1 Child Rehabilitation Division, Ningbo Rehabilitation Hospital, 315040 Ningbo, Zhejiang, China
2 State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, 100875 Beijing, China
Abstract
Autism spectrum disorder (ASD) is a multifaceted neurodevelopmental disorder marked by impaired interactions and restricted interests, the pathophysiology of which is not fully understood. The current study explored the potential therapeutic effects of transcranial direct current stimulation (tDCS) on the neurophysiological aspects of ASD, specifically focusing on the brain’s excitatory/inhibitory (E/I) balance and behavioral outcomes, providing scientific guidance for ASD intervention.
Forty-two children with ASD were randomly divided into either an active tDCS or sham tDCS group. Electroencephalography (EEG) recordings were conducted before and after stimulation to assess E/I changesusing EEG markers including α oscillations and the aperiodic exponent, and average spatial phase synchronization (ASPS) analysis and detrended fluctuation analysis (DFA) were performed. Behavioral changes were evaluated using the Autism Behavior Checklist (ABC) and the Social Responsiveness Scale (SRS).
Active tDCS resulted in significant increases in α oscillation power, reductions in α bandwidth, and improvements in γ-band ASPS and DFA values. Furthermore, participants in the active tDCS group exhibited improvements in behavioral scores on the ABC and SRS, with enhancements in social communication, sensory processing, and adaptive behavior. We found no significant changes in the sham group.
These findings suggest that tDCS intervention effectively reduced brain excitability and improved E/I balance and behavioral outcomes in children with ASD. The results warrant further investigation into the efficacy and underlying mechanisms of tDCS for ASD treatment.
No: ChiCTR2400092790, https://www.chictr.org.cn/showproj.html?proj=249950.
Keywords
- autism spectrum disorder
- brain modulation
- electroencephalography
- excitatory/inhibitory balance
- transcranial direct current stimulation
1. Transcranial direct current stimulation (tDCS) can effectively regulate the brain excitability of children with autism spectrum disorder (ASD) and optimize their excitation/inhibition balance (E/I balance).
2. After tDCS intervention, children with ASD demonstrated a remarkable decline in behavioral scale scores, which evidently indicated an improvement in their behavioral performance.
3. Electroencephalography (EEG) markers were capable of effectively mirroring the alterations in the E/I balance and can be utilized to assess the efficacy of tDCS.
Autism spectrum disorder (ASD) is a multifaceted neurodevelopmental condition marked by deficits in communication, repetitive behaviors and limited interests, typically manifesting in early childhood [1]. The etiology of this disorder is multifactorial, involving a complex interplay of genetics and environment [2]. Despite the increasing prevalence of ASD, its pathogenesis remains elusive. Currently, interventions for ASD children mainly involve traditional rehabilitation therapies designed to modify psychological states and correct abnormal behaviors through psychological and behavioral approaches. However, targeted therapies for ASD are actively being researched [3].
Traditional rehabilitative therapies for ASD include behavioral analysis, occupational therapy, and speech therapy. These methods are often time-consuming and have limited efficacy, placing significant burdens on families and caregivers [4]. Therefore, exploring new targeted treatments for ASD is of great importance. As neuromodulation technologies develop, transcranial direct current stimulation (tDCS) has emerged as a bright non-invasive neuromodulatory tool [5]. tDCS is a safe, inexpensive, non-invasive technique using a low DC voltage to brain’s cortex through electrodes placed on the scalp, thereby modulating brain excitability [6, 7]. Increasing evidence suggests that when tDCS is applied to the frontal regions, attention, learning, memory, alertness, and neurotransmitter levels can be significantly modulated [8, 9, 10, 11]. These effects make tDCS a hopeful tool for the treatment of ASD.
One of the most widely targeted areas for tDCS intervention was the dorsolateral prefrontal cortex (DLPFC), a critical region for executive functions that plays a crucial role in cognitive control, learning, planning, attention, and motivation [12, 13, 14]. However, research on the effects of tDCS in ASD has largely focused on behavioral assessments, which show positive effects on cognitive, social communication, and speech skills, but research on the direct effects of tDCS on the brains of children with ASD remains limited.
Study results have indicated that the brain’s excitatory/inhibitory (E/I) balance is crucial for understanding the pathophysiology of ASD [15]. Rubenstein and colleagues [16] identified a close relationship between changes in neuronal E/I balance and ASD, a finding that has been confirmed by several genetic studies. E/I balance is an intrinsic measure of excitability that does not depend on external stimuli [17]. A study has indicated that certain forms of ASD might be associated with an elevated E/I ratio, potentially specific to certain brain regions, reflecting local E/I imbalances and homeostatic regulation [18]. Other evidence suggests that the global deficits associated with ASD can also be described as neuronal hyperexcitability or hypo-excitability, emphasizing the importance of E/I balance [19].
Measuring E/I balance presents challenges, both directly and indirectly. Techniques like positron emission tomography (PET), magnetic resonance spectroscopy (MRS), and transcranial magnetic stimulation (TMS) are useful indicators of receptor occupancy and neurotransmitter activity, but they are limited by poor temporal resolution and high cost [19, 20]. On the other hand, electroencephalography (EEG) can capture real-time dynamics influenced by E/I fluctuations. High-density EEG systems also address spatial-resolution issues, allowing for precise source localization [21]. To assess E/I function, EEG markers are needed to capture both global and local E/I dynamics. Several methods, such as corrected
In conclusion, this study was designed to use EEG surrogate markers to capture the E/I change in the resting-state brain of ASD children after receiving tDCS stimulation on the left DLPFC. Additionally, we combined behavioral assessments to provide a more comprehensive insight into the therapeutic potential of tDCS in modulating the neurophysiological basis of ASD.
Forty-two ASD children were divided into two groups at random, each with 21 participants: one group received active tDCS stimulation, and the other group received sham stimulation. Basic information about the participants is shown in Table 1. All participants were diagnosed by professional doctors based on DSM-5 criteria [29]. Written informed consent was obtained for all participants or their legal guardians. Their parents were thoroughly briefed on the entire experimental procedure before participation. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Ningbo Rehabilitation Hospital (Approval Number: 2023006; Date: 24 March 2023).
| Group | Number of children | Male/female | Age |
| Real stimulation | 21 | 13/8 | 5.4 |
| Sham stimulation | 21 | 13/8 | 5.9 |
SD, standard deviation.
