1 Laboratory of Biodiversity of Microorganisms, Institute of Microbiology, Academy of Sciences of the Republic of Uzbekistan, 100128 Tashkent, Uzbekistan
2 Faculty of Biology, National University of Uzbekistan, 100174 Tashkent, Uzbekistan
Abstract
Background: Due to the constant and improper use of chemicals,
including pesticides, many substances, and their degradation products can
accumulate in the soil and negatively affect its organisms. Methods: In
this study, morphological methods, Gram-staining, and Matrix-Assisted Laser Desorption/Ionzation Time of Flight Mass Spectrometry
(MALDI-TOF MS) methods were
used to isolate bacteria from agricultural soils, while genetic identification
was conducted using 16S rRNA. The density of bacteria was determined using the
spectrophotometric method, and the residual amount of cypermethrin was determined
and analyzed using Gas chromatograohy-mass spectrometry (GC-MS) methods. Results: Nine isolates were obtained
from various agricultural soils. Isolate No. 3 showed the greatest effectiveness
against cypermethrin and was selected for further research. Isolate No. 3 was
identified as the Ochrobactrum intermedium strain PDB-3 and was
registered in the National Center for Biotechnology Information (NCBI) database
(GenBank: OL587509.1). Using this strain, the influence of various external
factors on the degradation of cypermethrin was studied. This bacterium
demonstrated 100% degradation of cypermethrin in 20 days under optimal
conditions (temperature: 30 °C; optical density (OD) = 0.2; cypermethrin concentration: 80
Graphical Abstract
Keywords
- Ochrobactrum intermedium
- cypermethrin
- degradation
- 16S rRNA
- GC–MS
Pyrethroids are insecticides that contain an ester bond formed by an alcohol and an acid [1]. These insecticides are synthetic derivatives of pyrethrin compounds produced by chrysanthemum plants [2]. This group of pesticides has been used in agriculture for more than 40 years and is widely used in forestry, horticulture, healthcare, home care, and for protecting textiles and buildings [3, 4]. A complete or partial ban on using organochlorine and organophosphate pesticides contributes to the wider use of pyrethroids in agriculture and households, accounting for about 25% of the global pesticide market [5, 6].
Cypermethrin ((
Soil samples were collected from various farm sites (where multiple plants were planted) in the Samarkand region of the Republic of Uzbekistan—where pesticides (chlorpyrifos and cypermethrin) have been used for many years. Soil samples were taken from depths of 0–20 cm using the envelope method and placed in sterile bags. Under laboratory conditions, the soil was cleared of all additional parts (large stones, plant debris, etc.) and filtered through a stainless-steel sieve with a diameter of 2 mm.
The cypermethrin standard (purity 97), acetone, and all other substances and
reagents were chemically pure and commercially available. Stock solutions (40
The collected soil samples were mixed and additionally contaminated with cypermethrin, then kept in a thermostat at 30 °C for 2 months. After a month, 10 g of soil was added to Erlenmeyer flasks containing nutrient broth with cypermethrin and incubated on a rotary shaker at 30 °C with a rotation speed of 150 rpm for 48 hours. On day 5, appropriate dilutions were prepared from the enrichment culture and added to agar + cypermethrin plates. The grown individual colonies were subcultured on MSM plates supplemented with higher concentrations of cypermethrin.
Identification of bacteria was carried out by studying morphological and cultural properties, Gram-staining, and Matrix-Assisted Laser Desorption/Ionzation Time of Flight Mass Spectrometry (MALDI-TOF MS) analysis. The 16S rRNA method determined the nucleotide sequences to ensure a more accurate identification.
First, a nutrient broth was prepared, and a bacterial culture was added. Then,
the broth was grown at 30 °C for 12 hours. Next, bacterial DNA was
isolated from the bacterial culture grown in 10 mL nutrient broth using the
RIBO-prep (InterLabServis, Moscow, Russia) reagent kit. DNA extraction was
performed according to the protocol provided in the kit. Extracted DNA samples
were analyzed on 0.9% agarose and a spectrophotometer before storing at –20
°C. PCR was used to amplify the 16S rRNA gene, which had been selected
for molecular genetic identification of bacterial cultures. The following primers
were used to amplify the 16S gene: 5
The PDB-3 strain was introduced into soil containing 80
A test culture was grown for 24 hours at a ratio of 50:1 and added to sterile
soil contaminated with cypermethrin. The cell titer of the culture fluid was
10
Studies on the biodegradation of cypermethrin by the isolated culture were
conducted using sterile soils. An initial cypermethrin concentration of 80
Cypermethrin concentrations were determined using an Agilent 8890B gas chromatograph mass spectrometry with split and splitless evaporators, which was used in conjunction with an Agilent 5977B series GC/MS in SIM, SCAN, and electron impact ionization (EI) modes. Analysis conditions: Gas chromatography analysis parameters. Analytical column HP: 5 ms; Ultra Inert: 30 m • 250 µm • 0.25 µm. Injection volume of 1 µL; Splitless injection mode. Evaporator temperature 280 °C UI liner, splitless, single throat, fiberglass Sputtered Gasket Gold plated, The evaporator temperature was 280 °C. Hydrogen was used as the Ccarrier gas Hydrogen, and a constant flow, of 1.2 mL/min. The thermostat program was 60 °C for 1 minute, then 40 °C/min to 170 °C, then 10 °C/min to 310 °C, then hold for 2 minutes. The temperature in the transport line was 280 °C. Data collection mode: SIM, SCAN. Gain factor 1.00. Source temperature: 250 °C. Quadrupole temperature: 150 °C.
Statistical analysis and exponential curve fitting were performed using Origin
8.6 software (Microcal Software Inc., Northampton, MA, USA). Results were
expressed as the mean
To isolate microorganisms resistant to the insecticide cypermethrin, we used
soil from various agricultural plots in the Samarkand region, which had been
treated with pesticides chlorpyrifos, cypermethrin, and others for many years.
Under laboratory conditions, soil samples were also contaminated with
cypermethrin at 40
The results showed that isolate 3 was the most effective at degrading cypermethrin. This microorganism was isolated from soil brought from an orchard. In further studies, this isolate was selected to determine the effective degradation of high concentrations of cypermethrin.
