1 Unit of Heart-Brain Axis: Cellular and Molecular Mechanisms, Centro Cardiologico Monzino IRCCS, 20138 Milano, Italy
2 Department of Biology and Biotechnology, University of Pavia, 27100 Pavia, Italy
3 Scuola Universitaria Superiore, IUSS, 27100 Pavia, Italy
†These authors contributed equally.
Academic Editor: Graham Pawelec
Abstract
Background: Platelet-derived extracellular vesicles (PEVs) are small
vesicles released by activated platelets that are gaining growing interest in the
field of vascular biology. The mode of platelet activation is a critical
determinant of PEVs release, phenotype and function. However, only very limited
information is available concerning the impact of the platelet purification
procedure on PEVs release. Methods: Washed or isolated platelets were
separated by differential centrifugations. For washed platelets, the platelet
pellet was washed by resuspension in PIPES buffer and finally resuspended in
HEPES buffer. Isolated platelets were obtained by directly resuspending the
platelet pellet in HEPES, skipping the washing steps in PIPES buffer. PEVs
release was induced in washed or isolated platelets by stimulation with different
agonist and analysed by Nanoparticle Tracking Analysis. Results:
Isolated platelets showed a higher release of PEVs upon adenosine diphosphate
(ADP) stimulation compared to washed platelets, whereas PEVs released upon
stimulation with strong agonists (thrombin, collagen, A23187, U46619) were
similar in the two groups. This different responsiveness to ADP was also observed
as a higher
Keywords
- platelets
- platelet-derived extracellular vesicles
- platelet preparation procedure
Platelet-derived extracellular vesicles (PEVs) are small vesicles released by activated platelets, that are gaining growing interest in the field of vascular biology. PEVs are the most abundant EVs in the circulation and they are extensively studied for their roles in a wide range of physiological and pathophysiological processes, including inflammation, cell communication, coagulation, and cancer metastasis [1].
The mechanism supporting platelet activation is a critical determinant of PEVs release, phenotype and function. Several studies have explored this aspect, clearly demonstrating that different stimuli display a different potency in inducing the release of PEVs. Importantly, PEVs release in response to both physiological and pathological stimuli present remarkably distinctive functions, indicating that the mechanisms supporting vesiculation likely influence the composition of PEVs [2]. In this context, only very limited information is available concerning the impact of the platelet purification procedure on PEVs release.
In platelet studies, the isolation protocol is critical since it strongly influences platelet response [3]. The ideal experimental conditions would involve the study of platelets in their physiological environment, however, platelet separation from other blood components is essential to dissect the biology of these cells at molecular and functional levels.
The most common separation procedure involves the washing of platelets. By repeated steps of differential centrifugation and platelet resuspension in specific buffered solutions, platelets are recovered and separated from other blood cells, plasma components and the anticoagulant used for blood withdrawal. Being widely used, the platelet washing procedure is often laboratory customized. Despite some guideline articles and book chapters have been published on this topic to support the authors who approach to the study of platelets, some major differences in the description of the platelet separation protocols adopted are found in literature [4, 5, 6, 7]. Although the influence of platelet washing procedure on platelet functionality has been extensively investigated, the information about the impact of platelet separation protocol on the release of PEVs is still limited.
In this work we aimed at investigating this important aspect focusing on two platelet separation procedures used in different published studies.
