1 Department of Psychiatry, Sleep Medicine Center, Nanfang Hospital, Southern Medical University, 510515 Guangzhou, Guangdong, China
2 Key Laboratory of Mental Health of the Ministry of Education, Southern Medical University, 510515 Guangzhou, Guangdong, China
†These authors contributed equally.
Abstract
Patients with narcolepsy experience excessive daytime sleepiness (EDS) and cognitive impairment. However, studies on the circadian variability associated with cognitive impairment in narcolepsy patients are scarce. This study aimed to explore circadian cognitive performance in narcolepsy patients compared with patients with obstructive sleep apnea (OSA) and EDS (OSA-with-EDS).
A total of 62 participants, 29 with narcolepsy and 33 with OSA-with-EDS completed the study. The assessments were done using questionnaires, polysomnography (PSG), the multiple sleep latency test (MSLT), and cognitive-behavioral tasks at different time points (20:00, 08:00, 10:00, 12:00, 14:00, 16:00, and 18:00) including the psychomotor vigilance task (PVT), the Stroop color-word task, and the 2-back task to separately assess the circadian variations of vigilant attention, inhibitory control, and working memory respectively.
Narcolepsy patients showed significant within-subject circadian variations in vigilant attention (p < 0.001), inhibitory control (p = 0.016), and working memory (p < 0.001) in the time domain. Overall, vigilant attention in narcolepsy patients presented a pattern with optimal performance observed at 20:00 on the previous night followed by deterioration in the morning (08:00~14:00) and improvement in the afternoon (14:00~18:00). Inhibitory control displayed a pattern of “enhancement in the morning (08:00~12:00) followed by a decline in the afternoon (12:00~18:00)”, while working memory displayed a trend of improvement during daytime hours, with these two measures showing their poorest performance at 20:00 on the previous night.
Circadian variations were prominently observed in vigilant attention, inhibitory control, and working memory performance among patients with narcolepsy. Except for EDS, the intrinsic disease specificity may play an important role in the cognitive impairments associated with narcolepsy.
Keywords
- narcolepsy
- circadian variations
- cognitive performance
- excessive daytime sleepiness
1. The cognitive function of patients with narcolepsy shows rhythmic changes during the day.
2. Compared with obstructive sleep apnea (OSA)-with-excessive daytime sleepiness (EDS) patients, patients with narcolepsy performed poorly in vigilance attention tasks, but performed better in inhibitory control and working memory tasks.
3. Intrinsic disease specificity plays an important role in the cognitive impairments associated with narcolepsy.
Narcolepsy is a central disorder characterized by hypersomnolence, resulting from the loss or dysfunction of hypothalamic neurons that regulate the neuropeptide hypocretin [1]. This chronic neurological condition affects approximately 0.025% to 0.05% of the general population [2]. Furthermore, almost half of these people experience cognitive impairments, which are the second most prominent symptoms after excessive daytime sleepiness (EDS) impacting their daily functioning [3]. Patients with narcolepsy frequently report cognitive impairments across distinct domains of attention, executive functions, and memory [4]. These impairments typically impose a lifelong burden that adversely affects their quality of life, productivity, as well as educational and employment outcomes [5].
Research has demonstrated that cognitive function exhibits a circadian rhythm [6], typically peaking in the morning, declining in the afternoon, and recovering during the evening [7]. Additionally, cognitive performance among patients with narcolepsy varies at different time points [8]. For instance, vigilant attention is notably poorer immediately after awakening compared with the previous night but shows improvement by late morning [9]. Inhibition control in narcoleptic patients tends to be most impaired 1–2 hours after waking [10]. An early investigation into the temporal course of attention in narcoleptic patients showed a distinct U-shaped pattern [8]. However, previous studies have primarily focused on limited time points and have been unable to comprehensively delineate various aspects of cognitive function among patients with narcolepsy. Consequently, it is imperative to investigate the circadian variations in cognitive impairment within this population to provide a scientific basis for patients to arrange their study and rest reasonably.
Previous studies have predominantly compared individuals with narcolepsy with healthy controls [9, 11]. To date, there has been little research comparing patients across different diagnostic categories of sleep disorders that share similar symptoms of EDS [8]. It is known that obstructive sleep apnea (OSA) is associated with EDS [8, 12]. Using OSA-with-EDS patients as a control group can mitigate the impact of EDS and yield valuable insights into the characteristics of cognitive impairment in narcolepsy patients.
Therefore, this study aimed to investigate the circadian variability of vigilant attention, inhibitory control, and working memory through multiple time point cognitive assessments among patients with narcolepsy and compare that with OSA-with-EDS patients.
Participants were patients who initially visited the Sleep Medicine Center of Nanfang Hospital, Southern Medical University due to EDS from 2023 to 2024. They all underwent polysomnography (PSG) and multiple sleep latency tests (MSLT).
A multi-observation repeated measurement comparative design was used in this investigation. A total of 29 patients with narcolepsy (18 diagnosed with type 1 and 11 diagnosed with type 2) and 33 OSA-with-EDS patients were included in the analysis. The inclusion and exclusion criteria for the two groups were as follows.
Inclusion criteria for the narcolepsy group: (A) Participants meeting the diagnostic criteria of the International Classification of Sleep Disorders, Third Edition (ICSD-3) for narcolepsy [13]; (B) Participants with the capacity to comprehend and adhere to the research protocol; (C) Provide written informed consent prior to participation.
Exclusion criteria for the narcolepsy group: (A) Patients with unresolved physical or mental disorders; (B) Shift workers, including night shift employees, and frequent cross-time zone travelers (such as international flight crews); (C) Pregnant or lactating women; (D) Any additional circumstances that render an individual unsuitable for study participation.
The diagnostic inclusion criteria for the OSA-with-EDS group were Apnea-Hypopnea
Index (AHI)
PSG recordings were performed according to the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events guidelines [15]. Nocturnal PSG recording was conducted starting at 22:00 and ending at 07:00 in this study.
All participants underwent the MSLT following the nocturnal PSG monitoring according to the AASM Practice Parameters for Clinical Use of the MSLT [15]. The test included five 20-minute naps spaced 2 hours apart, starting at 09:00. Both PSG and MSLT recordings were scored manually by sleep specialists.
The sleep and psychological states of the participants were assessed through self-rating scales.