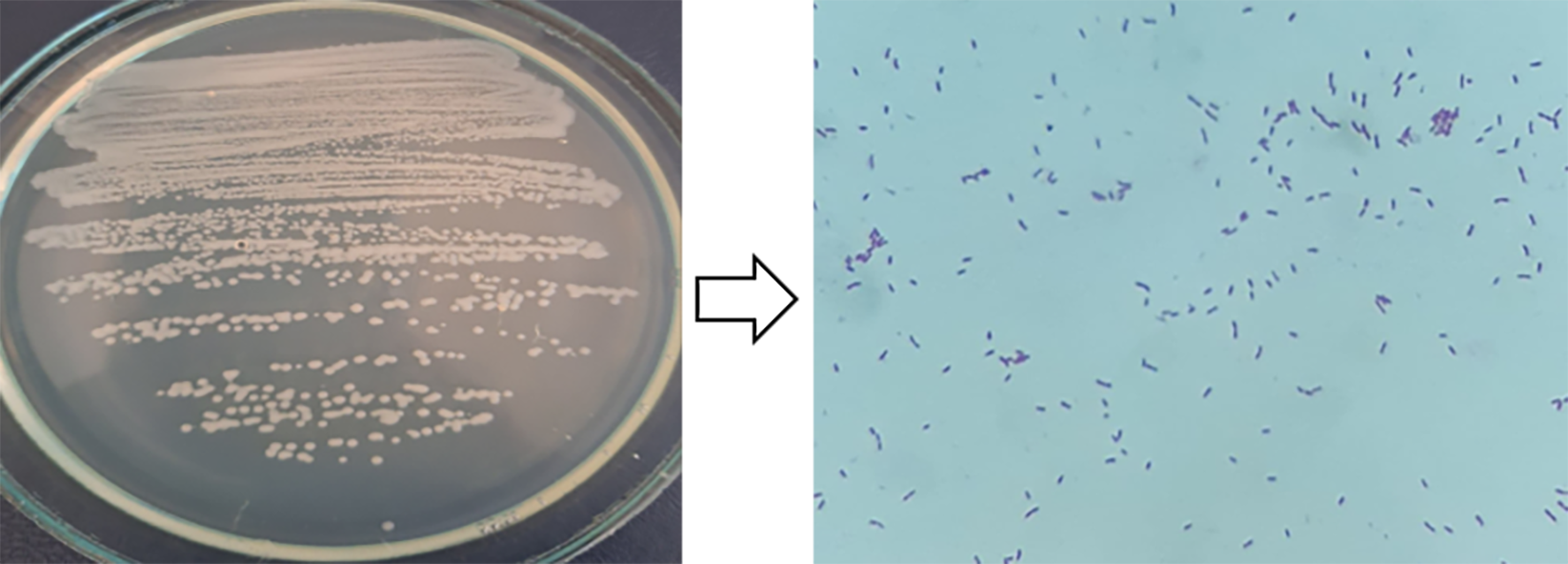
Under laboratory conditions, morphological and cultural properties, some physiological and biochemical properties, and the movements of living cells were studied under a microscope. The cells are rod-shaped, size 1.2–2.0 µm, solitary, and on MPA, they form smooth convex, shiny colonies, are white–gray in color, 2–2.5 mm in diameter, do not form spores, and have a pH of 6.9–7.2. Gram-staining indicated that this strain is a Gram-negative bacterium (Fig. 1). MALDI–TOF MS analysis was also performed.
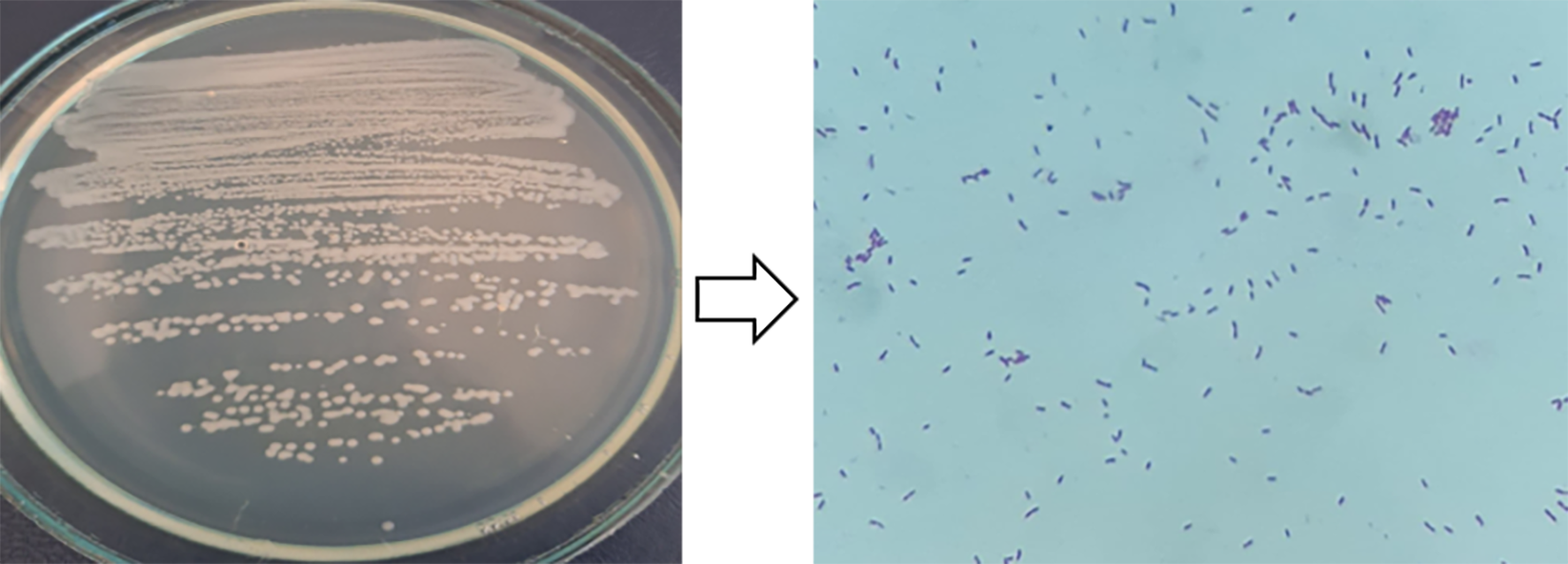 Fig. 1.
Fig. 1.Gram-staining.
