1 Department of Botany, Mohanlal Sukhadia University, 313 001 Udaipur, Rajasthan, India
2 Department of Botany, Government Science College, Pardi, 396125 Valsad, Gujarat, India
3 Department of Chemistry, Mohanlal Sukhadia University, 313 001 Udaipur, Rajasthan, India
4 Metagenomics and Secretomics Research Laboratory, Department of Botany, Dr. Harisingh Gour Central University, 470003 Sagar, MP, India
5 Diatom Nanoengineering and Metabolism Laboratory (DNM), School of Applied Sciences, Dr. Harisingh Gour Central University, 470003 Sagar, MP, India
Abstract
Algae possess a considerable potential as bio-refinery for the scale-up production of high-value natural compounds like—carotenoids. Carotenoids are accessory pigments in the light-harvesting apparatus and also act as antioxidants and photo-protectors in green cells. They play important roles for humans, like—precursors of vitamin A, reduce the risk of some cancers, helps in the prevention of age-related diseases, cardiovascular diseases, improve skin health, and stimulates immunity. To date, about 850 types of natural carotenoid compounds have been reported and they have approximated 1.8 billion US$ of global market value. In comparison to land plants, there are few reports on biosynthetic pathways and molecular level regulation of algal carotenogenesis. Recent advances of algal genome sequencing, data created by high-throughput technologies and transcriptome studies, enables a better understanding of the origin and evolution of de novo carotenoid biosynthesis pathways in algae. Here in this review, we focused on, the biochemical and molecular mechanism of carotenoid biosynthesis in algae. Additionally, structural features of different carotenoids are elaborated from a chemistry point of view. Furthermore, current understandings of the techniques designed for pigment extraction from algae are reviewed. In the last section, applications of different carotenoids are elucidated and the growth potential of the global market value of carotenoids are also discussed.
Keywords
- Astaxanthin
- Biosynthesis
- Carotene
- Fucoxanthin
- High-Value compounds
Carotenoids comprehend a group of naturally occurring lipophilic (fat-soluble)
pigments. C
About 850 kinds of carotenoids have been reported up to 2018 [7]. They are
broadly grouped into two categories either on basis of functional properties or
chemical structure. Functionally, they can be either primary having a vital role
in photosynthesis or secondary having a role in stress conditions [8]. Based on
chemical structure, carotenoids having pure carbon skeleton and are referred to
as carotenes (cyclized or uncyclized, e.g.,
Chloroplast—the green organ of photosynthetic tissue of higher plants, is not
only the site of photosynthesis, but also plays an important role in biosynthesis
and accumulation of carotenoid. Stanely and Yuan have reported many nuclei
encoded membrane proteins, their synthesis in the cytoplasm as polypeptide
precursor with amino terminus extension, directed to the chloroplast, for the
biosynthesis of carotenoids [17]. The carotenogenesis pathway is under strict
gene control and acts as a chemotaxonomic marker [18]. On the flip side, this
pathway is equally prone to stress periods and affected by physical and
environmental factors like salinity, temperature, irradiance, nutrition, and
growth factors [19]. The foremost, premier, and rate-limiting step of the
biosynthetic pathway is the condensation of two GGPP (Geranyl geranyl
pyrophosphate), to originate phytoene (colorless carotenoid) in presence of PSY
(Phytoene synthase) enzyme [20]. Subsequently, an array of sequential
desaturations results in the production of all-trans lycopene. Major enzymes
involved are Phytoene desaturase (PDS),
Carotenoids can be stored inside or outside the chloroplast according to their
functional role. Primary pigments are stored inside while secondary pigments
remain outside the chloroplast in lipid globules. Green tissue conserves the
accumulation of carotenoids while the levels in non-green tissues may vary
according to the developmental stage. Though, cell storage capacity, catabolism,
and degradation rate may alter the carotenoid profile [20]. The phenomenal
process of photosynthesis on the whole needs chlorophyll as a pre-dominant
pigment, while carotenoids play a donative role in the overall mechanism of
energy transport and conversion [23]. Carotenoids majorly play
a dual role, primarily; they act as accessory light-harvesting pigments in
photosystem, thereby extending the range of solar radiation (wavelength) which is
not absorbed by chlorophyll and hence, drive the process of photosynthesis to a
greater peak. Secondly, the noteworthy role of carotenoids is photo protective by
dissipating extra energy and scavenging toxic oxygen molecules. In this way,
carotenoids stabilize pigment-protein complexes; and maintain the integrity of
membranes necessary for cell survival and development [24]. Secondary carotenoids
like astaxanthin and canthaxanthin, accumulates in high amount in cytoplasmic
lipid globules under stress conditions. Begum et al. [25] have reported,
the presence of characteristic pink/red color of some stressed algae due to
carotenoid accumulation as a protective layer. In non-photosynthetic bacterium
(Deinococcus-Thermus) and fungi, carotenoid plays a proficient
photo-protective role [26]. The overall stability and functionality of the
photosynthetic apparatus can be attributed to the antioxidant property these
pigments own, which can prevent photo-oxidative damage [27]. Another role of
carotenoids is a requirement to form prolamellar bodies (PLB’s) in etiolated
seedlings to speed up photo-morphogenesis [27, 28]. Their role as a precursor of
phytohormone ABA (abscisic acid) and SL (strigolactone) has been reported [29, 30]. Cazzonelli and Pogson have reported the role of
Unlike higher plants and other conventional sources, algae have a small life cycle with a speedy growth, cover less area for cultivation purposes, and are more efficient at biomass production and therefore serve as a better source for carotenoid production that too in cost-effective way. Different algae have been explored and utilized for the production of different carotenoids, which are listed in Table 1 (Ref. [31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51]). In the next few sections of this paper, we have highlighted the biosynthesis pathway for different carotenoids with special reference to algae. Further chemical aspects, extraction of synthesized carotenoid and their application and global market scenario are also discussed.
