1 Department of Rehabilitation Medicine, Affiliated Hospital of North Sichuan Medical College, 637000 Nanchong, Sichuan, China
2 Department of Digestive Endoscopy Center, Digestive Disease Center, Suining Central Hospital, 629000 Suining, Sichuan, China
3 College of Sports Medicine and Rehabilitation, North Sichuan Medical College, 637000 Nanchong, Sichuan, China
4 Department of Rehabilitation Engineering, China Rehabilitation Science Institute, 100037 Beijing, China
†These authors contributed equally.
Abstract
The aim of this study was to develop and validate a machine learning model to predict the risk of comorbid depression in asthma patients.
We conducted a retrospective study of 2464 asthma patients with comorbid depression using National Health and Nutrition Examination Survey (NHANES) data. Feature selection was conducted using the Boruta algorithm and the Least Absolute Shrinkage and Selection Operator (LASSO). Eight machine learning algorithms, namely Decision Tree (DT), k-Nearest Neighbors (KNN), Light Gradient Booster Machine (LGBM), Logistic Regression (LR), Random Forest (RF), Support Vector Machine (SVM), eXtreme Gradient Boosting (XGBoost), and Multilayer Perceptron (MLP), were trained using 5-fold cross-validation methodology. Model performance was evaluated through various metrics such as area under the curve (AUC), accuracy, sensitivity, specificity, F1 score, and decision curve analysis (DCA). Interpretation was conducted using SHapley Additive exPlanations (SHAP) analysis, highlighting feature importance.
The training set comprised 1724 participants, while the validation set included 740 participants, with a depression prevalence of 14.45%. Significant predictors identified included hypertension, chronic obstructive pulmonary disease (COPD), stroke, sleep questionnaire (SLQ) scores, smoking status, Poverty Index Ratio (PIR), and educational level. The XGBoost model demonstrated superior performance compared with alternative machine learning (ML) algorithms, achieving an AUC of 0.750, an accuracy of 69.1%, a sensitivity of 68.2%, a specificity of 73.8%, and an F1 score of 79%. The SHAP method identified SLQ, PIR, and education level as the primary decision factors influencing the ML model’s predictions.
The XGBoost model effectively predicts the risk of depression in asthma patients, serving as a valuable reference for early clinical identification and intervention.
Keywords
- asthma
- depression
- machine learning
- predictive model
Bronchial asthma is a chronic inflammatory airway disease that impacts 262 million people globally [1]. In the United States, approximately 26 million asthmatics are afflicted by this disease, and is accompanied by more than 3000 deaths and a significant health burden of around $81 billion annually [2, 3, 4]. Asthma not only severely affects patients’ quality of life but also triggers psychiatric symptoms. Up to 71.4% of patients experience poorer quality of life and are more prone to psychiatric manifestations such as anxiety and depression [5, 6, 7]. Among patients with asthma and depression, 40% will exhibit symptoms such as poor sleep and insomnia [8, 9]. These issues not only affect the efficacy of asthma control but also lead to refractory asthma, increase the healthcare burden, significantly reduce the quality of life, and raise the risk of death [8]. Studies have indicated that asthma-associated depression is closely related to low-income families, smoking, chronic obstructive pulmonary disease (COPD), hypertension, and stroke [5, 10, 11]. However, there is currently limited research on these risk factors, and clinical predictive models have not been adequately developed. Therefore, it is recommended that a risk prediction model for depression in asthma patients be developed to enhance the management of these risk factors, to reduce the incidence of these co-morbidities.
Machine learning (ML), as a powerful predictive tool, has been widely used in fields such as medicine and engineering [12]. Its predictive accuracy surpasses that of traditional statistical methods [13]. While previous study have developed simple regression predictive models, there is a notable lack of predictive model platforms, which limits their clinical practicality [14]. ML-based algorithms can analyze extensive datasets and uncover subtle predictive risk factors, thereby enhancing clinical predictive capabilities.
This study utilized the dataset of the National Health and Nutrition Examination Survey (NHANES, 2005–2020) to establish machine learning models for predicting the risk of asthma accompanied by depression. A comprehensive machine learning model construction process, including preprocessing, feature selection, model training, and evaluation, was designed. Eight machine learning models were compared to select the model with the best performance as the final prediction model. The aim was to establish an effective prediction platform based on machine learning to provide a theoretical basis for clinical practice and improve the overall health management of the risk of asthma with depression.
All participant information was collected independently by two researchers from the NHANES database (2005–2020), which is part of the Centers for Disease Control and Prevention (CDC) in the United States. The was a retrospective cohort study.
NHANES is a national programme to assess the health and nutritional status of adults and children living in the communities in the United States. The NHANES database collects and stores information on interviews and examinations and is updated every two years [15]. The Ethics Review Board of the National Center for Health Statistics approved this study. Written informed consent was obtained from participants prior to their inclusion in the NHANES database. Full details of the ethical application and written informed consent are available on the NHANES website (https://www.cdc.gov/nchs/nhanes). All continuous variables were standardized, while categorical variables were processed using categorical coding to ensure data consistency and usability [11]. Two researchers independently extracted information from the database on January 1, 2024. Pertinent data collected were as follows: Participant demographic data: age, sex (male and female), and race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black and Other Race). Examination data: glucose (GLU), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglycerides (TG), triglyceride-glucose index (TyG). Questionnaire data: marital (married/living with partner, widowed/divorced/separated, never married), diabetes (yes or no), hypertension (yes or no), heart failure (yes or no), COPD (yes or no), coronary artery disease (CAD) (yes or no), cancer (yes or no), stroke (yes or no), sleep disorders (SLQ) (yes or no), body mass index (BMI) status (under weight, normal weight, over weight), smoking (never smoker, former smoker, now smoker), ratio of income to poverty (PIR) (non-low-income, low-income), education (less than 9th grade, 9–11th grade, high school graduate, some college or AA degree, college graduate or above), drinking (1 to 5 drinks/month, 5 to 10 drinks/month,
During the process of data merging, cleaning and organizing, individual data were longitudinally linked through the unique identified sequence number (SEQN). Variables with missing values exceeding 20% were excluded. Meanwhile, the data with missing values were also deleted. We only analyzed the complete data. Participants needed to be 20 years old or older. They had to partake in the dietary interview, undergo fasting glucose and triglyceride tests. They were required to complete the self-reported asthma information. Owing to the exclusion criteria, which accounted for the non-asthma population and missing data, the sample size was reduced 74,032. Eventually, a total of 2464 individuals with asthma were included and randomly divided into training and validation groups in a 7:3 ratio [16] (Fig. 1). Subsequently, a comparative analysis of variables was carried out. Among them, 1724 participants were assigned to the training data, and 740 participants were assigned to the validation data. The workflow of this study is shown in Fig. 2.