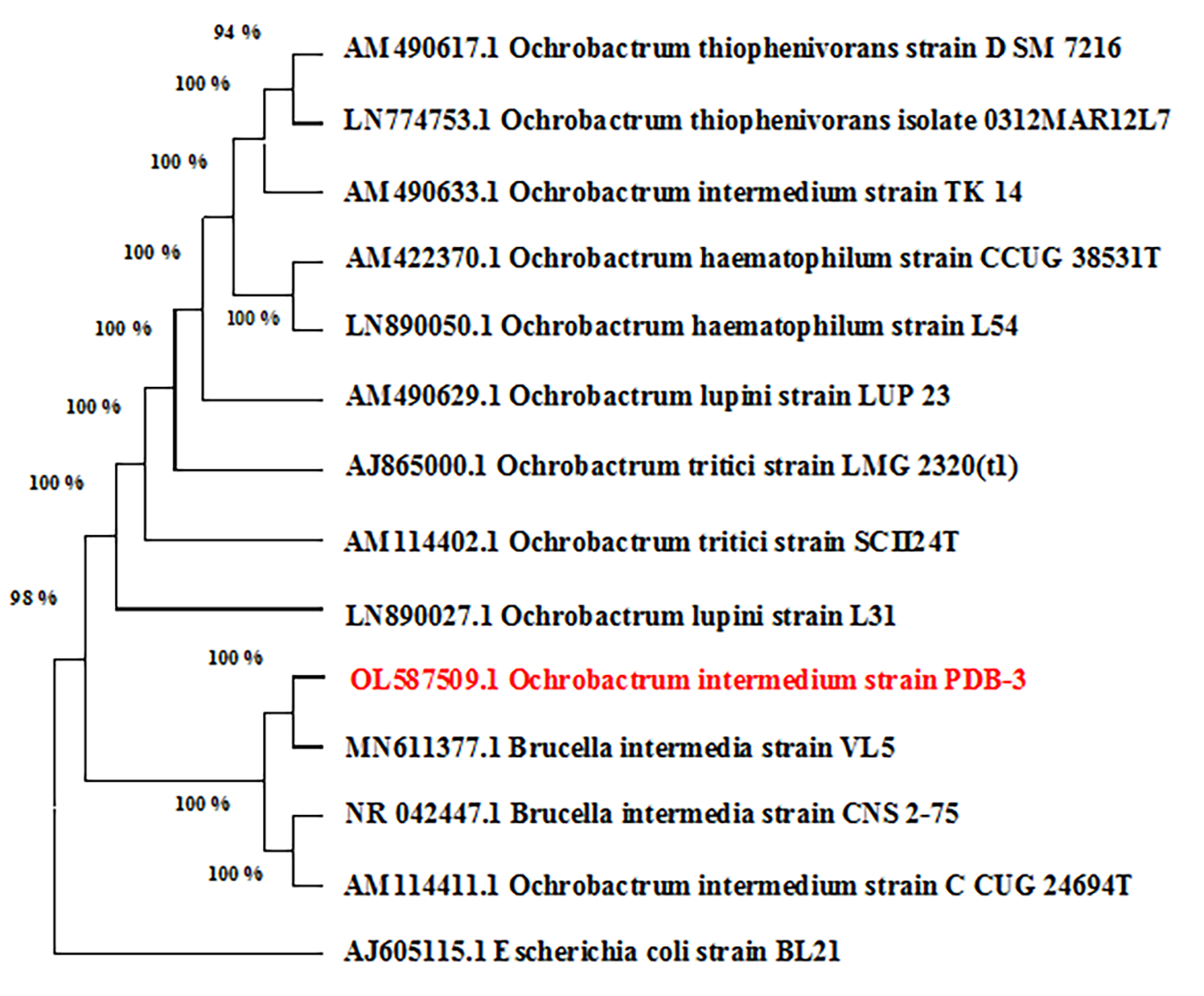
The obtained results tentatively suggest that this strain is Ochrobactrum intermedium. To ensure a more accurate identification, the 16S rRNA nucleotide sequence was determined. The results show 100% similarity to the species Ochrobactrum intermedium (Fig. 2).
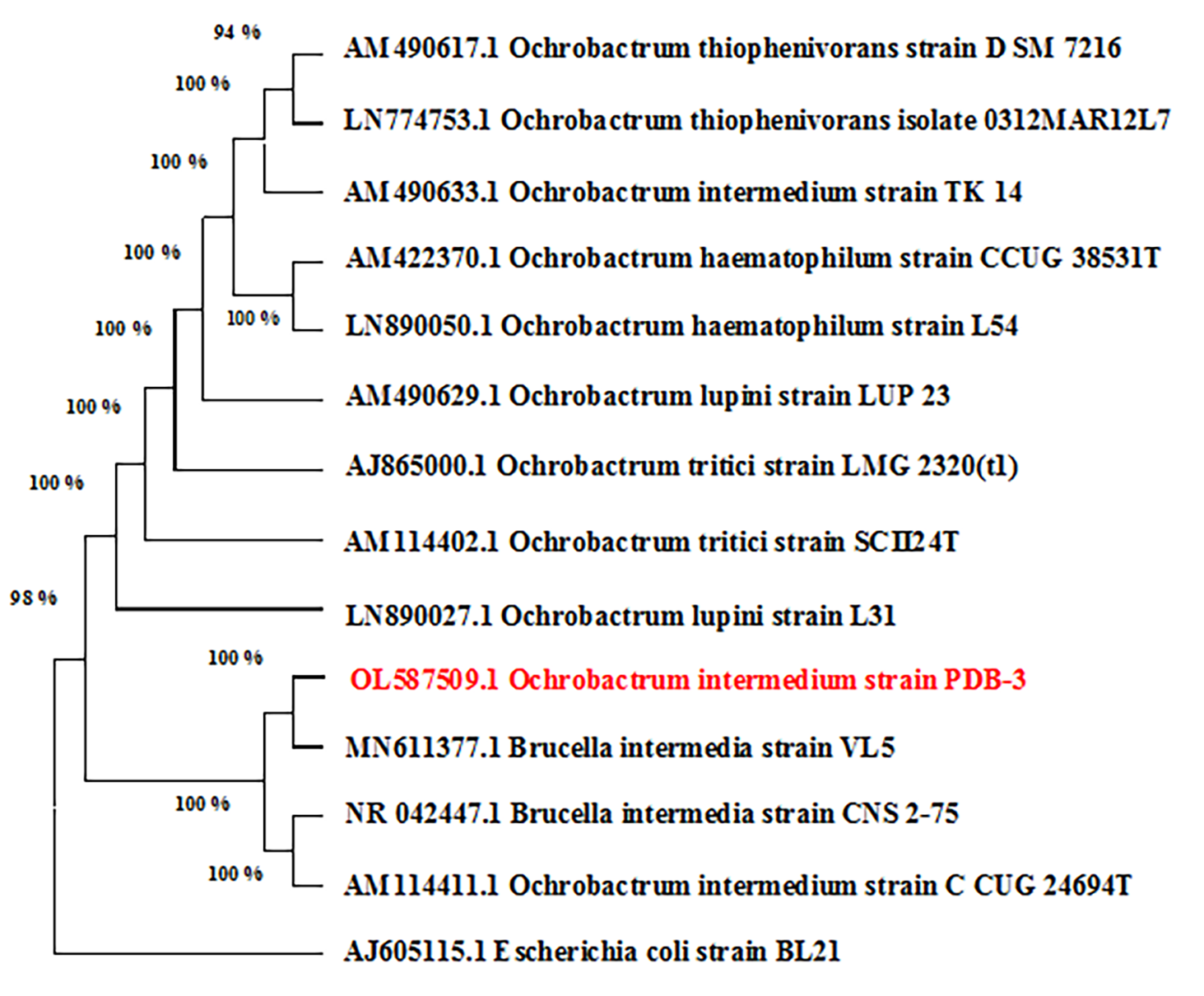 Fig. 2.
Fig. 2.Phylogenetic tree of Ochrobactrum intermedium strain PDB-3.
The 16S rRNA gene sequence data of Ochrobactrum intermedium strain PDB-3 were processed using UniGene Ver35 and Chromas software, and the low-confidence peaks were removed. Then, the DNA sequences were analyzed against the NCBI nucleotide database using BLAST. Highly similar DNA sequences were aligned using the MegaX program and the ClustalW algorithm. A phylogenetic tree was constructed using the Neighbor-Joining algorithm in the MegaX program.
