Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Behavioral Medicine, College of Medicine and Health Sciences, Sultan Qaboos University, Al-Khoud 123, Muscat, Oman
2 Ageing and Dementia Research Group, Sultan Qaboos University, Muscat, Oman
3 Department of Food Science and Nutrition, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Oman
4 Research and Policy Department, World Innovation Summit for Health (WISH), Qatar Foundation, P.O. Box 5825, Doha, Qatar
Abstract
Vitamin D deficiency has been estimated to affect roughly 30% to 50% of the global population and thus labeled as a silent pandemic. In addition to its role in skeletal and calcium homeostasis, vitamin D has been implicated in brain functioning across both preclinical research and human populations studies. These findings have also been extended to various neurodevelopmental and neuropsychiatric conditions. Furthermore, these individuals tend to display diminishing cognition symptoms. In this regard, this review is dedicated to address the relationship between vitamin D and dementia, mood disorders, and the various neuropsychological disorders of psychosis. The review takes both preclinical and clinical studies into consideration. While there are many literature suggesting the critical role of vitamins in cognition on the above said diseases, it is still premature to unequivocally postulate the role of vitamin D on cognitive symptoms. Further research is necessary to establish this association, including the need to increase the ecological validity of animal models, delineating the core cognitive symptoms associated with the disorders, and establishing the optimal source of vitamin D consumption.
Keywords
- Vitamin D
- Brain health
- Brain development
- CNS
- Neurological disorders
- Dementia
- Cognitive impairment
- Neuropsychological impairment
- Depression disorders
- Neuropsychological functioning
- Psychosis
- Schizophrenia
- Attention-deficit hyperactivity disorder
- ADHD
- Review
Vitamin D is a nutrient known to be critically involved in the metabolism of calcium and phosphate in living organisms (1). A primary goal of vitamin D is to ensure calcium assimilation by the body (2). Due to its crucial role in maintaining basic organism structure, i.e., musculoskeletal health, vitamin D is one of the few nutrients whose receptors are universally present throughout the body, including the brain (3). With regard to illness, there have been a number of studies linking an optimum amount of 20 mg/ml of vitamin D intake to overall health and wellbeing (4). A lower concentration of vitamin (e.g., < 20 ng/ml) has generally been considered to be suboptimal. However, these arguments may be harder to see through as there are dissenting views on what constitutes the optimal level of vitamin D (5).
In order to lay the structured groundwork for such synthesis, this review will initially focus on exploring the overall literature. We then review emerging evidence of the relationship between vitamin D and various neuropsychological impairments across four areas: dementia, major depressive disorders, psychosis and attention-deficit hyperactivity disorder (ADHD). The review will take into consideration both preclinical and clinical studies.
Due to the widespread distribution of vitamin D receptors throughout the brain, McCann and Ames (6) suggest consequent evidence for vitamin D's involvement in brain function and its ability to affect proteins in the brain known to be directly involved in learning and memory, maternal and social behavior and motor control. Through a vitamin D receptor (VDR), this vitamin is also known to regulate gene transcription. A specific nucleotide sequence in DNA binds together with VDR and between 200-2000 genes are regulated through this process thereby affecting the expression of genes having a variety of biological functions. Neurotrophic growth factor (NGF) and brain derived neurotrophic factor (BDNF) are two of the gene products that have specific relevance to cognitive and behavioral functions impacted by vitamin D. NGF, present mainly in the hippocampus and neocortex, enhances neurotransmission and has been suggested to be critically involved in memory and executive functioning. There is also the case of BDNF that affects the survival and differentiation of dopamine cells. Dopaminergic neurotransmissions have been hypothesized to be strongly associated with expression of many brain diseases (7).
There is also increasing evidence of the relationship between thyroid hormones and vitamin D, particularly glucocorticoids and androgens (7). Throughout the brain and spinal fluid, vitamin D potentiates the role of neurosteroids (7, 8). Vitamin D is a nuclear steroid transcription regulator and employs transcriptional control over a large number of genes. Exerting its influence over a vast number of genes, all of these agents have well-defined and intricate roles to play in brain development and biological function.
Several vitamin D metabolites are present in the brain and are present in specifically large numbers in the substantia nigra and hypothalamus. Vitamin D metabolites 25-hydroxyvitamin D3, 1,25-dihydroxyvitamin D3, and 24,25-dihydroxyvitamin D – are found in the cerebral spinal fluid and have the ability to cross the blood brain barrier. Both the hypothalamus and substantia nigra are responsible for motor functions and the presence of the vitamin D metabolites in these areas suggest that vitamin D is metabolized locally in the central nervous system.
Vitamin D receptors are found in the brain and are mostly seen in the cerebellum, basal ganglia, hypothalamus, thalamus, and hippocampus. Substantia niagra, one of the primary areas for dopamine production has the highest density of vitamin D receptor. The external granule cell layer of the prefrontal cortex and the hypothalamus (supra optic and paraventricular nuclei) have a significant portion of the receptors located in them. Two types of pathways – genomic and non-genomic – affect vitamin D functioning through: (i) a member of the steroid/thyroid hormone superfamily of transcription factors, the vitamin D receptor (VDR), acting in the nucleus and promoting protein synthesis, and (ii) the MARRS (membrane associated, rapid response steroid binding) receptor, also known as Erp57/Grp58 (9). The human brain has VDR and the enzyme associated with the synthesis of the active form of the hormone 1α-hydroxylase (CYP27B1). Vitamin D and its metabolites play a major role in the central nervous system (CNS) by actively being involved in the process of neuro-transmission and neuroplasticity and they can be metabolized and regulated in the active form in the CNS itself. Overall, there is increasing evidence that the vitamin D receptor is present in most cells and organs. All of them are able to produce 1,25-dihydroxyvitamin D and 24,25-dihydroxyvitamin D. They influence a large number of biological pathways and in turn are capable of regulating a wide variety of genes that have important functions in regulating cell growth (5). Vitamin D has an influence on neurogenesis and the expression of neurotrophic factors, detoxification and amyloid beta (Aβ) clearance, thereby creating a neuroprotective effect (6). There is a strong evidence to suggest that a diet deficient in vitamin D combined with inadequate sun exposure has potential to trigger many conditions, not only the well-known such as rickets and osteomalacia but also neurocognitive disorder, Amyotrophic Lateral Sclerosis (ALS), Alzheimer’s Disease (AD) and Parkinson’s Disease (PD). However, there is contradictory information regarding the implication of low vitamin D in health parameters (10). A large number of genes (0.8–5% of the total genome) is regulated directly and/or indirectly by vitamin D and is involved in DNA repair, a variety of cellular functions which involve growth regulation, metabolism, differentiation, apoptosis, membrane transport, cell adhesion, and oxidative stress (11), and an imbalance in vitamin D homeostasis can affect its functioning in neuroplasticity, and neuroprotection. Developmental vitamin D (DVD) deficiency can lead to dysregulation of 36 brain proteins involved in several biological pathways including oxidative phosphorylation, redox balance, cytoskeleton maintenance, calcium homeostasis, chaperoning, synaptic plasticity and neurotransmission (12).
Vitamin D plays a diverse role in the CNS and the integrity of CNS functioning. Studies have shown that deficiency of vitamin D has been suggested to be critically involved in the pathogenesis of many neurobehavioral disorders. Vitamin D and its potential role in CNS functioning and its deficiency can lead to a risk of neurodevelopmental and neurocognitive disarray. The aforementioned connexion between the CNS and integrity of vitamin D suggests that some of the common neuropsychiatric disorders are marked with dysfunctional vitamin D activity. In this regard, sections were dedicated to address the relationship between vitamin D and dementia, mood disorders and the various neuropsychological disorders of psychosis.
Aside from the numerous musculoskeletal, cardiovascular, and fertility and pregnancy-related complications, low serum 25-hydroxyvitamin D has recently been shown to impact and lead to impairment in ‘higher functioning’. These include those conditions that represent an impairment in brain and behaviour that manifest as cognitive impairments (13). Among the variety of conditions linked to deficits in vitamin D is dementia. There is currently an abundance of evidence, most significantly meta-analyses and systematic reviews that have advanced the view that dementia and deficiency in vitamin D have a temporal relationship (14). Since dementia is a condition characterized by a spectrum of neurobehavioral impairments, it is not clear whether the lower level of vitamin D has any bearing on the integrity of neuropsychological functioning. In order to fill gaps in the existing literature, the following sections examines the link between vitamin D and cognitive symptom.
What was once labelled as dementia has now metamorphosed into ‘major neurocognitive disorder’. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (15), diagnosis of a neurocognitive disorder warrants the presence of “significant cognitive decline from a previous level of performance in one or more of the cognitive domains including complex attention (sustained attention, divided attention, selective attention) and information processing speed, executive function (planning, decision making, working memory, responding to feedback, inhibition and mental flexibility), learning and remembering (memory), efficiency of language, among other impairment of cognitive domains, visual-spatial ability.” (15). As often is the case, the presence of a major cognitive disorder would lead to the impairment of self-directed behavior, quality of life (QoL) and meaningful existence (16). For the purpose of brevity, and since literature is yet to fully adopt the concept of major neurocognitive disorder, this review will employ the term “dementia” to encapsulate ‘major neurocognitive disorder’ as defined by DSM-5 (15).
Dementia often affects senior citizens but there are ‘atypical types’ of dementias as well (17). This “clinical syndrome” has been shown to commonly manifest as acquired loss of intellectual ability, impaired short-term memory, and sometimes the presence of aphasia, apraxia, and agnosia. Other deficits included in the DSM-5 are also common in dementia (15). The epidemic of dementia is associated with decreased life expectancy, lifestyle challenges and widespread factors with the potential to ‘insult’ the brain (18). Prolonged exposure to such agents has been suggested to set neuropathological processes that lead to the development of dementia into motion (19). Sadock, Sadock & Ruiz (20) have described factors that precede the development of dementia including degenerative processes, psychiatric issues such as cognitive decline associated with major depressive disorder, acquired brain injuries, tumours and the pressure they exert on the brain. Other factors include aberrant cardiovascular activities and anoxic events. Some infections such as HIV/AIDS have also been postulated to trigger the development of dementia.
The epidemiology of the varied spectrum of dementia has been suggested to qualify as a ‘silent epidemic’ with a new case of dementia being diagnosed every 4 seconds, i.e., 7.7 million cases per year (21). Despite its wide prevalence, if the type of dementia is not reversible, then it is likely to be classified as one of the neuro-progressive types. The currently available treatment strategies work only as symptomatic and till now there is no ‘cure’ for dementia, hence various alternative and complementary ‘medicines’ are often used to help people with dementia. Recently, there have been interest in role of vitamins in dementia. In the following paragraph, studies that have examined the efficacy of vitamin D are reviewed. Although dementia is often perceived to be neuroprogressive/neurodegenerative in nature (22), it is not necessarily progressive (23). A common misconception regarding dementia is the view that dementia is a disease in itself. There is no empirical support, however, to substantiate such a view (24). As defined by the Alzheimer’s Society (25), “dementia is an overall term that describes a group of symptoms associated with a decline in memory or other thinking skills severe enough to reduce a person's ability to perform everyday activities”. One of the most commonly diagnosed diseases under this banner of symptoms is Alzheimer’s disease (20). Aside from this, there are many other types/causes of dementia that have recently been operationalized as major and minor neurocognitive disorders. These, in turn, are sometimes labelled in terms of their location in the brain, etiological factors, natural history or mode of expressions including vascular dementia, lewy body dementia, and other subcortical and focal dementias (26).