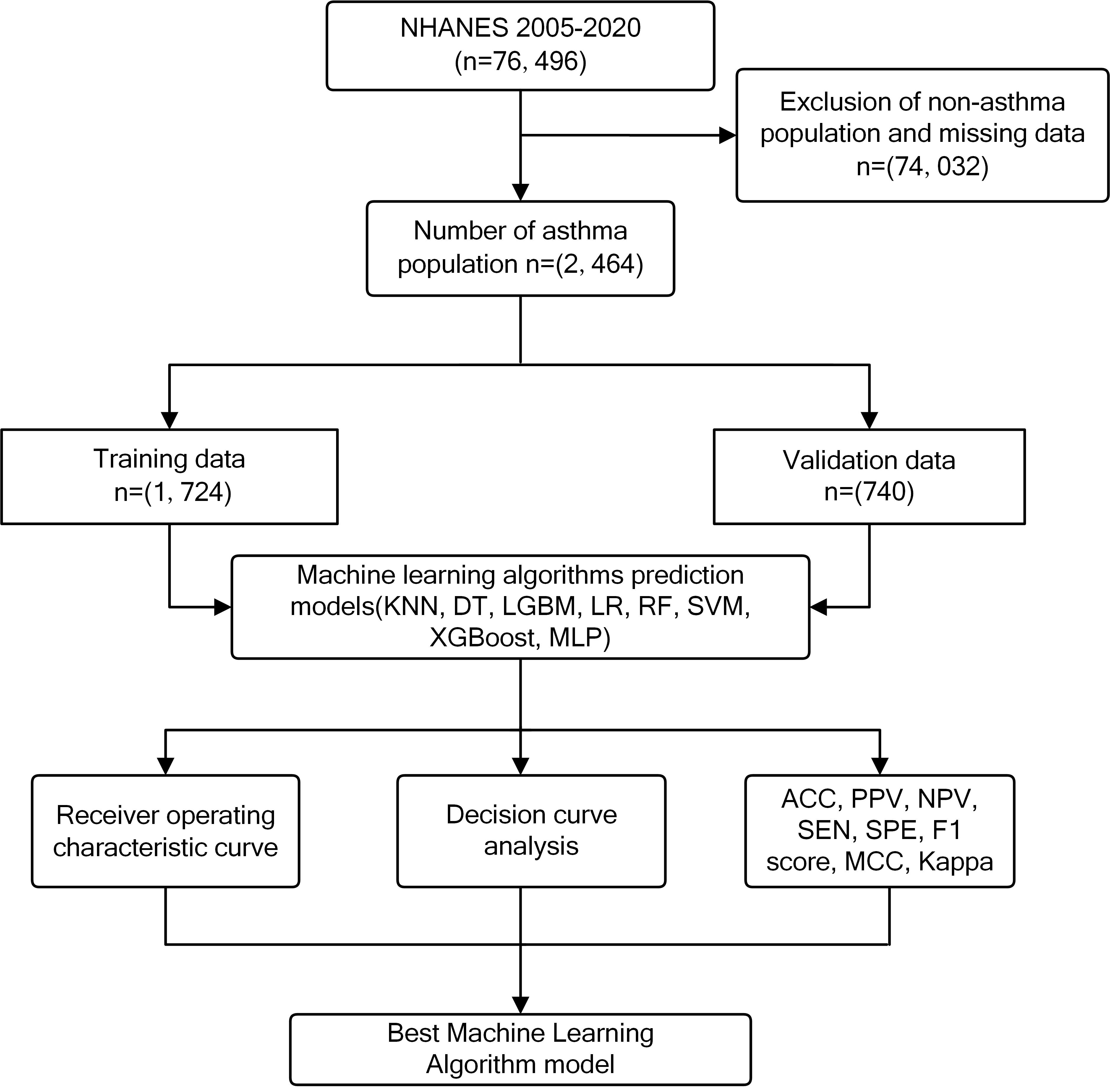 Fig. 1.
Fig. 1. Flow chart of the study. NHANES, National Health and Nutrition Examination Survey; KNN, k-Nearest Neighbor Algorithm; DT, Decision Tree; LGBM, Light Gradient Booster Machine; LR, Logistic Regression; RF, Random Forest; SVM, Support Vector Machine; XGBoost, eXtreme Gradient Boosting; MLP, Multilayer Perceptron; ACC, accuracy; PPV, positive predictive value; NPV, negative predictive value; SEN, sensitivity; SPE, specificity; MCC, matthews correlation coefficient.
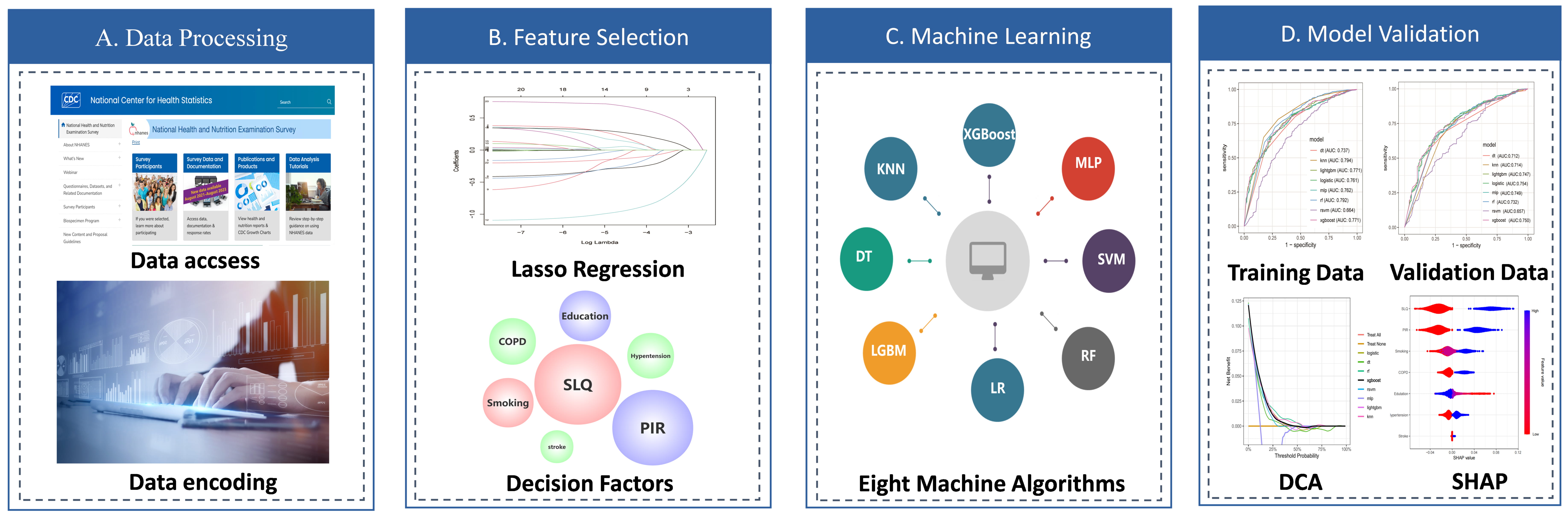 Fig. 2.
Fig. 2. Workflow for development and validation of the machine learning (ML) for predictions of the risk depression in patients with asthma. (A) Data accsess and encoding. (B) Using LASSO regression for feature selection of predictors. (C) Eight machine learning algorithms were used for model training and development. (D) 5-fold cross-validation was used to evaluate the model. DCA, decision curve analysis; SHAP, SHapley Additive exPlanations; SLQ, sleep questionnaire; PIR, ratio of income to poverty; COPD, chronic obstructive pulmonary disease.