Thus, strain PDB-3 Ochrobactrum intermedium showed very close similarity (100%) to strains of Ochrobactrum intermedium. This indicates that the strain under study belongs to the phylum Ochrobactrum intermedium. The strain was registered in the NCBI database: GenBank: OL587509.1.
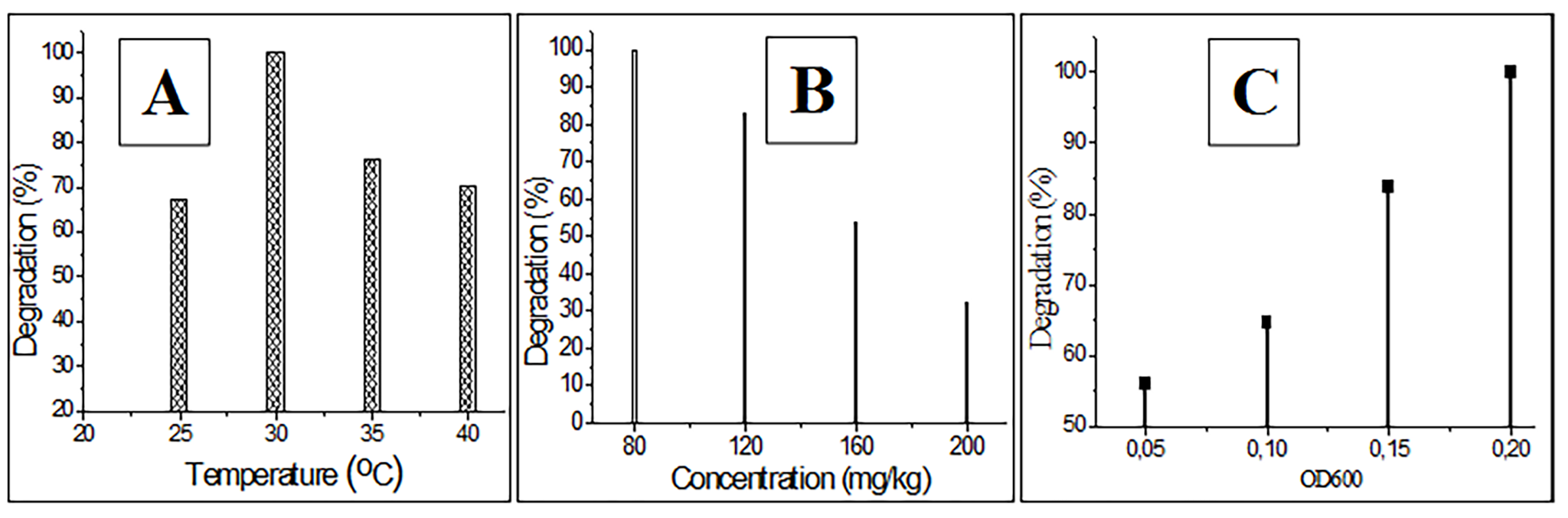
The influence of external factors on cypermethrin decomposition was studied
under laboratory conditions. Strain PDB-3 was added to soil containing 80
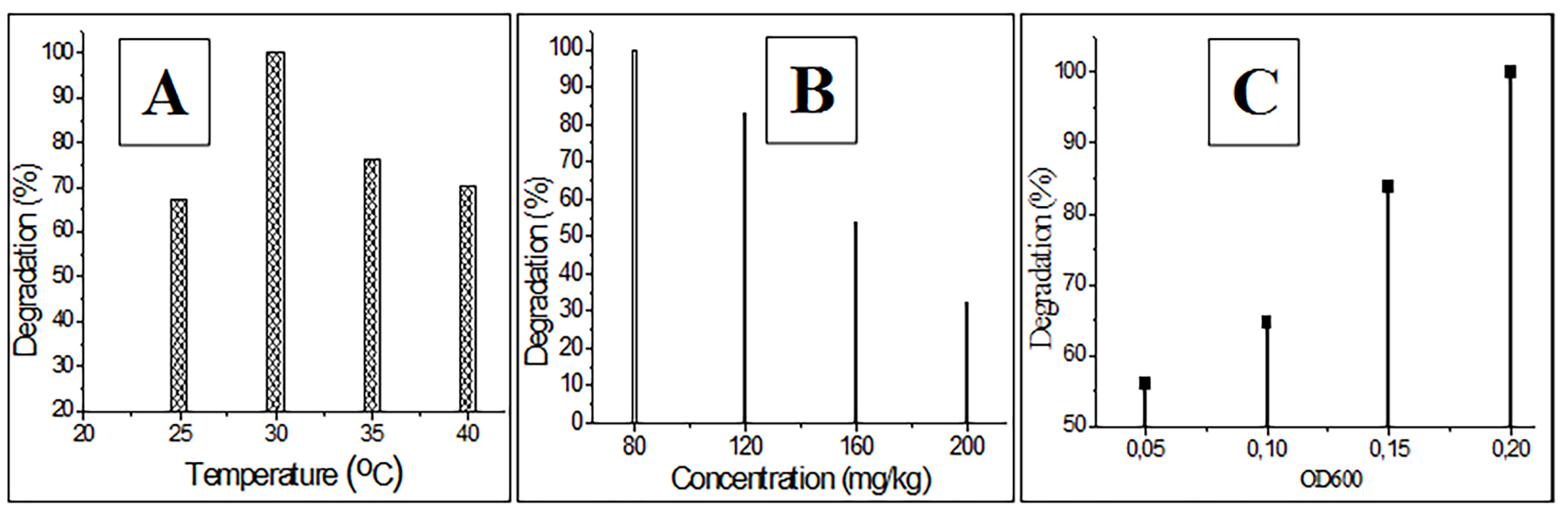 Fig. 3.
Fig. 3.Determination of the influence of some external factors on the degradation of cypermethrin. (A) Temperature. (B) Cypermethrin concentration. (C) Optical density.
The potential for maximum microbial degradation of pesticides depends entirely on optimal environmental conditions, such as initial concentration [27], pH, temperature, additional energy sources, and inoculum concentration [28]. Biotechnological processes based on microorganisms are usually influenced by environmental factors [29, 30]. Lysinibacillus cresolivuorans strain HIS7 was isolated from contaminated soil. Thus, the degradation process of cypermethrin was studied using this strain under the influence of various environmental factors. The optimal parameters for the biodegradation of cypermethrin were previously established as an incubation period of 8 days, inoculation volume of 3 mL, temperature of 35 °C, pH 7, and an additional source of carbon and nitrogen, which increased decomposition from 57.7% to 86.9%. In soil, the HIS7 strain degraded cypermethrin to 93.1% within 42 days [31].
Studies on the biodegradation of cypermethrin were carried out in sterile soils.
