1 Key Laboratory for Quality Evaluation of Bulk Herbs of Hunan Province, The School of Pharmacy, Hunan University of Chinese Medicine, 410208 Changsha, Hunan, China
2 Hunan Key laboratory of Vascular Biology and Translational Medicine, Medical School, Hunan University of Chinese Medicine, 410208 Changsha, Hunan, China
†These authors contributed equally.
Academic Editors: Marcus Franz and Alexander Pfeil
Abstract
Background: To investigate the effect and potential molecular mechanisms of Dipsacoside B (DB), an herb monomer extracted from Dipsacusasper or Lonicera macranthoides, on the migration and proliferation of vascular smooth muscle cells (VSMCs) and balloon-induced neointimal formation. Methods: In vivo, rat abdominal aorta balloon injury model was utilized to investigate the effect of DB on the neointimal formation. In vitro, cultured VSMCs were used to investigate the effect of DB on Angiotensin-II (Ang-II)-induced migration and proliferation of VSMCs. Western blot and immunofluorescence were used to measure PTEN expression. Results: As compared to vehicle control balloon-injury group, DB treatment significantly inhibited the neointimal formation together up-regulated the expression of phosphatase and tension homolog deleted on chromosome 10 (PTEN). Cell proliferations (MTT and Edu incorporation) assays and wound migration measurement further revealed that treatment with DB significantly blunted Ang-II-induced proliferation and migration potential of VSMCs. Western blot analysis exhibited that DB upregulated the expression of PTEN in vivo and in vitro. Conclusions: DB treatment suppresses the proliferation and migration of VSMCs and reduces neointimal formation by the mechanisms involving regulating the phenotype switch of VSMCs via upregulating PTEN expression.
Keywords
- dipsacoside B
- vascular smooth muscle cell
- phenotype switch
- balloon injury
- PTEN
Cardiovascular disease (CVD), which includes atherosclerosis, coronary heart disease, pulmonary hypertension and etc., is the major cause of death worldwide. The morbidity and prevalence remains very high and thus result in great burden on global healthcare [1, 2, 3]. Vascular remodeling (VR) is the underlying pathogenesis of CVD. As today, the close relationship between CVD and VR has been confirmed, and more attention has been paid to the strategies for the prevention and treatment of VR in cardiovascular events. However, medications utilized in clinical for VR treatment, such as renin-angiotensin-aldosterone system inhibitors, calcium antagonists, potassium channel openers, and so on, have limited efficacy [4]. Besides, the unpleasant side effects associated with the aforesaid medications, like coughing, rash, angiopaural edema and etc., restrict their applicability. Thus, the search for potential therapeutic agents for VR remains critical.
Intimal hyperplasia and arterial restenosis are two core parts existing in VR. Throughout the progression, vascular smooth muscle cell (VSMC) is the main contributor. Stimulated VSMCs underwent phenotype switch, proliferated and migrated from the media to the intima, thus causing the narrowing or restenosis consequence [5, 6]. Therefore, targeting hyper proliferating VSMCs and suppressing VSMCs’ phenotype switch can contribute to therapeutic outcome in vascular diseases [7]. Multiple studies [8, 9] have demonstrated that phosphatase and tension homolog deleted on chromosome 10 (PTEN) is the key regulator in VSMCs’ phenotype switch and sequentially alters behaviors in the migration and proliferation of VSMC. Down-regulation of PTEN promotes the migration and proliferation of VSMCs [10], whereas upregulation of PTEN suppresses the migration and proliferation of VSMCs, blunts neointimal formation, and alleviate stenosis [9, 11, 12]. All these clues implicate that PTEN may be a pivotal target in VR-related diseases. Herein, searching for medications that can block the development of VR by targeting PTEN becomes an effective strategy for CVD treatment.
In recent decades, natural components from Chinese herbs have exhibited
properties of suppressing VR with multiple targets and less negative effects
[13, 14, 15]. Triterpenoid saponins isolated from Chinese medicine have exhibited
attenuation effects in VR. Report has shown that astragaloside IV can inhibit the
proliferation and migration of rat VSMC by targeting CDK2 [16]. Besides, panax
notoginseng saponins present inhibitory effects in the phenotype transformation
of VSMCs and suppress abdominal aortic aneurysm progression [17].
Furthermore, ginsenoside Re inhibits VSMC proliferation induced by PDGF-BB via
the eNOS/NO/cGMP pathway [18]. Evidence is mounting that triterpenoid saponins
including Notoginsenoside R1, Astragaloside IV exert beneficial effects in oxygen
and glucose deprivation-induced injury [19] and myocardial infarction [20] via
PTEN expression. Nevertheless, it has not been reported that triterpenoid
saponins inhibit the phenotype transformation of VSMC in VR via PTEN. Dipsacoside
B (DB), triterpenoid saponins, is an herb monomer extracted from Chinese
medicine-Dipsacusasper or Lonicera macranthoides [21]. However,
the pharmacological effects of DB are rarely reported. Recent studies have
demonstrated that DB exerts beneficial effects on brain ischemic injury via the inhibition of mitochondrial E3 ubiquitin ligase 1 [21], suggesting the
therapeutic potential of DB in VR-related diseases. Our previous study [22] has
demonstrated that DB suppresses the proliferation of VSMCs by downregulating
TOP2
In the present study, we adopted rat abdominal aorta balloon injury model to evaluate the inhibitory effects of DB on neointimal formation in vivo. Then, we employed Ang-II to induce VSMCs’ proliferation and migration, investigated the effects of DB on the proliferation and migration of VSMCs and further examined the association between DB and PTEN in these effects. Our study may provide a new sight into the protective effect of DB in VR-related diseases.
Thirty-two male Sprague-Dawley (SD) rats (280–330 g) were purchased from
department of laboratory animal science of university of south China (Hengyang,
China). Animals were housed under controlled temperature (25
DB was obtained from Nanjing spring & autumn biological engineering co., td and dissolved in distilled water.