The frequency of depressive symptoms in the past two weeks was assessed in participants by the Patient Health Questionnaire (PHQ-9) [17]. This screening tool is based on the criteria of the Diagnostic and Statistical Manual of Depression, Fourth Edition (DSM-IV) [18]. The PHQ-9 consists of 10 questions. After answering all 9 questions in full, a total score is calculated, ranging from 0 to 27. Higher scores indicate more severe depressive symptoms. Individuals with a total score
Asthma was defined based on information from the NHANES questionnaire. A participant was considered to have asthma if they answered ‘yes’ to the question, ‘Has a doctor or other health care professional ever told you that you have asthma?’ The definition of asthma was defined if the subject answered ‘yes’ to the question, ‘Has a doctor or other health care professional ever told you that you have asthma?’ and also answered ‘yes’ to any of the following questions [20, 21, 22]:
(1) Have you had an asthma attack or flare-up in the last 12 months?
(2) In the past 12 months, have you visited an emergency room or urgent care center for asthma?
(3) In the past 3 months, have you taken any medication prescribed by a doctor for asthma?
(4) In the past 12 months, have you experienced wheezing or chest pain?
(5) In the past 12 months, have you taken medication prescribed by a doctor for wheezing or chest pain?
Data analysis was conducted using R version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). All data were assessed for normality. Continuous variables that followed a normal distribution were reported as mean
In the study, eight different machine learning models were used for training and testing: Decision Tree (DT), k-Nearest Neighbor Algorithm (KNN), Light Gradient Booster Algorithm (LGBM), Logistic Regression (LR), Random Forest (RF), Support Vector Machines (SVM), eXtreme Gradient Boosting (XGBoost), and Multilayer Perceptron (MLP). The dataset was divided in a 7:3 ratio, with 70% allocated to the training set and 30% to the validation set. Internal five-fold cross-validation was used, and the evaluation metrics included area under the curve (AUC) and decision curve analysis (DCA). AUC measures the model’s discriminative ability, while DCA assesses the net benefit threshold of the predictions. Additionally, the SHapley Additive exPlanations (SHAP) method was utilized to determine the importance of each variable, highlighting their relative contributions to the model. To facilitate the integration of machine learning into clinical practice, the research team created a predictive modeling website for assessing the risk of asthma-associated depression, accessible at (https://chunyuzhang28.shinyapps.io/asthma/).
A total of 2464 individuals with asthma were included in the analysis and randomly divided into a training set of 1724 (70%) and a validation set of 740 (30%) (Fig. 1). The participants had an average age of 47.66
| Variables | Total (n = 2464) | Training Cohort | Test Cohort | Statistic | p | |
| (n = 1724) | (n = 740) | |||||
| Age (years), Mean | 47.66 | 47.58 | 47.85 | t = 0.36 | 0.718 | |
| GLU (mg/dL), Mean | 110.27 | 110.20 | 110.42 | t = 0.14 | 0.892 | |
| HDL-C (mmol/L), Mean | 1.39 | 1.39 | 1.40 | t = 0.58 | 0.560 | |
| LDL-C (mg/dL), Mean | 111.37 | 112.37 | 109.03 | t = –2.11 | 0.035 | |
| TC (mg/dL), Mean | 188.52 | 189.70 | 185.80 | t = –2.24 | 0.025 | |
| TG (mg/dL), Mean | 116.16 | 117.69 | 112.59 | t = –1.76 | 0.079 | |
| TyG, Mean | 8.58 | 8.59 | 8.56 | t = –1.16 | 0.245 | |
| Depression, n (%) | 0.525 | |||||
| No | 2108 (85.55) | 1480 (85.85) | 628 (84.86) | |||
| Yes | 356 (14.45) | 244 (14.15) | 112 (15.14) | |||
| Gender, n (%) | 0.719 | |||||
| Male | 1049 (42.57) | 738 (42.81) | 311 (42.03) | |||
| Female | 1415 (57.43) | 986 (57.19) | 429 (57.97) | |||
| Martial, n (%) | 0.441 | |||||
| Married/Living with Partner | 1345 (54.59) | 927 (53.77) | 418 (56.49) | |||
| Widowed/Divorced/Separated | 579 (23.50) | 410 (23.78) | 169 (22.84) | |||
| Never married | 540 (21.92) | 387 (22.45) | 153 (20.68) | |||
| Diabetes, n (%) | 0.113 | |||||
| No | 1950 (79.14) | 1379 (79.99) | 571 (77.16) | |||
| Yes | 514 (20.86) | 345 (20.01) | 169 (22.84) | |||
| Hypertension, n (%) | 0.283 | |||||
| No | 1359 (55.15) | 963 (55.86) | 396 (53.51) | |||
| Yes | 1105 (44.85) | 761 (44.14) | 344 (46.49) | |||
| Heart Failure, n (%) | 0.709 | |||||
| No | 2311 (93.79) | 1619 (93.91) | 692 (93.51) | |||
| Yes | 153 (6.21) | 105 (6.09) | 48 (6.49) | |||
| COPD, n (%) | 0.854 | |||||
| NO | 1854 (75.24) | 1299 (75.35) | 555 (75.00) | |||
| Yes | 610 (24.76) | 425 (24.65) | 185 (25.00) | |||
| CAD, n (%) | 0.665 | |||||
| No | 2351 (95.41) | 1647 (95.53) | 704 (95.14) | |||
| Yes | 113 (4.59) | 77 (4.47) | 36 (4.86) | |||
| Cancer, n (%) | 0.326 | |||||
| Yes | 273 (11.08) | 184 (10.67) | 89 (12.03) | |||
| No | 2191 (88.92) | 1540 (89.33) | 651 (87.97) | |||
| Stroke, n (%) | 0.837 | |||||
| No | 2331 (94.60) | 1632 (94.66) | 699 (94.46) | |||