| S. No. | Algae | Pigment | Reference |
| 1 | Haematococcus pluvialis | Astaxanthin | [31] |
| 2 | Chlorella vulgaris | Astaxanthin | [32] |
| 3 | Chlorella striolata var. multistriata | Astaxanthin | [33] |
| 4 | Botryococcus braunii | Astaxanthin | [31] |
| 5 | Chlorella zofingiensis | Astaxanthin | [34] |
| 6 | Dunaliella salina | β-Carotene | [35, 36] |
| 7 | Dunaliella bardwal | β-Carotene | [37] |
| 8 | Coelastrella striolata var. multistriata | β-Carotene | [33] |
| 9 | Dunaliella salina | Bixin | [37] |
| 10 | Phaeodactylum tricornutum | Fucoxanthin | [38] |
| 11 | Isochrysis aff. galbana | Fucoxanthin | [39] |
| 12 | Odentella aurita | Fucoxanthin | [40] |
| 13 | Cylindrotheca closterium | Fucoxanthin | [38] |
| 14 | Isochrysis galban and Amphidinium carterae | Peridinin | [41, 42] |
| 15 | Phaeodactylum tricornutum | Zeaxanthin | [43] |
| 16 | Chlorella ellipsoidea | Zeaxanthin | [44] |
| 17 | Haematococcus pluvialis, Chlorella zofingiensis | Canthaxanthin | [45] |
| 18 | Chlorella vulgaris | Canthaxanthin | [46] |
| 19 | Botryococcus braunii and Dunaliella tetriolecta | Violaxanthin | [37] |
| 20 | Chlorella prothecoides | Lutein | [47] |
| 21 | Chlorella pyrenoidosa | Lutein | [48] |
| 22 | Chlorella vulgaris | Lutein | [49] |
| 23 | Chlorella salina, C. zofingiensis and D. salina | Lutein | [50] |
| 24 | Muriellopsis sp. | Lutein | [51] |
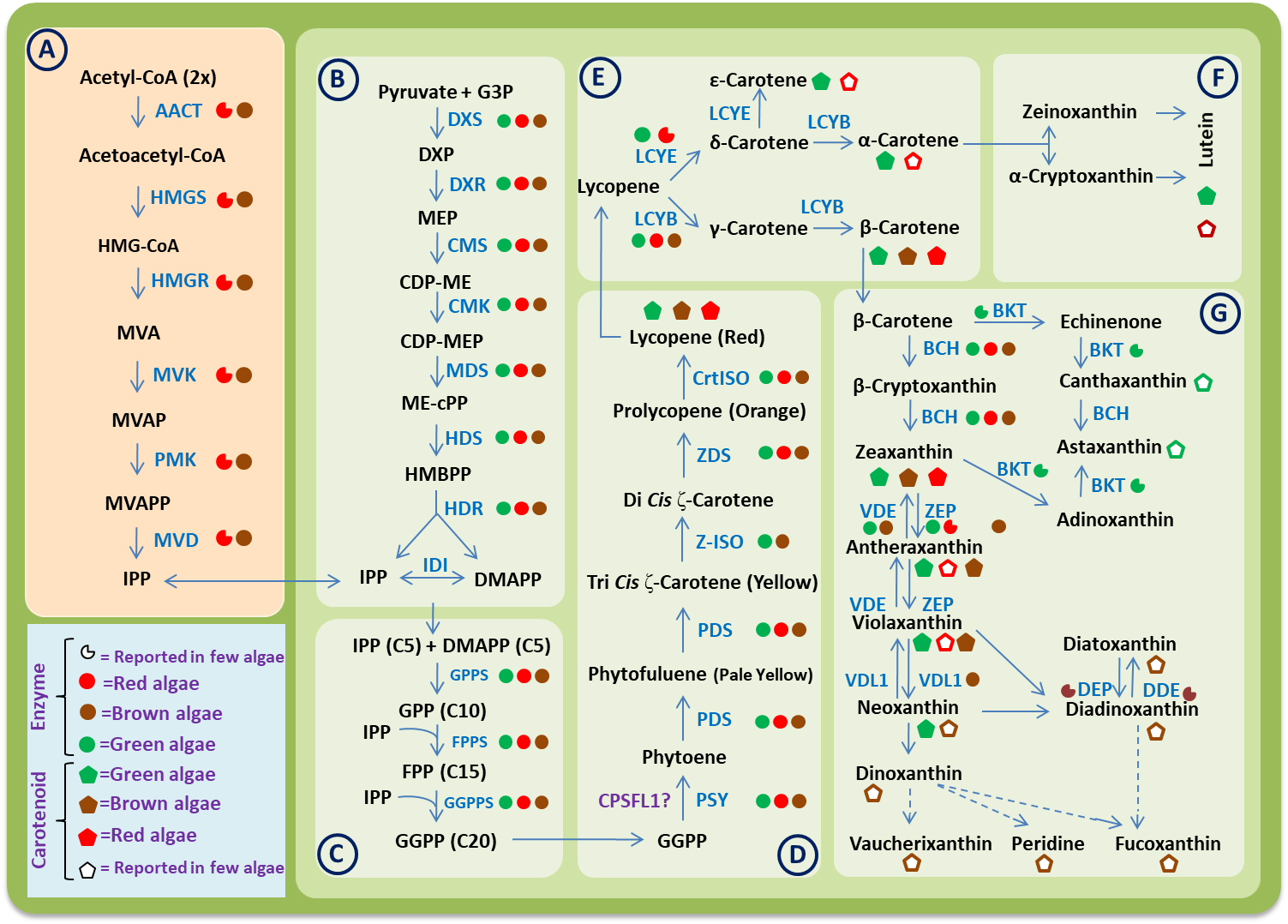 Fig. 1.
Fig. 1.A consensus carotenoid biosynthesis pathway in algae. Different
boxes represent the different steps involved in biosynthesis process as follows:
(A) Mevalonate (MVA) pathway. (B) Methylerythritol phosphate (MEP) pathway. (C)
Biosynthesis of geranylgeranyl diphosphate (GGPP). (D) Biosynthesis of phytoene
and lycopene. (E) Biosynthesis of carotenes. (F) Biosynthesis of xanthophylls
derived from
The basic carotenoid biosynthesis pathway seems to be the same in algae and streptophytes. Based on genome and transcriptome-wide studies, now it is clear that the evolution of carotenoid biosynthesis pathways in algae have involve various genetic mechanism like gene duplications, gene loss, gene transfer etc. Due to these genetic events, the carotenoid biosynthesis pathways in algae became more complex than that of terrestrial plants [52]. The biochemical and molecular mechanism for carotenoid biosynthesis has been studied in detail for microorganism and higher plants. But there are fewer reports in different groups of algae. In 1997, Hirschberg et al. [53] reported some genes and enzymes involved in the carotenoid biosynthesis pathway in algae and plants. Recently, Wang et al. [52] analyses the transcriptome of 22 red algae and 19 brown algae and then combine it with the data available publicly at different databases. Based on this study, they identified some important genes of the carotenoid biosynthetic pathway in algae. In 2019, Negre et al. [54] sequenced the genome of Saccharina japonica and Cladosiphon okamuranus and have proposed the model for carotenoid biosynthesis pathway in these brown algae using genome-scale metabolic networks (GSMNs). Based on available research data, the whole carotenoid biosynthesis pathway in algae can be divided into the following six steps (Fig. 1).
Isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are
five-carbon (C
MVA is a specific intermediate of IPP biosynthesis. This classical MVA pathway
is a multistep-cytosolic pathway, which begins with the condensations of three
acetyl-CoA molecules and comes to an end with the synthesis of one IPP molecule
(Fig. 1). In the initial two steps, one molecule of HMG-CoA (
Biochemical, ESTs and genomic research-based data elucidated that, MEP pathway
evolved due to cyanobacterial ancestry in algal cells. That is why, this pathway
operates in plastids of algal cells in contrast to the MVA pathway which operates
in the cytosol [62]. D-glyceraldehyde-3-phosphate and pyruvate participate as a
substrate in this pathway [63]. This pathway consists of eight steps, which start
with the conversion of G3P and pyruvate to DXS (1-deoxy-D-xylulose-5-phosphate
synthase) and end with the formation of IPP and DMAPP. A total of seven different
enzymes (DXS, DXR—1-deoxy-D-xylulose-5-phosphate reductoisomerase,
MCT—2-C-methyl-D-erythritol 4-phosphate cytidylyl transferase, CMK—4-
(cytidine 5’-diphospho)-2-C-methyl-D-erythritol kinase,
MDS—2-C-methyl-D-erythritol 2,4- cyclodiphosphate synthase,
HDS—4-hydroxy-3-methylbut-2-enyl diphosphate synthase, and
HDR—4-hydroxy-3-methylbut-2-enyl diphosphate reductase) participate in this
pathway. These enzymes are encoded by nuclear genes and guided by N-terminal
transit peptide sequences for their transport into plastids [64]. Out of these
enzymes, three enzymes: DXS, DXR and HDR catalyze the rate-limiting steps of the
pathway. DXS is a thiamin dependent enzyme which catalyzes the first step of this
pathway. In this step, 1-deoxy-D-xylulose-5-phosphate (DXP) is synthesized by the
decarboxylation of pyruvate and subsequent condensation reaction between
resultant and glyeraldehyde-3-phosphate [62]. It is a main rate-limiting enzyme,
its over expression enhance the rate of carotenoid synthesis [20]. Sun and Li
[65] also reported that, protein-protein interaction between DXS and PSY
(phytoene synthase) enzyme also regulates the carotenogenesis. The enzyme DXR
catalyzes the synthesis of 2-C-methyl-D-erythritol 4-phosphate (MEP) by
rearrangement and subsequent reduction of DXP [62]. This step might be considered
as the primary committed step of the MEP pathway. The activity of DXR enzyme can
be inhibited by fosmidomycin. The decreased activity of DXR affects the activity
of downstream enzymes like GGPS (geranylgeranyl phosphate synthase) and
ultimately interrupts the biosynthesis of carotenoids in algae. Du et
al. [59] cloned the cDNA of DXS and DXR genes from Pyropia haitanensis
(red alga). The HDR is another key enzyme, which catalyzes the reductive
dehydration reaction in the final step of the MEP pathway. As a result, HMBPP
(4-Hydroxy-3-methyl-but-2-enyl pyrophosphate) is converted to C
Geranylgeranyl diphosphate (GGPP) is an immediate metabolic predecessor of the carotenoid biosynthesis pathway. Formation of GGPS (geranylgeranyl phosphate synthase) is a three-step process. In the initial step, 10-carbon compound sesquiterpene is synthesized by the addition of one IPP and one DMAPP molecule. In subsequent steps, FPP is synthesized by the addition of one IPP molecule to sesquiterpene and then GGPP is formed by the addition of one more IPP molecule to FPP (farnesyl pyrophosphate) [67, 68]. This process involves three different types of enzymes: GPPS—geranyl diphosphate synthase, FPPS—farnesyl diphosphate synthase, and GGPPS—geranylgeranyl diphosphate synthase, sequentially [65]. Ruiz-Sola et al. [69] reported 12 paralogues genes for GGPPS in Arabidopsis. They also suggested that, out of different GGPPS isozymes, GGPPS11 isozyme behave like a hub isozyme and it interacts with other proteins required for the biosynthesis of carotenoids. These enzymes are rarely studied in algae in comparison to higher plants. Yang et al. [70] cloned and characterized the GGPP synthase gene (PuGGPS) in a red alga Pyropia umbilicalis (Bangiales). They reported that a polypeptide sequence of 345 amino acids with transit peptide sequence (N-terminal) is encoded by this gene. Lao et al. [67] reported that, GGPPS of an alga Haematococcus pluvialis (HpGGPPS) have tri-functional catalytic activities, which catalyzes all three steps of GGPP biosynthesis. Deng et al. [58] cloned and characterize the bfGGPPS from the red alga Bangia fuscopurpurea. They also report that GGPP is the only product of this enzyme and it also interacts with psy gene, which is the rate-limiting enzyme of the carotenoid biosynthesis pathway. Deng et al. [58] also performed the phylogeny analysis and suggest that the red algal and diatoms share a common ancestor for GGPPS but green algae and higher plants show an early divergence of GGPPS during evolution. It has been reported that, the supply of GGPP and its precursors decides the rate and subsequent flux of carotenoid biosynthesis in algae [68].
Biosynthesis of phytoene is the first entry step reaction towards the carotenoid biosynthesis. It is the first colorless carotenoid (40-carbon) compound, which is synthesized by condensation of two GGPP molecules. This reaction is catalyzed by enzyme phytoene synthase (PSY) [65, 71]. PSY is one of the important rate-limiting and key flux controlling enzyme, which decides the pool size of carotenoids. Some studies indicate that several isoforms of PSY exist in different plant species and these are regulated by alternative splicing and protein modifications under the influence of different abiotic and biotic signals [20]. Recently, various genomic and phylogenetic studies are conducted using genomic sequences of some algae belonging to different groups like red algae, green algae, brown algae and diatoms to identify the PSY genes [52]. Tran et al. [72] reported the two orthologous copies of the PSY gene in green algae Micromonas and Ostreococcus. These studies indicate the gene duplication events of the PSY gene during ancient evolution, which produces the two classes of PSY gene in algae. But presently, these two classes, PSY I and PSY II are only retained in members of Prasinophyceae (Chlorophyta). Green algae (other than Prasinophyceae) and higher plants have lost PSY II and reported to have only the PSY I class. In contrast to this, the members of algae belong to Rhodophyta, Heterokontophyta and Haptophyta have only the class PSY II gene [52]. Due to the major flux controlling enzyme of the carotenoid biosynthesis pathway, PSY has been recognized as a major target for metabolic engineering [73]. Recently a novel protein CPSFL1 has been identified, which is bound with phytoene and modulates the accumulation of carotenoids in the chloroplast [74].
Biosynthesis of lycopene is a multistep process in which phytoene is converted
to lycopene via sequential desaturation and isomerization reactions. This whole
process includes four conserved enzymes: PDS, Z-ISO, ZDS and CrtISO [75].
Phytoene, which is synthesized in the previous step, is desaturated to
Lycopene is the first link of carotenogenesis, which allows the biosynthesis of
both
Based on chemical, GSMN and proteomic studies, it has been elucidated that algae
are different in their lycopene cyclase enzyme compositions. Some algae contain
both LCYE and LCYB, while other algae have only one class of LCY. Cui et
al. reported that in green alga (except for C. reinhardtii (LCYE), and
Chlorella sp. NC64A (LCYE), H. pluvialis (LCYB), D.
salina (LCYB),) two distinct LCY (beta- and epsilon-type) enzymes are present,
on the other hand, heterokontophyta have only lycopene beta-cyclase (LCYB) [77].
Recently, Inoue et al. [78] also reported the LCYB activity in brown
alga Undaria pinnatifda. Macrophytic red algae have both LCYB and LCYE
enzymes while microphytic algae have only the enzyme LCYB [52]. Some other
studies also suggested that, synthesis of
In algae, different types of xanthophylls like: lutein, cryptoxanthin,
zeaxanthin, antheraxanthin, violaxanthin, neoxanthin, fucoxanthin,
diadinoxanthin, diatoxanthin, canthaxanthin, astaxanthin etc., are synthesized.
Types and nature of xanthophyll molecules differ in various algal groups. Both
Lutein is a derivative of
Zeaxanthin is a double hydroxylation derivative of
In the case of brown algae some commercially important carotenoids like fucoxanthin and diadinoxanthin are also synthesized. Scientists have proposed two different pathways for the biosynthesis of these carotenoid compounds. According to the first pathway, violaxanthin is converted to neoxanthin and then neoxanthin is used as a precursor molecule for the biosynthesis of both fucoxanthin. An enzyme neoxanthin synthase (NXS) catalyzes the conversion of violaxanthin to neoxanthin in higher plants. In the case of algae, recently, Dautermann et al. [85] reported a new enzyme violaxanthin de-epoxide-like (VDL), which is responsible for the conversion of violaxanthin to neoxanthin. They also reported that VDL is also involved in the synthesis of peridinin and vaucheriaxanthin. According to the second pathway, violaxanthin is used as a precursor for the biosynthesis of diadinoxanthin and then, the diadinoxanthin is converted into the fucoxanthin. The enzymes involved in this process are also still unknown. In some alga, diadinoxanthin can also be converted to diatoxanthin. This reaction is catalyzed by an enzyme de-epoxidase (DDE). This conversion of diadinoxanthin to diatoxanthin can be reversed by an enzyme diatoxanthin epoxide (DEP) under low light intensity. This pathway is called xanthophyll cycle-II. This cycle mainly occurs in members of Chrysophyceae, Bacillariophyceae, Phaeophyceae, and Xanthophyceae [54].