Balloon injury was administrated as follows: SD rats were anesthetized with isoflurane by aerosol inhalation and fixed on the simple operating plates. The skin around the neck was shaved, disinfected and paved with sterilized sheets. The platysma muscle and left sternocleidomastoid muscle were separated at the left of the midline to find left common carotid artery. The bifurcation between the left external and internal carotid artery was ligated with 1# silk suture, and then the proximal end of the left common carotid artery was ligated with vascular camp to block blood flow. Balloon catheter was inserted along left common carotid artery and put at the end of abdominal aorta, inflated with 8 Kpa and carefully pulled, repeated three times. After the intervention, penicillin was injected intramuscularly with 100,000 U a day for continuous 3 days to prevent infection. After 14 days with indicated administration, rats were sacrificed and tissue samples were obtained for the follow-up detection.
2
VSMCs were purchased from cell bank of central laboratory of Xiangya hospital
central south university (Changsha, Hunan, China). VSMCs were cultured in
Dulbecco’s Modified Eagle’s Medium (DMEM) (12800017, Gibco, Carlsbad, CA, USA),
supplemented with 10% fetal bovine serum (FBS) (P30 - 3302, PAN-Biotech, Dorset,
UK) and 1% penicillin-streptomycin solution (PB180120, Procell, Wuhan, China).
All cell lines were incubated in a incubating tank with CO
DB (purity
Briefly, cells were seeded (8
5-ethynyl-2’-deoxyuridine (EdU) incorporation assay (C0081S, Beyotime, Shanghai,
China) was performed according to the manufacturer’s instructions to detect the
proliferation of VSMCs. Cells were seeded (1
Cells were seeded (5
Cells were washed with PBS, harvested, and lysed in lysis buffer. Protein
samples of 20
Cells were fixed with 4% paraformaldehyde for 15 min, washed with PBS three
times, each for 5 min, blocked in the BSA (5%) for 60 min, incubated with the
indicated primary antibodies at 4 °C overnight, and then incubated in dark with
corresponding secondary antibodies at room temperature for 1 h. Cells were
stained with DAPI and washed with PBS three times, each for 5 min. Inverted
fluorescence microscope (ZKX53; Olympus, Tokyo, Japan) was used to obtain images.
Anti-OPN (1:200, ab8448), anti-SM22
2
All data were expressed as the mean
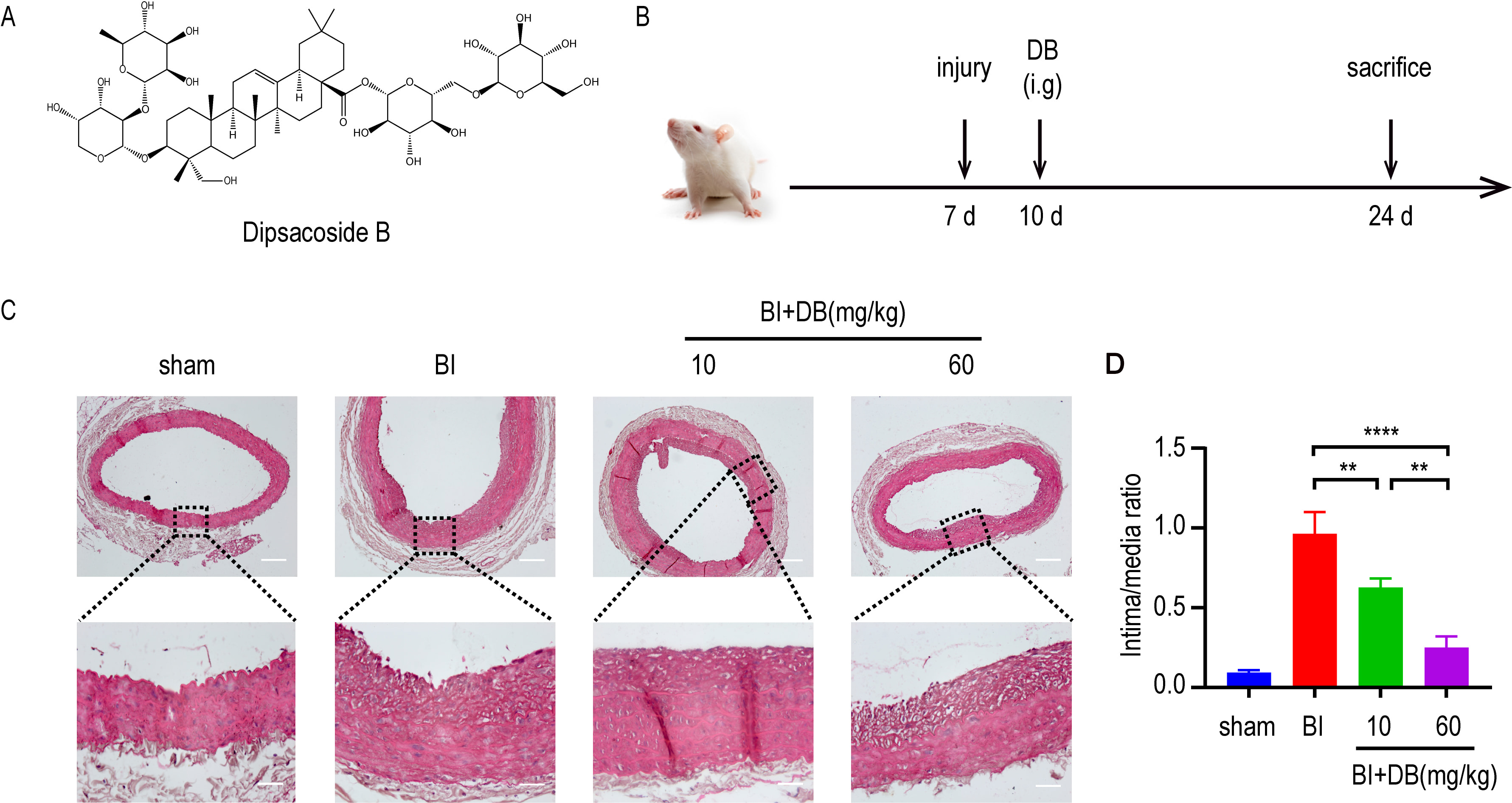
In the progression of AS, the proliferation and migration of VSMCs contributes to arterial restenosis. Given the fact, we induced a rat balloon catheter-injured abdominal aorta model to study the role of DB in vivo. HE staining showed that DB inhibits the intimal hyperplasia in rats with balloon catheter-injured abdominal artery (Fig. 1A,B).