| Yes | 133 (5.40) | 92 (5.34) | 41 (5.54) | |||
| SLQ, n (%) | 0.282 | |||||
| No | 1814 (73.62) | 1280 (74.25) | 534 (72.16) | |||
| Yes | 650 (26.38) | 444 (25.75) | 206 (27.84) | |||
| BMI status, n (%) | 0.888 | |||||
| Under weight | 43 (1.75) | 29 (1.68) | 14 (1.89) | |||
| Normal weight | 529 (21.47) | 372 (21.58) | 157 (21.22) | |||
| Over weight | 707 (28.69) | 501 (29.06) | 206 (27.84) | |||
| Obese | 1185 (48.09) | 822 (47.68) | 363 (49.05) | |||
| Smoking, n (%) | 0.649 | |||||
| Never smoker | 1186 (48.13) | 838 (48.61) | 348 (47.03) | |||
| Former smoker | 671 (27.23) | 470 (27.26) | 201 (27.16) | |||
| Now smoker | 607 (24.63) | 416 (24.13) | 191 (25.81) | |||
| PIR, n (%) | 0.554 | |||||
| Non-low-income | 1603 (65.06) | 1128 (65.43) | 475 (64.19) | |||
| Low-income | 861 (34.94) | 596 (34.57) | 265 (35.81) | |||
| Race, n (%) | 0.410 | |||||
| Mexican American | 209 (8.48) | 149 (8.64) | 60 (8.11) | |||
| Other Hispanic | 243 (9.86) | 164 (9.51) | 79 (10.68) | |||
| Non-Hispanic White | 1129 (45.82) | 789 (45.77) | 340 (45.95) | |||
| Non-Hispanic Black | 634 (25.73) | 436 (25.29) | 198 (26.76) | |||
| Other Race | 249 (10.11) | 186 (10.79) | 63 (8.51) | |||
| Education, n (%) | 0.351 | |||||
| Less than 9th grade | 149 (6.05) | 96 (5.57) | 53 (7.16) | |||
| 9–11th grade | 341 (13.84) | 229 (13.28) | 112 (15.14) | |||
| High school graduate | 565 (22.93) | 404 (23.43) | 161 (21.76) | |||
| Some college or AA degree | 882 (35.80) | 621 (36.02) | 261 (35.27) | |||
| College graduate or above | 527 (21.39) | 374 (21.69) | 153 (20.68) | |||
| ALQ, n (%) | 0.561 | |||||
| Non-drinker | 628 (25.49) | 438 (25.41) | 190 (25.68) | |||
| 1 to | 1332 (54.06) | 939 (54.47) | 393 (53.11) | |||
| 5 to | 173 (7.02) | 113 (6.55) | 60 (8.11) | |||
| 331 (13.43) | 234 (13.57) | 97 (13.11) | ||||
Note: t: t-test,
| Variables | Non-depression | Depression | Statistic | p | |
| (n = 2108) | (n = 356) | ||||
| Age, Mean | 47.45 | 48.89 | t = –1.57 | 0.117 | |
| GLU (mg/dL), Mean | 109.21 | 116.50 | t = –2.88 | 0.004 | |
| HDL-C (mmol/L), Mean | 1.40 | 1.36 | t = 1.64 | 0.102 | |
| LDL-C (mg/dL), Mean | 111.36 | 111.42 | t = –0.03 | 0.976 | |
| TC (mg/dL), Mean | 188.33 | 189.67 | t = –0.56 | 0.574 | |
| TG (mg/dL), Mean | 114.11 | 128.32 | t = –3.47 | ||
| TyG, Mean | 8.56 | 8.72 | t = –4.37 | ||
| Gender, n (%) | |||||
| Male | 931 (44.17) | 118 (33.15) | |||
| Female | 1177 (55.83) | 238 (66.85) | |||
| Martial, n (%) | |||||
| Married/Living with Partner | 1176 (55.79) | 169 (47.47) | |||
| Widowed/Divorced/Separated | 459 (21.77) | 120 (33.71) | |||
| Never married | 473 (22.44) | 67 (18.82) | |||
| Diabetes, n (%) | |||||
| No | 1700 (80.65) | 250 (70.22) | |||
| Yes | 408 (19.35) | 106 (29.78) | |||
| Hypertension, n (%) | |||||
| No | 1206 (57.21) | 153 (42.98) | |||
| Yes | 902 (42.79) | 203 (57.02) | |||
| Heart Failure, n (%) | 0.019 | ||||
| No | 1987 (94.26) | 324 (91.01) | |||
| Yes | 121 (5.74) | 32 (8.99) | |||
| COPD, n (%) | |||||
| No | 1641 (77.85) | 213 (59.83) | |||
| Yes | 467 (22.15) | 143 (40.17) | |||
| CAD, n (%) | 0.001 | ||||
| No | 2023 (95.97) | 328 (92.13) | |||
| Yes | 85 (4.03) | 28 (7.87) | |||
| Cancer, n (%) | 0.812 | ||||
| No | 1874 (88.90) | 318 (89.33) | |||
| Yes | 234 (11.10) | 38 (10.67) | |||
| Stroke, n (%) | |||||
| No | 2015 (95.59) | 316 (88.76) | |||
| Yes | 93 (4.41) | 40 (11.24) | |||
| SLQ, n (%) | |||||
| No | 1636 (77.61) | 178 (50.00) | |||
| Yes | 472 (22.39) | 178 (50.00) | |||
| BMI status, n (%) | 0.002 | ||||
| Under weight | 35 (1.66) | 8 (2.25) | |||
| Normal weight | 471 (22.34) | 58 (16.29) | |||
| Over weight | 619 (29.36) | 88 (24.72) | |||
| Obese | 983 (46.63) | 202 (56.74) | |||
| Smoking, n (%) | |||||
| Never smoker | 1071 (50.81) | 115 (32.30) | |||
| Former smoker | 577 (27.37) | 94 (26.40) | |||
| Now smoker | 460 (21.82) | 147 (41.29) | |||
| PIR, n (%) | |||||
| Non-low-income | 1455 (69.02) | 148 (41.57) | |||
| Low-income | 653 (30.98) | 208 (58.43) | |||
| Race, n (%) | 0.036 | ||||
| Mexican American | 188 (8.92) | 21 (5.90) | |||
| Other Hispanic | 194 (9.20) | 49 (13.76) | |||
| Non-Hispanic White | 965 (45.78) | 164 (46.07) | |||
| Non-Hispanic Black | 544 (25.81) | 90 (25.28) | |||
| Other Race | 217 (10.29) | 32 (8.99) | |||
| Education, n (%) | |||||
| Less than 9th grade | 110 (5.22) | 39 (10.96) | |||
| 9-11th grade | 271 (12.86) | 70 (19.66) | |||
| High school graduate | 476 (22.58) | 89 (25.00) | |||
| Some college or AA degree | 763 (36.20) | 119 (33.43) | |||
| College graduate or above | 488 (23.15) | 39 (10.96) | |||
| Drinking, n (%) | 0.219 | ||||
| Non-drinker | 523 (24.81) | 105 (29.49) | |||
| 1 to | 1144 (54.27) | 188 (52.81) | |||
| 5 to | 150 (7.12) | 23 (6.46) | |||
| 291 (13.80) | 40 (11.24) | ||||
Both groups exhibited statistically significant differences in terms of hypertension, COPD, stroke, SLQ, smoking, PIR, and education level (p
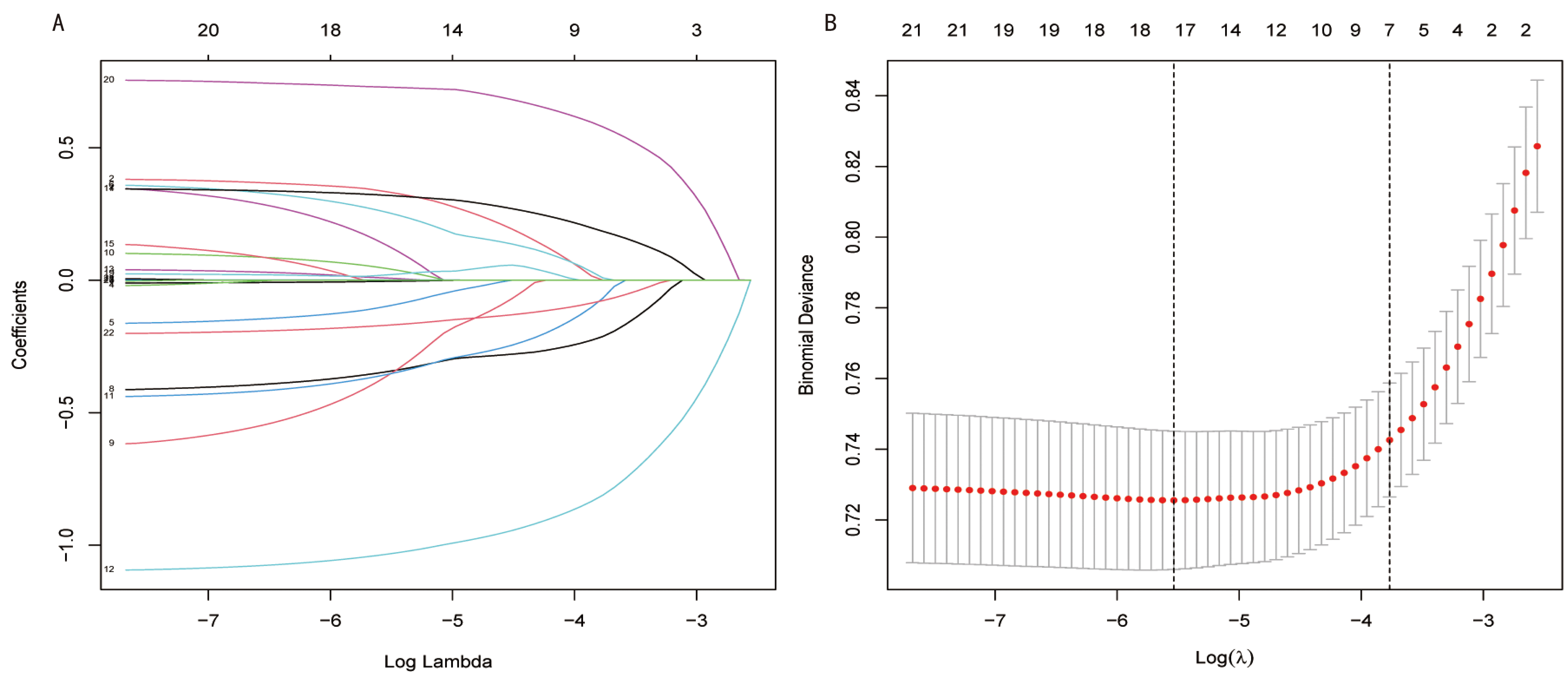 Fig. 3.