The soil was dried in laboratory conditions, and all unnecessary parts were
removed, e.g., large stones, plant debris, etc. Then, it was sterilized in an
autoclave at 121 °C for 45 minutes. Cypermethrin solutions were prepared
using acetone and added to a small part of the soil (approximately 15–20% of
the total soil mass); the soil was thoroughly mixed with the cypermethrin
solution (80
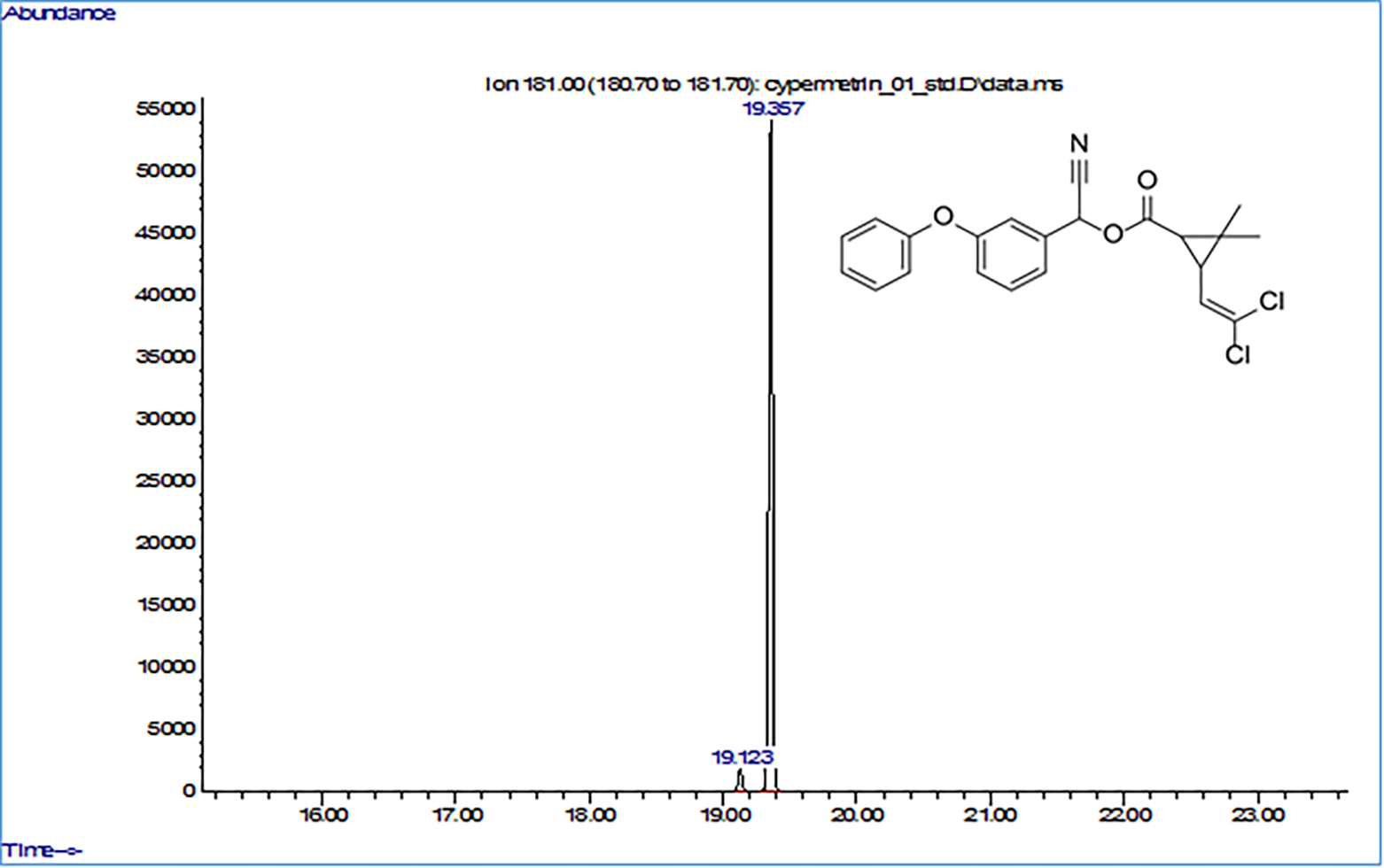
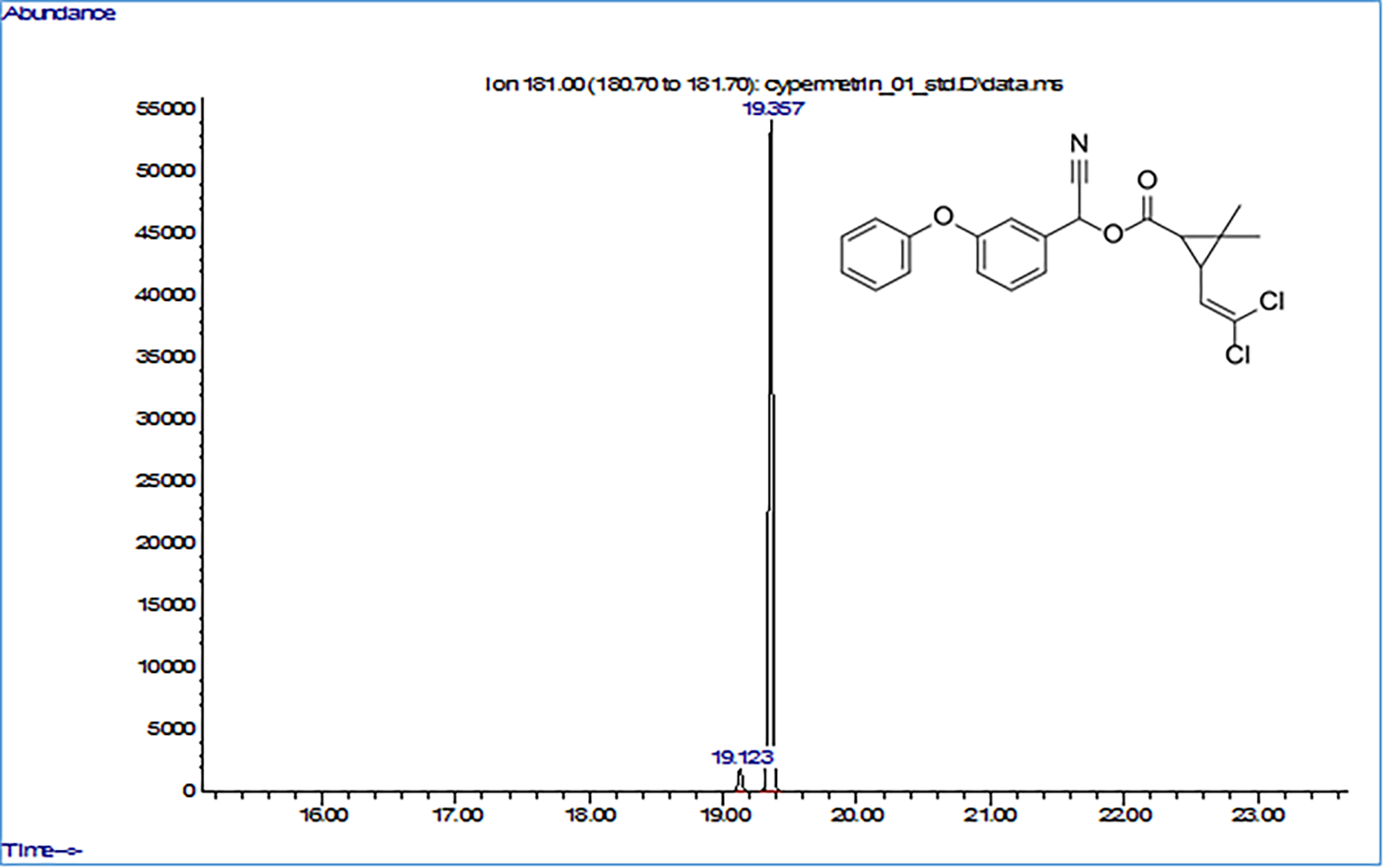 Fig. 4.