Thrombin, A23187, prostaglandin E
Washed human platelets, as well as isolated platelets, were prepared from
buffy-coat bags through a previously described protocol [7]. Briefly, the
buffy-coat was diluted with one fourth of its initial volume using a 9:1 solution
of HEPES buffer and citric acid/citrate/dextrose (152 mM sodium citrate, 130 mM
citric acid and 112 mM glucose) and spun at 120
Isolated platelets were obtained by directly resuspending the platelet pellet in
HEPES buffer plus 5.5 mM glucose, 1 mM CaCl
Platelet activation was assessed by flow cytometry analyses. Platelets (at 0.1
Washed and isolated platelets (2 mL at 0.5
Concentration and size distribution of particles in PEVs samples were measured with NanoSight (NS300) (Malvern Panalytical Ltd., Malvern, UK) equipped with NTA software (version 3.4; Malvern Panalytical Ltd., Malvern, UK). All samples were diluted to the appropriate concentration, and five videos of 60 s were recorded for each sample setting camera level to 14, viscosity to “water” (0.909–0.90 cP), at 23 °C. Videos were analysed using NTA software (version 3.4; Malvern Panalytical Ltd., Malvern, UK), with a Detection Threshold of 5. The settings were established according to the manufacturer’s software manual (NanoSight NS300 User Manual, MAN0541-01-EN-00, 2017).
Statistical analysis was performed using the GraphPad Prism 9.2 Software
(GraphPad Software Inc., San Diego, CA, USA). Continuous variables were expressed
as mean
In this study, we hypothesize that the platelet isolation protocol may influence the release of PEVs, since it is known to have a relevant impact on the general functionality of platelets [3]. We have selected two protocols commonly used in literature and described in the materials and methods section. The two platelet preparations were defined as isolated platelets and washed platelets.
To compare the effect of the separation protocol, PEVs were generated from
isolated or washed platelets, either under resting conditions or upon stimulation
with different physiological platelet agonists. Specifically, platelet agonists
known to induce abundant release of PEVs, including thrombin, collagen, the
thromboxane A
At basal conditions, PEVs released from isolated and washed platelets were
similar in terms of both concentration (isolated: 2.8
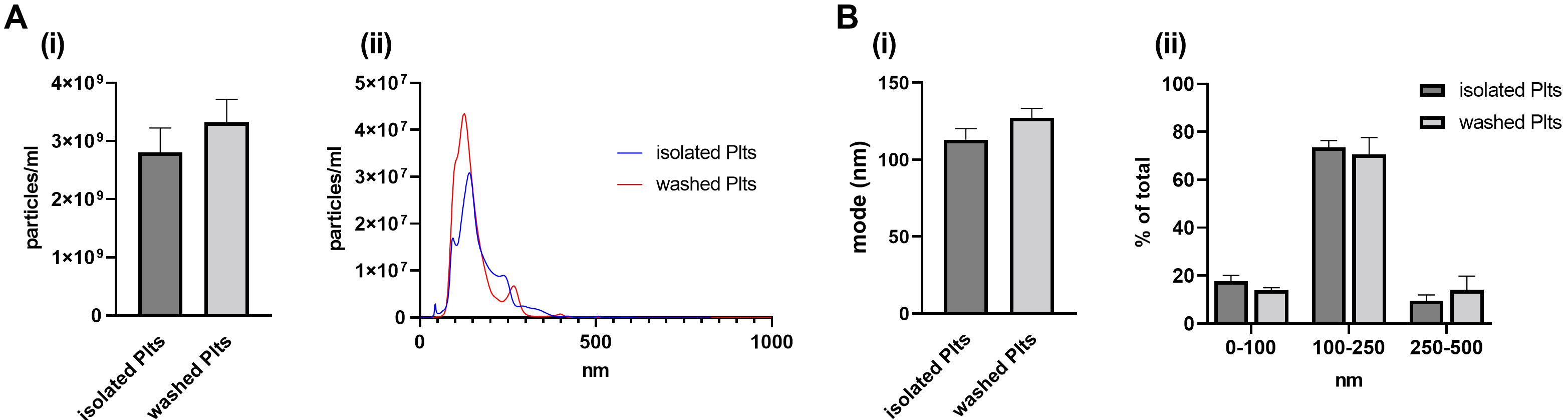 Fig. 1.