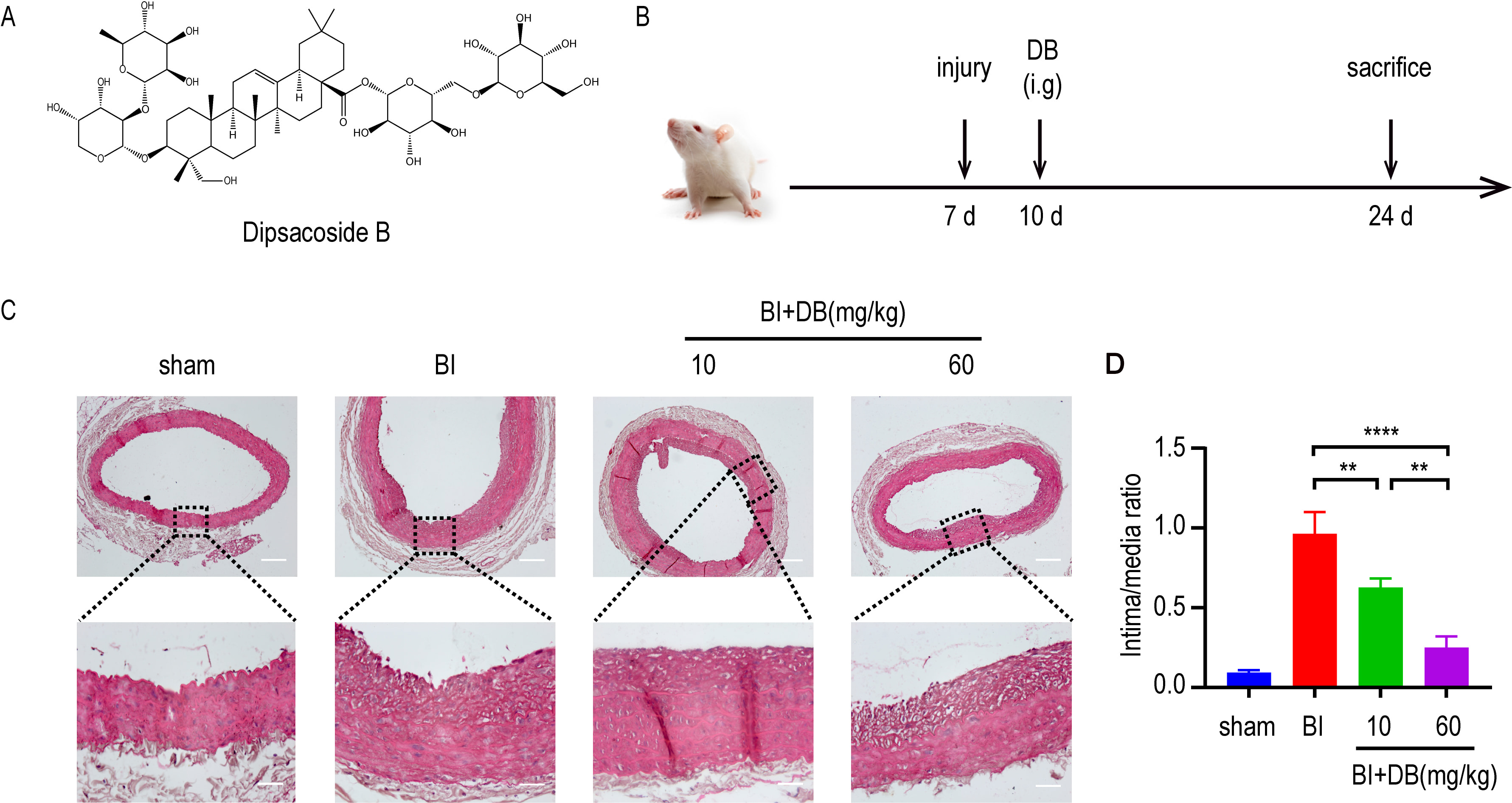 Fig. 1.