Inclusion criteria for ASD children were: (1) assessed by a professional child psychiatrist and diagnosed with autism; (2) age 4–6 years; (3) written informed consent was obtained. Exclusion criteria were: (1) children with neurological disease history (e.g., epilepsy, brain injury, and brain surgery); (2) children receiving any medications; (3) previous exposure to tDCS, transcranial magnetic stimulation, or neurofeedback.
EEG was recorded in a quiet room. Each child sat comfortably in a chair, wearing an EEG cap, and keeping their eyes open. Typically, a guardian and a specialist monitored the condition of the child and make sure that the data were of good quality, with the specialist gently reminding the child to minimize blinking. EEG recordings lasted approximately 5–10 minutes. EEG data were recorded using a 128-channel HydroCel Geodesic Sensor Net (GSN) system (Electrical Geodesics, Inc. [EGI], Eugene, OR, USA). Throughout the EEG collection, electrode impedance was maintained below 50 k
Offline data analysis was performed using MATLAB R2016a (Version 9.0.0.341360, MathWorks Inc., Natick, MA, USA) [30] and EEGLAB (Version 13.5.4b, SCCN/University of California San Diego, La Jolla, CA, USA) [31]. To increase the speed of operation, we first down-sampled the EEG data to 200 Hz and filtered the EEG signals using a 1–45 Hz bandpass filter according to research plan. Independent component analysis was applied to remove eye blinks, muscle artifacts, and myoelectric components from the collected EEG signals [32]. Then, visual inspection of the data was performed to remove noisy segments. Finally, all the channels were re-referenced to the mean reference. We chose 19 electrodes for further analysis based on the 10–20 International Electrode System (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2). This significantly decreased the complexity of data processing and reduced storage requirements. The 19 electrodes covered the brain regions we needed to analyze.
A direct-current stimulator (JX-tDCS-1, Huahengjingxing Medical Technology Co., Nanchang, Jiangxi, China) was applied to the scalp at a level of 1 mA. The impedance (7
We recorded scores on the Autism Behavior Checklist (ABC) [33] and the Social Responsiveness Scale (SRS) [34] for children with ASD both before and after tDCS stimulation. These scales were completed by caregivers who were familiar with the ASD children, in order to screen for various behaviors.
The ABC includes 57 items divided into 5 subcategories: sensory; social relating; body and object use; language and communication skills; and social and adaptive skills. The SRS consists of 65 items categorized into 5 dimensions: social awareness; social cognition; social communication; social motivation; and autistic mannerisms. Generally, higher scores on these scales indicate more severe behavioral issues.