Astaxanthin, is another important carotenoid compound, which has strong
antioxidant activity as compared to vitamin C and E, is synthesized by several
bacteria, fungi, algae and higher plants. In algae several species have been
reported which are involved in de novo synthesis of astaxanthin. The
scientist has identified the two biochemical pathways for the biosynthesis of
astaxanthin. According to the first pathway,
Carotenoids are naturally occurring chemically diverse pigments covering yellow,
orange, red, or dark green color, which are biosynthesized by diverse plants,
fungi, algae, and microorganisms [86]. Carotenoids possess various biological
functions, including light-catching, antioxidant activity, photoprotection from
harmful ionizing radiation and medicinal properties and they are used as
preventives against diseases such as cancer, diabetes, and cataract. Carotenoids
are also used in food supplements, cosmetics, and pharmaceuticals. These
compounds are largely isoprenoid chromophore-bearing polyene pigments having
3–13 conjugate double bonds containing two terminal rings. The presence of
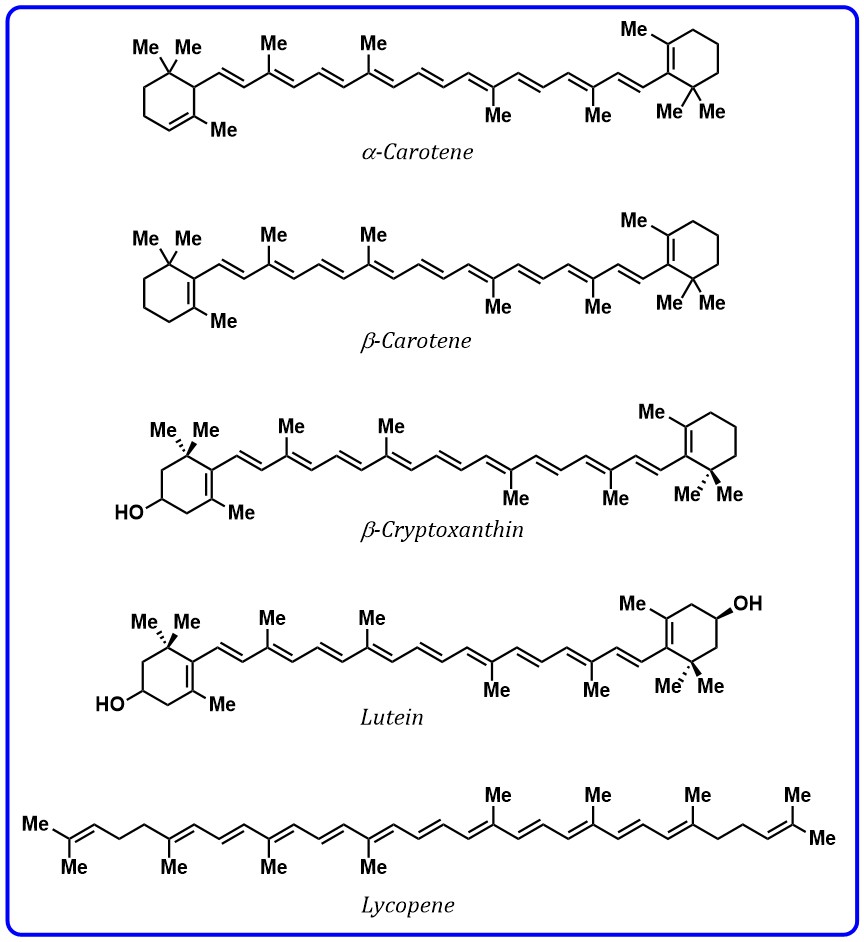 Fig. 2.
Fig. 2.Structures of carotenoids containing polyenes.
The unique spectroscopic property is mainly due to a strong symmetry allowed the
electronic transition from the electronic ground state, S
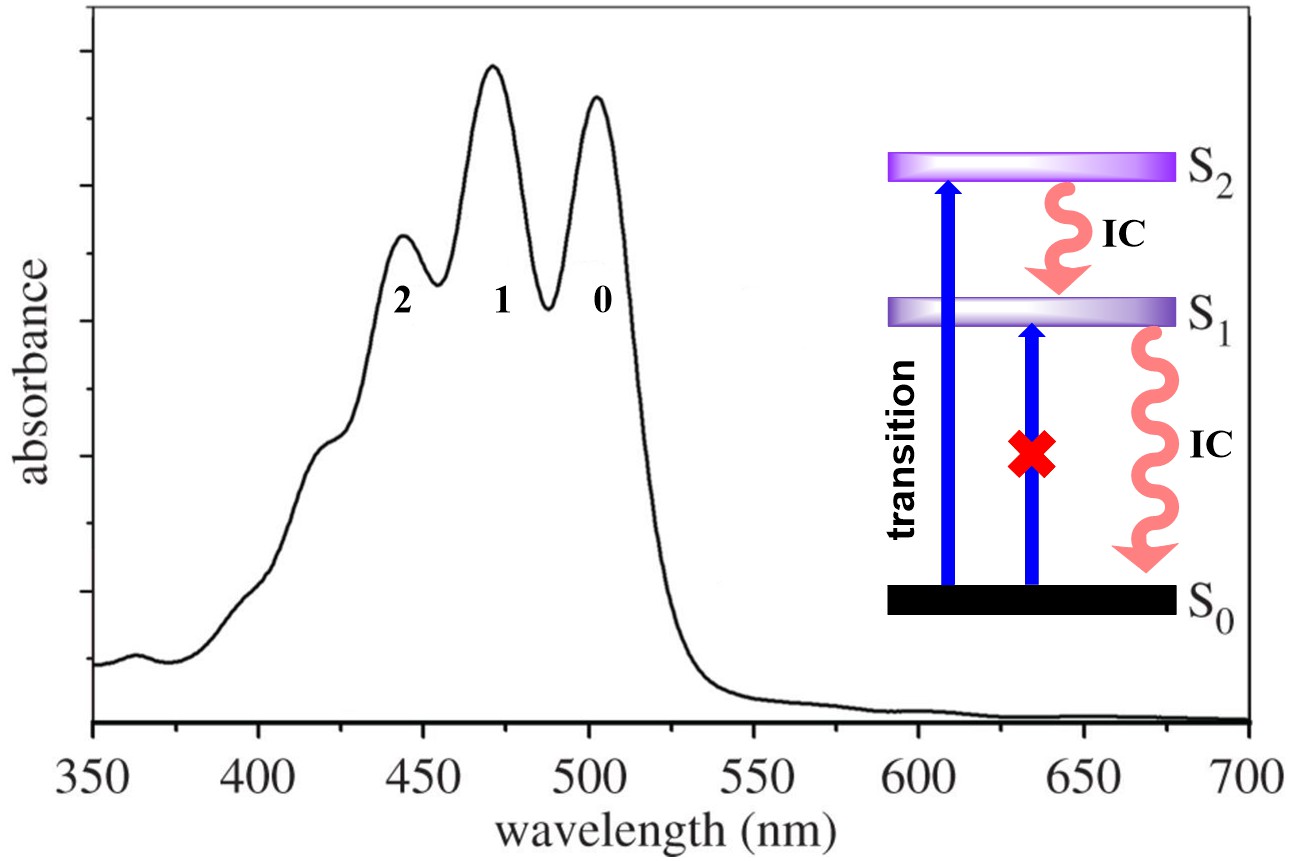 Fig. 3.
Fig. 3.Typical absorption spectrum of lycopene showing absorption spectrum, transitions in singlet state and relaxation of excited state by IC.