Although the impact of this cognitive disorder may take full effect only in the later stages of one’s life, the proper age of onset remains elusive. Burns & Iliffe (27) have highlighted a few major reasons for this. Firstly, “early recognition is not easy because of the insidious and variable onset of the syndrome, which emerges through the personality of the individual” (p. 405). Secondly, be it practitioners or family members, most people are apprehensive of diagnosing/reporting dementia due to its “largely unmodifiable nature” moreover, a certain social stigma that still exists around it (22) (p. 405). Borrowing from this, practitioners have also discouraged discussion of such diagnoses fearing potential triggering of patients’ reactions of depression, denial or even withdrawal from services altogether.
In the prodromal phases of dementia, the neuropsychiatric syndrome has been speculated to include disturbing emotions that manifest as dysphoria, impaired impulse control, negativistic behavior and disturbed wake cycles (28). Pseudo-dementia of depression is a psychiatric condition where there is a cardinal appearance of the presence of dementia (29). Longitudinal studies have indicated that pseudo-dementia tends to increase the development of irreversible dementia (30). In addition to neuropsychiatric symptoms, core features of the prodromal symptoms of cognitive decline have been suggested (31). A breakthrough in the study of patients with dementia opened a new avenue for potential early diagnosis: studying the process of cognitive decline. Cognitive decline refers to the gradual impairment of cognitive functions and abilities with age. Understanding the concept of cognitive decline has enabled the advancement of research on the identification of idiopathic, young-onset AD or atypical dementia often seen to occur among those not viewed as ‘senior citizens.’ Sometimes, if the cognitive disorder occurred as early as in their 40s, they are labelled atypical (32).
Despite the fact that dementia has been documented for over two centuries, there is no biological marker for the identification of the presence of cognitive decline. As of today, ‘bedside’ and neuropsychological testing are the only avenues available for the identification of the presence of cognitive decline. Most of the existing measures to detect the presence of dementia have been marred with confounders including intellectual capacity, premorbid education, effective state and language proficiency. These variables tend to inflate or deflate the score on most available instruments to quantify the presence of dementia (33). Furthermore, there are controversies about what constitutes cognitive decline and how it can be identified accurately. Some studies have advanced the view that memory impairment is the first fallout among people with cognitive decline. This view constitutes the memory hypothesis (34). On the other hand, there are suggestions that prodromal signs of dementia consist of impairment in executive functioning (35). However, from the neuropsychological perspective, it is difficult to disentangle the links between memory and executive functions as there are major overlaps between these highly interrelated cognitive domains (36).
Once the existence of cognitive decline is suspected, the next step is to then observe whether the affected individual is undergoing rapid decline or, conversely, if the observed cognitive impairment has plateaued. Ideally, the diagnosis of dementia should be confirmed via brain biopsy, but such an approach is deemed too invasive and only research-related, post-mortem examinations have been considered thus far (37). Neuroimaging data such as those deciphering functional activity (Single-Photon Emission Computed Tomography-SPECT, Positron Emission Tomography-PET, Functional Magnetic Resonance Imaging-fMRI) and structural changes in the brain (Magnetic Resonance Imaging-MRI, Computed Tomography-CT) are other ways of diagnosing dementia. The problems with such an undertaking are that the neural substrate of dementia has not been agreed upon yet (38). It has often been found that significant pathological changes in the brain like atrophy might be benign or simply a manifestation of age-related brain changes (39). The presence of amyloid plaques and neurofibrillary tangles have been advanced as unique neuropathology of the brain tissue of those with Alzheimer’s disease (40). However, recent evidence has dampened such enthusiasm that these plaques and tangles are ‘key players’ in the neuropathology of one of the cognitive disorders, namely Alzheimer’s disease (41). Studies now suggest that, if anything, amyloid plaques and neurofibrillary tangles might simply be ‘innocent bystanders’ when understanding the complex pathology of dementia (42).
One of the greatest challenges of this syndrome has been supporting both patients and caretakers. While there are a number of drug-based interventions that can be undertaken to manage dementia, ‘medicalization’ of dementia has not yielded expected results (43). Hence emerged the turn to more humanistic approaches to intervention: the main aim being, shifting perspectives from the “dementia sufferer’ to ‘the person with dementia” (44, p. 81). Research has shown that caretakers of those with neurocognitive disorders report worse mental and physical wellbeing than that of caretakers of patients with other chronic diseases (45). Supporting the development of positive coping strategies and providing caretakers with more problem-focused solutions with regard to the mental health of the patients they take care of, have proven to be effective interventions in this regard (22).
Neurocognitive disorders are increasingly recognized as triggering debilitating disorders and resulting in poor quality of life among afflicted individuals while also affecting their significant others. Hence, it tends to have many other adverse social implications. The prevailing view is that the existing model of health services for people with cognitive disorders – top-down, cure-oriented – is not designed to manage the host of problems found across such populations. Within such background, many approaches are being taken on behalf of dementia research, including those that examine the role of nutrition in the brain and whether vitamins have the potential to prevent or slow down pathological processes in the development of dementia. The decade long quest for ‘pharmacological armament’ has yet to provide a mechanism to understand the neurobiology of dementia. Unfortunately, however, very few compounds are able to play a significant enough role in preventing or mitigating the progress of dementia (46). ‘Big pharma’ has also recently announced a cessation of research on Alzheimer's disease (47). In the midst of all of this, various factors contributing to the pathology of dementia have been speculated. Given this context, the role of vitamin D is explored. The aim of the following sections is to advance the hypothesis that a lack of vitamin D has potential in contributing to the development of cognitive symptoms. The literature on both animals and humans are reviewed.
Neha et al (48) have identified various models of cognitive disorders that have a direct bearing on the clinical concept of dementia, including ‘senescence accelerated mouse models of dementia’, ‘chemically induced memory deficits’, ‘transgenic animal models of dementia’, ‘high fat diet induced dementia’, ‘hypoxia induced memory deficits’, ‘brain injury induced animal model’, ‘electroshock induced memory deficits’ and ‘thiamine deficiency induced animal models’. The conclusions made by Neha et al. (43) showing such diversity in animal models are important as some of the models tap into a few distinct subtypes of cognitive disorders linked to dementia.
With many animal models of dementia in existence, various studies have embarked on the exploration of the link between vitamin D and pathological processes that are closely related to the development of dementia. As part of the background, excitatory neurotransmitter (glutamate), and the inhibitory neurotransmitter Gamma-Aminobutyric Acid (GABA) might contribute to pathological processes that lead to the development of dementia (49). Krisanova et al. (50) have examined key transport characteristics of glutamate and GABA in the cortex of vitamin D3 deficient rats. The authors concluded that deranged glutamatergic and GABAergic neurotransmissions are strongly associated with the vitamin D deficiency experimental animal model. Although this study was not specifically equipped to examine cognitive functioning, the data do suggest that the pathological processes involved are likely to adversely affect information processing strongly related to domains such as attention and concentration, memory and executive functioning (3). Al-Amin et al. (51) have recently reported that experimentally induced low vitamin D activity is associated with diminution of activities of the hippocampus which concurrently affected the ability of the animal to learn and remember.
In their study, Molnár et al (52) showed a transgenic mouse breed to have a shorter lifespan and characteristic features of dementia, suggesting that vitamin D3 supplementation significantly increases the lifespan of transgenic animals. The problem with this study was that it had not been equipped to report whether a longer life span was associated with intact cognitive functioning.
Using the ‘Streptozotocin-induced sporadic Alzheimer's disease model’, Yamini, Ray & Chopra (53) examined the anti-inflammatory effects of vitamin D3. This study suggested two interrelated findings. Firstly, vitamin D3 had heightened the animal's ability to learn and remember and concurrently attenuate neuronal oxidative stress, mitochondrial aberrations. It also enhanced cholinergic neurotransmission. Second, the benefit of vitamin D3 intervention was the attenuation of pathological processes in brain regions critically linked to memory functioning such as the cortex and hippocampus.
Gezen et al. (54), drawing from the view that dementia might be due to neurochemical imbalance strongly associated with vitamin D, disrupted the functioning of vitamin D receptors in the brain of rats and observed that this disruption resulted in an increase in the indices of amyloid pathology and amyloid beta (Aβ) production. This study implied a critical link between the known pathology of dementia and integrity of vitamin D. Other studies are congruent with such view (55).
Patel and Shah (56), in their review, have suggested that Aβ deposition, often thought to be one of the primary pathologies in the development of cognitive disorders, can be slowed down using vitamin D supplementation. Similarly, Durk et al (57) have reported that VDR play a critical part in reducing amyloid-β (Aβ) peptides in mice and conversely, if the pathological processes are induced, it is possible to reverse them using vitamin D supplementation.
Bennett et al. (58) have examined whether vitamin D2-enriched mushrooms to thwart cognitive and biological abnormalities linked to dementia. The authors used transgenic (APPSwe/PS1dE9) mice and reported that animals fed with vitamin D showed improved cognition and reduction on the indices of pathological processes associated with dementia.
A study by Latimer et al (59) aimed to test if vitamin D could preserve or improve cognitive function with aged rodents. The authors divided the experimental groups based on the nutrition provided to each with different concentrations of vitamin D (‘deficient’ vs ‘sufficient’). The rodents with ‘sufficient’ concentration of vitamin D outperformed those with deficient concentration in the Morris water maze paradigm. In the ‘sufficient’ group, in addition to behavioral changes (improved memory), the rodents were noted to exhibit changes in synaptic transmission, cell communication, and G protein function. The authors hence concluded that “vitamin D-mediated changes in hippocampal gene expression may improve the likelihood of successful brain aging” (59, p. 43).
One of the neural pathologies viewed as a hallmark of dementia such as Alzheimer diseases is the formation of amyloid plaque. Yu et al. (60) have examined the neuroprotective role of vitamin D in the mitigation of plaque amyloid-β protein precursor (AβPP) transgenic mice. Accrued data suggested that vitamin D3-enriched diet impeded the accumulation of amyloid plaques, lowered the activities of Aβ peptides, inflammation and heightened NGF in the brain.
It is clear from the aforementioned literature review that there are various animal models of dementia with scientific merit. In essence, these animal models suggest that reduced level of vitamin D might work in tandem with other pathological processes to potentially trigger the development of dementia. Conversely, the data also suggest that increasing the activity of vitamin D has the potential to reverse or delay the development of dementia. If the animal model has heuristic value, then findings from human studies should complement animal literature. In this regard, the following section reviews existing clinical studies.