(1) Periodic and aperiodic components. To analyze the periodic and aperiodic components of the power spectrum, the power spectrum density (PSD) of the 19 channels was calculated by the Welch method (2s Hamming window, 50% overlap). This approach allowed for modeling the power spectrum as a combination of periodic and aperiodic components. The PSD at each frequency point f is expressed as:
Where
The aperiodic component
In this parameterization,
The periodic component can be considered as a combination of multiple Gaussian functions
Here,
The model-fitting was performed using the components described above, with the following settings: the peak width limits were set to 0.5–12 Hz, the range of fitting frequency was 1–40 Hz, aperiodic mode was set to ‘fixed’ with no inflection points. The periodic oscillatory component was obtained by subtracting the aperiodic component from the fitted spectrum. Final output of the algorithm included aperiodic parameters (exponent, offset) and periodic parameters (amplitude, bandwidth, center frequency).
(2) Average spatial phase synchronization (ASPS). This method has been demonstrated to be an effective indicator for monitoring E/I balance changes. It was computed across space rather than time and had been widely applied in neurology. In our study, we referred to commonly used frequencies for spatial phase synchrony and chose to calculate it in the
(3) Detrended fluctuation analysis (DFA). DFA was used to determine the self-similarity of signals. It calculated the detrended fluctuation of a signal, revealing its long-term correlations and nonlinear dynamic features. Increased DFA values indicated greater complexity and non-randomness in EEG signals. A previous study have shown that a higher DFA index reflected a greater E/I balance, with generally larger DFA indices corresponding to higher E/I ratios [28].
Initially, Shapiro-Wilk test was performed to assess the normality of the data distribution for all variables. Since the aperiodic exponent, offset, periodic
We calculated the aperiodic exponent and offset for 19 channels for active and sham tDCS stimulation groups. For the aperiodic exponent, Main effect of measurement time was significant (F = 131.390, p
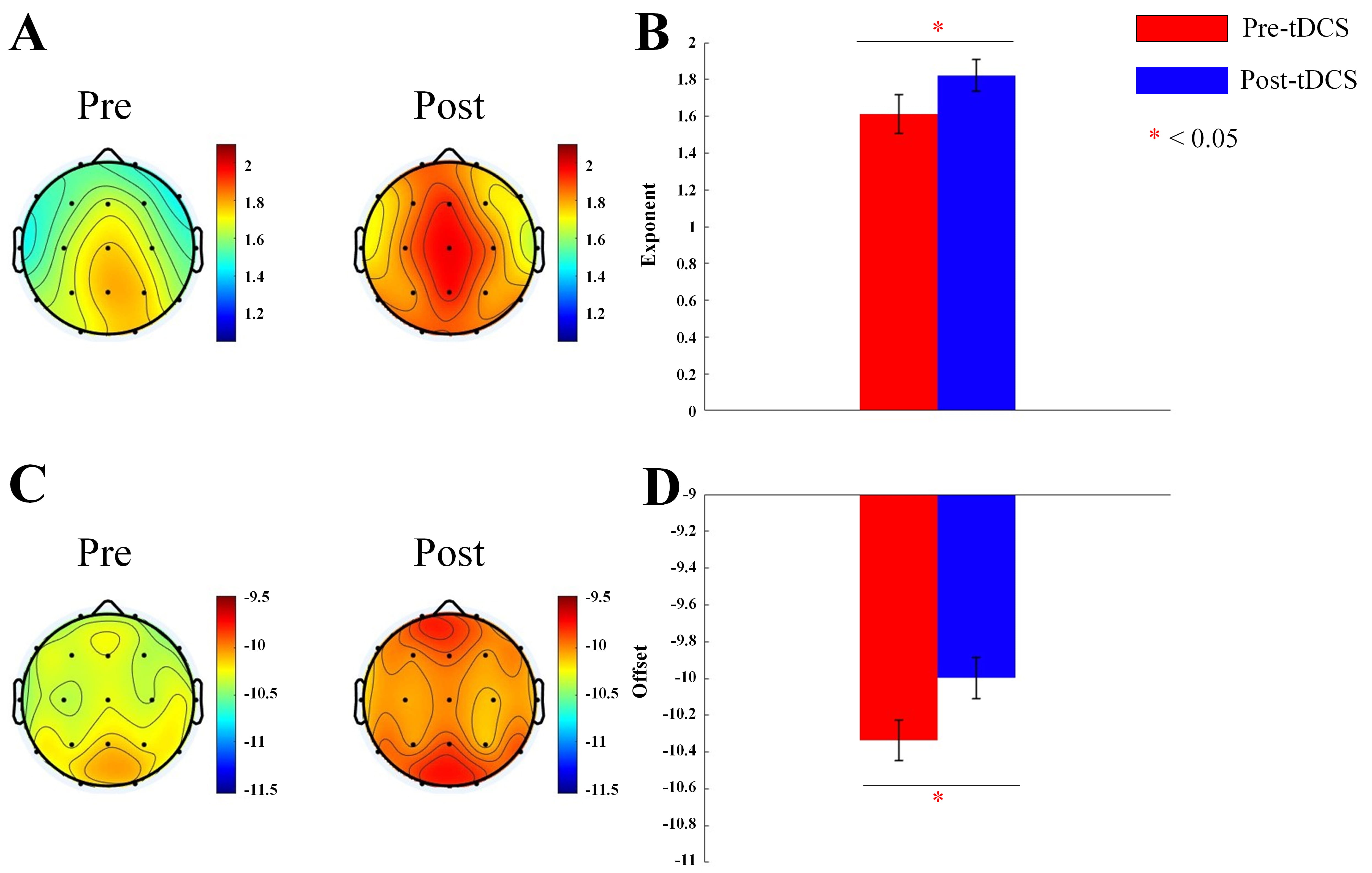 Fig. 1.
Fig. 1. Topographic map and bar graph results of aperiodic parameters. (A) Topographic map of the exponent before and after active tDCS stimulation. (B) Bar graph of the exponent before and after active tDCS stimulation. (C) Topographic map of the offset before and after active tDCS stimulation. (D) Bar graph of the offset before and after active tDCS stimulation. tDCS, transcranial direct current stimulation. *, p
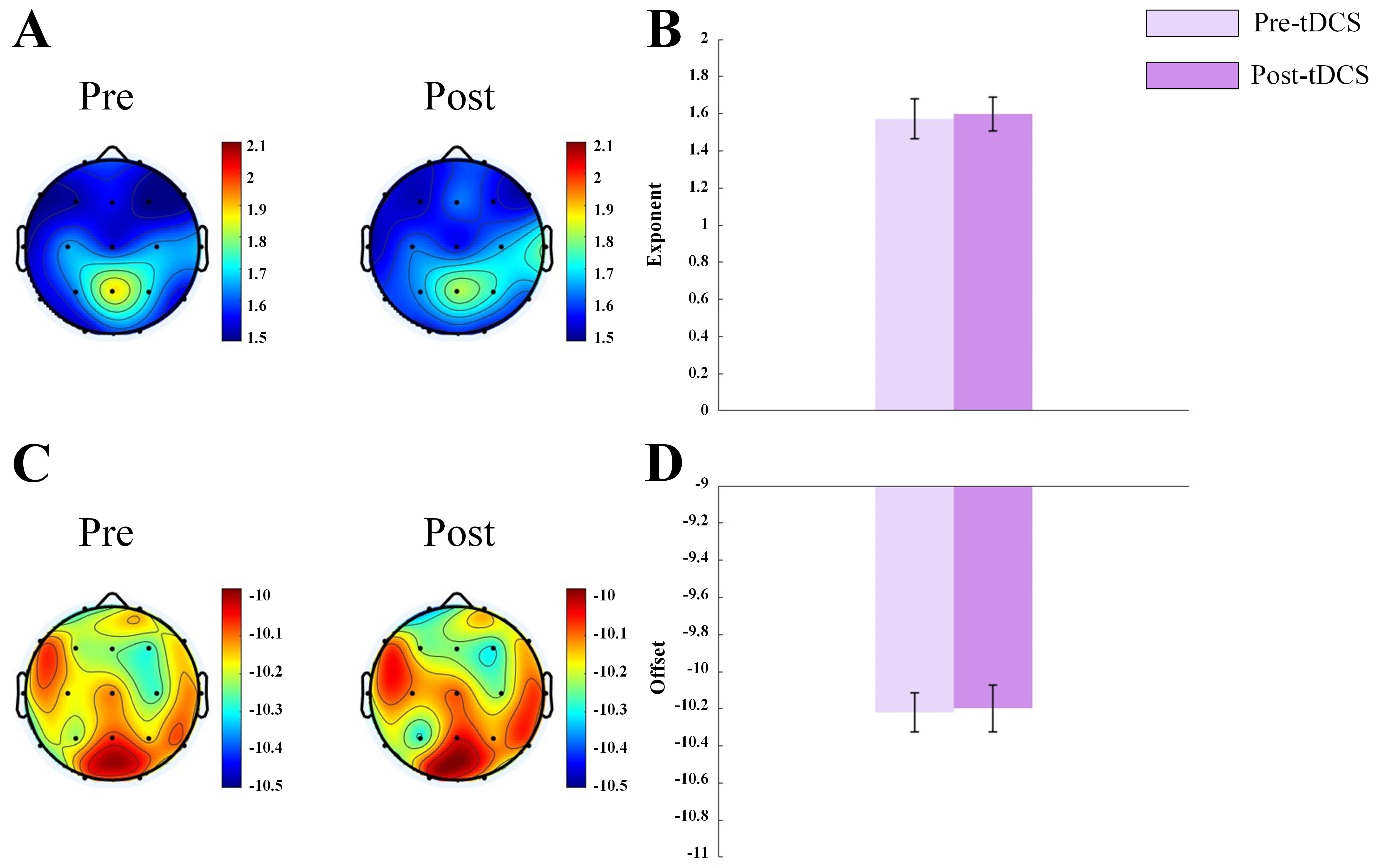 Fig. 2.