Many studies have demonstrated that the energy of the S
Although conjugation length governs most of the spectroscopic properties of
carotenoids, but it was reported that the presence of specific functional groups,
such as conjugated aryl ring, carbonyl group, can significantly affect the energy
of excited states. Aryl ring exhibiting carotenoids such as chlorobactene,
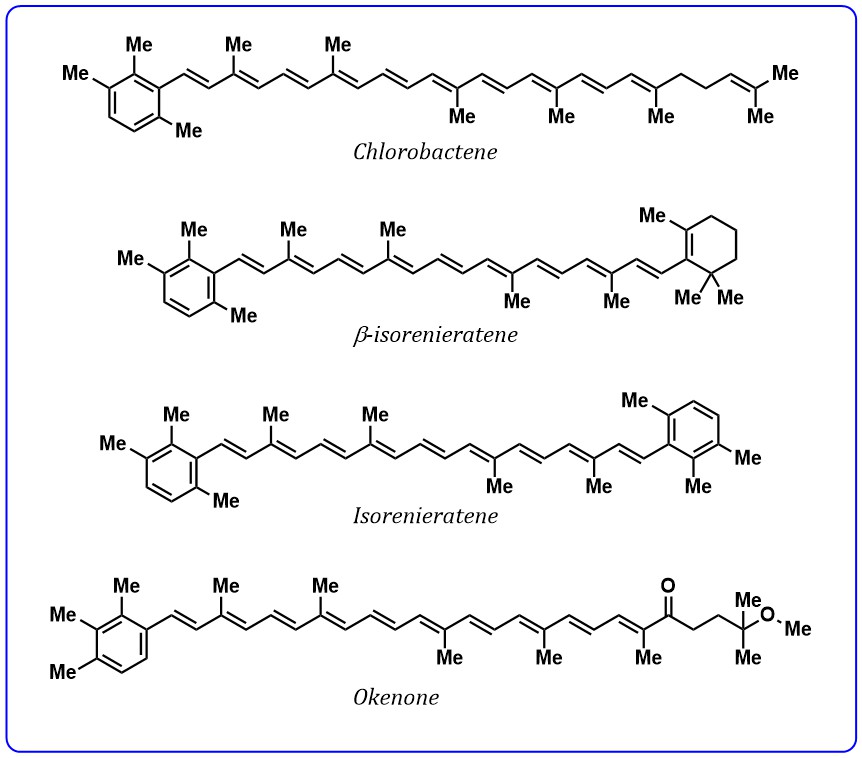 Fig. 4.
Fig. 4.Structures of carotenoids containing aryl ring.
Moreover, carotenoids with a conjugated carbonyl group are widely available pigments in plants and microorganisms and these carotenoids are most abundant in nature. Astaxanthin peridinin, fucoxanthin, and siphonaxanthin (Fig. 5) are carotenoids contain conjugated carbonyl groups found in algae and bacteria. These carotenoids are most studied as light-harvesting agents and excitation energy transfer agents to chlorophylls.
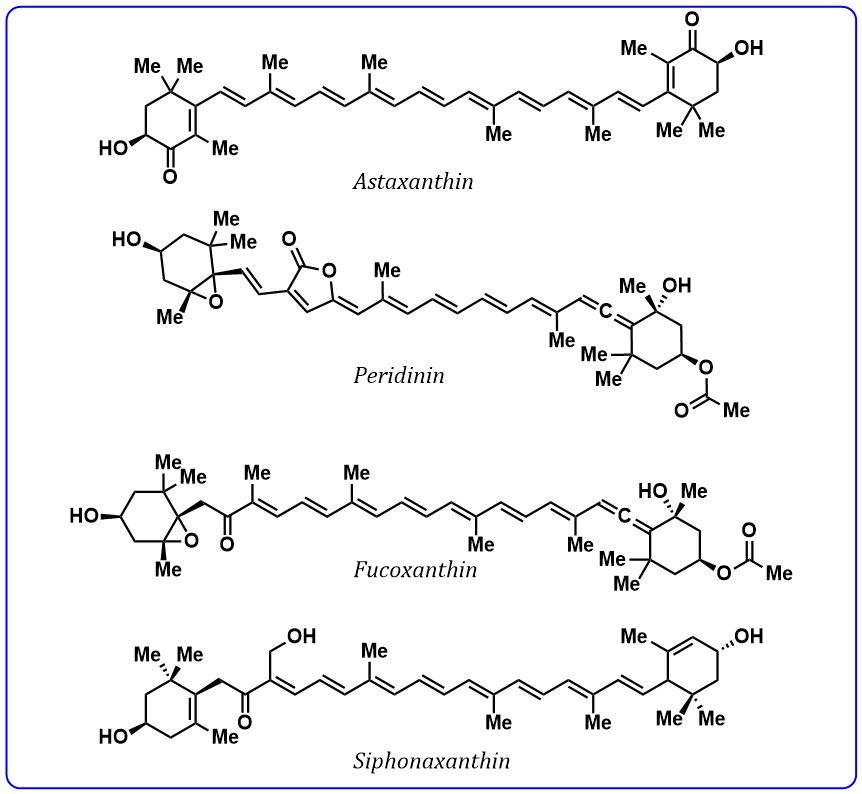 Fig. 5.
Fig. 5.Structures of carotenoids containing conjugated carbonyl group.
Carotenoids play a vital role in food industries as they offer natural color and
flavours to various foods. Carotenoid-derived aroma compounds have been detected
in leaf (tobacco, tea, and mate), essential oils, fruits (grapes, passionfruit,
starfruit, quince, apple, nectarine), vegetables (tomato, melon), spices
(saffron, red pepper), as well as coffee, oak wood, honey, seaweeds, etc. [94].
Degradation of carotenoids leads to different volatile flavor compounds [95].
Carotenoids produce a broad spectrum of aroma compounds (called apocarotenoids)
in plants by oxidative cleavage, giving rise to volatile compounds responsible
for the aroma of flowers, fruits, and leaves, as well as the well-known
phytohormones such as abscisic acid and strigolactones [96]. The important
volatile fragments of carotenoids with a 9–13 carbon skeleton frequently
detected in nature. The important carotenoids-derived aroma compounds are:
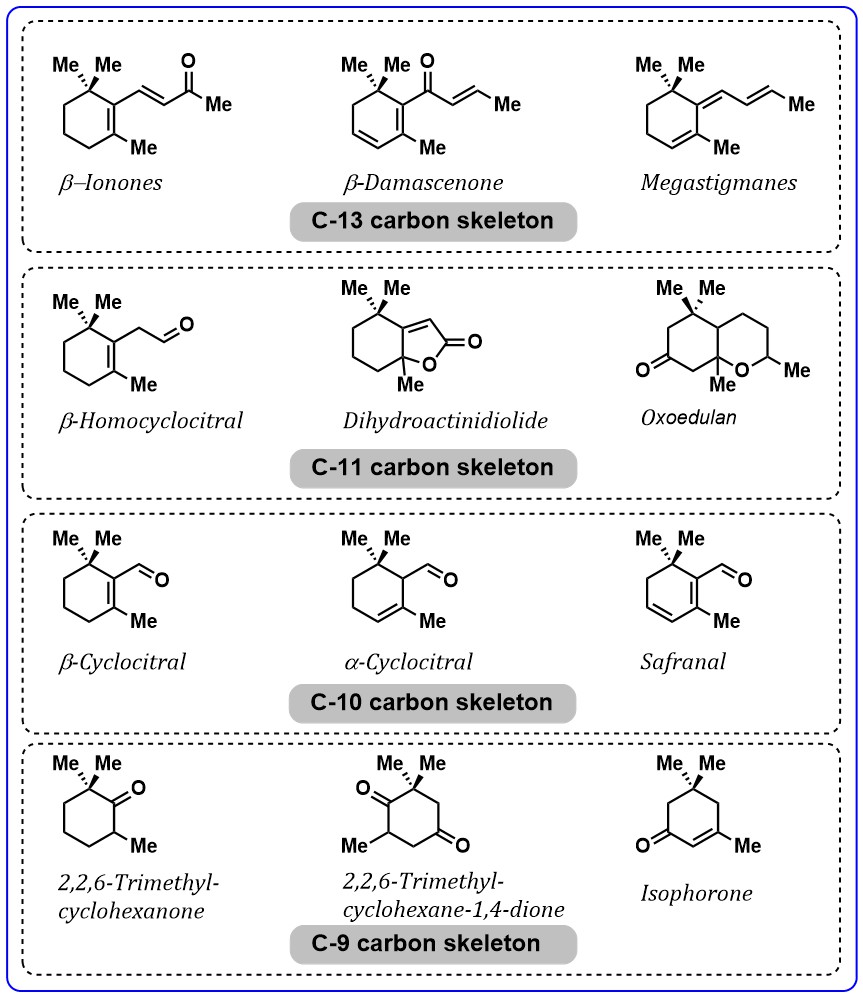 Fig. 6.