Fig. 1.DB inhibited neointimal formation. (A) The chemical structure
of DB. (B) Schematic illustration of animal experimental protocol. (C) H&E
staining of neointimal formation in arteries from rats underwent different
administration after balloon injury, magnification: upper
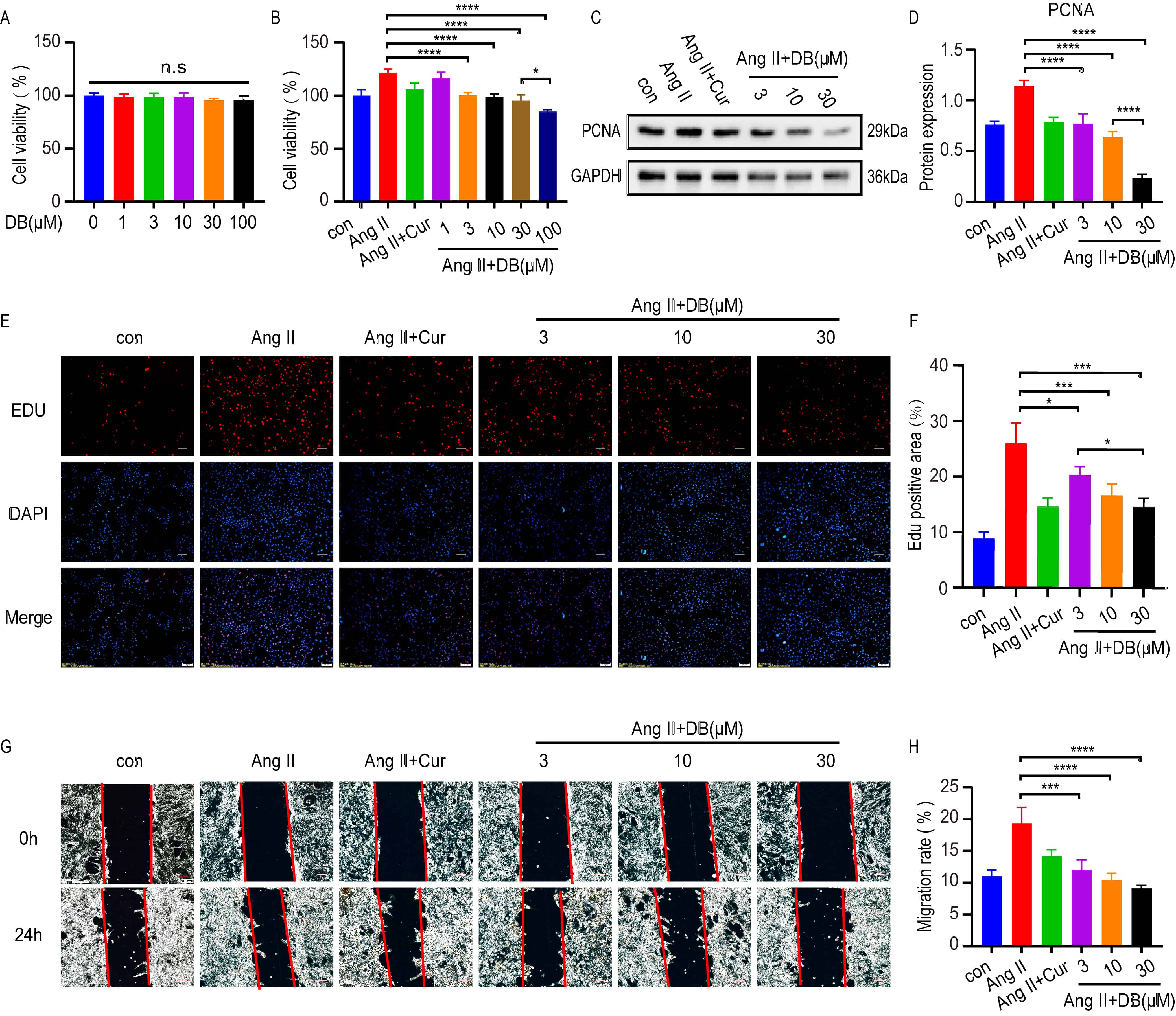
To further elucidate the underlying mechanism of DB in cultured VSMC, MTT, Edu
and wound migration assays were used to test the measurements of cell viability,
proliferation and migration in VSMCs treated with DB at indicated concentrations
for 24 h. MTT assays demonstrated that no cytotoxicity was observed at DB
concentrations up to 100 Mm (Fig. 2A). Ang-II significantly increased the cell
viability of VSMCs, which was inhibited by DB in a dose-dependent manner (Fig. 2B). Based upon the result, we chose the concentration of 3, 10, 30
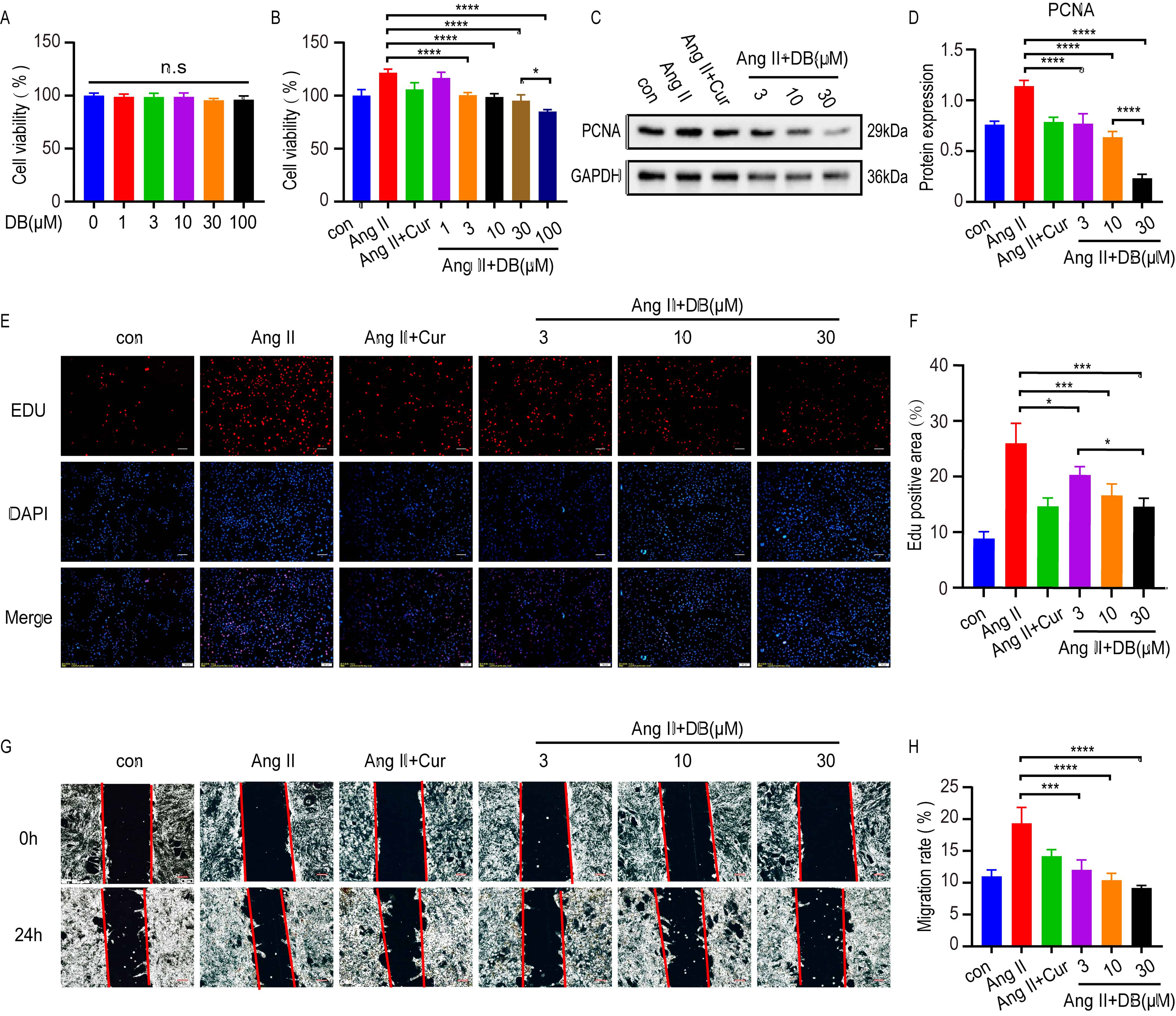 Fig. 2.