Fig. 1.Release of PEVs in basal conditions. PEVs released from
unstimulated (resting) platelets (30 min at 37 °C) were isolated by
ultracentrifugation and analysed by NTA. (A) (i) Vesicle concentration, assessed
by NTA, is reported as particles/mL and (ii) representative NTA traces. (B) (i)
Vesicle average size, measured as mode (nm), and (ii) vesicle
size distributions shown as percentages of the total EV populations analysed by
NTA. n = 8 independent platelet preparations per group. Data are shown as mean
Upon stimulation with the different agonists (thrombin, collagen, A23187,
U46619), an increase of PEVs ranging from 2- to 10-fold compared to respective
untreated controls was found in both isolated and washed platelets (Fig. 2A).
Under these conditions however, no relevant differences between the two isolation
methods were observed in the number of released PEVs (p = 0.26; Fig. 2A). In line with previously published results [2], Ca
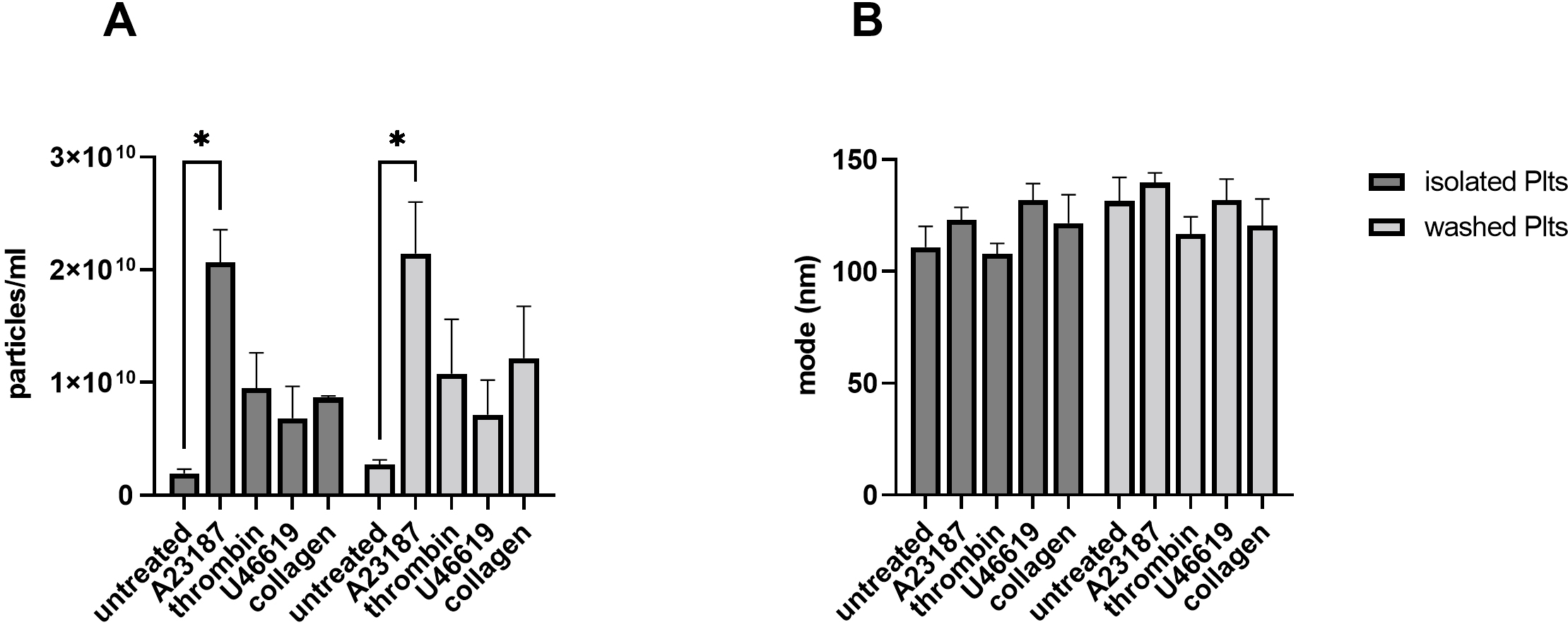 Fig. 2.