Fig. 2. Topographic map and bar graph results of aperiodic parameters. (A) Topographic map of the exponent before and after sham tDCS stimulation. (B) Bar graph of the exponent before and after sham tDCS stimulation. (C) Topographic map of the offset before and after sham tDCS stimulation. (D) Bar graph of the offset before and after sham tDCS stimulation.
By correcting for aperiodic activity and calculating the adjusted EEG power spectrum, we assessed the impact of the corrected 1/f-like aperiodic activity on power spectrum, as well as the characteristics of the corrected
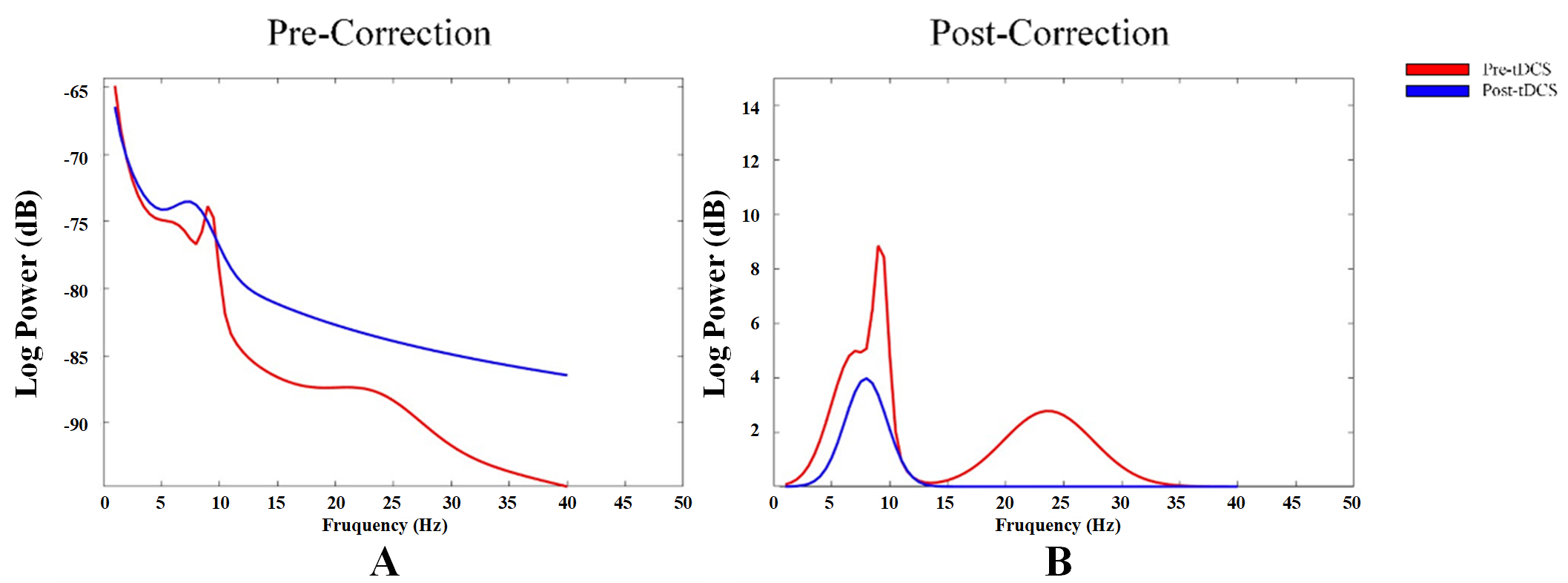 Fig. 3.
Fig. 3. Power spectrum changes in aperiodic neural activity before and after correction. (A) Power spectrum before correction of aperiodic neural activity. (B) Power spectrum after correction of aperiodic neural activity.
We obtained the periodic oscillatory components by subtracting the aperiodic components from the fitted spectrum. We analyzed power and bandwidth of
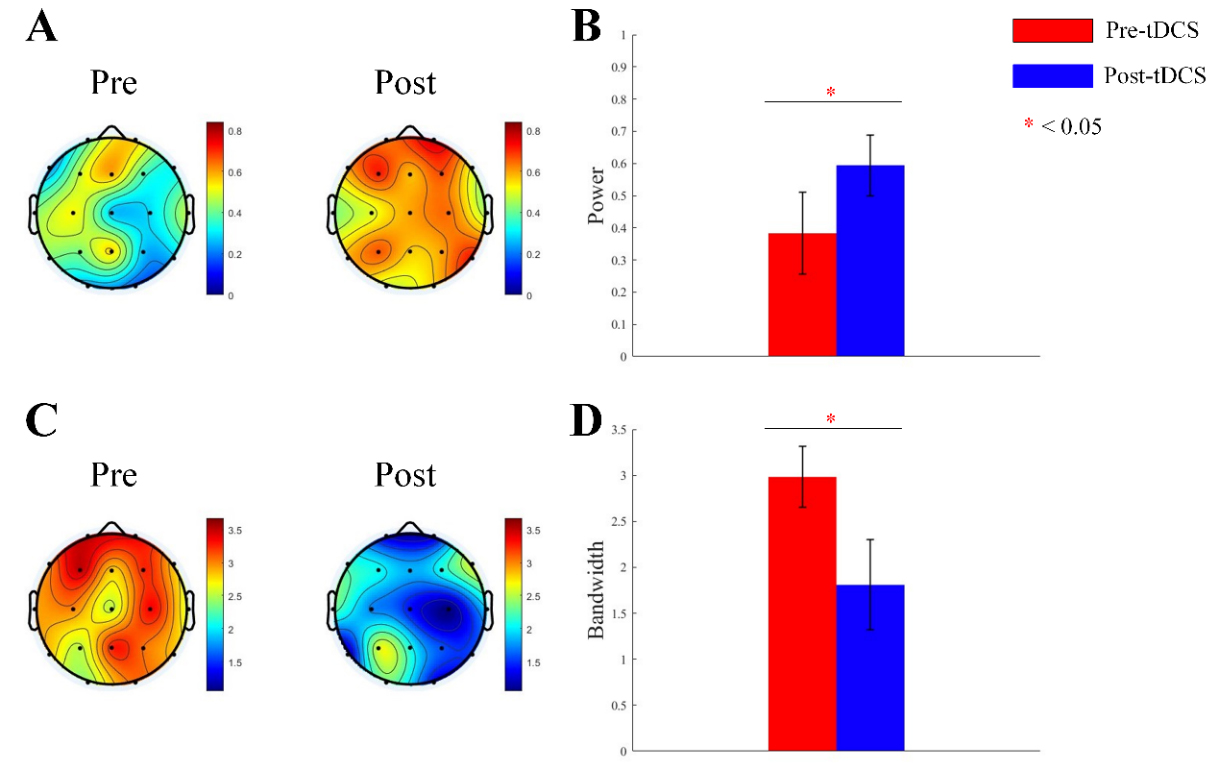 Fig. 4.