Current literature is replete with interventional studies on vitamin D and its effects on cognitive symptoms, some of which are shown in Table 1. Various meta-analyses and systematic reviews have examined the risk factors for dementia. Jayedi et al. (61) have examined the dose-response association of serum 25-hydroxyvitamin D and vulnerability to developing cognitive disorders. The study relies on a dataset till September 2017 and the authors found studies with 1953 participants with dementia and 1607 cases of AD. They concluded that higher levels of serum 25 (OH) D were associated with a lower risk of dementia and AD. In another systematic review and meta-analysis that looked at the risk factor that link between the two variables at hand, Cao et al. (62) have reviewed 43 trials that met the inclusion standard. The results suggested that low levels of vitamin D were strongly associated with impairment in neuropsychological functioning. A comprehensive study conducted on an elderly US-based population in 2011 showed that there is indeed a strong association between low vitamin D levels and increased odds of cognitive impairments (63).
| Research Design | Age |
Sample size | Outcome |
Instruments |
Serum concentration | Findings | Significance | Reference |
|---|---|---|---|---|---|---|---|---|
| Cohort | > 65 y/o (M = 79.7, SD = 8.4) | N = 858 | Global cognition, symptoms of dementia executive function, visual attention, sequential processing |
MMSE, Trial Making Test | Serum 25-hydroxyvitamin D (25 (OD)D) | Low levels of vitamin D associated with cognitive decline in the elderly population | Yes | Llewellyn et al. (2010) (63) |
| Cross-sectional | M = 77.6, SD = 7.3 | N = 225 | Global cognition, symptoms of dementia | MMSE | Serum 25- (OH)D3 level <50 nmol/l | Vitamin-D-sufficient patients had significantly higher MMSE scores | Yes | |
| RCT | ≥ 18 y/o |
N = 128, |
Working memory, response inhibition, cognitive flexibility, psychotic-like experiences, hallucinatory proneness, depressive symptoms, state anxiety, state anger |
N-Back task, stop signal task, set shifting task, Peters Delusion Inventory-21, white noise task, Beck Depression Inventory, State-Trait Anxiety Inventory, The State-Trait Anger Expression Inventory, The Treatment Emergent Symptom Scale | 25OHD3 (nmol/L) | Vitamin D supplementation does not influence cognitive or emotional functioning in healthy young adults | No | Dean et al. (2011) (140) |
| Cohort | MQ1= 74.6 |
N = 1604 | Memory, attention, concentration, psychomotor speed, cognitive shifting |
3MS and Trail Making Test Part B (Trails B) | 4 ng/mL for 25 (OH)D2 and 2 ng/mL for 25 (OH)D3 | No independent association be- tween vitamin D level and cognitive performance in the male cohort | No | Slinin et al. (2010) (13) |
| Cross-sectional | MB = 75.7, SDB = 8.6 |
N = 1081, |
Global cognition, symptoms of dementia, verbal intelligence, supraspan learning, auditory and visual retention, executive function/mental processing speed, visual construction/fluid reasoning, verbal fluency, and anxiety. | MMSE, NAART, WMS-III word list learning, WMS-III logical memory, digit symbol coding, trail-making test, mental alternations, WAIS-III block design, matrix reasoning, controlled oral word association, self-rated anxiety scale | Circulating 25 (OH)D concentrations; deficient (<10 ng/mL), in- sufficient (10–20 ng/mL), and sufficient (>20 ng/mL) |
25 (OH)D was positively associated with cognitive performance, particularly with measures of executive function in this elderly population | Yes | Buell et al. (2009) (3) |
Another set of systematic reviews and meta-analyses examined the association between cognitive impairment and vitamin D concentrations. Aghajafari et al. (64) have reported systematic reviews and meta-analyses of studies up to 2017 in order to shed light on the available evidence for the association of vitamin D with mental efficiency and impaired performance in people with cognitive disorders. The pool of five studies pointed to a link between low serum concentration of D and the emergence of cognitive disorders. Although the link between low serum concentration of 25-hydroxyvitamin D and cognition showed conflicting findings, the authors did also report that the studies suffered from poor methodology and low quality. Balion et al. (65) sought to tease out the association between cognitive function and dementia with vitamin D concentration in adults. They reviewed data available till August 2010 and included all study designs with a comparison group. The authors were able to unearth 37 studies that were deemed to fit the inclusion criteria and reported that lower vitamin D concentrations were correlated with impaired neuropsychological functioning. Zhao et al. (66) have scrutinized studies up to March 2012. The central question was regarding the concentration of vitamin D among levels of AD. The search resulted in the inclusion of six studies (n= 892) and the meta-analysis suggested that people with cognitive disorders under the rubric of dementia had lower concentration of vitamin D compared to healthy controls. The aforementioned literature review appears to suggest that status of vitamin D plays a critical role in the expression of cognitive disorders. But the ‘verdict is still out’ for such a link since a majority of the clinical population have been investigated for peripheral levels of vitamin D and their association with cognitive performance and it is not clear whether there is a link between the levels of vitamin D in the peripheral and central nervous system.
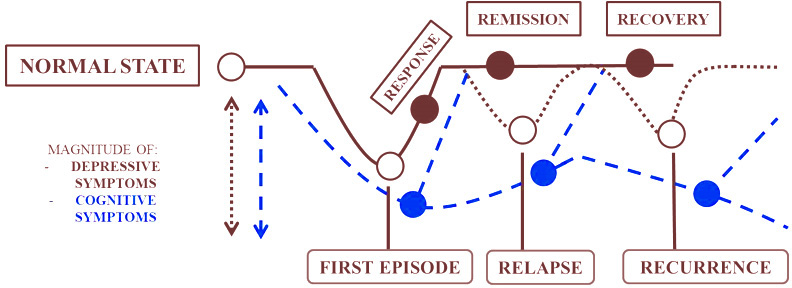
As per the DSM-5 (15), depressive disorders have been divided into different subtypes including ‘disruptive mood dysregulation disorder’, ‘premenstrual dysphoric disorder’, ‘persistent depressive disorder’, ‘substance/medication-induced depressive disorder’, ‘depressive disorder due to another medical condition’ and ‘other specified depressive’/’unspecified depressive disorder’ and ‘major depressive disorder’ (MDD). Although depressive disorders are thought to be marked under mood disorders mainly due to affective feelings, one of the characteristic features of depressive symptoms is the presence of cognitive impairment testified by the DSM criteria as ‘diminished ability to concentrate/indecisiveness’. This understanding of cognitive impairment has received scant attention with a few exceptions (15). Such state of affairs has been attributed to the view that cognitive dysfunction is typically confined to the chronic or refractory type of MDD. The acute form of MDD has been considered to be characterized by affective symptoms rather than cognitive symptoms. Being an episodic disorder, the cognitive symptom in MDD has thus been perceived as being transient and therefore benign. However, evidence also suggests that cognitive symptoms tend to persist despite the fluctuation of depressive symptoms (67). As shown in Figure 1, cognitive symptoms progress along the course of different MDD states when untreated and hamper remission (68).
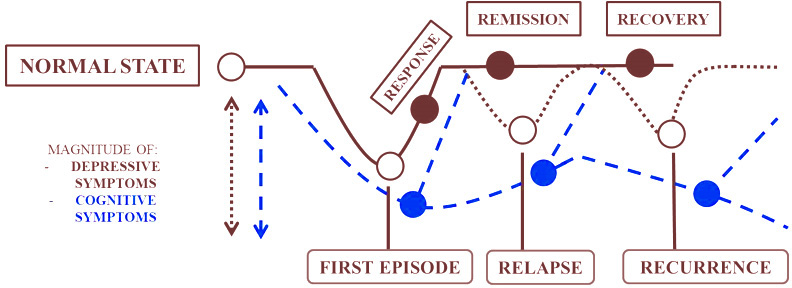 Figure 1
Figure 1Progression of cognitive symptoms along the course of MDD states.
Early onset of cognitive symptoms prior to the diagnosis of depression can also lead to susceptibility to chronic recurrent depression (68). Cognitive impairments are strong determinants of poor QoL. The presence of neuropsychological impairments tend to be associated with poor efficacy to antidepressant medications (69).
Despite their diverse clinical nature, the mainstay treatment of people with depressive symptoms is pharmacological and various brands of psychotherapeutic interventions. Other modalities that are known to ameliorate depressive symptoms include lifestyle modification (70) and what is sometimes known as complementary and alternative medicine (70). Similarly, there are also dietary supplementations and trace elements that have been increasingly marketed as one of the means to mitigate the presence of depressive symptoms. One such trace element that has attracted much attention is vitamin D. Vitamin D receptors appear to exist in the brain and be closely related to the activities of neuronal and glial cells in the brain, which includes the prefrontal cortex, hippocampus, cingulate gyrus, thalamus, hypothalamus, and substantia nigra (71). When their integrity is compromised, these brain regions, have been previously shown to be critically associated with a variation in the symptoms of depression disorder (72). While the emotional symptoms of depressive illness has received extensive coverage using pharmacological studies, there is a dearth of literature examining the link between vitamin D and neuropsychological impairment in people with depressive disorders. This section first examines depression disorder providing a brief overview followed by a consideration of the emerging evidence of vitamin D and cognitive functioning in depressive illness. Literature on both preclinical and human studies will be considered, in this regard.
Epidemiological studies carried out around the world suggest that debilitating and afflictive emotion or clinical depression/MDD affects approximately 3% of the global population (73). Other ‘minor’ but debilitating and intransigent depressive disorders such as dysthymic, minor depressive disorders and current brief disorder are even more common (59). For these reasons, depression has been speculated to surpass other longstanding ‘enemies of health’ such as cancer and cardiovascular diseases in terms of mortality, dependency and disability (74). Hence, it has been projected to cause higher burden as calculated by disability-adjusted life years (DALYs) (75).
Historically, neuropsychological impairments in depressive disorders have been deemed to be episodic and have often coincided with the exacerbation of the mood disorder. Notwithstanding such view, Hammar & Ardal (67) reported that cognitive dysfunction is a common occurrence during the acute phase of depressive symptoms. To reach such conclusions, the authors synthesized existing literature and in their analysis, Hammar & Ardal collapsed divergent neuropsychological profiles into four types: executive functions, attention, memory and psychomotor speed that are common in the acute phase of depressive symptoms. Subsequently, the review and meta-analysis were found to be congruent with such a view that depressive disorders are accompanied by cognitive impairment even during the acute phase (76). Three of the four neuropsychological profile types will be highlighted here.
There is ample evidence in the literature indicating that neuropsychological impairment of executive function in the acute phase of depressive disorder tends to persist even after amelioration of mood symptoms (69). The type of executive functions includes those related to the inability to shift mental set, working memory and planning. This disruption in the cognitive domain has been previously reported as frontal lobe dysfunction. Thus, the brain region that has been postulated to be critically involved in executive functions has been noted to have lower cerebral perfusion (77).
In addition to executive functions, impaired attentional capacity is one of the common neuropsychological impairments found in people with depressive disorder. Impaired attentional capacity includes impaired integrity of processing speed, selective attention and automatic processing. Some studies have narrowed the impairments in attentional capacity among people with depressive disorder down to include the most significant ones that tap into executive functions and effortful attention (processing speed plus selective attention). In contrast, automatic processing was found to be intact among such people.
The third cognitive domain that has been frequently associated with depressive disorder is memory. It has been estimated that 50-70% of people with depressive disorders tend to display depressive pseudo-dementia (78). Memory essentially presents one’s ability to learn and remember. Neuropsychological literature defines memory to include the processes of encoding, storage/consolidation and retrieval. Memory impairment is thought to occur whenever there is a fault in any of these three operations. Integrity of memory can manifest verbally or visually. Tulving (79) has divided memory into ‘declarative memory’ and ‘non-declarative memory. Most of the studies on memory impairment in people with depressive disorder have focused on declarative memory and its subtypes such as episodic memory and semantic memory (80). It appears therefore that memory impairment in people with depressive disorders include verbal and visual memory, verbal delayed memory. The centrality of executive functions and memory in depressive disorders have been supported by neuro-imaging studies. In vivo brain scans during the acute phase of depressive disorders are associated with attenuated cerebral blood flow in the region critically involved in executive functions and memory (81).