Fig. 6.Important aroma compounds related to carotenoid’s degradation.
Carotenoids are extracted from microalgae utilizing conventional solvent extraction methods using organic solvents. Conventional extraction using organic or aqueous solvents depends on the polarity, solubility, and chemical stability of carotenoids to be extracted. Therefore, the selection of a suitable solvent system is necessary which can selectively and efficiently extract carotenoids with high purity. Non-polar solvents (n-hexane, dichloromethane, dimethyl ether, diethyl ether) and polar solvents (acetone, methanol, ethanol, biphasic mixtures of several organic solvents) can be used based on the polarity of the target carotenoid. The use of green solvents (environmentally safe and non-toxic solvents) such as ethanol, limonene, and biphasic mixtures of water and organic solvents has been investigated for the recovery of carotenoids from microalgae. However, the commercial reality of carotenoid extraction from microalgal species is still challenging due to the high cost of production, and usage of enormous amounts of solvents. The uses of non-conventional extraction methods are therefore gaining interest in recent years. These non-conventional extraction methods have several advantages including rapid extraction, low solvent consumption, better recovery, and higher selectivity. These different extraction approaches for various carotenoids along with their relative yield are mentioned in Table 2 (Ref. [97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112]).
| Microalga | Pigment | Technical approach | Carotenoid yield | Reference |
| Haematococcus pluvialis | Astaxanthin | Integrated ultrasound-assisted liquid biphasic flotation | Maximum recovery yield, extraction efficiency, and partition coefficient of astaxanthin were 95.08 ± 3.02%, 99.74 ± 0.05%, and 185.09 ± 4.78, respectively | [97] |
| Haematococcus pluvialis | Astaxanthin | Biocompatible protic ionic liquids-based microwave-assisted liquid-solid extraction | high purity (97.2%) of free astaxanthin was achieved | [98] |
| Haematococcus pluvialis | Astaxanthin | cell permeabilizing ionic liquids | More than 70% | [99] |
| Haematococcus pluvialis | Astaxanthin, lutein, β-carotene and canthaxanthin | Supercritical carbon dioxide extraction | 92% recovery of carotenoids was obtained at the pressure of 300 bar and the temperature of 60 °C, using ethanol as a co-solvent | [100] |
| Haematococcus pluvialis | Astaxanthin and lutein | Supercritical carbon dioxide extraction | 98.6% and 52.3% recovery of astaxanthin and leutin respectively, was achieved at 50 °C and 550 bars | [101] |
| Haematococcus pluvialis | Astaxanthin and lutein | Supercritical carbon dioxide extraction | highest astaxanthin and lutein recoveries were found at 65 °C and 550 bar, with 18.5 mg/g dry weight (92%) astaxanthin and 7.15 mg/g dry weight (93%) lutein | [102] |
| Haematococcus pluvialis | Astaxanthin | Pressurized extraction solvent | extraction yield of 20.7 mg/g dry weight | [103] |
| Chlorella vulgaris | Leutin | Pulse electric field | the concentration of lutein was around 4.5-fold higher when the fresh biomass was previously electroporated at 40 °C by a PEF of 25 kV/cm for 75 μs | [104] |
| Chlorella sorokiniana MB-1 | Leutin | Pre-treated by bead-beating and high-pressure cell disruption methods, followed by harvesting with reduced pressure extraction method | Extraction with tetrahydrofuran as solvent resulted in high lutein recovery efficiencies of 99.5% (40 min) at 850 mbar and 25 °C. In contrast, using ethanol as the solvent, 86.2% lutein recovery was achieved under 450 mbar, 35 °C and 40 min extraction | [105] |
| Tetradesmus obliquus | α-tocopherol, canthaxantin, γ-tocopherol, lutein, phylloquinone, phytofluene, retinol, and menaquinone-7 | supercritical fluid extraction (SFE) | The highest extraction of alpha-tocopherol, gamma-tocopherol, and retinol was achieved at a pressure of 30 MPa and a temperature of 40 °C | [106] |
| Chlorella zofingiensis | Cantaxanthin | High-speed counter-current chromatography (HSCCC) | The recovery of canthaxanthin was 92.3%. Canthaxanthin at 98.7% purity from 150 mg of the crude extract | [107] |
| Phaeodactylum tricornutum | Fucoxanthin | maceration, ultrasound-assisted extraction, soxhlet extraction, and pressurized liquid extraction | ethanol provided the best fucoxanthin extraction yield (15.71 mg/g freeze-dried sample weight). Fucoxanthin content in the extracts produced by the different methods was somewhat constant (15.42–16.51 mg/g freeze-dried sample weight) | [108] |
| Phaeodactylum tricornutum | Fucoxanthin | microwave-assisted treatment | ethanol was preferable for the extraction of fucoxanthin than other solvents in terms of the fucoxanthin yield (ethanol/methanol, 48.01 ± 0.35%; ethanol/acetic acid, 53 ± 0.46%) under the continuous microwave-assisted treatment time of 1 min | [109] |
| Cylindrotheca closterium | Fucoxanthin | Microwave assisted extraction, vaccum microwave assisted extraction and ultrasonic assisted extraction | Extraction yield UAE: 4.95 ± 0.27 mg/g; Rt soaking in acetone for 60 min: 7.48 ± 0.21 mg/g; hot soaking in acetone for 30 min: 9.31 ± 0.44 mg/g; MAE: 8.65 ± 0.29 mg/g; VMAE (vacuum-microwave assisted extraction): 5.25 ± 0.04 mg/g) | [110] |
| Dunaliella salina | Carotenoids (not specified) | Supercritical carbon dioxide | highest carotenoids extraction yield (115.43 mg/g dry algae) was obtained at pressure of 400 bar and temperature of 55 °C | [111] |
| Haematococcus pluvialis | Astaxanthin | Solvent extraction hydrochloric acid pretreatment followed by acetone extraction (HCl-ACE), hexane/isopropanol (6 : 4, v/v) mixture (HEX-IPA), methanol extraction followed by acetone extraction (MET-ACE), and soy-oil extraction | HCl-ACE method yielded the highest astaxanthin content (19.8 ± 1.1%) | [112] |
Microalgal cells are difficult to disrupt due to algaenan and sporopollenin
within their cell wall [113]. Further, conventional techniques used for cell
disruption and extraction methods have low efficiencies. MAE is an efficient
method that takes advantage of microwave irradiation to accelerate the extraction
of a diversity of compounds from natural matrices. MAE generates high-frequency
waves (ranging from 300 MHz to 300 GHz) with wavelengths of 1 mm to 1 m.
Microwave radiation when applied at a frequency near 2.45 GHz causes vibration of
polar molecules resulting in inter and intra-molecular friction. The friction,
together with the movement and collision of a large number of charged ions,
results in the rapid heating (within few seconds) of the matrix. Intracellular
heating leads to the breakdown of cell walls and membranes and therefore there is
a faster transfer of the compounds from the cells into the extracting solvent.