EDS was assessed using the ESS, an eight-item patient-reported outcome measure
to assess the likelihood of falling asleep under various conditions. Each item
scored from 0 (would never doze) to 3 (high chance of dozing), with a total score
ranging from 0 to 24. A score
The participants’ subjective sleep quality was evaluated by the Pittsburgh Sleep Quality Index (PSQI), a validated instrument comprising seven components. Each component was scored on a scale from 0–3, with the total score ranging from 0–21; where a higher score describes poorer sleep quality. A total PSQI score greater than 5 has been validated as being highly sensitive and specific in distinguishing good from poor sleepers across several populations. Study have shown that Cronbach’s alpha for each item of the PSQI was 0.875 [17].
Chronotype was assessed using the Morningness-Eveningness Questionaire-5 (MEQ-5), which contained five items. The total score ranged from 4 to 25, where 18 to 25 points were defined as the morning chronotypes, 12 to 17 points were defined as the neutral chronotypes, and 4 to 11 points were defined as the evening chronotypes. Chronotype was examined in this study as a continuous score, with lower scores indicating greater eveningness. Cronbach’s alpha coefficient in this study was 0.74 [18].
Anxiety and depression symptoms were assessed using the Hospital Anxiety and
Depression Scale (HADS), a self-rating scale containing two subscales measuring
symptoms of anxiety (HADS-A) or depression (HADS-D). A score of
Vigilance attention assessment was done using the psychomotor vigilance task
(PVT) [20] using the computer-administered E-prime program. Subjects were
instructed to press a button immediately after a stimulus (a red dot in the
center of a screen on a black background) appeared. The reaction time of visual
stimuli presented at random intervals of 2 seconds to 10 seconds over 10 minutes
was recorded. E-prime program metrics were response speed (1/reaction
time) and lapses in attention (trials
Inhibitory control assessment was done using the computer-administered Stroop color-word task in the E-prime 3.0 (Psychology Software Tools, Inc., Pittsburgh, PA, USA). Inhibitory control refers to the ability of an individual to consciously suppress interference or reaction tendencies in goal-oriented activities [21]. Subjects were asked to indicate the stimulus color presented by 80 randomly ordered individual, consistent, and inconsistent stimuli. The monitoring index was the difference in reaction time between the consistent stimulus and the inconsistent stimulus when the response was correct. The smaller the difference, the better performance of inhibitory control.
Working memory was assessed using the computer-administered 2-back test. Working memory refers to temporarily storing and processing information in various complex cognitive activities [22]. Subjects were asked to judge whether the current stimulus (letter) was consistent with the second stimulus (letter). The E-prime program includes a practice part and a test part, and the index is the accuracy of the test part.
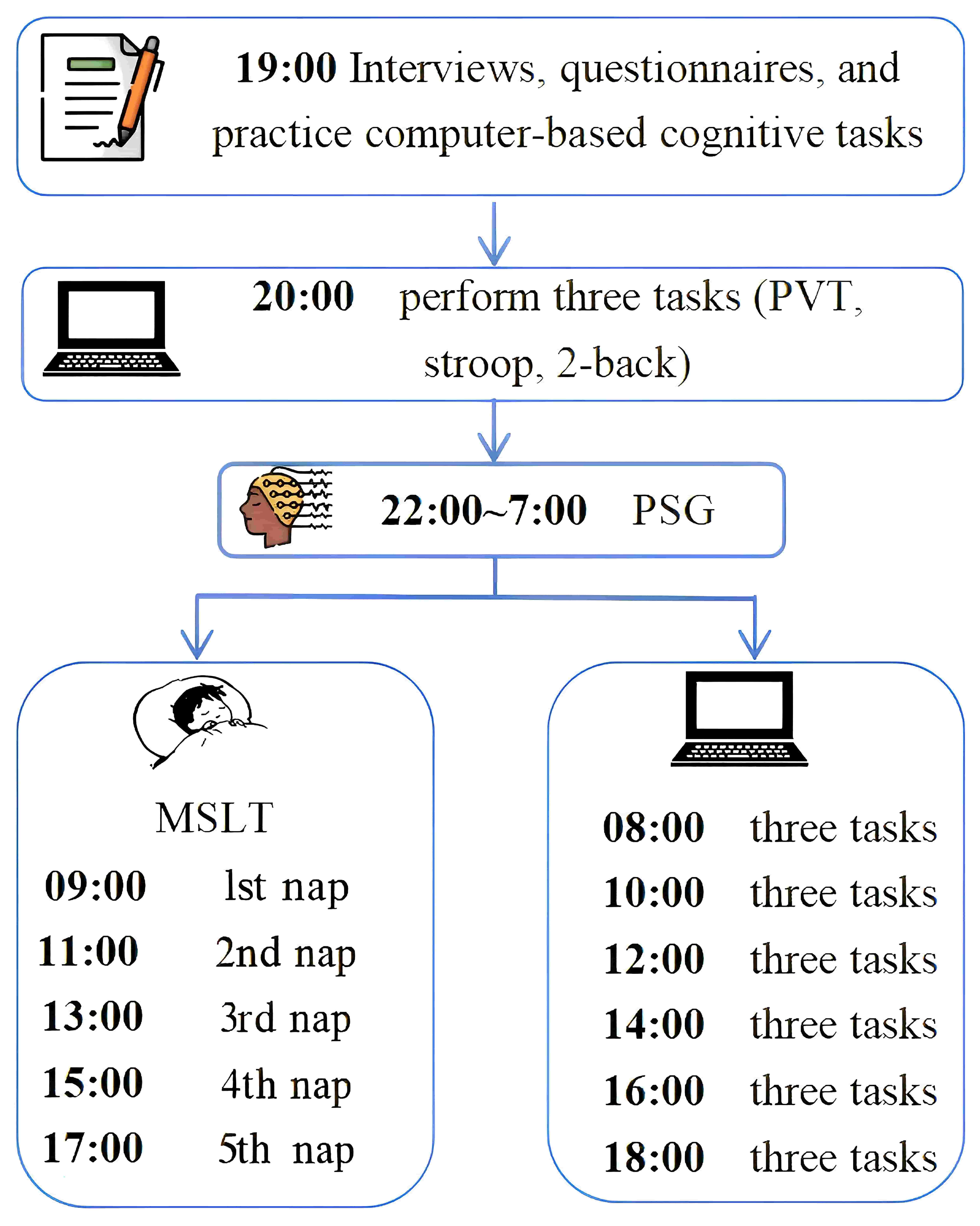
When the participants of this study arrived at the Sleep Medicine Center of Nanfang Hospital, Southern Medical University, two trained researchers collected their general information through 20-minute structured interviews and guided them to complete the self-rating scales. Then, the nocturnal PSG followed by the MSLT with five naps were carried out. At the start of the nocturnal PSG as well as before and after each MSLT nap, participants completed computer-administered cognitive-behavioral tasks, including a 10-minute PVT, a 5-minute Stroop color-word task, and a 2-minute 2-back test. In addition, a pre-training procedure was set for each cognitive behavioral task before each formal test to reduce the influence of the learning effect; participants could enter the formal test only when their performance was stable. The specific experimental procedure is shown in Fig. 1.