In terms of localization of neuropsychological impairment in depressive disorder, four interrelated brain regions have been strongly implicated including limbic–frontal circuitry (82), amygdala (83), hippocampal-prefrontal circuit (84) and catecholaminergic system (85). Firstly, one hypothesis suggests that limbic cortical dysregulation might play a crucial link between cognitive impairment in depressive disorders (86). Some review articles on neuroimaging (MRI, PET and SPECT scans) have attempted to find the linkage between the pathophysiology of depressive illness (87). These reviews have shown many brain regions and circuits to have caused people with depressive disorder to experience dysfunction, including those that are often known to constitute limbic–frontal circuitry (82).
Secondly, the role of amygdala is gaining increasing attention among studies in relation to depressive symptoms (88). Functional connectivity of the right middle occipital gyrus to bilateral amygdala was found to be altered and this indicated the presence of cognitive impairments rather than severity of depression. Specifically, attention, episodic memory and executive function were affected. One of the observed neuropsychological impairments in people with depressive disorders is their inability to recall specific positive emotional memories (81). It was found that training people with depressive illness to hold ‘positive autobiographical memories’ in their minds enhances blood flow in the amygdala (89). Such an undertaking concurrently improved depressive symptoms. This suggests that, in addition to its therapeutic implication for people with depressive disorder, what was previously perceived as ‘unhelpful thinking’ common in people with dysphoria is likely to be intertwined with memory functioning linked to the hippocampus.
Thirdly, the hippocampal-prefrontal circuit has been found to play a pivotal role in the pathology of depressive symptoms (89). The hippocampus has been suggested to be associated with memory functioning (90) while the prefrontal cortex has been associated with executive functioning (91). Thus, the hippocampal-prefrontal circuit might be crucially involved in underpinning impairment in neuropsychological functioning among people with depressive disorders. Among rodents, dorsoventral segmentation along the hippocampal axis was found to have dual functionality with dorsal hippocampus regulating cognitive function and ventral hippocampus partaking in mood regulation (92). Similar findings were replicated in primates.
Besides brain circuit, neurochemical circuits have also been implicated in the expression of depressive disorders and most significantly biogenic amine that include norepinephrine, serotonin and dopamine (89). These transmitters have constituted the basis for existing pharmacological treatments for people with depressive disorders. There is evidence to suggest that biogenic amine might be strongly involved in neuropsychological function (93). There is also evidence to suggest that the interface between emotion and cognition are an integral part of this system. For example, disruption of these biogenic amine could result in cognitive biases such vicious cycles of negative thinking triggered by negative expectations (94). According to the Roiser and Sahakian (95), cognition is strongly influenced by one’s emotional state. Negative emotion tend to dent cognition and it has been found that executive functioning is often affected by negative emotion (96) and can be modulated with biogenic amine (97).
In addition to limbic–frontal circuitry, the amygdala, the hippocampal-prefrontal circuit and catecholaminergic system, there is increasing interest in the role of vitamin D. There have been a number of studies linking the level of vitamin D and intestine, kidney, and bone function. There is also substantial evidence suggesting both vitamin D activating enzyme and vitamin D receptors are expressed in the human brain (12). This raises the possibilities of a link between deficiency of vitamin D and diseases of the brain (12) and, for the present discourse, its role in depressive disorder and neuropsychological impairment. In the ensuing paragraphs its link to depressive disorder and cognitive impairment are considered in both preclinical and clinical studies.
Krishnan & Nestler (98) have stated that “diagnosis of depressive episodes is made when patients display a certain number of vaguely defined clinical symptoms”. In the absence of more objective diagnostics such as neuroimaging, genetic variations, biomarkers, or biopsies, this rudimentary “symptom-counting” approach creates obvious limitations for the development of animal models (p.2). Despite such caveat, there are a few animal models of depression in literature which, in simple language, are geared towards capturing two behavioral aspects of depressive symptoms: experimentally induced ‘defeat’ and the ‘despair’ paradigm. These two modes are genetically produced in the experimental animal and they are also used as models to environmentally induce depression-like symptoms. Some of the animal models are designed to reduce locomotor activity, appetitive behavior as well as something akin to anhedonia. These models have been intended to answer whether deficiency of vitamin D during the prenatal period might be involved in the development of neurological and neurocognitive disorders. The paradigm also deciphers whether vitamin D has potential to heighten cognitive reserves during childhood and adolescence and whether vitamin D has the potential to reduce or reverse neuropsychological impairment in senescent rats.
Al-Amin et al. (46) have been credited to show how the level of vitamin D is associated with structure and function of the brain. Specifically, the study explored the impact of vitamin D deficiency on connectivity and volume of hippocampal region and cognitive functioning (spatial learning). After 10 weeks of being vitamin D deprived, the rodents have shown significant decline in their ability to performa in indices of spatial learning compared to a control group and when histologically examined, cognitively declined rodents also showed reduction in connectivity and volume of hippocampus.
In their review on animal model of depression and vitamin D, Harms, Burne, Eyles & McGrath (7) have stated that there is substantial evidence to suggest that animals experimentally deprived of vitamin D in utero or after birth and those lacking functional receptors undergo structural changes in the brain including increased size of the brain and behavior deficits that mimic depressive symptoms and cognitive impairments. On the other hand, some studies have suggested that supplementation of vitamin D has the potential to reverse neuro-inflammations and other pathological process in the brain that could have adversely affected different behavior repertoires including cognition (99).
Some studies have examined that the integrity of certain neurotransmitters have been clinically attributed to modulate the depressive symptoms. Some studies have suggested that the level of vitamin D has a direct bearing on the expression of gene encoding as a precursor of neurotransmitters that have been interrelated to depressive illness and cognition (100). Similarly, there is a recent evidence suggests that the induction of neurotoxins in the brain that destroys the activity of neuro-transmission and is strongly involved in cognition and depression, can be prevented or reversed within treatment of vitamin D (101). Interestingly, the regions that appear to have robust effects appear to be those that have been clinically linked with cognition and depressive symptoms (101).
Some studies have examined the link to hippocampus, the damage of which tends to result in impairment in something akin to memory in humans. Vitamin D receptors have been widely documented in the hippocampal region of the brain (12). Interestingly, hippocampal neurons can be modulated with vitamin D and offer neuroprotection (102,103).
While vitamin D levels have been widely reported to reduce age-related brain atrophy, specifically grey matter volume (104). There was no clear evidence that such undertaking stems directly from the mechanism within the brain or mediated via peripheral pathways. Brewer et al. (102) indicated that vitamin D directly confers neuroprotection while Latimer et al. (54) have suggested that vitamin D hampers with cognitive decline and enhances hippocampal synaptic function in animals with memory deficits associated with senescence.
Cholecalciferol derived from sunlight has been postulated to be source of vitamin D since time immemorial (105). If this assumption is correct, then among people who are living in latitudes or parts of the world where the sun is abundant, the likelihood of vitamin D deficiency should be less as they get more exposure to the sun. There is a caveat to such a view. Firstly, globally, the rate of vitamin D deficiency is common in countries that are expected to have sunshine throughout the year and this has been attributed to ‘sun-avoidance habits’ in people coming from certain geographical regions (106). The question then arises regarding the link between latitude and vitamin D deficiency. Existing data suggests that there is a strong link between high prevalence of depressive disorders and residence in regions that have less exposure to the sun (107).
In support of this view, some have hypothesized lack of sun exposure to have the potential of triggering depressive disorder (108). In phenomenological studies of seasonal affective disorder, there is evidence to suggest that there is a temporal relationship between onset of depressive symptoms and seasonality, specifically the time of the year where exposure to the sun is less common (e.g. fall or winter). Studies have found that those diagnosed with seasonal affective disorder have impaired spatial memory and learning along with significantly slower reaction time (108). In support of this view, supplementation of D3 to women during the winter, serum D levels rose significantly and this was associated with the attenuation of depressive symptoms (109). Another line of research showing a temporal relationship between exposure to the sun and neuropsychological impairment is the one reported among people with cognitive decline. In a study by Wilkins et al. (110), it was found that although the primary source of vitamin D can be the cutaneous synthesis of ultraviolet exposure, the efficiency of this metabolism is significantly affected among the elderly, hence making them more vulnerable to vitamin D deficiency. Those with insufficient (10-19.9 ng/ml) and deficient (less than 10 ng/ml) status of vitamin D had significantly associated risk of depression. They also scored poorly in a cognitive measure of memory, orientation and attention. Among African-American and European American elderly, both groups performed significantly worse in cognitive measures when associated with low levels of vitamin D. Further study suggests that the African American elderly could possibly have more proneness to cognitive impairments as it was found that three-fourth of the sample had abnormally low level of vitamin D (110). In addition to naturalistic observation, there are intervention studies that have emerged, paving the way for the link between deficiency of vitamin D and neuropsychological impairment among people with depressive symptoms. On one hand there is indirect evidence in meta-analysis and systematic reviews to suggest that vitamin D supplementation has the potential to reduce depressive symptoms (111). Suggested mechanisms could be oxidative stress, neuroinflammation theory and systemic inflammatory markers (111). On the other hand, there is also a view that some of the cognitive impairments that have been previously seen in people with depressive symptoms are strongly linked to the variant in vitamin D (112). There is also a direct study, though limited by being cross-sectional, suggesting a link between low vitamin D level and neuropsychological impairment in depressive disorders. Belzeaux et al. (113) recruited 91 drug naive participants (aged 18–65) with diagnosis of depressive disorder. The result suggested that those patients with deficiency of vitamin D concurrently performed poorly on indices of executive functioning. The link between the status of vitamin D and neuropsychological functioning in depressive illness is shown in Table 2.
| Research Design | Age | Sample size | Cognitive Domains Affected | Deficient Serum concentration | Assessment Method and Criteria | Findings | Significance of relationship between vit. D and cognition | Reference |
|---|---|---|---|---|---|---|---|---|
| Cross-sectional | M= 38.5 years | 91 | Cognitive Inhibition/interference | <50 nmol/L (20ng/ mL) | Non-Interventional; Non-medicated patients in current episode of major depression | HypoVD increases cognitive interference in tasks | Yes | Belzeaux et al (2018) (113) |
| Experimental | M= 62.1 years | 84 | Working memory capacity, speed of information processing, language/fluency | <37nmol/L with serum PTH >6.8 pmol/L (less than 51 years) and >7.5 pmol/L (more than 50 years) | Non-Interventional; patient with secondary hyperparathyroidism without renal failure | Cognitive dysfunction and higher depression score is found among those with persistent serum PTH | Yes* | Jorde et al (2006) (224) |
| Cross-sectional Cross-sectional | M= 74.79 years M= 70-79 years | 80 155 | Orientation Memory Concentration (Bedside tests) Orientation Memory Concentration (Bedside tests) | <10ng/mL (deficient); 10-19.9ng/mL (insufficient) <20ng/mL | Non-interventional; patient with Alzheimer’s and control Non-interventional; Elderly | Patients are found to have active mood disorder and poor cognitive performance Significant correlations were found between blood level of calcium and depression; blood level of vitamin D and cognitive functioning | Yes* Yes* | Wilkins et al (2006) (110) Lee & Kim (2017) (225) |
| Retrospective | M= 52 years | 548 | Orientation Memory Concentration (Bedside tests) | 10-24ng/mL (moderate hypovitaminosis D); <10ng/mL (severe hypovitaminosis D) | Non-interventional; Case Records of patients in adult psychiatric inpatient wards | No significant association between hypovitaminosis D and depressive symptoms or global cognition. | No* | Leedahl et al (2013) (226) |
Psychosis, a common but unpredictable characteristic of many psychiatric and neurodevelopmental disorders, is considered to be the defining feature of Schizophrenia Spectrum Disorders (114). For the purpose of this review, schizophrenia will be addressed as the main feature of psychosis. According to the DSM-5, schizophrenia is characterized by two (or more) of the following: “delusions, hallucinations, or disorganized speech” (15). While the focus has been on delusions, hallucinations, or disorganized speech, people with schizophrenia are also known to have impairments in cognition, which in turn, have a direct bearing on social and occupational functioning (115).