Fig. 2.DB inhibits the viability, proliferation and migration of
VSMCs. (A) MTT assay analysis of cell viability rate of VSMCs treated with DB of
different concentrations (0, 1, 3, 10, 30, 100
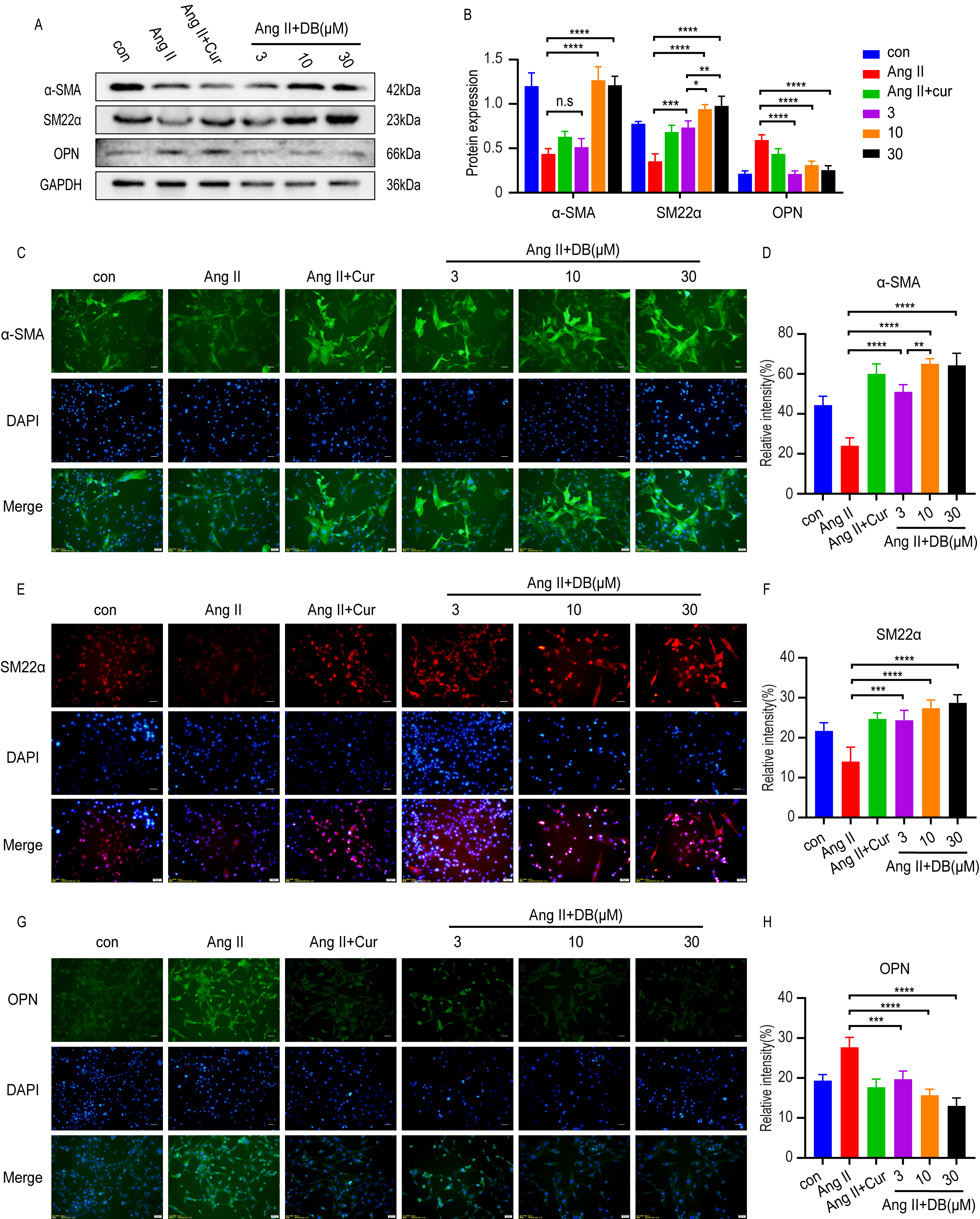
Increasing evidence [5, 23] has shown that the phenotype switches of VSMCs from
contractile phenotype to synthetic ones secrets growth factors, and promotes the
proliferation and migration of VSMCs. To investigate the interaction between DB
and phenotype switch, we employed western blot and immunohistochemistry analysis
to measure the expression of phenotype switch related protein-alpha smooth muscle