Fig. 2.PEVs generation with different strong
agonists. Isolated and washed platelets were left untreated or stimulated with 5
Overall, we did not find significant procedure-dependent differences when PEVs
were generated upon stimulation with strong inducers of vesiculation. Thus, we
next focused our attention on the release of PEVs induced by ADP, which is
considered as a weak platelet agonist [9, 10]. ADP is a physiological platelet
activator mediating its effects via purinergic receptors and playing a central
role in thrombus formation. It has been previously reported that responsiveness
to ADP is reduced in washed platelets [11]. As shown in Fig. 3, ADP stimulation
induced a significant different PEVs release in isolated compared to washed
platelets (isolated: 4.99
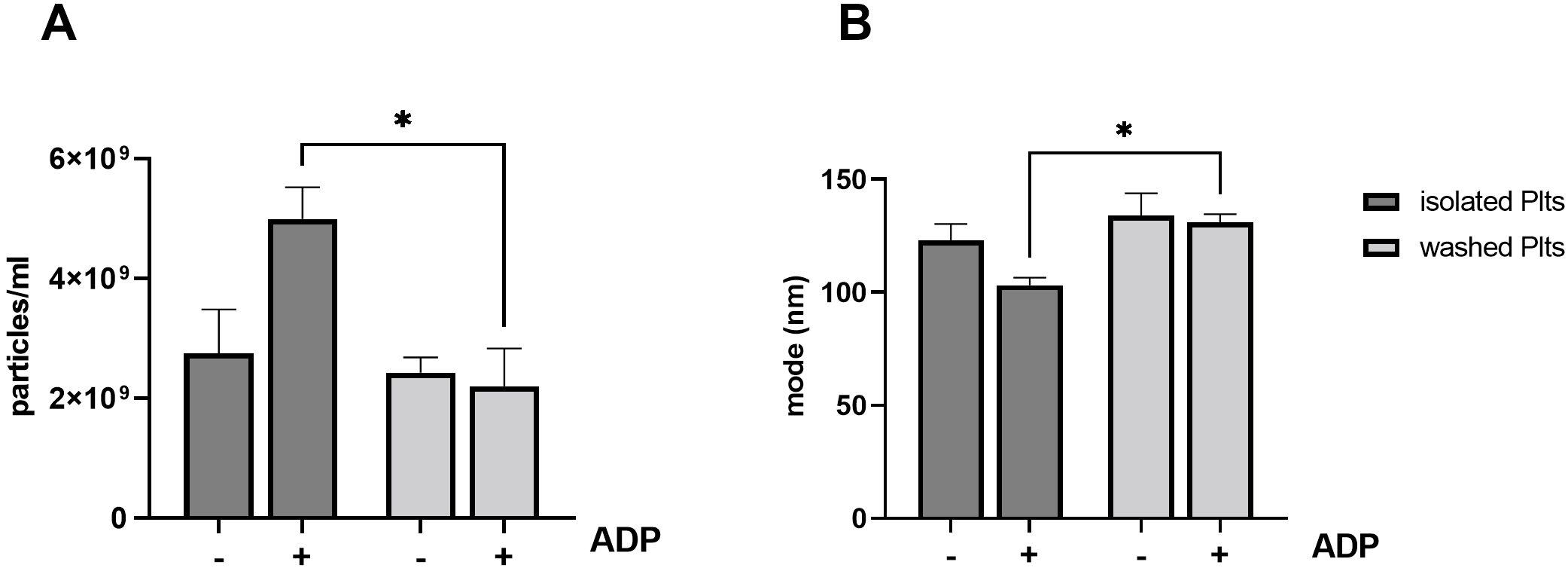 Fig. 3.