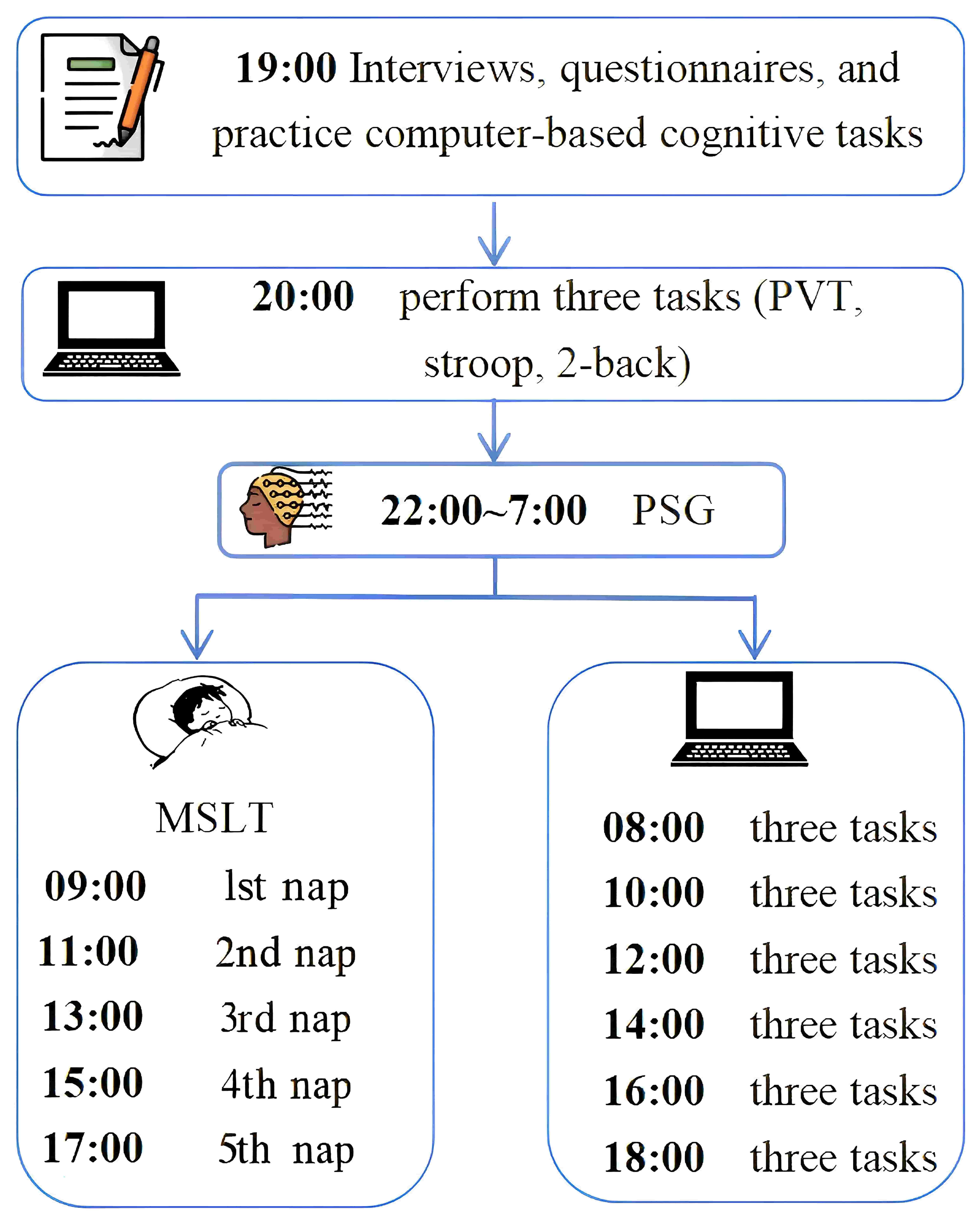 Fig. 1.
Fig. 1.
Experimental procedure. PVT, psychomotor vigilance task; Stroop, Stroop color-word task; 2-back, 2-back working memory task; PSG, polysomnography; MSLT, multiple sleep latency test.
For continuous variables, the differences between groups were compared using a
two-independent sample t-test or Mann-Whitney U test. For categorical
variables, the chi-squared test was used to compare the differences between
groups. The Shapiro-Wilk test was performed to check normality and Levene’s test
was used to check for homogeneity of variance for continuous variables
(normality: p
Patients with narcolepsy had a significantly higher proportion of females
(p
| Narcolepsy (n = 29) | OSA-with-EDS (n = 33) | p-value | ||
| Sex, female, n (%) | 13 (44.8%) | 2 (6.1%) | ||
| Age, years | 18.93 |
41.55 |
||
| BMI, kg/m2 | 23.14 |
27.49 |
||
| Questionnaires | ||||
| PSQI (0–21) | 6.00 (4.25–8.00) | 9.00 (6.75–13.25) | 0.003 | |
| MEQ-5 (0–25) | 13.21 |
13.73 |
0.596 | |
| HADS (0–21) | 11.00 (7.25–17.25) | 12.00 (5.75–17.00) | 0.979 | |
| ESS (0–24) | 15.50 |
16.03 |
0.706 | |
| PSG | ||||
| Total sleep time, min | 430.46 |
431.54 |
0.961 | |
| Sleep latency, min | 5.00 (2.10–10.45) | 4.50 (1.00–8.95) | 0.882 | |
| REM latency, min | 72.55 (9.33–165.00) | 113.20 (76.05–210.70) | 0.017 | |
| Wake after sleep onset, min | 40.65 (13.39–88.90) | 77.00 (31.88–130.75) | 0.052 | |
| Sleep efficiency, % | 87.01 |
82.69 |
0.171 | |
| REM sleep percentage, % | 22.92 |
19.79 |
0.094 | |
| N1 sleep percentage, % | 10.20 (6.60–17.45) | 23.00 (15.40–47.70) | ||
| N2 sleep percentage, % | 45.82 |
45.36 |
0.887 | |
| N3 sleep percentage, % | 18.40 (13.30–24.00) | 2.30 (0.00–13.90) | ||
| Arousal index | 14.10 (10.70–17.95) | 36.50 (19.70–61.00) | ||
| Apnea-hypopnea index | 2.40 (0.45–5.05) | 49.50 (20.45–71.20) | ||
| MSLT | ||||
| Sleep onset in REM periods, n | 3.50 (2.00–5.00) | 1.00 (0.00–2.00) | ||
| Mean sleep latency, min | 2.15 (1.40–3.75) | 4.90 (2.20–7.95) | 0.022 | |
BMI, body mass index; PSQI, Pittsburgh Sleep Quality Index; HADS, Hospital Anxiety and Depression Scale; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; EDS, excessive daytime sleepiness; REM, rapid eye movement; MEQ-5, Morningness-Eveningness Questionaire-5.