actin
Western blot and immunohistochemistry analysis demonstrated that Ang-II
down-regulated the expression of contractile protein-
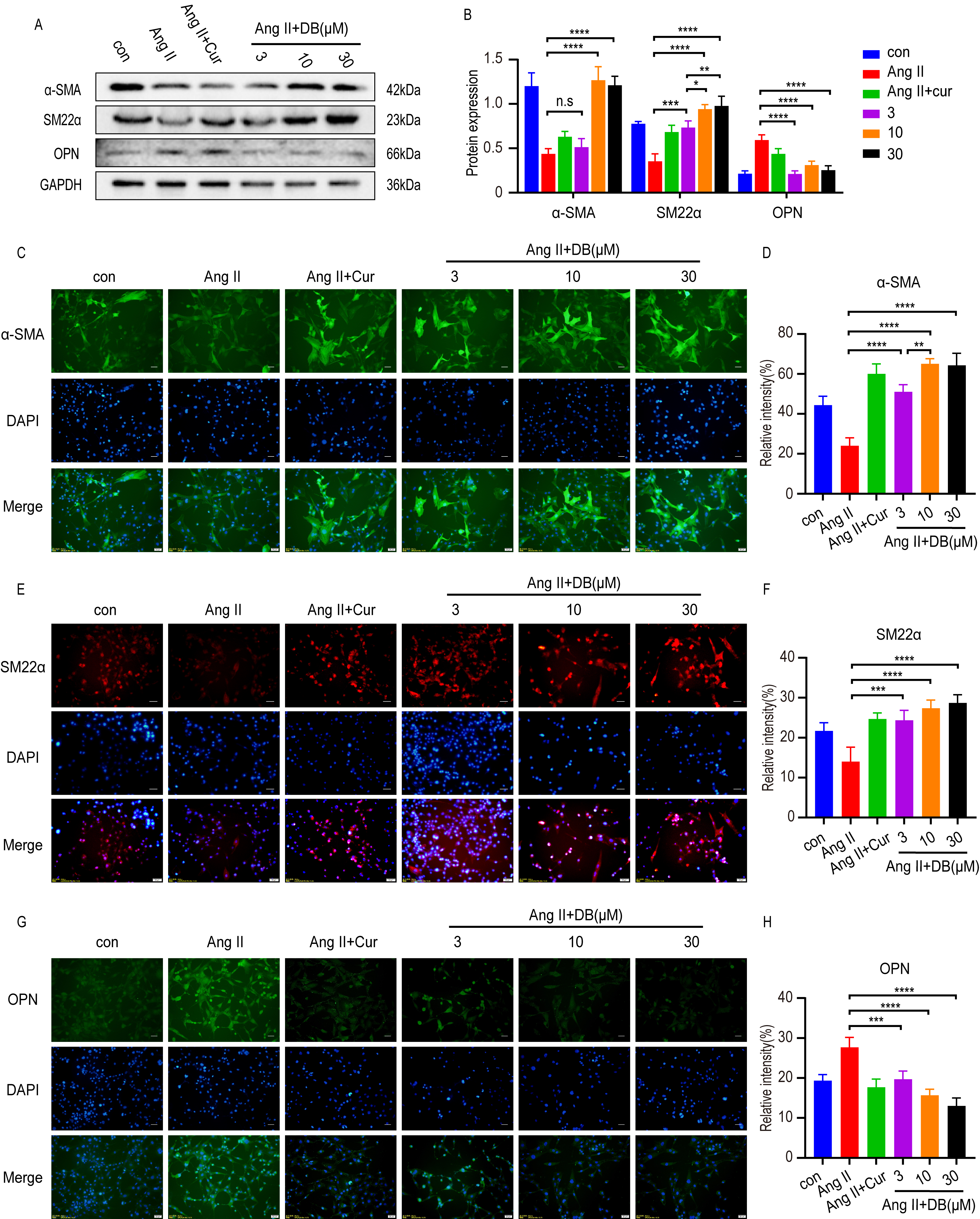 Fig. 3.
Fig. 3.DB exerts its role by modulating phenotype switch of VSMCs. (A)
Western blot analysis of
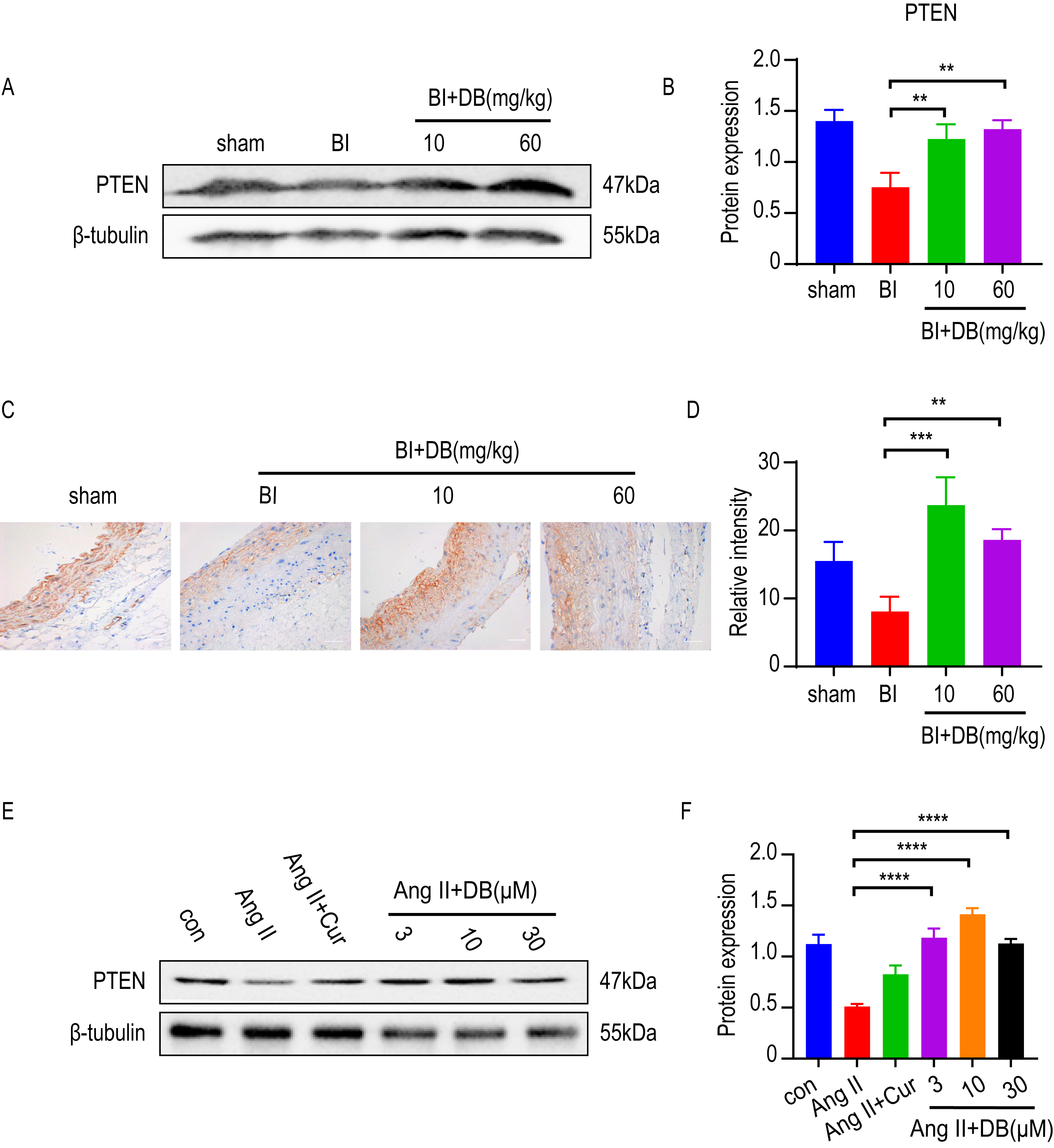
Increasing evidence [8, 11, 24] showed that PTEN was a potent regulator in inhibiting the proliferation and migration of VSMCs. To determine whether PTEN was involved in the activity of DB, we employed western blot and immunohistochemistry to measure the expression of PTEN in vivo. The results