Fig. 3. Least Absolute Shrinkage and Selection Operator (LASSO) regression plot. (A) Plot of LASSO coefficient profiles. (B) Plot of partial likelihood deviance.
| Variables | Univariate OR (95% CI) | p | Multivariate OR (95% CI) | p | |
| Hypertension | |||||
| No | 1.00 (Reference) | 1.00 (Reference) | |||
| Yes | 1.77 (1.41 | 1.36 (1.05 | 0.018 | ||
| COPD | |||||
| No | 1.00 (Reference) | 1.00 (Reference) | |||
| Yes | 2.36 (1.86 | 1.48 (1.14 | 0.003 | ||
| Stroke | |||||
| No | 1.00 (Reference) | 1.00 (Reference) | |||
| Yes | 2.74 (1.86 | 1.55 (1.01 | 0.046 | ||
| SLQ | |||||
| No | 1.00 (Reference) | 1.00 (Reference) | |||
| Yes | 3.47 (2.75 | 3.08 (2.40 | |||
| Smoking | |||||
| Never smoker | 1.00 (Reference) | 1.00 (Reference) | |||
| Former smoker | 1.52 (1.13 | 0.005 | 1.16 (0.85 | 0.351 | |
| Now smoker | 2.98 (2.28 | 1.98 (1.47 | |||
| PIR | |||||
| Non-low-income | 1.00 (Reference) | 1.00 (Reference) | |||
| Low-income | 3.13 (2.49 | 2.20 (1.70 | |||
| Education | |||||
| Less than 9th grade | 1.00 (Reference) | 1.00 (Reference) | |||
| 9–11th grade | 0.73 (0.46 | 0.168 | 0.71 (0.44 | 0.169 | |
| High school graduate | 0.53 (0.34 | 0.004 | 0.60 (0.38 | 0.030 | |
| Some college or | 0.44 (0.29 | 0.54 (0.34 | 0.007 | ||
| AA degree | |||||
| College graduate | 0.23 (0.14 | 0.41 (0.24 | 0.001 | ||
| or above | |||||
Abbreviations: OR, odds ratio.
The AUC values for the eight models in the training set are as follows: DT (0.737), KNN (0.794), light GBM (0.771), LR (0.761), MLP (0.762), RF (0.792), SVM (0.664), and XGBoost (0.771) (Fig. 4A). In the validation set, the AUC values are DT (0.712), KNN (0.714), light GBM (0.747), LR (0.754), MLP (0.749), RF (0.732), SVM (0.657), and XGBoost (0.750) (Fig. 4B). Overall, The XGBoost model demonstrated superior performance compared with alternative ML algorithms, achieving an AUC of 0.750, an accuracy of 69.1%, a sensitivity of 68.2%, a specificity of 73.8%, and an F1 score of 79% (Fig. 4 and Table 4).
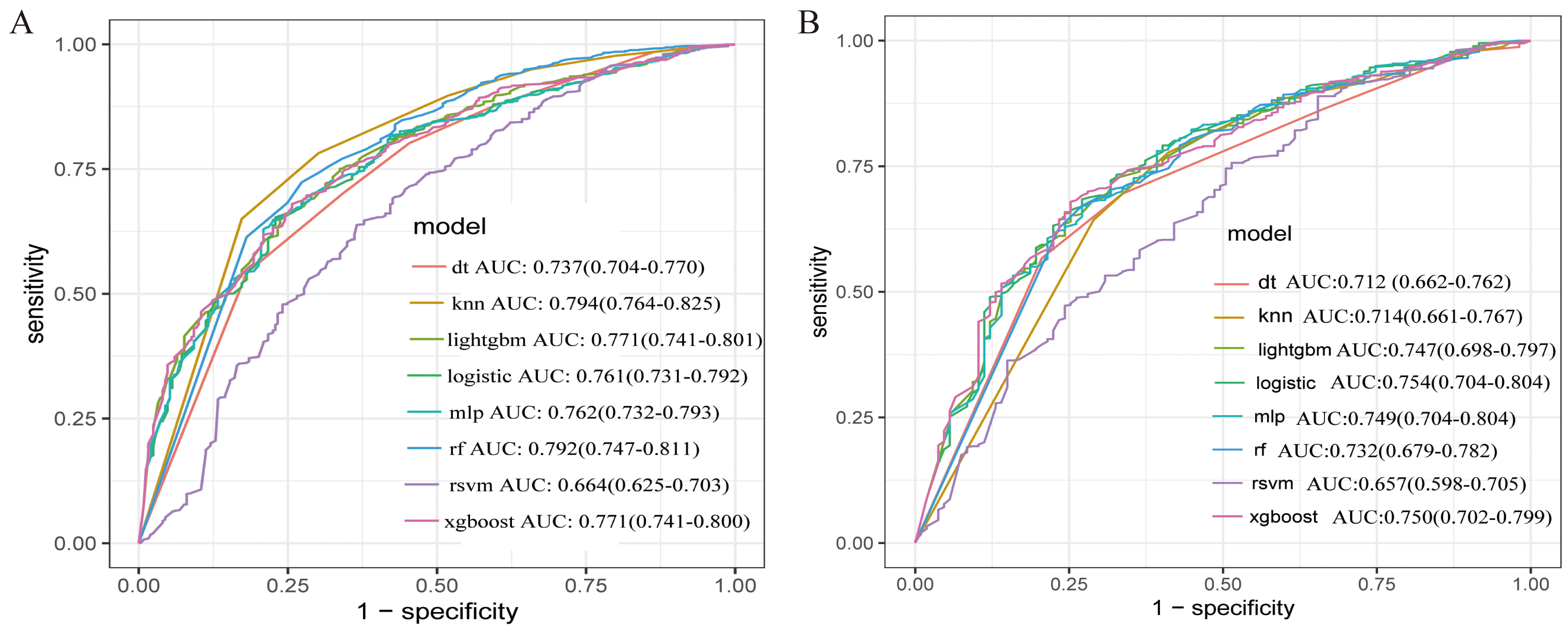 Fig. 4.