Fig. 4. Topographic map and bar graph results of periodic parameters. (A) Topographic map of
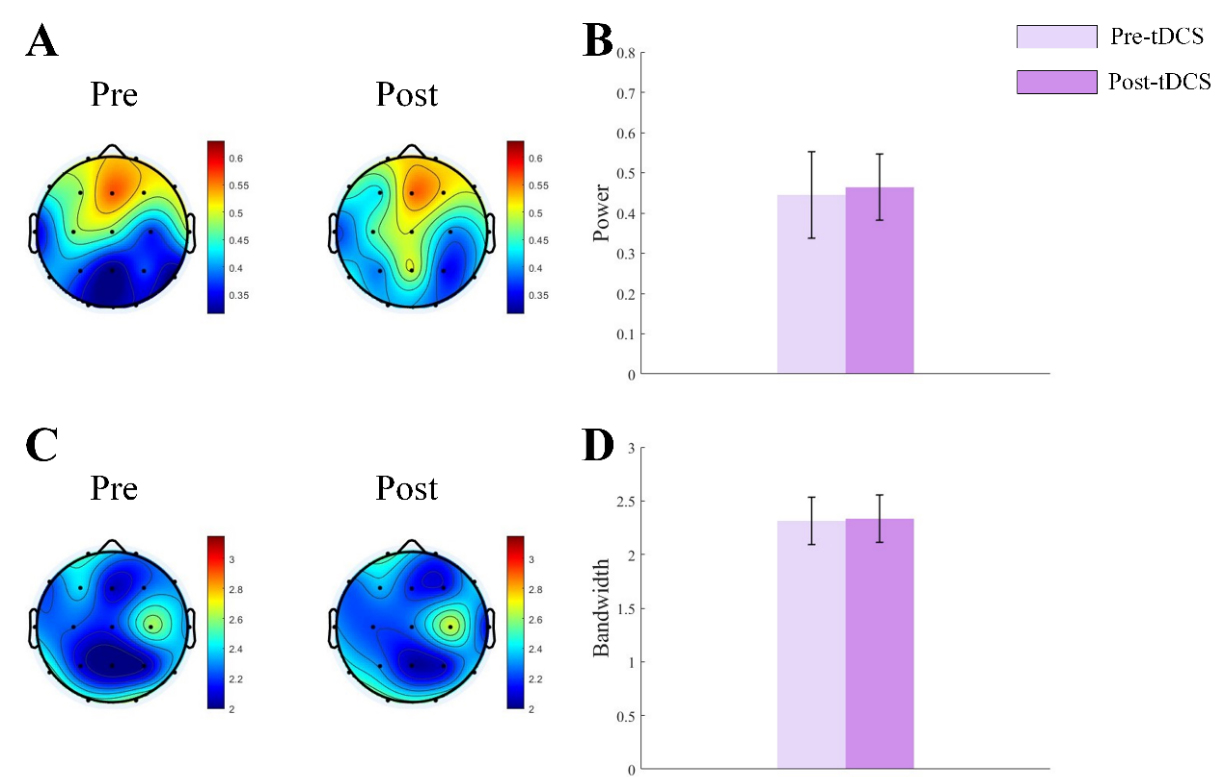 Fig. 5.