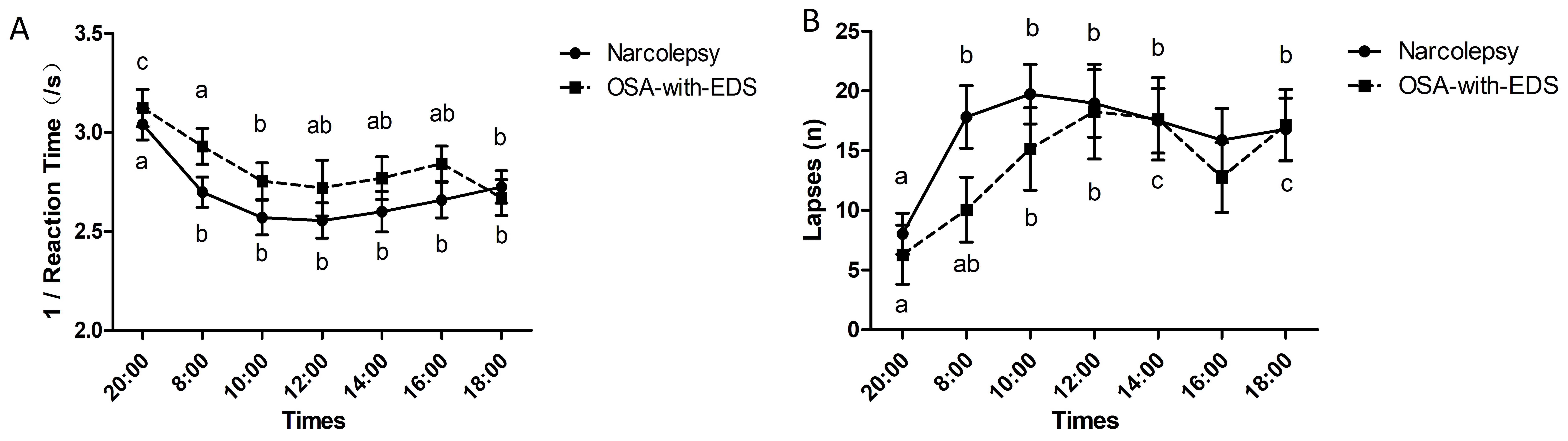
A repeated measures ANOVA with a Greenhouse-Geisser correction determined that
the response speed (1/reaction time) between the narcolepsy and OSA-with-EDS
groups was not statistically significant (pgroup = 0.248) but
differed significantly between time points (ptime point
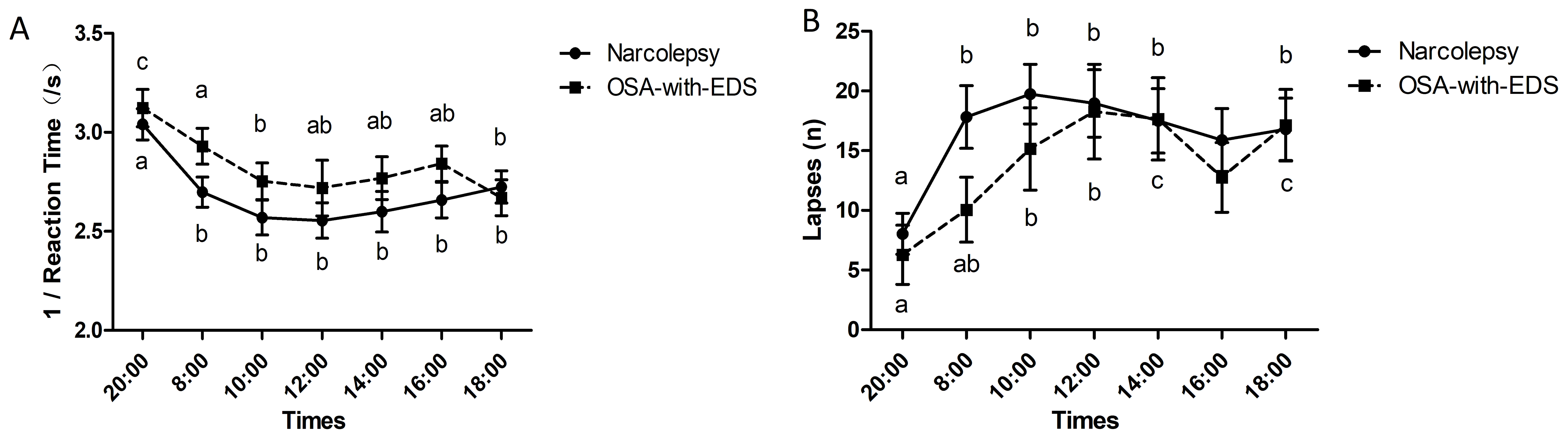 Fig. 2.
Fig. 2.