Fig. 4. Receiver operating characteristic curve (ROC) curve analysis of the eight machine learning algorithms for predicting short-term prognosis of asthma with depression patients. (A) Training data. (B) Validation data.
| Characteristics | DT | KNN | LGBM | LR | RF | SVM | XGBoost | MLP |
| AUC | 0.712 | 0.714 | 0.747 | 0.754 | 0.732 | 0.657 | 0.750 | 0.749 |
| Accuracy | 0.600 | 0.750 | 0.728 | 0.659 | 0.701 | 0.618 | 0.691 | 0.699 |
| Sensitivity/recall | 0.567 | 0.777 | 0.744 | 0.641 | 0.708 | 0.626 | 0.682 | 0.705 |
| Specificity | 0.794 | 0.589 | 0.636 | 0.766 | 0.664 | 0.579 | 0.738 | 0.664 |
| PPV | 0.942 | 0.918 | 0.924 | 0.942 | 0.926 | 0.898 | 0.939 | 0.925 |
| NPV | 0.237 | 0.309 | 0.296 | 0.265 | 0.277 | 0.207 | 0.282 | 0.275 |
| F1 score | 0.708 | 0.842 | 0.824 | 0.763 | 0.802 | 0.737 | 0.790 | 0.800 |
| MCC | 0.254 | 0.288 | 0.288 | 0.291 | 0.275 | 0.147 | 0.305 | 0.272 |
| KAPPA | 0.182 | 0.266 | 0.257 | 0.229 | 0.235 | 0.117 | 0.252 | 0.232 |
Abbreviations: AUC, area under the curve.
DCA was conducted on the eight machine learning models in the validation set to evaluate the net benefit of the best models in clinical decision-making, defined as the minimum probability of illness necessitating further intervention. Fig. 5 shows the net benefit at various threshold probabilities. The orange line represents the scenario where all patients receive the intervention, while the yellow line indicates all the patients do not receive it. Here, “Treat all” overlaps with “MLP”. Given the heterogeneous nature of the study population, the treatment strategy provided by any of the machine learning-based models outperformed the default strategy of intervention or no intervention for all patients, with the XGBoost model outperforming the other models in terms of net benefit.
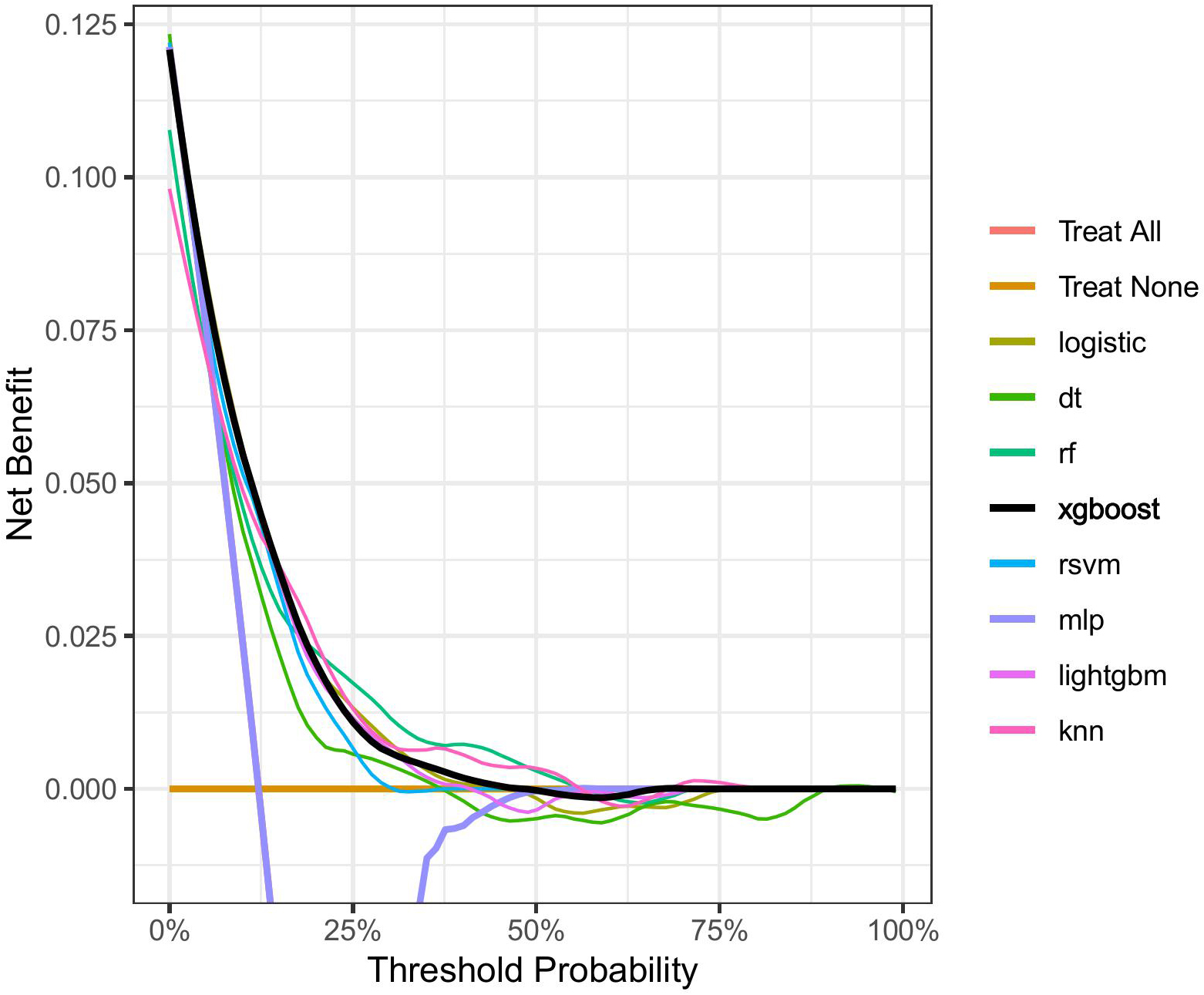 Fig. 5.
Fig. 5. Decision curves for each model.