Fig. 3.Release of PEVs from ADP stimulated platelets. Isolated and
washed platelets were left untreated or stimulated with 5
To investigate whether the observed differences resulted from a different
general platelet sensitivity to ADP, we assessed agonist-stimulated
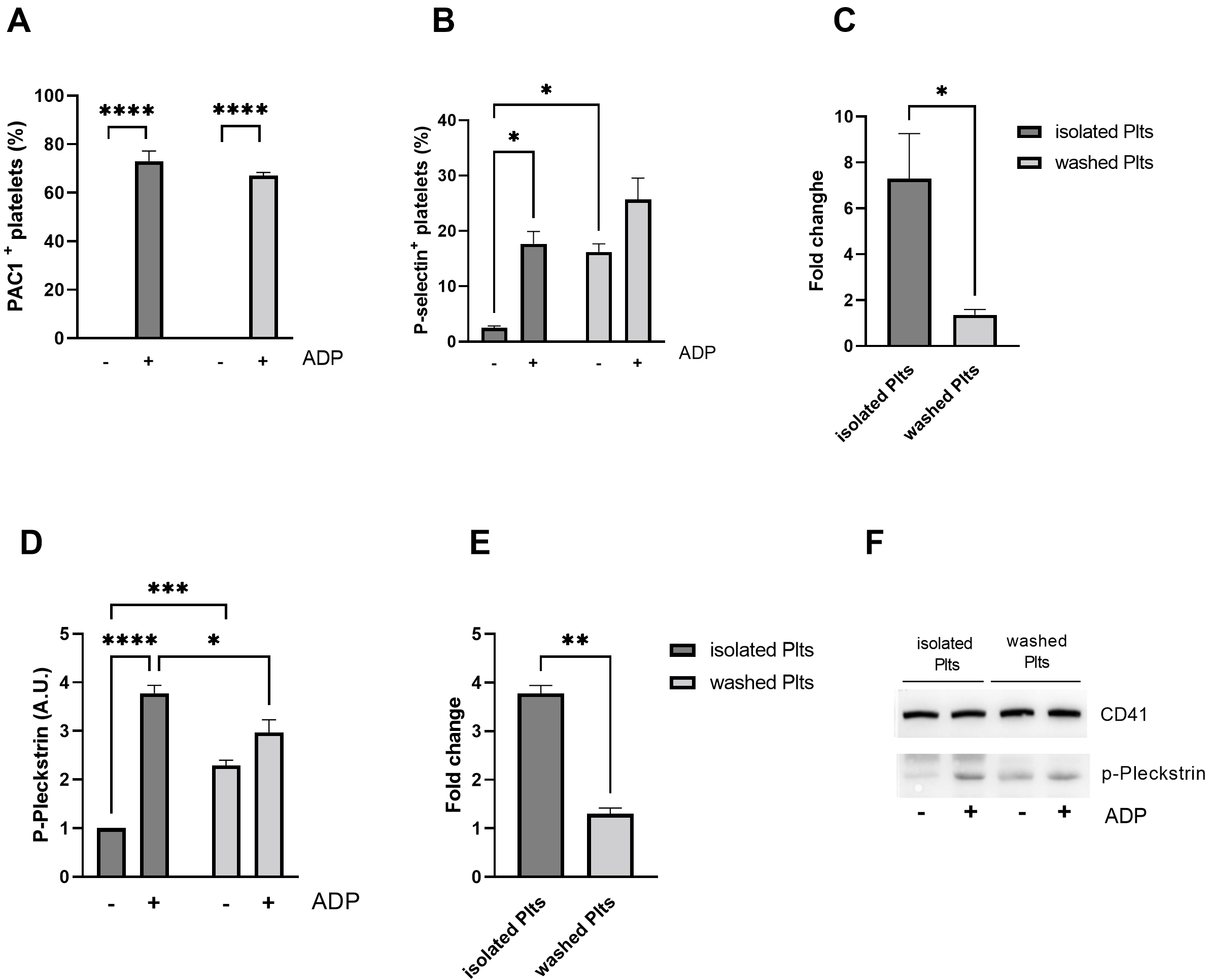 Fig. 4.