There are two major types of microwaves; closed and open vessels. In open vessels
microwave application is performed at atmospheric pressure while in closed
vessels, samples are irradiated by microwave under controlled pressure and
temperature. The extraction temperature depends on the polarity of the solvent.
Solvents with higher dielectric constant (
Ultrasonic assisted extraction is based on ultrasonic cavitation. Ultrasonic
extraction has been used to extract bioactive compounds like vitamins,
polyphenols, pigments and other phytochemicals. UAE is cost-effective and
significantly reduces the extraction time, whilst resulting in increased
extraction yields. The ultrasound can be divided into two distinct categories:
low intensity-high frequency (100 kHz–1 MHz) and high intensity-low frequency
(20–100 kHz). Ultrasonic extraction is achieved with high intensity and
low-frequency ultrasound waves. Ultrasound waves when traveling through liquid
creates alternating high-pressure and low-pressure cycles resulting in the
production of cavitation bubbles in the solvent. Cavitation bubbles form in the
liquid during the expansion phase. The ability to cause cavitation depends on the
frequency of the ultrasound wave, the solvent properties, and the extraction
conditions. During the compression cycle cavitation bubble implodes on the
surface of the matrix (cell, tissue or any particle) and a high-speed micro-jet
is created leading to the generation of effects like surface peeling, particle
breakdown, sonoporation and cell disruption. Sonoporation (perforation in cell
walls and membranes) exerts a mechanical effect, allowing greater penetration of
solvent into the sample matrix. This leads to increased extraction efficiency in
less time. Using ultrasound-assisted extraction, 4.66 mg
Electrotechnologies, such as pulsed electric field (PEP), moderate electric
field (MEF), high-voltage electric discharges (HVED) are emerging, non-thermal,
and green extraction techniques for targeting intracellular compounds from
bio-suspensions. In the pulsed electric field (PEP), the sample matrix is exposed
to repetitive electric frequencies (Hz–kHz) with an intense (0.1–80 kV/cm)
electric field for very short periods (from several nanoseconds to several
milliseconds). In the moderate electric field (MEF), the sample matrix is exposed
to low electric fields (between 1 and 1000 V/cm) with electric frequencies in the
range of Hz up to tens of kHz. While high-voltage electric discharges (HVED)
typically have 40–60 kV/cm for 2–5
The main purpose of using PLE is that it allows rapid extraction and reduces
solvent consumption; therefore, it is sometimes referred to as accelerated
solvent extraction. PLE involves the extraction using solvents at elevated
temperature and pressure but always below their critical points. This normally
falls in the ranges of 50–200
SFE involves extraction using supercritical fluids i.e., fluids at a temperature
and pressure above its critical limit. Supercritical fluids provide better
solvating and transport properties than liquids due to their low viscosity and
high diffusivity. For the selective extraction of a broad range of compounds, the
solvating power of supercritical fluid can be adjusted by manipulating the
temperature and pressure of the fluid. Carbon dioxide is the preferable solvent
which can easily achieve supercritical conditions and has benefits like high
purity, low toxicity and low flammability compared to other fluids. Supercritical
carbon dioxide is non-polar and its polarity can be modified by using
co-solvents. Besides CO
Subcritical fluid extraction is similar to SFE, where subcritical (liquefied)
fluids are used as extraction solvent. Subcritical fluid extraction works at
relatively low temperature and pressure than supercritical fluid extraction. In
various studies, subcritical CO
Microalgal cells are difficult to disrupt, therefore, a physical or enzymatic
pre-treatment before extraction can be opted to promote the recovery of
carotenoids. High-pressure homogenization (HPH) is one such method, where, cell
disruption is achieved by applying high intensity fluid stress (50–400 MPa). In
comparison with other physical milling processes, it offers significant
advantages such as ease of operation, commercial applicability, reproducibility
and high throughput. High-pressure homogenization (HPH) found to be very
effective in microalgae with a recalcitrant cell wall such as
Nannochloropsis [130]. Cell disruption by high-pressure homogenization
has been shown to increase carotenoid and
Non-photosynthetic organisms (humans and animals) are unable to synthesize carotenoid. However, they intake them through their food metabolize them for normal physiological functions [133]. Carotenoid possess boundless and expansive applications and therefore have commercial significance. Further, health is paramount to the consumer, which triggers the large-scale production of these pigments. In this section application of different carotenoid is discussed along with their commercial implications.
Astaxanthin is generally known as ‘Super Vit-E’ due to its compelling antioxidant properties [134]. Kishimoto et al. [135] bestowed it with ‘King of antioxidants’ because of its better antioxidant properties as it emulates Vit C, resveratrol, CoQ10, green tea catechins, and Vit E. Lipid peroxidation, LDL oxidation, and peroxide-induced cytotoxicity, etc. are inhibited by astaxanthin [136]. Their roles as anti-inflammatory, hepatoprotector and protective influence on retinal, brain, and spinal cord neurons and in the prevention of cardiovascular disease have been reported [137]. Aquaculture feed; poultry and food industries use this tremendously as red colorant [138]. Astaxanthin favorably lowers the level of plasma reactive protein C, down regulate inflammatory cytokines, modulate mitogen-induced lymphoproliferation, and boost the immune response [139]. It protects the skin from the detrimental effects of UV-A photo ageing and is therefore widely used in cosmetics [140]. The inability of astaxanthin to convert into Vit A in the human body safeguards from hypervitaminosis A toxicity even after its overconsumption [141].
The role of
Fucoxanthin has well-marked use as antioxidant, anti-angiogenic,
anti-inflammatory, photo- and neuroprotective, which makes it commercially
valuable. Peng et al. [145] reported its role in hindering DNA damage
and H
The role of zeaxanthin in ophthalmological fields is significant and crucial. It is naturally present in the central macula of the eye, and protects the visionary organ from blue, near UV radiations, age-related macular degeneration [149]. Its promising property as natural color mark it use in pigmentation of poultry and fishes, in food, and as an antioxidant in cosmetics [44]. Regular consumption of zeaxanthin might prove useful in avoiding lung and pancreatic cancers in diabetic patients and found to be effective in cardiovascular problems [150]. This xanthophyll has the ability to trigger apoptosis in melanoma cells and therefore useful in adjuvant therapy.
Lycopene has antioxidant, photo-protective properties. It proves to be anti-cancerous against cell lines from the human colon, breast, prostate, liver, and lymphocytes [151]. It prevents from early arthrosclerosis and high blood pressure problems by boosting endothelial functioning and lowering oxidative stress. Lycopene, also act as hypolipidemic in a way similar to statin and maintain blood cholesterol levels [152]. The sufficient daily doses of lycopene strengthen the skeleton system, enhance gap junction intercellular communications, and maintain glucose homeostasis, which in turn prevent type-2 diabetes. Viuda-Martos et al. [151] reported lycopene-induced inhibition of tumor metastasis because of modulated cell cycle progressions. The poultry farms use this as feed to improve the health of poultry birds and it also relieves heat stress in commercial poultry. Lycopene is commercially available in form of capsules, tablets, and in gel forms. It has profound role in cosmetics and coloring agent in food sectors.
Marigold is the chief source of lutein production at the industrial level. Like zeaxanthin, it protects eye from pernicious UV, blue light and therefore, referred to as eye vitamin. It lowers the level of plasma factor D and in turn plays a defensive role against age-related macular degeneration and cataract. It is effectual as a chemotherapeutic agent [153]. It is an active anti-tumor substance against prostate, breast, and colon cancer. Lutein consumption can also lessen the chance of early atherosclerosis and lung cancer as well. It alleviates the effects of neurodegenerative disorders arise due to inflammation. It has prodigious use in poultry as yolk color enhancer, feather coloration through feed and as an additive in infant food, drug and cosmetics [154].
