increased
in DRG whether in the early or late stage of pathological pain following SNL.
This is similar to previous reports [34, 35, 36]. Innovatively, single administration
of AM
The increase of AM and RAMP2 may be a cause or consequence of neuropathic pain.
In consistence with previous reports [24, 48, 49], our results showed that CGRP
mRNA levels were upregulated in the spinal dorsal horn following SNL.
Importantly, the chronic administration of AM
Collectively, our results clearly demonstrate that the expression of AM dynamical changes with the progression of neuropathic pain and that blockade of AM receptor is an effective pharmacological intervention to inhibit mechanical allodynia in late-phase development of neuropathic pain. Furthermore, targeting AM can even abolish the increase of the important nociceptive mediator nNOS and CGRP. The present study suggests that AM receptor should be considered as a new therapeutic target for relieving chronic neuropathic pain.
DW designed the study; CW, YX and QL performed the research; YX and QL analyzed the data; YS and WT provided technical assistance and produced graphs; CW and DW wrote and revised the manuscript. All authors read and approved the final manuscrip.
Not applicable.
We thank the scientific research innovation program “Xiyuanjiang River Scholarship” of College of Life Sciences in Fujian Normal University.
This work was kindly supported by NSFC grants (81571084, 81400922) and Natural Science Foundation of Fujian Province (2020J05038).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.