Fig. 4.Platelet activation in response to ADP. Flow cytometric
analysis of activation of (A) integrin
Interestingly, the activation of Protein Kinase C (PKC), a key player in ADP
mediated granules secretion, is in line with
So far, we showed that washed and isolated platelets had a similar release of
PEVs at basal conditions as well as upon stimulation with a wide range of
agonists. By contrast, the two different platelet preparations displayed a clear
difference in response to ADP, in terms of PEVs release and platelet activation.
To explain these observations, we hypothesized that a residual contamination of
plasma components, rather than a limited response of washed platelets due to a
preceding undesired platelet activation during the washing procedure, was
responsible for the unresponsiveness of washed platelets to ADP in terms of
release of PEVs. We have previously demonstrated plasma contamination in the
final platelet preparation may have important consequences on platelet
functionality in terms of tumor cell-induced platelet aggregation [7]. The
analysis of the protein expression pattern, performed by Coomassie blue staining
upon SDS-PAGE separation, revealed that isolated and washed platelets display an
overall similar composition in terms of the most abundant proteins. However, a
strong band between 75 and 50 kDa, likely corresponding to albumin, was detected
in isolated platelets, suggesting the presence of a significant plasma
contamination. This observation implies that additional contaminant plasma
proteins remain in the isolated platelet preparation, although they could not be
detected by gel staining. We verified this possibility by immunoblotting
analysis, probing the plasma protein
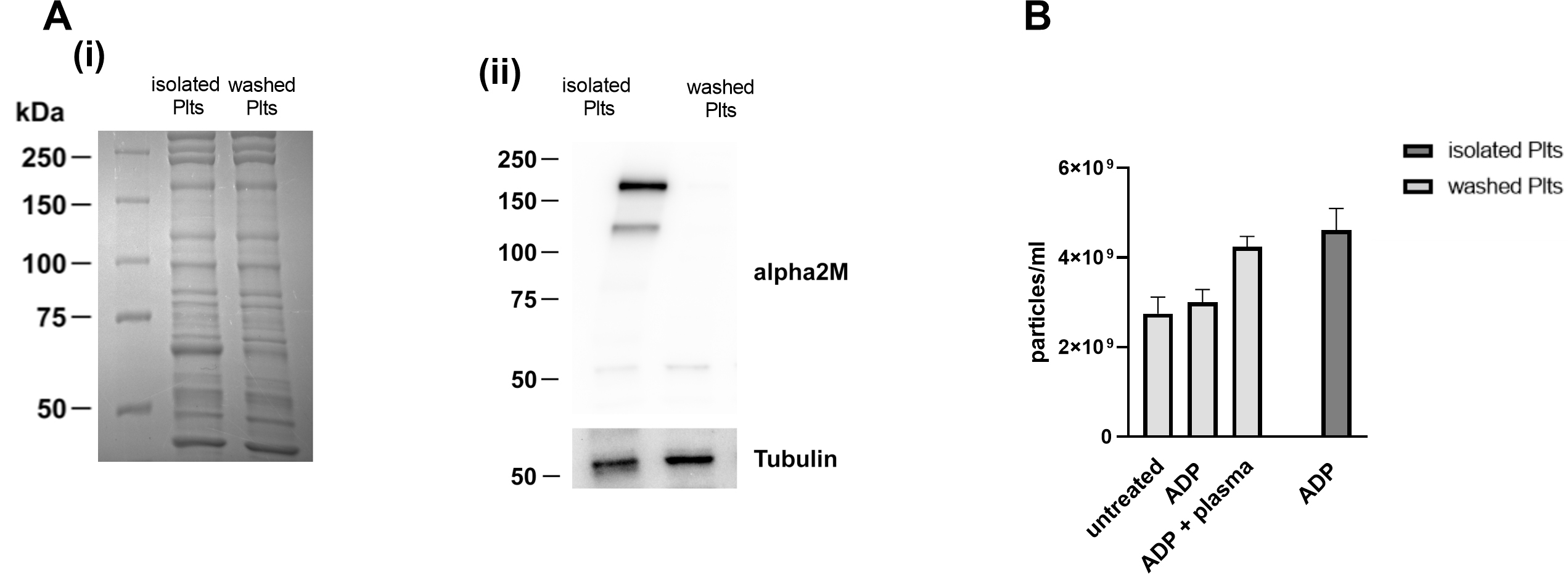 Fig. 5.