Canthaxanthin is a
The global monetary value of carotenoids depends upon its demand by the consumer
and its extraction cost. Major branches of the carotenoid market include feed,
food, nutraceutical, pharmaceutical, cosmetic, and aquaculture sectors [154]. An
amount of USD 1.5 billion market was expected at the global level but with
compelling demand it is expected to surge to USD 2 billion by 2022 with a
compound annual growth rate (CAGR) of 5.7%. Astaxanthin,
There are several desperate elements and factors, which govern the carotenoid market. From production point of view, the revamping of the selected entity depends upon their cultural conditions i.e., temperature, irradiance, pH, salinity, nutrients, and presence of oxidizing substances. Secondly, as consumers gaining interest and awareness to have an immune diet rather than muscle full supplement and balanced consumption is imperative in governing the carotenoid market and foster the overall demand and industrial production. The global carotenoid market estimates the major market value for the feed segment by 2023 because of less restricted regulations. Additionally, an increase in meat, poultry, and dairy products will accelerate the rise of the feed market. Feed segment is going to witness colossal demand because of a pandemic outbreak, animal diseases, and the requirement of quick-service restaurants both at the domestic and international level. The investment and participation of well-known cosmetic industries like Hindustan Unilever, L’Oreal, Henkel, and Beiersdorf may be lucrative for the growth of the European carotenoid market [9]. The high commercial demand and potential values inflate the market as per the demand and supply curve. Deficiency of raw material, efficient tools, and methods both for up streaming (extraction) and for the down streaming process (purification) attribute to high monetary value [158]. The presence of another compound along with required under amount substance make the large-scale production technically difficult and costly. Besides, legal and strict regulatory and approval checklist related to the health care issues, effect on environment and animals, constraint the market of carotenoids. The build-out of the carotenoid market and its constituent products depends on several ethical, social, commercial, and biotechnical factors. Biotechnologists are endeavouring for the burgeoning of production scale by developing green, renewable resources, efficacious technologies and companies are trying to propitiate consumers by sophisticated presentation and sale of their products. With the growing exigency for natural products and declining traditional resources, key commercial entities are investing in carotenoid production at a large scale and augmenting the carotenoid market competitiveness. The pre-eminent producers of carotenoid are listed in Table 3 along with their web address.
| S. No. | Name of company | Website address |
| 1 | Algae technologies | https://www.algatech.com |
| 2 | Mera pharmaceuticals | http://merapharma.com |
| 3 | Cyanotech corporation | Cyanotech.com |
| 4 | Valensa international | https://valensa.com |
| 5 | Fuji chemical industries Co, Ltd. | ww.fujichemical.co.jp |
| 6 | Nature beta technologies | www.nikken-miho.com |
| 7 | AlgaNova international | http://www.algeanova.com |
| 8 | Leili natutral products Co, Ltd. | www.leili.com |
| 9 | Koninklijke DSM NV | www.dsm.com |
| 10 | Sensient technologies corporation | https://www.sensient.com |
| 11 | Market young | https://www.youngsmarket.com |
| 12 | Novus international, Inc. | http://www.novusint.com |
| 13 | Seambiotic | www.seambitotic.com |
| 14 | Tanjin norland biotech Co, Ltd. | www.norlandbiotech.com |
| 15 | Jingzhou natural astaxanthin Inc. | www.jzxqs.cn |
| 16 | Nikken sohonsha corporation | www.nikken-miho.com |
| 17 | Lycored Ltd. | https://www.lycored.com |
| 18 | Divis laboratories | https://www.divislabs.com |
| 19 | FMC corporation | www.fmc.com |
| 20 | Naturex SA | https://www.naturex.com |
| 21 | Kemin industries Inc. | https://www.kemin.com |
| 22 | E.I.D parry Ltd. | https://www.eidparry.com |
| 23 | Brenntag | https://wwwbrenntag.com |
| 24 | D.D williamson and Co. Inc. | https://ddwcolor.com |
| 25 | Dohler group | https://www.doehler.com |
| 26 | ExcelVite SDN | https://www.excelvite.com |
| 27 | Cognis nutrition and health | www.na.cognis.com |
| 28 | Allied biotech | www.altratene.com |
| 29 | BASF corporation | https://www.basf.com |
| 30 | Omniactive | Omniactives.com |
| 31 | Chrysantis | www.chrysantis.com |
In this paper, the biosynthesis of carotenoids is discussed with special reference to algae. This discussion has brought to the notice that many genes are not yet characterized. A consensus pathway has been however drawn in this paper. This paper can be used as a framework to look in to the future research scope for more investigation into the carotenogenesis in algae. Molecular characterization of genes is essential for metabolic engineering of algae for enhanced production of carotenoids. Different extraction approaches revealed that a single method is not sufficient and different customized approaches are required for the extraction of different pigments. Increasing knowledge of the beneficial use of carotenoids has led to wider application and increase in the global market and there is still a huge potential for market growth in the future.
AK, VV and H conceptualize this review article. AKG, KS, KM and MM wrote the original draft. PKB wrote and interpreted about chemistry of different carotenoids. AK, VV and H edited the manuscript and prepared the tables. All authors read and approved the final manuscript.
Not applicable.
The authors are grateful Head, Department of Botany, Mohanlal Sukhadia University, Udaipur for providing necessary laboratory facility.
Part of this work is supported by a grant from the Department of Science and Technology, New Delhi (SERB File Number: EEQ/2020/000011). VV is thankful to DST-Nanomission (Govt. of India) project number (SR/NM/NT-1090/2014(G) for financial support.
The authors declare no conflicts of interest.
AACT, acetoacetyl-CoA thiolase; ABA, abscisic acid; BCH, β-carotene hydroxylase; BKT, β- carotene ketolase; CAGR, Compound annual growth rate; CMK, 4- (cytidine 5ʼ-diphospho)-2-C-methyl-D-erythritol kinase; Crt-ISO, carotenoid isomerase; DMAPP, dimethylallyl diphosphate; DXP, 1-deoxy-D-xylulose 5-phosphate; DXR, 1-deoxy-D-xylulose-5-phosphate reductoisomeras; DXS, 1-deoxy-D-xylulose-5-phosphate synthase; EHY, ε-carotene hydroxylase; ESTs, expressed sequence tags; FPPS, farnesyl diphosphate synthase; GGPP, geranyl geranyl pyrophosphate; GGPPS, geranylgeranyl diphosphate synthase; GPPS, geranyl diphosphate synthase; GSMN, genome scale metabolic network; HDR, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase; HDS, 4-hydroxy-3-methylbut-2-enyl diphosphate synthase; HMG-CoA, β-Hydroxy β-methylglutaryl-CoA; HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; HMGS, 3-hydroxy-3-methylglutaryl-CoA synthase; IDI, isopentenyl diphosphate isomerase; IPP, isopentenyl diphosphate; LCYB, lycopene β-cyclase; LCYE, lycopene ε-cyclase; MCT, 2-C-methyl-D-erythritol 4-phosphate cytidylyl transferase; MDS, 2-C-methyl-D-erythritol 2,4- cyclodiphosphate synthase; MEP, methyl erythritol phosphate; MVA, mevalonate; MVD, mevalonate diphosphate decarboxylase; MVK, mevalonate kinase; NXS, neoxanthin synthase; PDS, phytoene desaturase; PMK, phosphomevalonate kinase; PSY, phytoene synthase; SL, strigolactone; VDE, violaxanthin de-epoxidase; ZDS, ζ-carotene desaturase; ZEP, zeaxanthin epoxidase; Z-ISO, ζ-carotene isomerase.