There are some major, persisting views in the literature on people with psychotic disorders. Despite the fact that schizophrenic-like conditions have been documented since the ancient Egyptians, and schizophrenia has remained at the ‘heartland’ of psychiatry in the past 100 years (116), between 15% and 30% of schizophrenics fail to respond to antipsychotic drugs (117). For this reason, with an increase in interest in alternative and complementary medicine, there is an interest in whether deficiency of trace elements might play part in the pathogenesis of psychotic illnesses. Accompanying such interest is the widespread dissent towards strict reliance on descriptive psychiatry that strongly hinges on the ‘symptom checklist’ as defined in the DSM (15) or the International Classification of Diseases (ICD) (118). Such dissenting views have given birth to the surge of interest in exploring neuropsychological functioning among people with schizophrenia. Earlier, there was a discussion on whether neuropsychological deficits should be considered a characteristic cognitive impairment in the diagnostic criterion for schizophrenia DSM-5 (119). However, such changes were not embraced under the pretext of there being a lack of specificity of cognitive impairments in schizophrenia.
Considering the aforementioned discussion on the rising interest in trace elements and the development of schizophrenia along with the emerging data on the neuropsychological deficits in schizophrenia, the aim of the present discourse is to highlight emerging evidence of the link between variations in vitamin D and the neuropsychological deficits in schizophrenia. In the present review, the first aim is to focus on the link between schizophrenia and the activities of vitamin D. Related to this question is the aim of examining whether there is a link between the neuropsychological domain and vitamin D.
People with schizophrenia are known to be deprived of quality of life and it has been estimated to rank among the top 25 leading causes of disability worldwide (120). While still considered a low-prevalence disorder, there has been an increase in the number of cases of schizophrenia around the world (121). Although the development of schizophrenia is complex, recent literature indicates that schizophrenia develops through a combination of genetic, environmental, lifestyle and physical factors such as increased body mass index and inactivity, psychological factors, changes in neurodevelopmental activity, and low prenatal exposure to vitamin D (122). Vitamin D deficiency has become a global concern with one billion individuals suffering from this problem worldwide (122). While it is difficult to come to a consensus regarding the optimal serum levels of vitamin D, inadequate vitamin-D status is commonly defined as constituting serum concentrations < 25 nmol/L (123).
There is a significant temporal relationship between the development of schizophrenia with season of birth, geographical locations and human migrations. As for the season of birth and geographic location, research indicates that the prevalence of psychotic disorders increases in colder climates (born in Winter/Spring) and higher latitudes; suggesting that sun exposure or a lack of it is a potential risk factor for psychotic disorders (12). As for migration, studies have indicated that when migrants from the tropics move further north, their rate of psychotic illness appears to increase (124). Although there are likely to be pre-existing factors among migrants that render them more vulnerable to risk factors for the development of psychotic illness, there is a hypothesis pointing that lack of the “sunshine vitamin” might play a role in the overrepresentation of statistics on schizophrenia in Western Europe (125). Lally et al. (123) have reported that migrants with psychosis in the UK (people with darker complexation or African/Caribbean heritage) had lower vitamin D levels. However, this condition was reversed when they spent time outside to get the required amount of sunlight.
Yee et al. (126) conducted a study examining the levels of vitamin D and first-episode psychosis among patients from a population that received regular sunlight exposure throughout the year. Results from their study illustrated a significant relationship between low levels of vitamin D and negative symptoms of schizophrenia, indicating that symptoms such as isolation leads to less time spent outdoors, and therefore, less sunlight exposure and absorption of vitamin D.
Relevant to this, with increasing urbanization, often characterized by the sprawling ‘concrete jungle’ and pollution, there is also an increase in the number of people with lower status of vitamin D (127). Epidemiological studies have suggested that schizophrenia and their outcomes are more common in urban than rural areas (128). Therefore, there is evidence to suggest that increased rates of psychotic disorders found among migrants, during a certain season, and urbanity lend some credence to the role of vitamin D in schizophrenia.
Support for the link between vitamin D and psychosis have also emerged in clinical trials and the resultant systematic review and meta-analysis (122,129).
This is further emphasized in research where it was suggested that individuals who have not received enough vitamin D supplementation as infants would be at risk for developing schizophrenia as adults (7). Moreover, a large cohort study of 33,623 adult Swedish women was conducted, findings from which indicated that there was a significant relationship between high intake of vitamin D and fish, and lower rate of psychotic-like symptoms (130), signifying that optimal level of vitamin D is essential across all age groups.
There have been many animal studies focusing on vitamin D where it has been suggested that vitamin D plays an important neuroprotective and neurodevelopmental roles (131). Similarly, vitamin D has been postulated to be a critically involved neurotransmitter in an animal model of psychosis (132). Vitamin D receptors, that are critically associated with areas of the brain related to learning and remembering as well as higher order functions like executive functioning along with the pathology of schizophrenia (133), were identified in the hippocampus and prefrontal lobe (132). It was shown through pre-clinical trials that these receptors are directly involved in the regulation of dopaminergic-associated gene expression (12). Dopamine has been suggested to be one of the important neurotransmitters involved in the pathology of schizophrenia (134). Interestingly, there are some suggestions regarding the link between dopaminergic dysfunction and its causal link to vitamin D by animal developmental vitamin D-deficient models (135).
Vitamin D deficiency negatively alters brain activity, particularly those brain regions that have been speculated to play a role in cognitive impairments found in schizophrenia (132). In a controlled laboratory study, brain development evaluation was carried out on neonatal rats whose mothers had a vitamin D-deficient diet and were deprived of sunlight (12). Surprisingly, results indicated that vitamin D deficiency could alter the structure and function of the brain (132), and lead to cortical thinning – both of which are associated with schizophrenia. In addition to its effect on function and structure of the brain, rats deprived of vitamin D in utero showed increased activity on the elevated plus maze and significantly impaired latent inhibition, considered prominent features of schizophrenia-related impairments in attention (12). Research also shows that transient prenatal vitamin D deficiency has an adverse impact on the efficiency of learning and remembering in animals (136).
In the aforementioned literature on animal models of psychosis and its links to the integrity of vitamin D status, the evidence is tantalizing but must be accepted with caution, as there is disjunction between animal models and clinical reality. Animal models seem to be in support of implicating the role of vitamin D in the mechanisms that are thought to be strongly related to the expression of psychotic and cognitive symptoms in schizophrenia. Despite the fact that studies on animal experimentation do not always make headway to clinical application, there is heuristic value to such models
Despite the fact that schizophrenia has been the ‘heartland’ of psychiatry (116), little progress has been made in both basic and clinical sciences in shedding light on its central pathology, prevention and treatment. Rather than banking on ‘descriptive psychiatry’ that strongly hinges on ‘symptom checklists’ as defined in the DSM (15) or ICD (118), a paradigm shift is required to further our understanding of this intransigent and debilitating malady. In the past years, it has been increasingly recognized that neuropsychological dysfunctions are likely to play a central role in the severity, disability and prognosis of mental disorders. They are liable in shaping the quality of life among people with psychotic illnesses (137).
In the past decade, data has been amassed to suggest that people with psychotic illnesses are marked with various domains of neuropsychological impairments. Among many varied neuropsychological impairments, it has been found that processing speed appears to be a key characteristic of cognitive deficits in schizophrenia (114). Deficits in processing speed also lends merit to other observed cognitive impairments often found in schizophrenia. For instance, commonly observed deficits in schizophrenia such as memory impairment and de-executive dysfunction are thought to be linked to impaired processing speed (115). Therefore, processing speed has been understood to be a fundamental deficit in people with schizophrenic disorders (138). The question then arises on the link between neuropsychological functioning in psychotic patients and the activities of vitamin D. Valipour, Saneei & Esmaillzadeh (139) have reported that prevalence of vitamin D deficiency amounted to 65.3% among the people with schizophrenia from the data obtained from 9 studies for systematic review.
Some studies have indirectly shown the link between vitamin D and neuropsychological functioning. One such study was by Dean et al. (140) who used randomized controlled trials to decipher the effects of vitamin D supplementation on neurocognitive functioning known to be common in people with psychotic illness among healthy young adults. Their results showed that vitamin D supplementation had no significant effect as such on neuropsychological functioning. In another study, McGrath et al. (141) examined the levels of vitamin D within the community and its association with neuropsychological functioning that has been linked to psychotic illnesses. It was concluded that no causal relationship could be found between neurocognitive performance and levels of vitamin D. These studies imply that neuropsychological domains that are linked to psychosis are not amenable with vitamin D supplementation.
A cross-sectional study conducted by Nerhus et al. (142) on a young population of patients with diagnosed psychotic disorders (n= 225) were selected to evaluate the effect of vitamin D deficiency on four cognitive domains: processing speed, verbal learning, verbal memory, and executive functioning. Findings indicated that vitamin D deficiency was significantly associated with decreased processing speed and verbal memory in patients with psychotic disorders. As noted above, processing speed in particular has previously been shown to be a specific marker for cognitive deficits in schizophrenia. However, it is important to note that Nerhus et al. (142) did not find any significant associations between low vitamin D levels and verbal memory.
Another cross-sectional study was performed by Graham et al. (87) on a sample of 40 patients with first episode psychosis and it compared their neuropsychological performance with 20 matched healthy controls. This result suggested that the low status of vitamin D is strongly related to impairment across all cognitive domains explored: verbal fluency, attention, processing speed and working memory.
Zoghbi et al. (143) reported results from their cross-sectional study among people with schizophrenia (n= 196). The authors examined status of vitamin D and neuropsychological measures using the Brief Cognitive Rating Scale (BCRS). The results suggested strong association between level of vitamin D and neuropsychological performance.
Krivoy et al. (144) executed a randomized, double-blind, placebo-controlled clinical trial in order to tease out whether vitamin D supplementation will have an effect on psychosis severity, affective range, neuropsychological functioning and metabolic profile. The study found that vitamin D supplementation slightly elevated neuropsychological status but had no bearing on other outcome measures.
Lerner, Sharony & Miodownik (122) carried out a systematic bibliographical research on various disorders including schizophrenia. The review failed to decipher any link between effect of vitamin D and neuropsychological dysfunction in people with schizophrenia.
In addition to intervention studies, Shivakumar et al. (145) had examined the relationship between vitamin D status and integrity of brain regions that have been postulated to be critically involved in memory functioning (46). After controlling for age and education, the status of vitamin D was significantly related to hippocampal volume. In a related study, Gurholt et al. (146) showed that supplementation of vitamin D decreased volume of people with psychosis.