Circadian variations of vigilant attention in the psychomotor
vigilance task. (A) Circadian variations of response speed in the psychomotor
vigilance task between the narcolepsy and OSA-with-EDS groups. (B) Circadian
variations of lapses in attention in the psychomotor vigilance task between the
narcolepsy and OSA-with-EDS groups. Time points sharing the same letter (a, b, c) on a curve are not significantly different from each other
(p
| 20:00 | 08:00 | 10:00 | 12:00 | 14:00 | 16:00 | 18:00 | p-value | ||
| 1/reaction time of PVT | Narcolepsy (n = 29) | 3.04 |
2.70 |
2.57 |
2.55 |
2.60 |
2.66 |
2.72 |
pgroup = 0.248 |
| OSA-with-EDS (n = 33) | 3.12 |
2.93 |
2.75 |
2.72 |
2.77 |
2.84 |
2.67 |
ptime point | |
| pgroup × time point = 0.089 | |||||||||
| Number of PVT lapses | Narcolepsy (n = 29) | 8.03 |
17.82 |
19.74 |
18.97 |
17.50 |
15.89 |
16.80 |
pgroup = 0.487 |
| OSA-with-EDS (n = 33) | 6.28 |
10.06 |
15.15 |
18.27 |
17.67 |
12.76 |
17.14 |
ptime point | |
| pgroup × time point = 0.070 | |||||||||
| Differential reaction time of inhibition control | Narcolepsy (n = 29) | 58.45 |
44.75 |
39.07 |
25.59 |
30.15 |
35.77 |
39.14 |
pgroup = 0.009 |
| OSA-with-EDS (n = 33) | 77.08 |
63.78 |
65.40 |
57.05 |
57.05 |
59.75 |
61.85 |
ptime point = 0.016 | |
| pgroup × time point = 0.969 | |||||||||
| 2-back accuracy | Narcolepsy (n = 29) | 65.00 |
67.97 |
71.12 |
72.20 |
72.32 |
74.11 |
75.99 |
pgroup = 0.308 |
| OSA-with-EDS (n = 33) | 59.78 |
61.34 |
66.33 |
70.22 |
70.46 |
72.33 |
72.27 |
ptime point | |
| pgroup × time point = 0.462 | |||||||||
The number of PVT lapses in attention was not statistically significant between
the two groups (pgroup = 0.487), while with a Greenhouse-Geisser
correction, a notable time series effect was present (ptime point
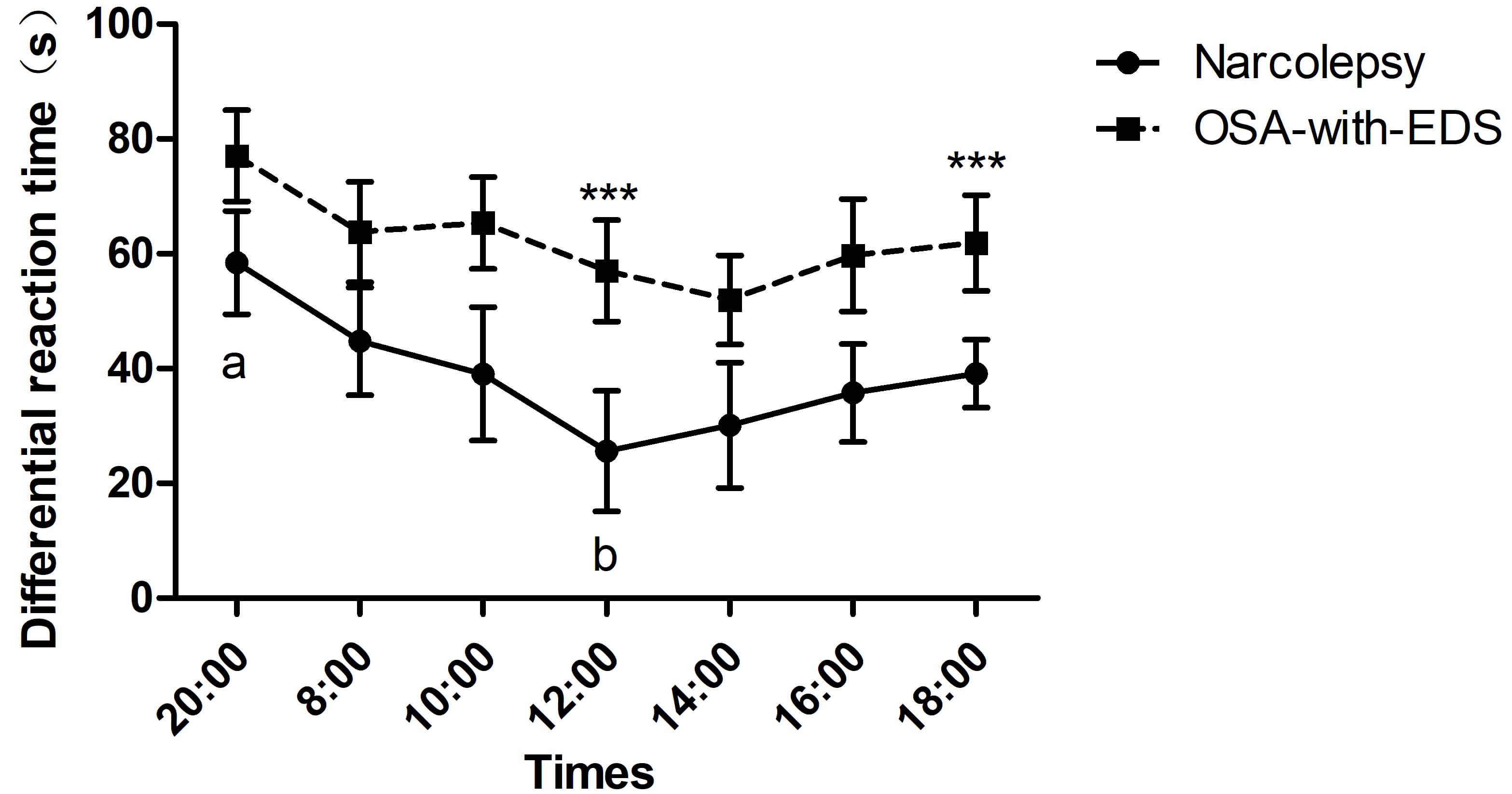
The differential reaction time of inhibition control between the narcolepsy and OSA-with-EDS groups displayed marked group effects (pgroup = 0.009), and post hoc comparisons showed that the differences between the two groups were significant at 12:00 (p = 0.025) and 18:00 (p = 0.033). ANOVA with a Greenhouse-Geisser correction showed a significant difference in time points (ptime point = 0.016) (Table 2). Additionally, an increased difference in reaction time was seen among the narcolepsy group at 20:00 relative to 12:00 (p = 0.049), but no difference in differential reaction time was seen in the OSA-with-EDS group (Fig. 3).