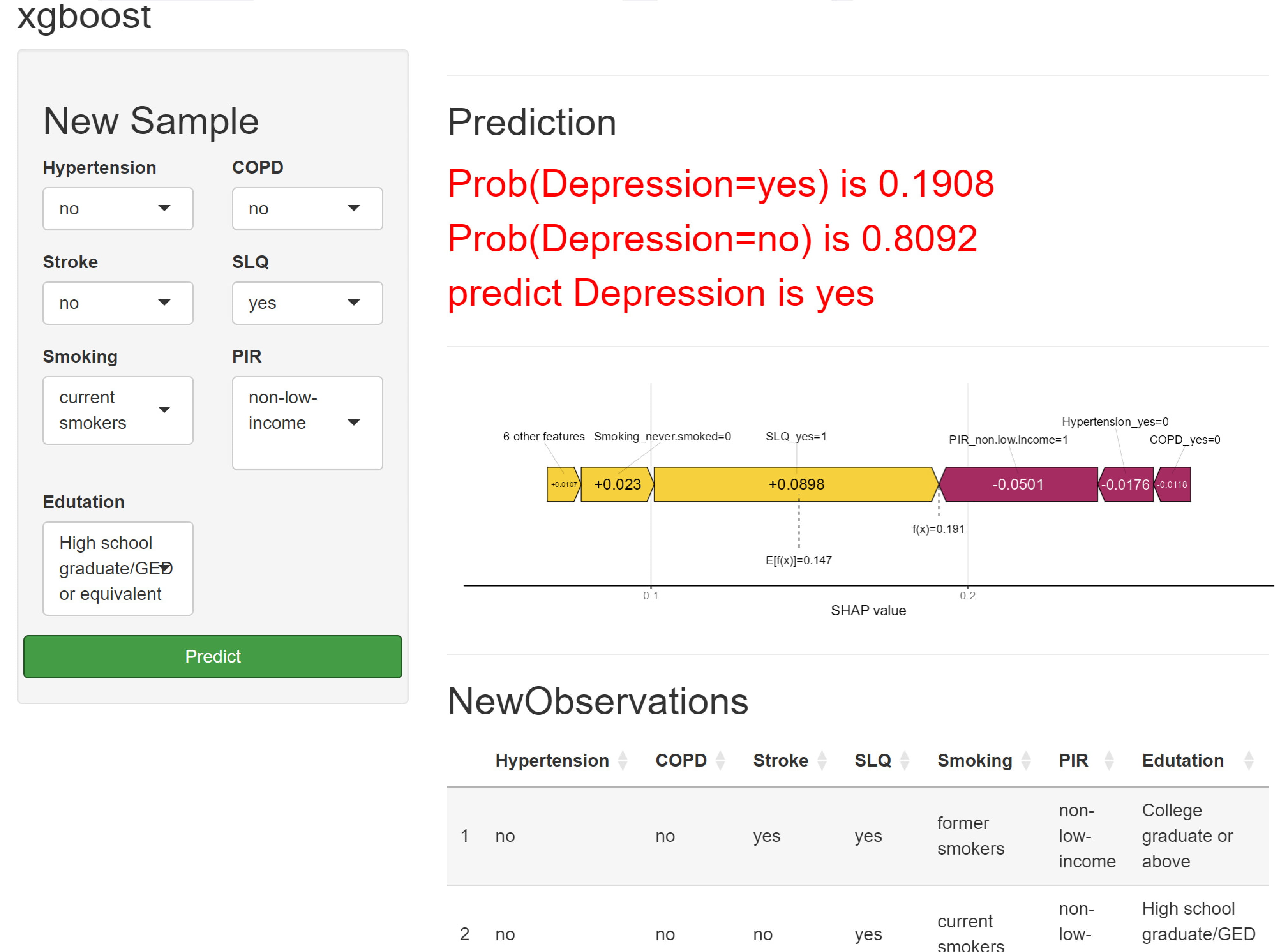
We visualized the importance of each predictor variable in the XGBoost model predictions using SHAP plots. Sleep disturbance emerged as the most significant predictor, followed by PIR and education level. The effect of each variable on the outcome is illustrated by the magnitude of the SHAP value (indicated by color changes) and the trend along the horizontal axis (which represents the probability of a poor outcome). For instance, individuals with severe sleep disorders (shown in blue) are more likely to experience depression than those without sleep disorders (shown in red). Similarly, those with lower household incomes face a higher risk of depression compared to individuals with higher incomes, and asthma patients with lower education levels may also have an increased risk of depression (Fig. 6). In addition, we have developed a predictive modeling website for evaluating the risk of asthma-associated depression. Medical staff can use this website to quickly assess the risk of depression in asthma patients within a short period of time. It can be accessed via the following link: (https://chunyuzhang28.shinyapps.io/asthma/) (Fig. 7). For instance, consider an asthma patient who does not suffer from hypertension, coronary heart disease, or stroke. This patient has a history of sleep disorders and smoking, comes from a high-income family, and belongs to a highly educated demographic group. Following the construction and application of a machine learning predictive model, it is determined that the probability of this individual having depression is 0.1908. Such a result implies a certain predisposition to depression, and thus clinicians can carry out targeted interventions on this patient based on this finding.
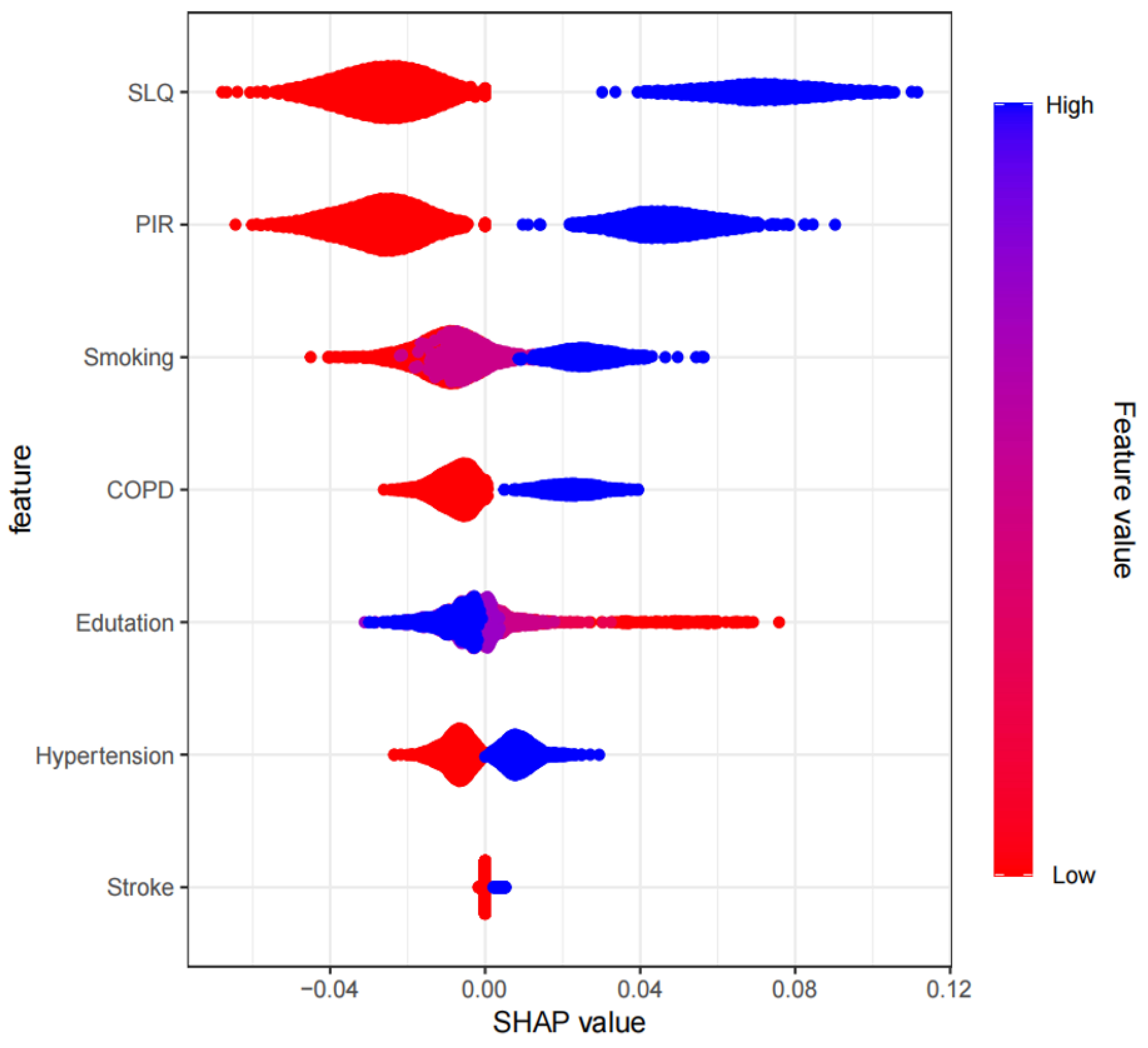 Fig. 6.