Fig. 5. Topographic map and bar graph results of periodic parameters. (A) Topographic map of
In the active group, ASPS in the
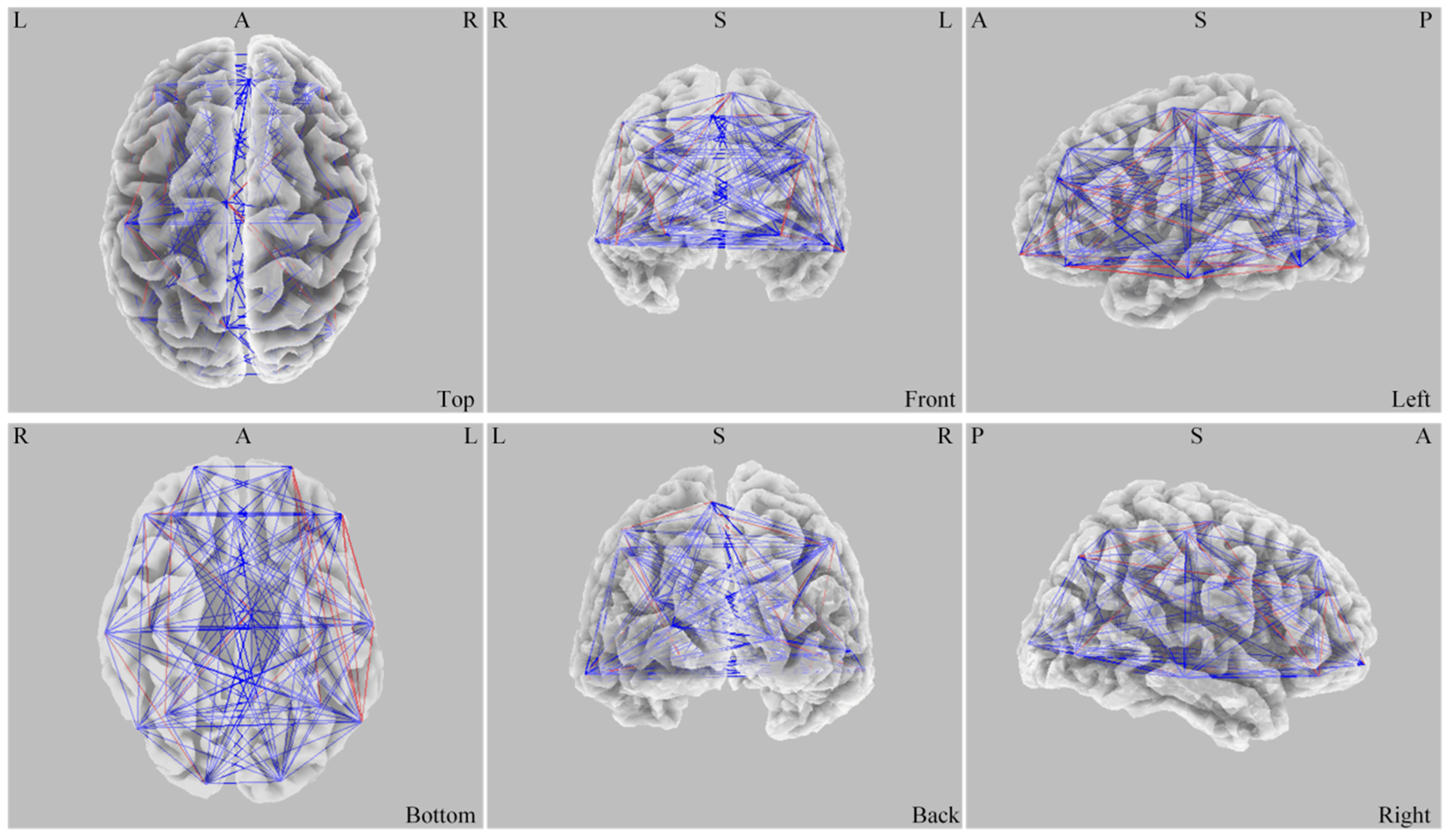 Fig. 6.
Fig. 6. Difference of ASPS before and after stimulation. L, left; R, right; A, anterior; P, posterior; S, superior; ASPS, average spatial phase synchronization.
In the
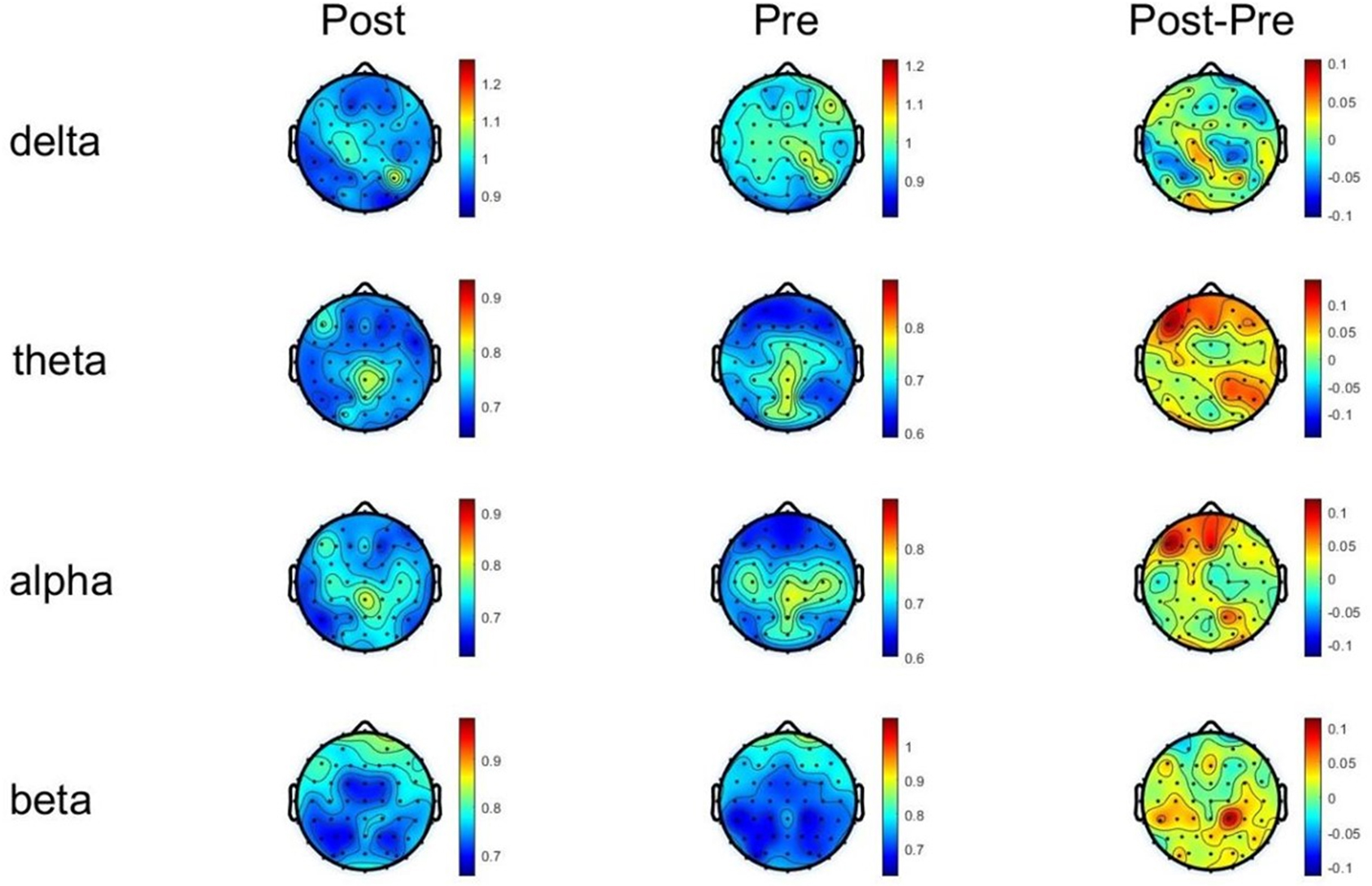 Fig. 7.