In summary, studying vitamin D levels has begun regaining some ground in understanding neuropsychological functioning of people with psychosis. However, these studies are still limited by their cross-sectional nature. Some interventional studies which show that supplementation of vitamin D is capable of impacting neuropsychological functioning have also started to emerge. Nevertheless, such studies are few. Final evidence has been derived from studies examining the function and structure of neural substrate that has been critically linked to cognition. These studies have indicated that structure and function of such neural substrate are strongly influenced by status of vitamin D. At the very top of the hierarchy of evidence-based medicine, systematic reviews and meta-analyses have failed to detect the link between status of vitamin D and neuropsychological functioning in people with psychosis. Therefore, the jury is still out to provide convincing evidence of the influence of vitamin D on the cognition of people with schizophrenia. Table 3 recapitulates the study linking vitamin D status and neuropsychological functioning in schizophrenia.
| Research Design | Sample Size |
Mean Age | Serum Concentrations (Vit.D Deficiency) | Cognitive Domains Assessed | Assessment Method (s) | Findings | Sig. of Association between Vitamin D & Cognition | Reference |
|---|---|---|---|---|---|---|---|---|
| Cross-sectional | N=384 (n1=225, n0=159) | M1 = 30.2y |
S-25 (OH)D<25 nmol/L | Processing speed, verbal memory, verbal learning, working memory | Neurocognitive test battery, clinical protocol (SCID-1 & PANSS), physical examinations (blood tests, etc.) | Vit.D deficiency sig.associated with decreased processing speed and verbal memory in schizophrenic patients | Yes | Nerhus et al. (2017) (142) |
| Cross-sectional | N=40 (n1=20, |
M1 = 23y |
25OHD <30 ng/mL-1 | Verbal fluency, attention, processing speed, visual working memory, executive function | Neurocognitive test battery, clinical test evaluation (PANSS & CDRS), physical examinations | Lower Vit.D levels associated with overall cognitive deficits | Yes | Graham et al. (2015) (87) |
| RCT | N = 128; |
M1 = 21.4y |
Low serum concentrations at 25OHD3 at baseline (<75.00 nmol/L) | Working memory, response inhibition, cognitive flexibility | Working memory (N-back,) response inhibition (stop signal task), cognitive flexibility (set shifting task), clinical evaluation (BDI, PDI-21, STAI, STAXI-2, & White noise task) | Vit.D supplementation has no influence on cognitive functioning or psychotic-like experiences in a sample of healthy young adults | No | Dean et al. (2011) (140) |
| RCT | N=47 (n1=24, |
M1 = 39.4y |
Insufficient Vit.D levels: (<75 nmol/L) |
Delayed recall (memory), attention | PANSS, CDS, MoCA, metabolic parameters, safety measurements | Vit.D supplementation is associated with improved cognition in schizophrenic patients | Yes | Krivoy et al. (2017) (144) |
| Cross- sectional | Adolescents (n=1676); Adults (n=4747); Elderly (n=4809) | Adolescents age range= 12-17y; Adults= 20-60y; Elderly= 60-90y | Serum 25-hydroxyvitamin D3 (25 (OH)D) | Neuropsychological functioning (learning, memory, etc.) | Psychometric and cognitive measures | Lower Vit.D levels do not influence neurocognitive performance | No | McGrath et al. (2007) (141) |
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder that is often marked with inattention, hyperactivity and impulsivity. Based on the criteria of the DSM-5, ADHD can present as three different subtypes: the inattentive subtype, the hyperactive-impulsive subtype and the combined subtype where at least six symptoms need to be present for a period of six months to be labelled as ADHD. ADHD is usually referred to as a childhood disorder, diagnosed after the age of 7, where symptoms appear to interfere with functioning in two or more contexts, such as at home and in school (15). Conversely, ADHD may persist into adulthood where the symptom of inattention is more common compared to hyperactivity (147).
While the symptoms of ADHD are largely gleaned from descriptive psychiatry with an emphasis on signs and symptoms as testified by psychiatric nomenclature such as the DSM-5 and ICD (118), there are vast neuropsychological studies suggesting that ADHD has many facets of cognitive disorders (148).
Firstly, one of the core deficits in ADHD is hyperactivity/impulsivity. Neuropsychological literature suggests that hyperactivity/impulsivity, often characterized by failure to regulate impulsivity or thought before action, is likely to fall within de-executive syndrome (149). In support of such view, a myriad of studies has indicated that children with ADHD tend to display impairment in executive functioning frequency (150).
A second core symptom of ADHD is poor attention. A seminal work in neuropsychological literature has stated that when attention or its integrity is compromised, it results in inattention. Various attentional paradigms and neuroimaging data have been complied with the view that inattention is one of the subtypes of neuropsychological dysfunction in children and adults with ADHD (151). Various authors have reported that ADHD tends to come with neuropsychological deficits like impaired sustained attention, poor response inhibition, and problems with working memory (148). This may lead to impaired daily functioning including poor time-management which, in turn, has repercussions on education, employment attainment and the quality of life (152).
There are various pharmacological and non-pharmacological approaches that have been employed to mitigate the symptoms of ADHD (153). Along with this, there has been an increased interest in using alternative and complementary medicine and dietary and nutritional supplements as ADHD is progressively becoming a ‘lifestyle disease’ (154). There is also an interest in the role of trace elements in the etiology and pathogenesis of ADHD. Among many varied types of trace elements, vitamin D appears to have received a bulk of empirical attention in the past decades (155). The aim of the present discourse is to review the link between ADHD and vitamin D with a particular focus on neuropsychological or cognitive symptoms. In order to lay the groundwork for such a review, we first provide an overview of ADHD and the global trends associated with it. We will then examine literature highlighting the link between vitamin D status and neuropsychological functioning in ADHD using preclinical and clinical studies.
The prevalence rate of ADHD has been estimated to range from 1-2% to 6-7 % of children and adolescents depending on which psychiatric nomenclature is used: DSM or ICD (156). Approximately 65% of those diagnosed continue to face impairments in adulthood (157). Nationwide trends show a prevalence of 9.9% in the UK and a prevalence of 5% in the US (156). These trends suggest that ADHD is more prevalent in children and adolescents than adults, but these rates have recently changed as more adults are being diagnosed with ADHD (157). Additionally, prevalence rates seem to vary based on gender, where boys are almost twice more likely to be diagnosed with ADHD than girls (157). This may be due to boys typically presenting with externalizing symptoms such as hyperactivity and impulsivity that are more likely to be deemed as unacceptable and therefore easily detected. On the other hand, females are likely to display more internalized symptoms such inattention (158), which may be construed as forgetfulness and being dumbfounded. As the general characterization of adult ADHD are symptoms of inattention, females are more likely to be diagnosed with ADHD in adulthood making the gender gap in adulthood less evident (159).
The causes of ADHD are multifactorial, resonating with the ‘nature-nurture’ debate. Various twin studies have contributed to research establishing that genetic factors influence this disorder. It has been speculated that genetic factors may even play a role in up to 80% of the cases (160). Researchers have also found seven candidate genes that have shown a significant association with ADHD using the genome scan and candidate gene approach: DRD4, DRD5, DAT, DBH, 5-HTT, HTR1B and SNAP-25 (161). In addition, researchers have also suggested that neurological abnormalities may contribute to the etiology of ADHD, probably precipitated and exacerbated by both nature and nurture (162). Children diagnosed with ADHD have been shown to have decreased volume of the prefrontal cortex and parietal cortex. Some specific brain circuits have been associated with ADHD, including prefrontal-striatal-cerebellar and prefrontal-striatal-thalamic circuits (163). In vivo neuro-imaging studies are largely congruent with structural studies (164). Other influences may also include environmental factors such as prenatal factors, psychosocial adversity, history of acquired brain injury and diet (165,166). Over usage of modern technology has also been suggested to contribute to the development of the etiology of ADHD (167).
In their research, Sontag, Tucha, Walitza & Lange (168) claimed that “all drugs that are found to be therapeutically effective in ADHD affect central catecholaminergic neurotransmission, namely the dopaminergic and noradrenergic systems” (p. 2). Such neuro-stimulants are increasingly becoming the main pharmacological armaments to ameliorate the symptoms of ADHD (169). There is indication that some of the pharmacological treatments are effective up to 14 months (170). Molina et al. (171) have followed up with children for nearly a decade and have concluded that children with ADHD tend to persist with many social deficits regardless of whether treatment was used or not. This suggests that ADHD is a refractory condition despite many critiques of its existence (172).
A recent meta-analysis has suggested the diet and nutrition may play role in the pathogenesis of ADHD and may even, conversely, have the potential to prevent it (166). Alongside such discussions, there has also been some vested interest in trace elements such as vitamin D and their effects on neurodevelopmental disorders. As such, several hypotheses have emerged linking vitamin D to neurodevelopment prenatally and in children (12) where researchers have also suggested that deficiency in vitamin D may be a risk factor of ADHD in children (173). In the following section, the role of vitamin D and its association with neuropsychological symptoms of ADHD are explored. First the focus will be animal literature after which we move on to human studies.
Leffa et al. (174) in their literature review, have identified several models that would have heuristic value in studying ADHD, including Wistar–Kyoto hyperactive rat, Naples high-excitability rat, dopamine transporter knockout mouse, 6-hydroxydopamine-lesioned rat, and spontaneously hypertensive rat. While animal models of ADHD appear to exist, not many of them have examined the role of vitamin D. It has also been suggested that such models tend to be limited by the fact that they tend to capture ADHD-like symptoms or certain ADHD subtypes rather than the actual, complex behavioral disorder clinically defined as ADHD (168).
Some animal models have attempted to chart out brain regions that are likely to be critically involved at the behavioral level in what is clinically called ADHD. Ko, Burkert, McGrath & Eyles (175) examined maternally deprived experimental animals with vitamin D and reported that such animals tended to be marked with less apoptotic cells including those brain regions that have been previously linked to attention and goal-directed behavior that has been shown to be underfunctioning in ADHD. In consonance with human literature, both the prefrontal cortex, a brain region modulating thought and action and the hippocampus play significant roles in learning and memory, including attention, tend not work in synchronicity in animal models of ADHD (176).
Al-Amin et al. (46) examined the role of integrity of vitamin D in synchronicity of the prefrontal cortex and hippocampus. In animals deprived of vitamin D, significant reduction of volume of brain regions of interest was observed along with poor performance in spatial learning as well. Impairment in spatial learning, sometimes related to what is known as “working memory” that closely resembles attention, is one of the major neuropsychological features of children with ADHD (177). In addition to working memory, some studies have specifically examined the status of vitamin D and attention (15); particularly the types of inattention that hamper with one’s ability to filter irrelevant or intrusive thoughts (178). An experimental task known as pre-pulse inhibition has been used frequently to study the attentional system in animals (179). Some studies have explored whether such an experimental paradigm can be modulated by vitamin D. Burne et al. (180) have subjected rats to vitamin D deprivation during their prenatal and postnatal period. When they were tested as adults, they exhibited impaired pre-pulse inhibition. Another related model of pre-pulse inhibition is the latent inhibition paradigm, where pre-exposure to a stimulus with an irrelevant stimulus prevented new conditioned associations with that stimulus (181). It has previously been used as a model of ADHD with studies showing that disrupted latent inhibition mediates inattention and hyperactivity in mice (182). Similarly, developmental vitamin D DVD-deficient rats have been shown to perform poorly on the tests of latent inhibition including the shuttle-box paradigm (136). In addition, Burne et al. (180) found that prenatal vitamin D depletion in adult rats resulted in hyperlocomotion when performing the hole board test and the elevated plus maze test. Hyperlocomotion has also been associated with animal models of ADHD, where the effects of hyperlocomotion in mice were found to be reversible by methylphenidate, a first-line psychostimulant for ADHD (183). Taken together, these findings highlight that prenatal vitamin D depletion may alter ADHD-like cognitive impairments. However, not all studies lend support to this association. For instance, Orsinni et al. (184) found no difference in serum levels of vitamin D in mice purported to have a gene defect implicated with ADHD compared to controls. Thus, although existing animal literature implies that vitamin D may be implicated in the cognitive impairments of ADHD, the extent of this association remains unclear.