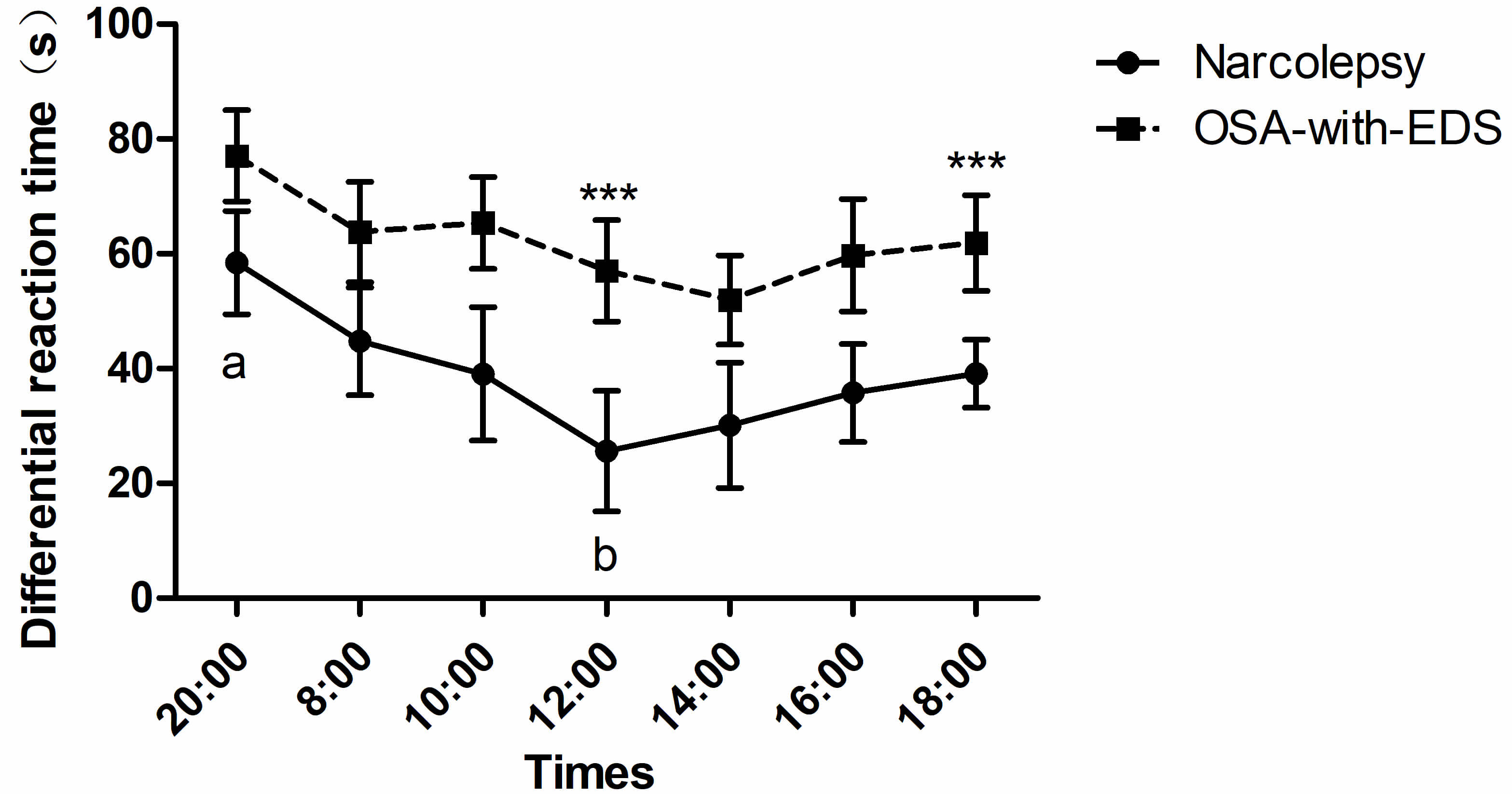 Fig. 3.
Fig. 3.
Circadian variations of inhibitory control in the Stroop
color-word task. Time points with statistically significant differences between
groups are marked with asterisks (***). Time points labeled with different letters (a, b) on the same curve are statistically different
(p
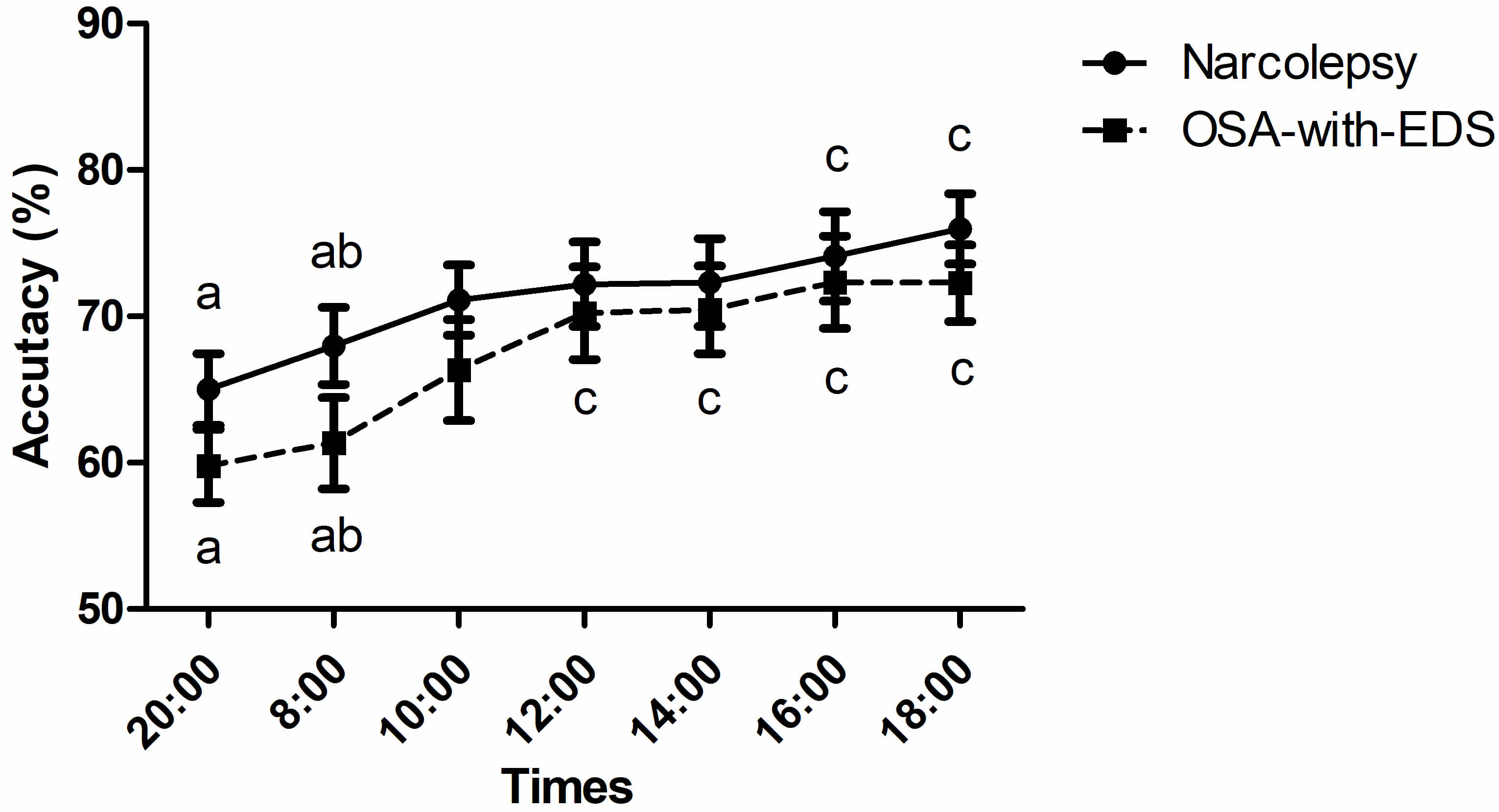
The 2-back accuracy between the narcolepsy and OSA-with-EDS group had no
statistically significant between-group differences (pgroup =
0.308), however, the Greenhouse-Geisser corrected time series effect was
significant (ptime point
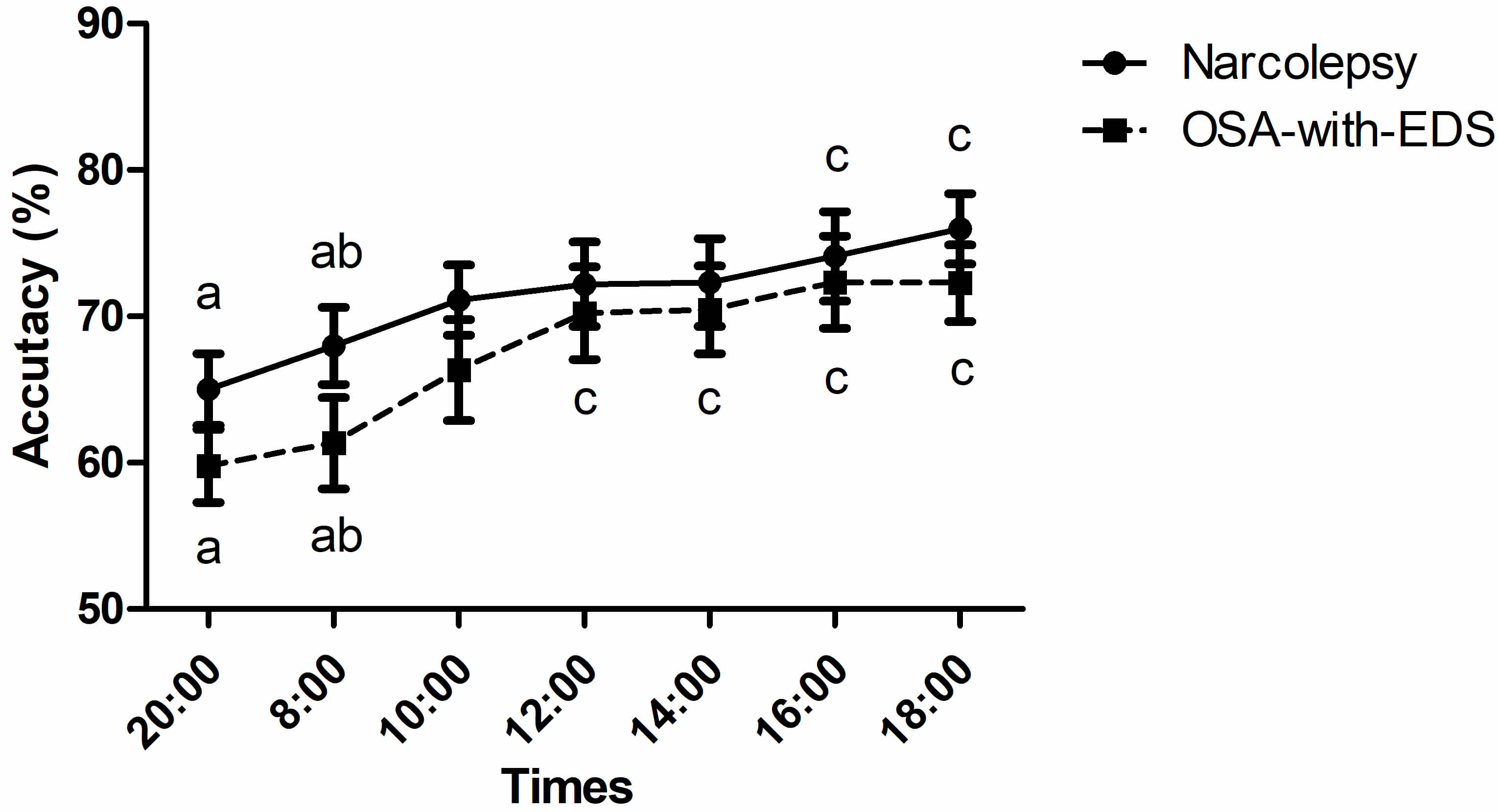 Fig. 4.
Fig. 4.
Circadian variations of 2-back working memory. Time points sharing the same letter (a, b, c) on a curve are not significantly different from each other (p
Our study revealed that patients with narcolepsy exhibited significant circadian variations in vigilant attention, inhibitory control, and working memory. The circadian variations of cognitive performance in the narcolepsy group showed similar trends in diurnal variation as those of patients with OSA-with-EDS. However, patients with narcolepsy performed poorly in vigilant attention tasks but performed better in inhibitory control and working memory tasks.