Fig. 7. DFA changes of each frequency band before and after tDCS stimulation. DFA, detrended fluctuation analysis.
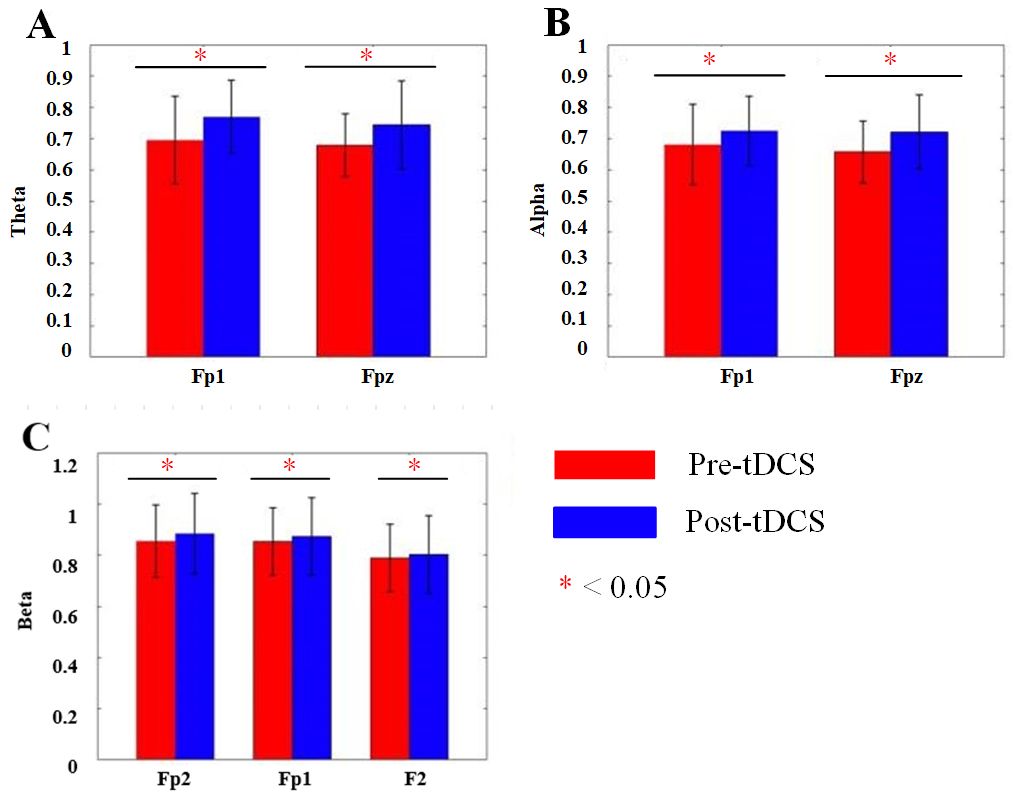 Fig. 8.
Fig. 8. Bar charts of DFA changes before and after tDCS in each frequency band. (A) Bar chart of DFA values for the Fp1 and Fpz channels in the
We collected ABC and SRS scores of ASD children. In the ABC scale of active stimulation, for sensory scores, Main effect of Measurement Time was significant (F = 6.362, p = 0.023). Main effect of Group was significant (F = 15.201, p = 0.001). Interaction effect was significant (F = 16.602, p
In the SRS scale of active stimulation, for social-awareness scores, Main effect of Measurement Time was significant (F = 13.422, p = 0.001). Main effect of Group was significant (F = 38.428, p
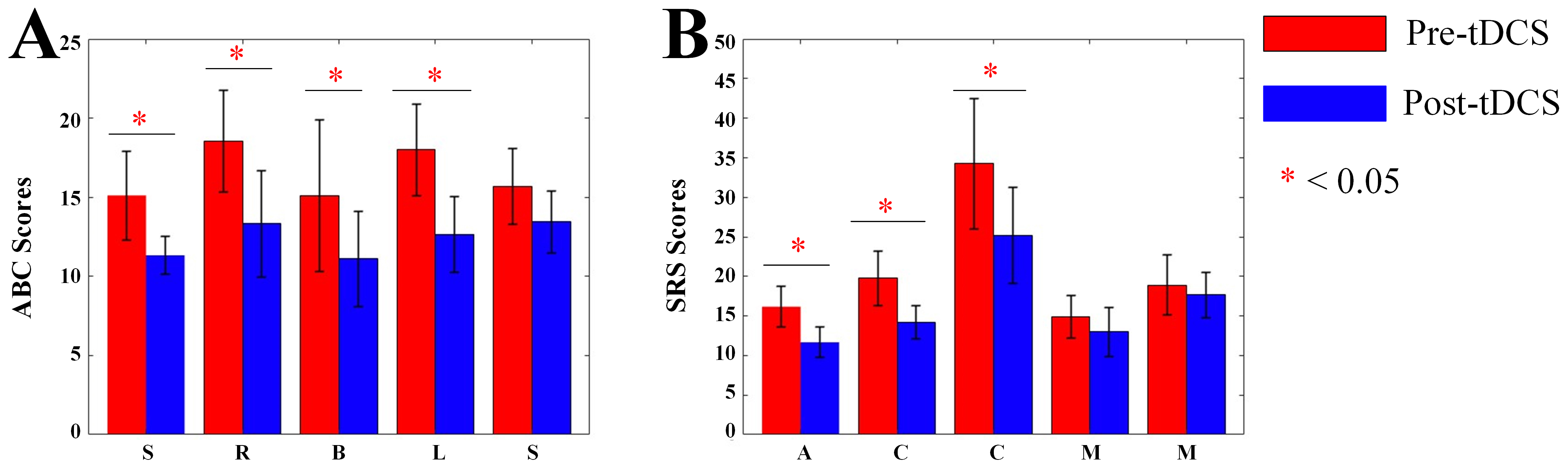 Fig. 9.