It has been argued that low levels of prenatal vitamin D may be associated with cognitive impairments similar to those found in ADHD. DVD deficiency has been modelled in mice by feeding females a vitamin D-deficient diet prior to mating and during gestation (7). Looking at cognitive behaviors in mice that have a DVD-deficiency, Harms et al. (7) assessed attentional processing and response inhibition using the five-choice serial reaction task and the five-choice continuous performance task, respectively. Although DVD-deficient mice showed no difference in performance on tests of altered attention, male DVD-deficient mice showed impaired performance on tests of response inhibition as they made more preservative errors, which have been highly implicated in ADHD (185). The findings imply that vitamin D deficiency may have resulted in alterations in impulse-control systems that govern perseverative responses. Impaired impulsivity, sometimes labelled as an integral part of executive functioning, is often thought to be one of the core impairments in ADHD and, in particular, the hyperactivity symptoms (186). In another study, DVD-deficient mice were found to have impaired performance on tests of learning, where they showed reduced performance in an operant conditioning task (187). Similar results were also found in rats with prenatal vitamin D deficiency, where subtle impairments in measures sensitive to higher functioning such as working memory, executive functioning and temporal organization of behavior were observed (136). These deficits correspond to neuropsychological impairments that have previously been documented to be common among people with ADHD.
The catecholaminergic system comprising of adrenalinergic, and dopaminergic neurotransmitters, has so far remained one of the few options for counteracting the symptoms of ADHD (188). In a pre-treatment with vitamin D, subsequent experimental depletion of catecholamine neurotransmission, induced by 6-hydroxydopamine (6-OHDA), did not compromise ventral mesencephalic dopaminergic activity (189). This region has also been implicated to play part in the expression of the ADHD subtype (190).
The first evidence linking human population with the level of vitamin D was derived from ecological observations, where there was an association between people living in certain latitudes and the epidemiology of ADHD. Since one of the major sources of vitamin D is exposure to the sun (191), this would imply that there is likely to be a relationship between latitude and level of vitamin D deficiency among populations dwelling in these particular regions. Data from the U.S Centers for Disease Control and Prevention (CDC) illustrate that the lowest ADHD prevalence rates are shown by states with high solar intensity (192). Similarly, cross-national data trends depict lower prevalence rates of ADHD in countries with higher sun-exposure including Mexico, Spain and Lebanon and higher prevalence rates in countries with lower sun-exposure such as France, Germany and the Netherlands (193). This effect was attributed to the possibility of improved circadian rhythms, but may have also been influenced by increased exposure to sunlight-induced vitamin D. Furthermore, altitude has also been thought to be a predictor of ADHD, where it was shown that regions at higher altitude had lower ADHD prevalence rates (194). Collectively, these findings propose that geographical latitude may contribute as a risk factor in the development of ADHD. In addition to latitude, there is also evidence to suggest that prevalence of ADHD is more common in the urban, rich and industrialized countries of North America and Western Europe (195). One plausible explanation is that the ‘concrete jungle’, typically characteristic of urban environments, disallows many from being exposed to the main source of vitamin D, namely, the sun. In other neuropsychiatric conditions, there is a hypothesis that connects season of birth with the development of neuropsychiatric disorders (196). Since season of birth has been equated with winter weather denoting limited sun exposure, this may indicate that ADHD might be critically influenced by lack of sun-exposure, implying a lack of vitamin D (197). However, there are limited studies linking ADHD with this hypothesis, these types of studies do not enable a clear disentanglement of cause and effect.
Literature reporting the status of vitamin D during pregnancy, the subsequent development of the fetus and post-natal developmental of the child does exist. Gale et al. (198) examined the effect of having optimal status of maternal vitamin D concentrations during pregnancy on indices of intellectual and behavioral functioning. The study suggested an optimal level of vitamin D has no negative effect on the development of the child. Some studies have suggested that sub-optimal level of vitamin D could trigger the development of ADHD. Morales et al. (199) conducted a prospective community study among 1,650 mothers at 13 weeks of gestation and later (4-5 years) examined the presence of ADHD symptoms on children. The conclusion drawn from this study was that elevated levels of vitamins could be strongly related to lower risk of developing ADHD-like symptoms.
Kamal, Bener and Ehlayel (200) examined the status of vitamin D in children and adolescent (5-18-year-old) who have been previously diagnosed with ADHD. Compared to matched-controls, children and adolescence with ADHD were observed to have lower level of vitamin D. This study could be criticized on the grounds that it did not take into account the amount of sun-exposure or the dietary intake. Goksugur et al. (173) also examined the status of vitamin D among 7-18-year-old with ADHD. Compared to typically developing children, they found ADHD children to have lower status of vitamin D. While a follow-up systematic review and meta-analysis of existing observational studies by Khoshbakht, Bidaki & Salehi-Abargouei (201) are congruent with such view, there are also dissenting views. Since diet also plays part in defining the level of vitamin D, Holton, Johnstone, Brandley & Nigg (202) have examined dietary intake of school and college-going students with ADHD. The indices of nutrients explored were reported as not being different between the cohort with ADHD and those designated as controls.
Tolppanen et al. (203) looked at the association between vitamin D and the presence of behavioral problems in childhood such as those commonly found in children with ADHD. Their data suggest no link between the status of vitamin D levels and indices of ADHD. Similar results were also found by Whitehouse et al. (204), where in addition to measuring behavioral and emotional outcomes, they measured language outcomes of the children sporadically from ages 2-17. Interestingly, they found that maternal vitamin D deficiency influenced language development in children, where these children were twice as likely to develop language impairments. Although the findings do not address ADHD symptoms per se, language development plays an important role in cognition, and language impairment has been frequently associated with children with ADHD (205). In another study, Gustafsson et al. (155) analyzed the vitamin D levels in blood samples from the umbilical cord collected at birth in both ADHD children and matched controls. The study found no difference in vitamin D between the two groups, suggesting that levels of vitamin D may have no influence on neurodevelopmental factors. However, it is noteworthy to mention that the study lacked statistical power to detect an association, which may have limited its findings. Despite such caveat, a more recent systematic review and meta-analysis appears to suggest that prenatal exposure to adequate vitamin D has the potential to lower the risk of ADHD (206). Similarly, Kots, Kotsi & Perrea (207) have conducted a meta-analysis on existing observational studies examining the status of vitamin D and ADHD. The author included 8 studies (n= 11,324 children). The chosen studies appear to endorse the view that ADHD individuals seem to have lower serum concentrations of vitamin D compared to controls.
A number of intervention studies have recently been conducted to shed light on the status of vitamin D and ADHD and some of these are summarized in Table 4. It is worthwhile to note that some of these studies have not specifically employed neuropsychological measures. However, in this review, symptoms of inattention and impulsivity are operationalized to be neuropsychologic in nature, a view in consonance with the previous suggestion that neuropsychological deficits are ‘markers of the disorder’ (208). In this context, inattention often reported in ADHD represents neuropsychological entity (209). Similarly, what is often viewed as a symptom of disruptive and impulse control disorders is frequently viewed as an integral part of executive functioning (208). Interestingly, neuropsychological impairments have been shown to be strong predictors of academic success and the resultant meaningful existence and quality of life (148).
| Research Design | Age |
Sample size | Cognitive Domains Affected | Serum Concentration | Significance of Association between Vitamin D and Cognition | Reference |
|---|---|---|---|---|---|---|
| RCT | 6-13 | 70 | Attention, Executive function | 25OHD |
Yes | Naeini et al. (2019) (210) |
| RCT | 5-12 | 54 | Attention, Executive function | 25OHD |
Yes |
Mohammadpour et al. (2018) (211) |
| Case-Control | 15-18 | 2,662 | Attention, Executive function | 25OHD |
Yes | Kamal et al. (2014) (200) |
| Cross-sectional | 7-18 | 90 | Attention, Executive function | 25OHD |
Yes | Goksugur et al. (2014) (173) |
| Prospective | Infant | 202 | Attention, Executive function |
25OHD3 |
No | Gustafsson et al. (2015) (155) |
In a double-blind randomized-controlled trial, Naeini, Fasihi, Ghazvini and Hasanzadeh (210) examined the effects of daily doses of vitamin D supplements (1000 IU) compared to placebo given to 6-13-year-olds with ADHD and matched controls for a period of three months. Symptoms of ADHD were assessed at baseline and after the completion of the intervention. The results show reduced impulsivity post intervention, while no differences in attention and response inhibition were detected. These findings highlight that vitamin D supplementation is effective in reducing some but not all symptoms of ADHD, and thus may be proposed as an adjunct to pre-existing treatments. In fact, there are studies where vitamin D was used as part of adjunct therapy. Assessing the outcomes of using vitamin D supplementation, Mohammadpour et al. (211) conducted a randomized, double blind, placebo-controlled trial where 5-12-year-old children with ADHD were given methylphenidate with either 2000 IU vitamin D or a placebo for a period of 8 weeks. ADHD symptoms were assessed at baseline, mid-intervention and at the end of the intervention using various indices of emotional, cognitive and behavioral functioning. Although both groups showed a significant decrease in ADHD symptoms following the intervention, vitamin D combined with methylphenidate resulted in greater improvements on evening out the symptoms. However, no difference in symptoms was found between both groups. Taken together, these results suggest that although vitamin D supplements may be beneficial in reducing some symptoms of ADHD, they are not effective on ADHD symptoms as a whole.
In the past decades, most of our understanding of disorders, disabilities and illnesses affecting cognition, behavior and emotion have been derived using a phenomenological approach. More recently, a wealth of research has emerged shedding light on the relationship between brain and behavior using a neuropsychological approach that aims to decipher the biological sub traces of thought. Existing research has suggested a direct relationship between thought, cognition and information processing in the mind and brain function. The neuropsychological assessment reflects cognitive function and its relationship with brain function (212).