Fig. 6. SHAP dendrogram of features of the logistic regression model.
Depression is a significant global mental health issue. Genetic, biological, and environmental factors can contribute to comorbid depression in asthma patients. Early prediction and intervention for depression in these individuals are particularly important. ML-based methods have demonstrated promising applications in the diagnosis and treatment of depression. In this study, we developed a predictive model for asthma-related comorbid depression and created an interactive visual prediction platform. Our goal was to offer personalized counseling and enhance medical decision-making, ultimately leading to more effective management strategies in clinical practice. Compared to traditional prediction models, such as nomogram plots, this platform provides more efficient support for clinical applications.
This study referred to previous studies on common risk factors associated with depression in asthma patients, including hypertension, COPD, stroke, SLQ, smoking, PIR and educational level [20, 23]. In a study of 100 patients with bronchial asthma, Zhang et al. [24] found that age
Behavioral factors such as sleep disturbance and smoking are strongly associated with the onset of depression, particularly in people with asthma. Studies have shown that asthmatics with sleep disturbances are more likely to experience severe depressive symptoms [25, 26]. Approximately 90% of patients experience coughing or wheezing at night, which is associated with nocturnal bronchial hyperresponsiveness and decreased lung capacity [7, 25]. The prevalence of comorbid anxiety and depression is as high as 20% in patients with asthmatic insomnia [9, 27]. Rhoads et al. [5] showed that insomnia not only exacerbates anxiety symptoms in patients with asthma, but can also lead to depressive symptoms such as trouble falling asleep, difficulty staying asleep, and early awakening, which can further exacerbate depressive symptoms [28, 29]. In turn, chronic insomnia can lead to mood problems by affecting brain function and reducing mood regulation through mechanisms that may be related to an imbalance of neurotransmitters involved in mood regulation (e.g., serotonin, norepinephrine and dopamine) and may lead to abnormalities in cortisol secretion, which can induce chronic stress and depression [30]. Similarly, smoking, a risk factor for acute asthma exacerbations and limited irreversible airflow, not only exacerbates asthma symptoms but may also induce persistent limitation airflow [31]. Although the nicotine in smoke may improve mood in the short term, long-term smoking exacerbates asthma and depressive symptoms [32, 33]. Toyama et al. [34] analyzed 1962 asthma patients and found that smokers had a significantly higher prevalence of asthma than non-smokers and that depressed mood had a significant negative effect on asthma control scores. These findings highlight the importance of smoking cessation in the health management of asthma patients.
Socioeconomic factors such as PIR and education level play a key role in the health status of asthma patients [35]. Low-income families face greater economic pressures, including medical, educational and living expenses, and this chronic economic pressure increases the risk of depression in asthma patients [10]. Moreover, education level has a significant impact on managing depression in asthma patients. Tarraf et al. [36] found that less than 30% of asthma patients had good control and that the level of control was closely related to smoking frequency, lower educational level and inappropriate use of medication. Asthmatics with lower education levels often lack awareness of mental illness and effective coping strategies, making them more susceptible to negative emotions like anxiety and depression. Therefore, attention should be paid to the family income and the education level of asthma patients to reduce the risk of depression through early detection and prevention.
Disease factors such as COPD, hypertension and stroke may negatively affect the mental health of asthma patients and increase the risk of depression mood [11]. These chronic diseases not only lead to symptoms such as dyspnea, but also reduce the quality of life of patients, which increases the risk of depression. Chronic health problems may also increase psychological stress [37, 38]. Notably, these diseases may be interrelated, for example, patients with COPD are often associated with complications such as hypertension, which may further exacerbate depression. Therefore, it is recommended to enhance chronic disease management in patients with asthma to reduce the risk of depression.
XGBoost is an integrated decision tree-based learning algorithm widely used in risk prediction, feature selection and clinical decision support, which has the advantages of fast speed, good results, high flexibility and high predictive accuracy. In this study, it was found that the prediction model of XGBoost performed the best in terms of synthesis and performance compared to algorithms such as DT, KNN, LGBM, LR, RF, SVM, MLP, etc., and was especially optimal in predicting asthma-combined with depression. Li et al. [39] found that the Cat Boost model was suitable for depression screening in older adults using data from the China Health and Retirement Longitudinal Study (CHARLS). Su et al. [40] predicted risk factors for depression in older adults using eight ML models and found that self-rated health, marital status, arthritis, and number of cohabits were key factors. Hatton et al. [41] confirmed that the XGBoost model outperformed the traditional logistic regression model in predicting depression in elderly patients. Xia et al. [42] also confirmed that the prediction of depression by XGBoost was superior to that of DT, SVM, Multivariate Adaptive Regression Splines (MARS), Artificial Neural Network (ANN), BT, RF, KNN and other models, supporting the findings of this study.
In conclusion, prevention of depression in asthma patients should focus on those patients with sleep disturbances, lower family economic income and lower educational levels. XGBoost has significant advantages in predicting depression in asthma patients, and by incorporating seven independent risk factors, it helps in the prevention and management of depression improve their mental health.
This study has several limitations: First, all participants were Americans aged
The interpretable XGBoost prediction model we developed performs optimally in assessing the risk of depression in asthma patients. This interpretable machine learning approach accurately identifies risk factors for depression in asthma patients and enhances physicians’ confidence in the prediction results, thus helping them to identify asthma patients at high risk of depression and provides them with the best treatment options.
These survey data are free and publicly available, and can be downloaded directly from the NHANES website (http://www.cdc.gov/nchs/nhanes.htm) by users and researchers worldwide.
Concept—QN, YXW, YLX, WL; Design—QN, XD, XC, TWL, WL; Supervision—YTL, XC, QSR, JJL, JYL; Resources—YLX, YXW; Materials—QN, YXW, YLX, WL; Data Collection and/or Processing—QN, XD, XC, TWL, WL, QSR, JJL, YTL, JYL, YXW, YLX; Analysis and/or Interpretation—QN, YXW, YLX, WL, XD, QSR, YTL, JYL, JJL; Literature Search—QN, YXW, YLX, JJL, JYL; Writing—QN, YXW, YLX, WL, TWL, XD; Critical Review—QN, YXW, YLX. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We are centrally grateful to C.Z., for his guidance and support in data analysis.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.