Fig. 9. Bar charts of ABC and SRS scores. (A) Bar chart of ABC scale scores. (B) Bar chart of SRS scale scores. S, Sensory; R, Social relating; B, Body and object use; L, Language and communication skills; S (the second), Social and adaptive skills; A, Social awareness; C, Social cognition; C (the second), Social communication; M, Social motivation; M (the second), Autistic mannerisms; ABC, Autism Behavior Checklist; SRS, Social Responsiveness Scale. *, p
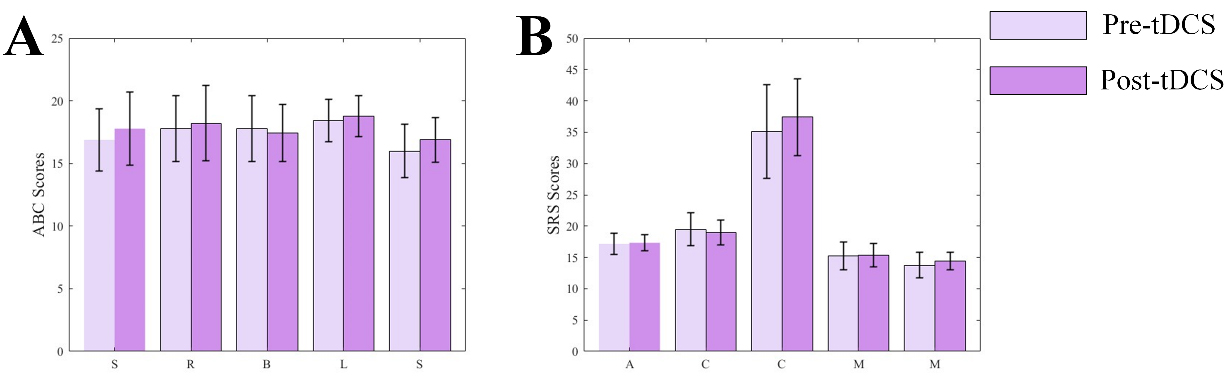 Fig. 10.
Fig. 10. Bar charts of ABC and SRS scores. (A) Bar chart of ABC scale scores. (B) Bar chart of SRS scale scores.
The current study explored the effects of tDCS on the E/I balance and behavioral symptoms in children with ASD. By using EEG-based surrogate markers of brain function, including aperiodic parameters,
One of the key findings in this study was the significant modulation of the aperiodic parameters of the EEG, which reflected the underlying excitability of the brain. Specifically, we observed that after active tDCS stimulation, the exponent and offset of the aperiodic component increased significantly, suggesting a shift toward a more balanced E/I ratio. This finding was consistent with previous research that has suggested that there is an elevated E/I ratio in individuals with ASD, reflecting neuronal hyperexcitability [16]. The increase in the aperiodic exponent and offset after active stimulation aligned with the notion that tDCS acts to normalize this imbalance, potentially reducing hyperexcitability and promoting neurophysiological homeostasis. These findings offered support for the hypothesis that neuromodulation through tDCS could be a valuable tool in restoring optimal brain excitability in individuals with ASD [35].
It is interesting to note that the sham stimulation group did not show any significant changes in the aperiodic exponent or offset, further validating the specificity of the effects of active tDCS. This result highlighted the potential of tDCS in directly modulating neural activity in a way that was not attributable to placebo effects. The absence of changes in the sham group suggested that the improvements observed in the active tDCS group were indeed related to the neuromodulatory effects of the stimulation, rather than to the general experience of participating in the study.
The
Another important finding was the significant increase in ASPS in the
The application of DFA, to assess the long-term correlations and complexity of EEG signals, provided additional insights into the effects of tDCS on brain function [39]. In the present study, DFA values significantly increased in the
In addition to the neurophysiological changes, the study also assessed behavioral outcomes using the ABC and the SRS. Active tDCS led to significant improvements in various behavioral dimensions, including sensory processing, social relating, language and communication skills, and social awareness. These results aligned with a previous study that has showed the efficacy of tDCS in enhancing cognitive and social outcomes in ASD children [40]. The improvements in ABC and SRS scores, particularly in those that relate to social communication and social awareness, suggested that neurophysiological changes induced by tDCS may have translated into functional improvements in social interaction and communication skills—core areas of difficulty for individuals with ASD. The absence of significant changes in behavioral scores in the sham group further supported the effectiveness of the active tDCS intervention. These findings highlighted the potential of tDCS not only as a tool for modulating brain activity but also as a promising approach to address the behavioral symptoms of ASD.
Although this study provided compelling evidence for the potential of tDCS in modulating neurophysiological activity and improving behavioral outcomes in children with ASD, there were several limitations that should be acknowledged. First, the sample size was small; future studies with larger cohorts are needed to confirm these findings and to further explore the generalizability of the results. Second, the long-term effects of tDCS remained unclear, and additional research is needed to investigate the sustainability of the observed improvements in both neurophysiological markers and behavioral symptoms. Finally, future studies should consider investigating other brain regions and stimulation parameters (e.g., current intensity, stimulation duration) to determine the optimal conditions for tDCS in the treatment of ASD.
The present study demonstrated that active tDCS applied to DLPFC significantly modulates E/I balance in the brains of ASD, leading to changes in neural synchrony and cortical excitability. These neurophysiological changes were associated with improvements in various behavioral domains, including social communication, sensory processing, and cognitive functioning. Given its non-invasive nature, low cost, and potential for enhancing brain function, tDCS holds promise as a therapeutic tool for children with ASD, offering a complementary approach to traditional behavioral interventions. It will also reduce the economic burden of ASD treatment on families and society. Further studies were needed to optimize the use of tDCS and explored its long-term impact on ASD symptomatology.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
JK, YL, WM, JW, and XL designed the research study. YL and WM performed the research. JK and JW provided help and advice on the experiments. XL analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Helsinki Declaration and was approved by the Ethics Committee of Ningbo Rehabilitation Hospital (Approval Number: 2023006; Date: 24 March 2023). Written informed consent was obtained for all participants or their legal guardians. Their parents were thoroughly briefed on the entire experimental procedure before participation.
Not applicable.
This research was funded in part by “Kechuang Yongjiang 2035” Key Research and Development Program (2025Z182).
The authors declare no conflict of interest.
During the preparation of this work the authors used DeepSeek in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.