of western blot and immunohistochemistry showed that rat subjected to balloon injury resulted in a significant reduction of PTEN expression. Furthermore, the inhibition of PTEN induced by balloon-catheter injury was dramatically attenuated by DB treatment (Fig. 4A,B). We then measured the expression of PTEN in cultured VSMCs. Interestingly, Ang-II significantly decreased the expression of PTEN while DB treatment significantly increased the expression of PTEN in Ang-II induced VSMCs (Fig. 4B), indicating that DB may exert its effects via up-regulation of PTEN expression.
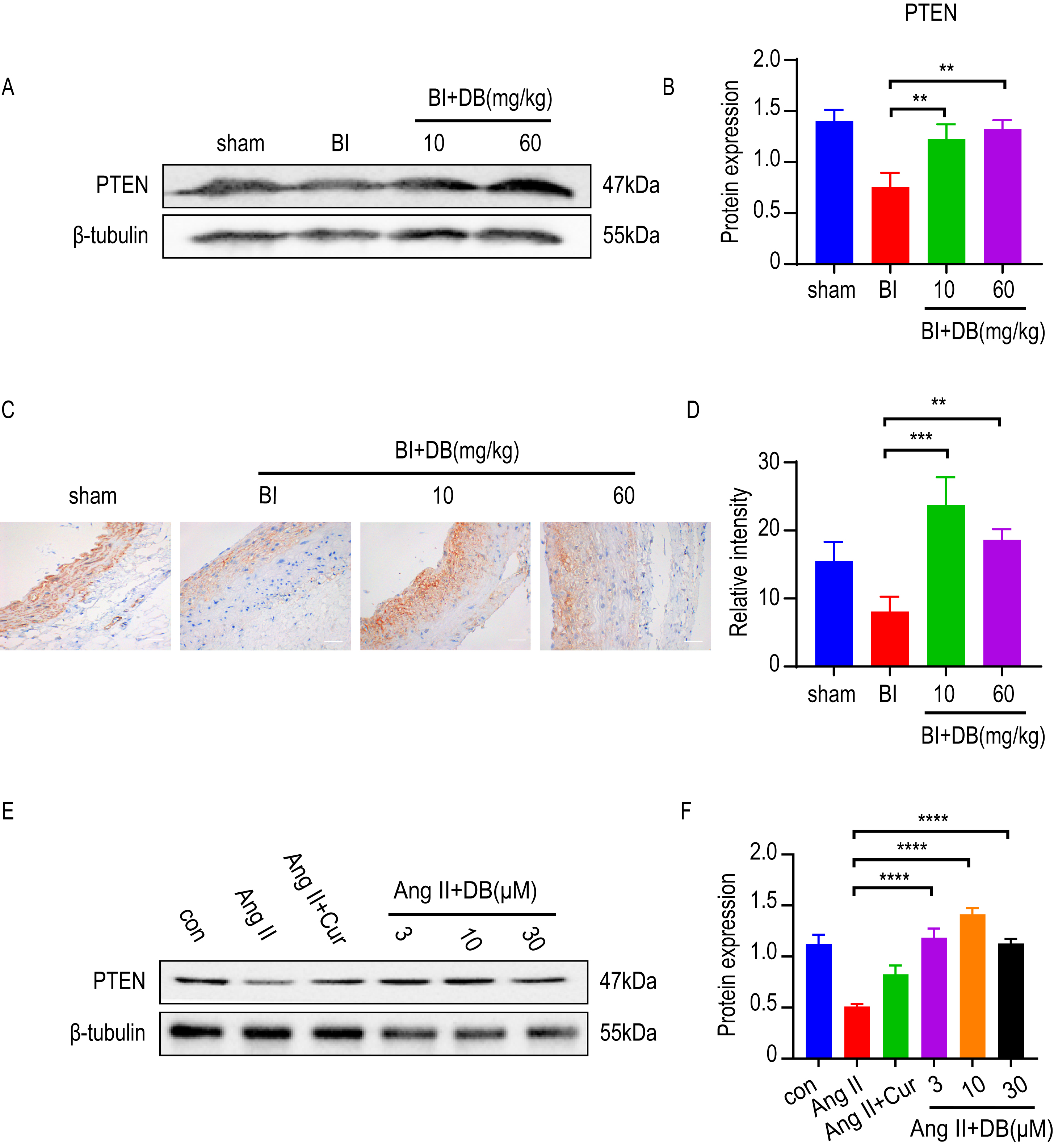 Fig. 4.