Fig. 4.Initial Gas chromatograohy-mass spectrometry (GC–MS) chromatogram of cypermethrin.
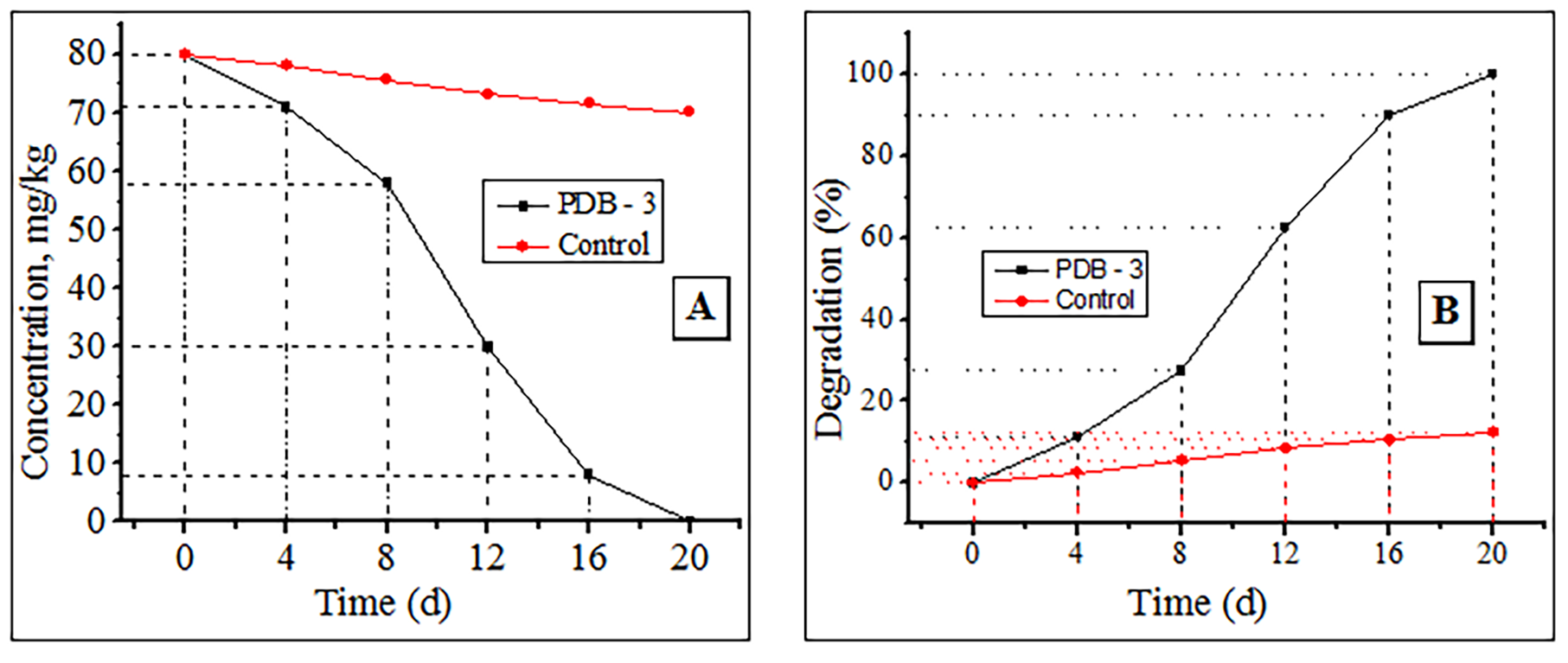
Thereafter, this peak gradually decreased and was undetectable in the soil samples on day 20. In the control sample, virtually no changes were observed during the experiment. Based on the chromatographical analyses, the decrease in the initial concentration of cypermethrin (Fig. 5A) and the percentage of degradation (Fig. 5B) were calculated.
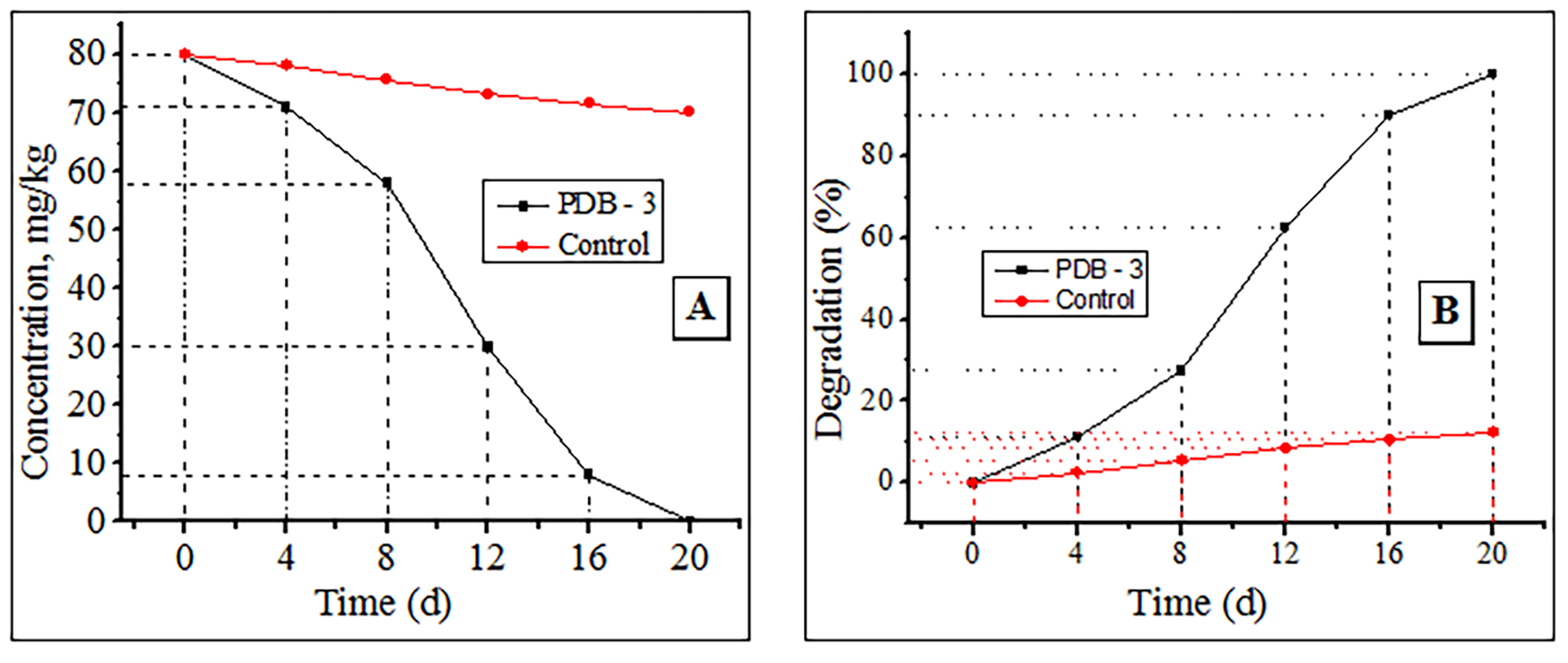 Fig. 5.