Fig. 5.Analysis of plasma contamination in the two platelets
preparations. (A) Lysates of isolated and washed platelets were separated on
7.5% and visualized by (i) Coomassie staining or (ii) by immunoblotting
analysis. Representative western blot of
To investigate whether such plasma contamination could contribute to PEVs release observed in isolated platelets in response to ADP, washed platelets were stimulated with ADP in the presence of small amount (0.05% V/V) of autologous plasma. Traces of autologous plasma were sufficient to rescue the release of PEVs in washed platelets to a level comparable to that observed in isolated platelets (Fig. 5B), suggesting that some plasma components could be responsible for the increased release of PEVs from isolated platelets.
Here, we show that platelet responsiveness, in terms of release of PEVs, is strongly influenced by the isolation procedure. The different platelet reactivity is a critical determinant for PEVs release when induced by weak agonist such as ADP, whereas it has no effect upon stimulation with strong agonists. Indeed, any significant difference neither in number of released vesicles, nor in size distribution was observed when isolated and washed platelets were stimulated with thrombin, collagen, thromboxane A2 and calcium ionophore.
It has been previously demonstrated that the composition of PEVs is largely dictated by the stimulus that induce their release [12]. It can be speculated that the platelet purification protocol may influence the mechanism of cargo selection, leading to different vesicle composition in terms of proteins, nucleic acids, and small molecules. Since residual plasma contamination appeared to be essential for the ability of platelets to release PEVs in response to ADP, it is expected that major differences in the cargo composition could be observed when isolated and washed platelets are activated by weak agonists. However, it cannot be excluded that the presence of plasma contaminants could also affect the composition of vesicles released upon stimulation of platelets with stronger agonists. To evaluate this possibility, further investigations exploiting proteome/transcriptome analyses are going to be performed.
Such omics approaches, coupled to bioinformatic analyses, will also allow to predict whether PEVs released by isolated versus washed platelets may also display different functional roles physiological and pathological contexts, such blood coagulation, thrombosis, and cancer. The results collected within these studies will aid the understanding of the complex interplay occurring between platelets, PEVs and the blood microenvironment.
Our results also indicate that the washing procedure may cause a partial
preactivation of platelets. Indeed, we detected a higher activation of washed
platelets at basal level, implicitly suggesting that using different purification
protocols, may lead to different interpretation of the results of
In conclusion, our study strongly suggests that procedure by which platelets are isolated is a critical determinant of PEVs release upon ADP stimulation. Future studies are required to in-depth investigate the molecular mechanisms by which plasma influence PEVs release and functionality.
ADP, Adenosine diphosphate; alpha2M,
SSB and GFG designed the research study. MZ, GDD, MV performed the research. PA, SMGT, LS provided help and advice on manuscript preparation. MZ analysed the data. MZ, GFG, SSB wrote the first version of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final version of the manuscript.
Not applicable.
Not applicable.
This research was supported by the Italian Ministry of Education, University and Research (MIUR): Dipartimenti di Eccellenza Program (2018–2022) - Dept. of Biology and Biotechnology “L. Spallanzani”, University of Pavia; and by the Italian Ministry of Health, Rome, Italy (Ricerca Corrente RC 2021, RC2022).
The authors declare no conflict of interest. MZ and SSB are serving as the Guest Editors of this journal. We declare that MZ and SSB had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to GP.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2705161.