Studies have suggested that neuropsychological functioning plays a crucial role in the quality of life and meaningful existence. Thus, compromised neuropsychological functioning has direct bearing on “employment status and associated societal costs, and also adversely affects driving safety, household task completion, social activity, physical independence, rehabilitation progress, coping, treatment adherence and mental health” (p. 891) (213). The present review and synthesis have examined the association between vitamin D status and cognitive symptoms in dementia, depression, schizophrenia and ADHD. The review studies suggest that vitamin D receptors and catalytic enzymes, localized in the multiple brain areas, are strongly involved in higher functioning (3). Evidence suggests that vitamin D plays a critical role in the integrity of the neuropsychological functioning and when this integrity is compromised, it results in various cognitive symptoms. In the current context, the presence of cognitive symptoms in dementia, depression, schizophrenia and ADHD were explored. A related aim was to shed light on the link between vitamin D and cognitive symptoms in dementia, depression, schizophrenia and ADHD. Dementia, with an increase in elderly population and a lifestyle that compromised cognitive functioning, are becoming common in all parts of the world. In terms of the link between dementia and vitamin D, the bulk of our evidence is derived from preclinical literature. However, the extrapolation of findings from animal literature to human studies has not yet provided us with the expected results. It is not clear whether complex cognitive impairment in people with dementia can be captured adequately in animal models (214). In addition, the prevailing views hold that senile plaques, neurofibrillary tangles and synapse loss are some of the hallmarks of dementia. While vitamin D has been linked to the development of senile plaques, neurofibrillary tangles and synapse loss (215), its role has not been well charted. Furthermore, oxidative/nitrosative stress, decreased antioxidants, mitochondrial damage and other factors have been implicated in the pathological mechanisms involved in the development of dementia. Therefore, the role of vitamin D in cognitive symptoms in dementia is yet to yield robust results.
Depressive symptoms are frequently reported in the general as well as the clinical population. Depression outstrips other conditions when considering its negative impact on the QoL and meaningful existence (216). There is an indication that the deficiency of vitamin D may serve as a precursor for the development of depressive disorder and conversely, intervention to enhance the activity of vitamin D has potential to mitigate the cognitive symptoms. There are three major neuropsychological impairments associated with major depression: executive function, attention and memory. These impairments can be localized in various brain regions such as the hippocampal pre-frontal circuit, concurrently found to be a site for vitamin D receptors. While there is an abundance of cross-sectional studies at our disposal, they are yet to be equipped to establish a cause-and-effect relationship between vitamin D and the cognitive symptoms in depressives illness. There are also conflicting views in meta-analytic reviews on what constitutes clinical decision levels for vitamin D deficiency (217). Overall, the evidence is not yet robust to implicate the role of vitamin D in cognitive symptoms among people with depressive illness. More evidence is therefore warranted.
Humanity has been grappling with psychosis for a long time, but there is still no known cure. Many hypotheses have been suggested to play part in the aetiology of psychosis. In addition to psychotic symptoms, people with schizophrenia are known to be marked with cognitive symptoms. Neuropsychological impairments are increasingly recognized to be one of the core deficits in people with schizophrenia. Specifically, processing speed has consistently been viewed as the central cognitive symptom in people with schizophrenia and this has been largely replicated in an animal model wherein the role of vitamin D is implicated. In human literature, there is a growing body of evidence suggesting the association of vitamin D deficiency with an increased risk of developing schizophrenia and compromising neuropsychological functioning. In studies on developing foetuses, the role of vitamin D in the development of neuropsychological impairment in schizophrenia has been suggested, highlighting the effects of vitamin D deficiency during pregnancy. There is merely suggestive evidence of the potential benefits of vitamin D supplementation on improving psychotic symptoms (123). Intervention studies are still in their infant stages and hence it is difficult to draw any conclusions on the effect of vitamin D and cognitive symptoms of schizophrenia. Lack of such intervention studies contributes to the view that the verdict is out to implicate the role of vitamin D in the cognitive symptoms of psychotic illnesses.
ADHD is a neurodevelopmental disorder that was once thought to be limited to children representing western populations. However, it is now being increasingly recognized in many parts of the world. In preclinical research, findings do suggest that vitamin D is associated with cognitive impairments implicated in ADHD, but evidence drawing a clear link between ADHD and vitamin D appears to be lacking and further research to develop experimental grounds is required. There is some debate on whether existing animal models tap into ADHD-like symptoms, some subtypes of ADHD or a broad spectrum of ADHD symptoms. Some studies have highlighted the role of prenatal vitamin D in neurocognitive development which, in turn, would imply the critical role of vitamin D in mediating cognitive impairments and ADHD. There is, however, a dearth of studies to further elaborate on such a link and intervention studies are also lacking. Furthermore, there is a concern regarding how vitamin D might be employed. Some studies have implied it could be used complementary to pharmacotherapy, while the suggestion of its usage as alternative has also been put forward. Overall, the specific cognitive symptoms in ADHD and their relationship to vitamin D has yet to be established.
While more studies are needed to shed light on the relationship on variation in vitamin D and dementia, depression, psychosis and ADHD, there are the various conceptual and methodological problems that need to be contemplated if the view that vitamin D has direct bearing on cognitive symptom is to gain more ground. Some of these issues include considerations surrounding the utilization of animals to shed light on the relationship between vitamin D and these conditions. The second issue is regarding the heterogeneity of cognitive symptoms. The third is regarding the question of the optimal source of vitamin D to be used on these conditions. Finally, there are still issues pertinent to the preventive and therapeutic aspects of vitamin D. These four points are discussed below in tandem.
Animal models of cognitive disorders. Various models of dementia, depression, psychosis and ADHD currently exist in the literature. There is a concern about whether animal models can be used to model neurocognitive symptoms in humans. Most of the animal models discuss the sub-types rather than addressing the complex spectrum of clinically cognitive symptoms related to this. It has been widely established that animal experimentation does not always make headway to clinical application.
The heterogeneity of cognitive symptoms. As the above review suggests, the scrutinized disorders—dementia, depression, psychosis and ADHD—have been implicated to have cognitive symptoms and the cognitive symptoms have been linked with a variation in the integrity of vitamin D. One source of attrition on this is regarding the heterogeneity of the cognitive symptoms identified between and within the disorders. It is possible that cognitive symptoms are different in each of these disorders despite being interconnected to variation in vitamin D. However, in order to heighten the scientific merit of exploring cognitive symptoms in each of these disorders, it would be necessary to establish a broad spectrum of cognitive domains that implicate vulnerability to this disorder. The integrity of vitamin D to cognitive symptoms of these disorders also needs to be established firmly.
The optimal source of vitamin D. Humans can acquire their supply of vitamin D from exposure to the sun or via diet. The subtypes of vitamin D are often classified as cholecalciferol (D3) and ergocalciferol (D2) (218). However, there is a dearth of experimental designs showing how these two forms actually influence the concentration of vitamin D and how this impacts the manifestation of cognitive symptoms. There is also growing concern over what constitutes the optimum dose of vitamin D. Serum vitamin D levels tend to fluctuate with seasonal variations that are specific to each geographical and latitudinal location. In humans, the most important type of vitamin D falls under the umbrella cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2) which has been linked to poor nutrition and spending less time outdoors. In the literature pertinent to cognitive symptoms and disorders such as those under scrutiny in this review—dementia, depression, psychosis and ADHD—it is not clear whether vitamin D deficiency is due to poor nutrition or the lack of exposure to sunlight. Related to this, although vitamin D can be obtained dietarily (ergocalciferol or D3), there are few diets that are known to be a rich source of vitamin D (219). The second type of vitamin D is cholecalciferol, a natural source of vitamin D derived from sun exposure (5). The role of ‘sunshine vitamin’ has been used to support whether there are links between geography and cognitive symptoms in people with dementia, depression, psychosis and ADHD. Thus, populations lying closer to parts of the earth receiving abundant sunshine (lower latitudes) have reported lower prevalence of dementia (220). Conversely, the high prevalence of dementia has been suggested to occur in the region where there is less exposure to the sun (221). Lower indices of vitamin D have been reported in different parts of the world. It has been viewed as ‘silent’ pandemic and estimated that one billion people have been affected by it (5). However, the view that cholecalciferol is linked to latitude has been questioned within the view that the presence of sunshine is not always associated with adequate vitamin in the serum. Populations that live with abundant sunshine have not been necessarily found to have an adequate level of vitamin D (222). This argument was further corroborated in research conducted on Middle Eastern countries that receive sufficient sun exposure, yet still suffer from vitamin D deficiency (227).
The role of vitamin D in prevention or treatment. There are plenty of studies supporting the view that variation in vitamin D might have direct bearing on the development of dementia, depression, psychosis and ADHD. It is not clear, however, whether the impact of vitamin D is on the cognitive symptoms under scrutiny here. Most of the existing research has defined the role of vitamin D in terms of clinical improvement rather than the amelioration of cognitive symptoms. It needs to be firmly established whether vitamin D is a part of the pathogenesis or if the observed pathology of vitamin D is just an innocent bystander. Given such background, there is a need to lay groundwork on whether to employ vitamin D as adjunct, complementary or alternative form of treatment. Some studies have suggested the role of vitamin D in these disorders should be directed toward risk factor modification prior to the onset of the disease; other studies have suggested the role of vitamin D prevention and still some others have suggested vitamin D be used as treatment for these conditions. Further research is recommended to answer questions of causality, the effect of vitamin D on the pathogenesis of neurocognitive disorders, and to explore the role that vitamin D plays in the prevention/treatment of the progressive course of dementia, depression, schizophrenia and ADHD. Larger scale randomized control trials to determine the efficacy of vitamin D as an alternative or complementary treatment option are also required.
The aforementioned discussion has charted existing literature on the link between cognitive symptoms in dementia, depression, psychosis and ADHD. An abundance of literature shedding light on such a link has emerged. However, current evidence is at a premature stage and hence cannot be sought to attribute the cognitive symptoms of these conditions to the under- or over-functioning of vitamin D. If this line of research is to have meaningful implication and not render the role of vitamin D into another “phlogiston theory”, more studies are needed to increase validity and ecological reliability of the animal models that examine cognitive symptoms in dementia, depression, psychosis and ADHD. The second issue to tackle is the reduction of the heterogeneity of cognitive symptoms associated with these conditions. The third is to establish an optimal source of vitamin D that could be utilized to properly study its effects on cognitive symptoms. Finally, studies establishing the role of vitamin D in the prevention and treatment of these conditions are essential.
Our final research and policy thoughts in order to heighten such link are, firstly, more studies are needed to increase the ecological validity of the animal models purporting to shed light on cognitive symptoms associated with hypovitamin D. Secondly, there is need for delineating core cognitive symptoms associated with each of these disorders. Thirdly, consensus is needed to establish the optimal source of consuming vitamin D. Fourthly, studies would be needed to determine whether vitamin D should be utilized for preventive or therapeutic purposes. Finally, guidelines are needed to suggest whether vitamin D should be used as an alternative or complimentary therapy.
The authors thank their institutions for their continued support. Nithila Mariam Roy, Lara Al-Harthi and Neela Sampat contributed equally to this paper. The technical and language editing support provided by The Editing Refinery, MD, USA is gratefully acknowledged. This research was not supported by grant from any funding agency or the public, commercial, or not-for-profit sectors. The support provided by SQU as PhD bench fees for Neela Sampat is highly acknowledged. The authors declare no conflict of interests.
ADHD
Attention deficit hyperactivity disorder
Amyloid Beta
Amyloid-ß protein precursor
Amyotrophic lateral sclerosis
Alzheimer's disease
Brief cognitive rating scale
Brain derived neurotrophic factor
Centres for disease control and prevention
Central nervous system
Computed tomography
Disability adjusted life year's
Diagnostic and statistical manual of mental disorders
Developmental vitamin D
Functional magnetic resonance imaging
Gamma aminobutyric acid
International classification of diseases
Membrane associated rapid response steroid binding receptor
Major Depressive disorder
Magnetic Resonance imaging
Neurotrophic growth factory
Parkinson 's disease
Positron emission tomography
Quality of life
Single-photon emission computed tomography
Vitamin D receptor
6-Hydroxydopamine