Narcolepsy patients showed the lowest vigilant attention performance at noon followed by recovery and the best vigilant attention performance was seen in the evening. A previous study revealed similar results, in that sustained attention showed fluctuations in performance in patients with narcolepsy, with declines in the morning and at noon [23]. Other studies aimed at vigilant attention in patients with EDS performed poorly at 07:00 [24], even lower at 13:00, and improved by 18:00 [25]. Research has also indicated that the attention of normal individuals shows relatively consistent circadian variability compared with those of patients with narcolepsy. It has been reported that attention is relatively low in the morning, improves throughout the midday period, experiences a decline post-lunch, and subsequently improves during the afternoon and early evening hours [8, 26]. Furthermore, as for vigilant attention between different groups, studies have found that patients with narcolepsy performed worse compared with healthy controls [9, 27]. In summary, the circadian variations of vigilance attention in narcolepsy show similar time trends in diurnal variation implying similar regulatory mechanisms for the circadian rhythm of cognitive performance.
The inhibitory control of patients with narcolepsy showed a pattern of improvement in the morning and a decline in the afternoon, which is consistent with previous studies. A study aimed at narcolepsy patients indicated that inhibitory control was at its lowest 1–2 hours after waking [10]. A previous functional magnetic resonance imaging study found that inhibitory control activation and neural activity in associated brain regions exhibited time-dependent changes [28]. Importantly, evidence suggests that inhibitory control performance is associated with the 24-hour activity rhythm [29], and the circadian rhythm plays a fundamental role in regulating inhibitory control [10]. Moreover, a study comparing narcoleptics with healthy controls demonstrated that narcolepsy was associated with attenuated medial prefrontal cortex (mPFC) responses during inhibitory control [11]. Preclinical research has also shed light on how this impaired cognition may be a consequence of reduced hypocretin signaling in the mPFC systems [30]. In the present study there was a between-group difference in the inhibitory control task, where narcolepsy subjects performed better than the OSA-with-EDS group. This result necessitates replication prior to drawing any conclusions about the significant differences in task performance between the two groups.
The working memory of patients with narcolepsy demonstrated a gradual improvement throughout the day. A previous neuroimaging study has consistently monitored participants at multiple time series during the 2-back task, revealing that activation of the cortical executive network is diminished in individuals with narcolepsy [31], which may account for the observed changes in working memory performance in this study. Furthermore, one study reported that while attention performance was poorer in narcolepsy patients compared with controls, their memory may not be objectively impaired [32]. Conversely, another investigation identified significant group differences in procedural memory among patients with narcolepsy compared with controls [33]. This fact that the intercept was not significantly different for the 2-back task strongly suggests that group differences are not so pervasive that they can be reliably assessed by a single test run of a performance test. This may explain to some extent why results from earlier studies with working memory performance measures in narcolepsy patients were inconsistent or contradictory.
Generally, cognitive impairment of narcolepsy is assumed to be a consequence of EDS [4, 34]. It is presumed that vigilance levels associated with EDS contribute to impairments in higher-order cognitive functions [35]. Another hypothesis posits that cognitive impairment in narcolepsy may also involve disease-specific components [36], such as the depletion of hypocretin neurons in various brain regions, including the mPFC and hippocampus [37, 38]. Our study demonstrated that patients with narcolepsy exhibited poorer vigilant attention compared with OSA-with-EDS patients while showing significantly better performance in higher-order cognitive tasks (e.g., inhibitory control). Therefore, we propose that disease specificity is an integral factor contributing to cognitive impairment.
This study has the following limitations. Firstly, the sample size of this study is relatively small, thereby potentially restricting the statistical power and augmenting the risk of incidental findings. Furthermore, owing to the constraint of the sample size, we were incapable of fully adjusting for the potential confounding effects of baseline variables in the analysis, which might have resulted in a bias in the results estimation. Further verification of the robustness of the results of this study, through larger-scale studies, is required in the future. Finally, although we screened the participants, the medication history and comorbidities of the participants were not recorded in detail, which could have influenced the final results.
Circadian variations are evident in cognitive performance among patients with narcolepsy across essential cognitive functions, including vigilant attention, inhibitory control, and working memory. Furthermore, when compared with the OSA-with-EDS group, intrinsic disease specificity may play an important role in the cognitive impairments associated with narcolepsy. The results of this study provide a scientific basis for optimizing the scheduling of academic and occupational activities for patients.
EDS, excessive daytime sleepiness; OSA, obstructive sleep apnea; PSG, polysomnography; MSLT, Multiple Sleep Latency Test; PVT, psychomotor vigilance task; ICSD-3, International Classification of Sleep Disorders, Third Edition; AHI, apnea/hypopnea index; ESS, Epworth Sleepiness Scale; AASM, American Academy of Sleep Medicine; PSQI, Pittsburgh Sleep Quality Index; MEQ-5, Morningness-Eveningness Questionnaire-5; HADS, Hospital Anxiety and Depression Scale; BMI, body mass index; REM, rapid eye movement; MSL, mean sleep latency; SOREMP, sleep onset rapid eye movement period; mPFC, medial prefrontal cortex.
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
YC: made contributions to the conception and design of the work, data acquisition, analysis, interpretation, and have drafted the manuscript. RF: made contributions to the conception and design of the work, to data acquisition, analysis, interpretation, and revised the manuscript. LF: made contributions to data interpretation and revised the manuscript. DP: made contributions to data interpretation and revised the manuscript. YX: made contributions to data acquisition. AW: made contributions to data interpretation and revised the manuscript. SL: made contributions to data interpretation and revised the manuscript. JJ: made contributions to data acquisition. YW: made contributions to data acquisition. DZ: made contributions to data acquisition. BZ: made contributions to funding support, data interpretation and revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was approved by the Ethical Committee of Nanfang Hospital, Southern Medical University (grant number: NFEC-2022-307) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participating patients, with legal guardians providing consent on behalf of minor participants.
We thank all the patients who participated in the study.
This work was funded by the National Key R&D Program of China (grant number 2021YFC2501500), and Nanfang Hospital Clinical Research Project of Southern Medical University (grant number 2021CR009) to Bin Zhang. The sponsor had no role in the design or conduct of this research.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.