Fig. 5.Cypermethrin degradation using PDB-3. (A) Decrease in initial concentration. (B) Degradation percentage.
Fig. 5 shows that strain PDB-3 successfully decomposes 80
Cypermethrin has been widely used in agriculture and households since the late
1980s [7]. However, due to its lower bioavailability, it persists in soil for a
long time; its half-life ranges from 100 to 200 days [32]. Many studies examining
the toxicity and persistence of cypermethrin indicate the need to develop methods
for removing it and its metabolites from the environment. Biodegradation based on
the use of microorganisms is the preferred method for breaking down pesticides
into simple inorganic chemicals since it is environmentally friendly and
cost-effective [33]. Numerous studies have been conducted on isolating,
characterizing, and selecting pesticide-degrading microorganisms [34, 35]. In our
study, strain PDB-3 was isolated and identified from soils exposed to pesticides.
Morphological and cultural studies and MALDI-TOF MS and 16S rRNA analyses showed
that this strain belongs to the bacterial species Ochrobactrum intermedium. This
type of bacteria was first described in 1998 [36]. Based on recent genome
comparison studies, the genus Ochrobactrum has been reclassified, and its species
are included in the genus Brucella [37, 38]. Ochrobactrum intermedium
degrades many pollutants [39], including pesticides [40, 41, 42]. For example,
Ochrobactrum intermedium SP9 degraded the pesticide cypermethrin to 69.1% within
8 days [43]. Ochrobactrum anthropi has been used to degrade
2,4-dichlorophenoxyacetic acid (2,4-D) [44]; Ochrobactrum anthropi
strain L1-W successfully degraded air pollutant Di-2-ethylhexyl phthalate (DEHP)
[45]. The effectiveness of bacteria in decomposing pesticides depends on the
following environmental factors: pH and temperature [33], initial concentration
of pesticide [46, 47], optical density of microorganisms, etc. We conducted
studies to determine the influence of temperature, initial pesticide
concentration, and optical density of the PDB-3 strain. The optimal parameters
for degrading cypermethrin using the PDB-3 strain were established: temperature:
30 °C; time: 20 days; concentration: 80 mg/kg; OD: 0.2. It was shown
that changing these parameters negatively affects the bacterial growth phases
(soil microbial analysis showed a decrease in bacterial colonies under such
conditions). This, in turn, slows down the degradation process. Our results
confirm the data from previous studies on the biodegradation of the pyrethroid
insecticide fenvalerate by the strain Stenotrophomonas sp. ZS-S-01. It
was inactive at temperatures below 25 °C or above 35 °C, at
which significant cell death was observed and degradations of 46.5% and 56.6%,
respectively, were achieved [35]. The degradation percentage was 99% at a
temperature of 30 °C, which is the optimal temperature for ZS-S-01 [35].
It is also reported that the initial pesticide concentration plays a significant
role in the degradation process. Various concentrations of cypermethrin were
tested, ranging from 20 to 125 mg/L. At a concentration of 40 mg/L, cypermethrin
decomposed up to 81%, up to 51% at 80 mg/L, and up to 22% at 125 mg/L [48].
The Ochrobactrum lupini strain DG-S-01 absorbed approximately 87% of
the
Studies on the degradation of cypermethrin in laboratory conditions were carried out using selected optimal parameters, and the decomposition of cypermethrin was calculated using the following formula [50]:
where X is the decomposition of the pesticide; Cx is the pesticide concentration (mg/kg) in an environment with microorganisms that decompose cypermethrin; Cck is the concentration of cypermethrin (mg/kg) in a microorganism-free medium.
It has been reported that a new bacterial strain, BSF01, identified as
Bacillus subtilis, was isolated from activated sludge. It showed high
efficiency in the degradation of cypermethrin, which degraded 89.4% at a
concentration of 50 mg/L [18]. A fungal strain, Aspergillus niger YAT,
was also isolated from Chinese brick tea and could degrade 54.83% of
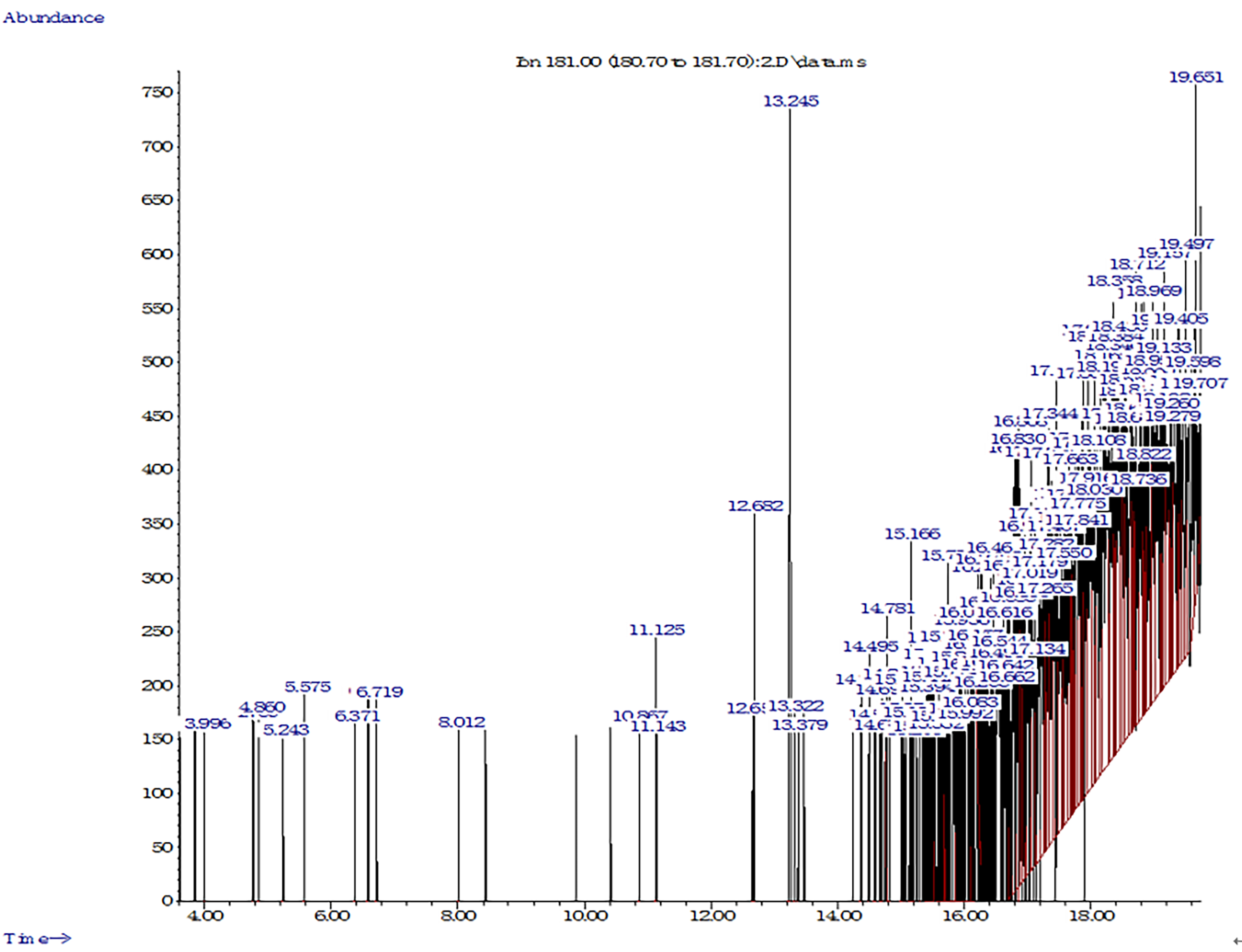
During our research, new intermediate metabolites were discovered (Fig. 6).