Fig. 4.DB exerts its role via up-regulation of PTEN expression. (A)
Western blot analysis of PTEN expression in arteries obtained from rats underwent
balloon injury (sham, BI, BI + 10 or 60 mg/kg DB treated groups). (B)
Quantification of PTEN levels in (A). (C) Immunohistochemistry analysis of PTEN
expression in arteries obtained from rats underwent balloon injury (sham, BI, BI
+ 10 or 60 mg/kg DB treated groups), magnification:
The excessive proliferation and migration of VSMCs is the basic pathogenesis in VR-related diseases, such as atherosclerosis, hypertension and etc. [25, 26]. Thus, it is urgent to find a promising medicine that can suppress the proliferation and migration in an effective way. Previous studies [27, 28] have demonstrated that triterpenoid saponins can inhibit the neointimal formation caused by balloon injury. As a member in triterpenoid saponin family, the effect of DB on neointimal formation remains unknown. Therefore, rat balloon injury animal model was employed to investigate the in vivo activity of DB. In vivo experiments showed that DB treatment significantly decreased the intima to media ratio, indicating that DB blunted neointimal formation in rats underwent balloon injury.
Ang-II is an important inducer in the proliferation of VSMC. To find out whether
DB has an influence on the proliferation and migration of VSMCs, we investigated
the role of DB on the Ang-II induced proliferation and migration of VSMCs. CCK-8
assay showed that when the concentration of DB came up to 100
Phenotype switch of VSMCs accompanied by the transition of protein markers is
the key process in the proliferation and migration of VSMCs [29]. Among the VSMCs
transformative process from contractile type to synthetic type, the expression of
synthetic protein marker like OPN increased while the contractile protein marker
like
Based on the effect of DB on anti-proliferation and phenotypic regulation in vivo and in vitro, we then further dissect the underlying mechanism. Increasing evidence [9, 32] revealed that PTEN is a powerful target that can regulate VSMCs’ proliferation and migration, which shows close association with vascular fibrosis and remodeling [33, 34]. Studies have proven that Ang-II decreased PTEN expression in SMC [33]. The low level of PTEN in turn promotes the proinflammatory SMC phenotype [35], and subsequently improves vascular remodeling [36]. The above results demonstrated that DB suppressed neointimal formation, and inhibited Ang-II induced migration and proliferation of VSMC. However, whether DB interfered with PTEN remains to be further elucidated. To investigate whether DB can affect PTEN expression, immunofluorescence and western blot was utilized to measure the effect of DB treatment on PTEN expression. Our data showed that DB significantly upregulated PTEN expression in vivo and in vitro, indicating DB may exert its role by targeting PTEN expression.
However, there are still a number of issues that need to be further elucidated. Firstly, many investigations regarding the interaction between PTEN stability and proteasome has shown that proteasome has an impact on PTEN protein stability [37]. Through the ubiquitin/proteasome pathway, treatment that modulates PTEN stability can suppress cell proliferation and migration [38]. But the interaction among DB, PTEN and proteasome remains unknown. Then, it is well known [20, 24] that PTEN can suppress the proliferation of VSMCs by dephosphorylat-ing phosphatidylinositol (3, 4, 5) triphosphate (PIP3) and preventing the activation of the PI3K/Akt signaling pathway. We hypothesize that DB plays its role through suppressing the PI3K/Akt pathway based on our observations of the elevation effects of DB on PTEN protein levels and the strong link between PTEN and PI3K/Akt pathway, and these effects will be shown in the follow-up research.
In this study, we first demonstrated that DB treatment blunted the intimal formation in balloon injured abdominal aorta. Furthermore, we confirmed that DB treatment suppressed the proliferation and migration of VSMCs. Then, we examined PTEN expression in balloon injured abdominal aorta and cultured VSMCs, and found DB treatment upregulated PTEN expression, revealing that DB may exert its role by upregulating PTEN expression. Therefore, for the first time, we report a novel role of DB in the cardiovascular disease and sheds insights into the application of DB in arterial restenosis.
In conclusion, DB can suppress the proliferation and migration of VSMCs and neointimal formation by regulating the phenotype switch of VSMCs and targeting PTEN expression.
DL and QT designed the research study. JX, DY and ZS advised experimental design. YH and YC performed the research. WQ and YH analyzed the data and wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was approved by the Institute Research Ethics Committee of Hunan University of Chinese Medicine. The animal ethics number is LL2020022001.
Not applicable.
This research was funded by the Key Research and Development Projects of Hunan Provincial Science and Technology Department, grant number: 2022SK2011, the Science and Technology Innovation Program of Hunan Province, grant number: 2021RC4064, Key Projects of Hunan Provincial Department of Education, grant number: 20A379, Natural Science Foundation of Changsha, grant number: kq2202268, and Graduate Innovation Project of Hunan University of Chinese Medicine, grant number: 2021CX10 and 2021XJJJ010.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.