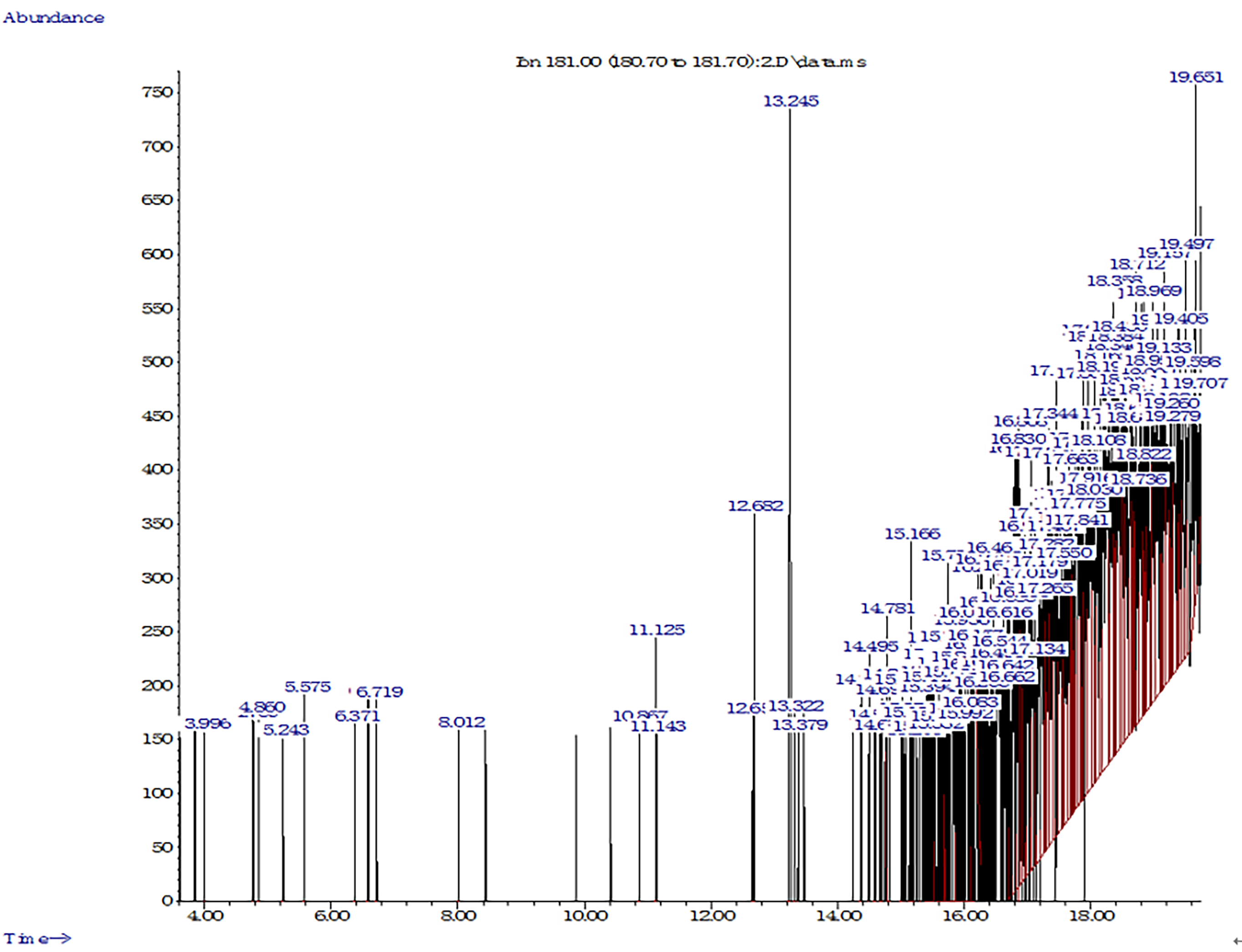 Fig. 6.
Fig. 6.GC–MS chromatogram of cypermethrin degradation in soil by PDB-3.
The results obtained using the GC–MS method confirmed the degradation of
cypermethrin by strain PDB-3. The formed metabolites appeared on day 16 and were
compared using a GC–MS library of compounds. Strain PDB-3 degraded cypermethrin
into compounds with lower molecular weights. The peaks noted at retention times
of 3.996 min, 5.575 min, 6.371 min, and 12.678 min were identified as phenol,
anethol, citral, and methyl stearate. Additionally, 2-hydroxy-3-phenoxy
benzeneacetonitrile (16.778 min), 3-phenoxybenzaldehyde (16.946 min), and
3-phenoxybenzaldehyde (17.775 min) were observed. Similar studies have confirmed
that cypermethrin is transformed into 3-phenoxybenzaldehyde and
In this work, the Ochrobactrum intermedium PDB-3 strain was isolated from agricultural soils contaminated with the pesticide cypermethrin. This strain completely degraded the initial amount of cypermethrin. Moreover, various intermediate metabolites were formed due to the decomposition of this pesticide. The obtained results serve as the basis for conducting field studies on the bioremediation of soils contaminated with cypermethrin.
All experiments and results obtained during this study are presented in this article. Additional information is available on request from the corresponding author.
DK participated in the isolation, selection, and inoculation of bacteria in the soil. DK, LZ, and AM participated in the identification of bacteria. DK and RE carried out GC–MS analyses of the soil, and NL and TK analyzed the results and worked with figures. All authors of the article bear equal responsibility for the results obtained in this study. All authors read and approved the final manuscript. All authors contributed to editorial changes in the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.