1 Department of Clinical Laboratory, Beijing Huilongguan Hospital, 100096 Beijing, China
Abstract
To analyze the correlation between interleukin-5 (IL-5), eosinophils (EOS), and immunoglobulin A (IgA) levels with schizophrenia, and assess their potential as auxiliary diagnostic markers for schizophrenia.
This study comprised 57 patients with first-episode schizophrenia and 340 patients with recurrent or chronic schizophrenia who were hospitalized at Beijing Huilongguan Hospital from March 2023 to August 2024, and 72 healthy volunteers were recruited as the control group. Fasting venous blood samples were collected from all participants on the second day after admission. For patients with first-episode schizophrenia, a second blood draw was performed after two months of treatment. Simultaneously, the Positive and Negative Symptom Scale (PANSS) was administered to assess the subjects. IL-5 and EOS levels were measured using flow cytometry; IgA levels were measured using immunoturbidimetry. SPSS v.29.0 was used to conduct t-tests, one-way ANOVA, correlation analysis and receiver operating characteristic (ROC) curve analysis.
The first-episode schizophrenia group and the recurrent/chronic schizophrenia group had elevated IL-5 levels relative to healthy controls; however, the increase in EOS levels was specifically observed in the recurrent/chronic schizophrenia group. After treatment, the IL-5 level in the first-episode group was markedly reduced. Correlation analysis revealed that in patients with schizophrenia, IL-5 levels were positively correlated with EOS (r = 0.338, p < 0.001), and EOS levels were positively associated with disease duration (r = 0.171, p < 0.05), the ROC curve analysis revealed that IL-5 had a sensitivity of 52.9%, specificity of 69.4%, and a cut-off value of 2.445 pg/mL for predicting schizophrenia.
In patients with schizophrenia, the elevated levels of IL-5 and EOS appear to be disease-related rather than medication-induced, suggesting their potential involvement in the inflammatory pathogenesis of schizophrenia. Furthermore, IL-5 exhibits greater predictive accuracy for schizophrenia compared to EOS, suggesting that IL-5 may serve as a valuable biomarker for auxiliary diagnosis and stratification analysis in schizophrenia.
Keywords
- schizophrenia
- interleukin-5
- eosinophils
- immunoglobulin A
Schizophrenia is a serious mental condition characterized by positive symptoms (e.g., delusions and hallucinations), negative symptoms (e.g., anhedonia and social withdrawal), affective symptoms, and cognitive dysfunction [1, 2]. The course of the illness is generally prolonged, with a tendency for relapse that can gradually progress to chronic deterioration of mental function. This condition inflicts varying degrees of harm on affected individuals, their families, and society [3, 4]. The exact pathogenesis of schizophrenia remains incompletely understood, although it is thought to be related to the interplay between environmental and genetic factors [5, 6]. Numerous hypotheses have been proposed regarding its etiology, including neurodevelopmental theories and neurochemical hypotheses associated with neurotransmitters, such as dopamine, serotonin, and glutamate [7, 8]. In recent years, accumulated evidence from an increasing amount of research has suggested that the onset and progression of schizophrenia are linked to immune inflammation [9].
Inflammation can lead to negative emotions and anxiety, as well as impact cognitive processes. Existing research indicates that pro-inflammatory cytokines in the central nervous system may contribute to increased depressive behaviors; neuro-inflammation enhanced by the activation of microglia (immune effector cells within the brain) affects endothelial cells of the blood-brain barrier and promotes the recruitment and transport of peripheral immune cells to stress-sensitive neural regions, which can also cause behavioral changes; sustained inflammation during brain development can potentially interfere with normal brain maturation [7, 10, 11]. In the study of schizophrenia, some patients exhibit varying degrees of systemic inflammatory states, specifically evidenced by enhanced activation of microglia in the brain, increased levels of pro-inflammatory cytokines such as interleukin-1
The administration of anti-inflammatory agents (e.g., non-steroidal anti-inflammatory drugs and N-acetylcysteine) has been reported to mitigate the severity of schizophrenia symptoms. Anti-psychotic medications have been shown to reduce the over-activated inflammatory response observed in psychiatric conditions. For example, haloperidol, risperidone, and aripiprazole effectively suppress the expression of pro-inflammatory markers on microglial cells, including CD16/32 and CD86; aripiprazole increases the expression of anti-inflammatory markers, such as CD206 and Arg-1, leading to reduced levels of pro-inflammatory cytokines (e.g., IL-1
IL-5 is a homodimeric glycoprotein secreted by T-helper type 2 (Th2) cells upon stimulation. This cytokine exerts its effects on target cells by binding to its specific receptor, IL-5R, which consists of a unique
Research on IL-5 has revealed that the colony-stimulating factor 2 receptor beta (CSF2R
Patient data was collected from March 2023 to August 2024 at the Huilongguan Hospital in Beijing. The study included 57 first-episode schizophrenia patients who had not received systematic pharmacological treatment, 340 patients with recurrent or chronic schizophrenia, and 72 healthy controls. The inclusion criteria were: (1) diagnosis of schizophrenia made by two senior physicians based on the relevant standards of the International Classification of Diseases, Tenth Revision (ICD-10); (2) patients had not recently used immunosuppressants, antibiotics, chemotherapeutic agents, or other medications that affect immune status; (3) no infections occurred within one month prior to inclusion. The exclusion criteria were: (1) presence of other severe neurological disorders; (2) existence of serious somatic disease; (3) female participants who were pregnant or breastfeeding; (4) patients who developed infections during the data collection period while hospitalized. The study was approved by the Ethics Committee of Beijing Huilongguan Hospital.
Blood was collected from all study subjects on the second morning of hospitalization after fasting. The 397 schizophrenia patients were categorized into two groups: 57 first-episode cases, and 340 recurrent or chronic schizophrenia cases. Additionally, blood for follow-up testing was collected from first-episode patients on the morning after a two-month systematic treatment regimen.
Three mL of venous blood was collected from each patient in a fasting state and transferred to a tube containing 10% ethylenediaminetetraacetic acid (EDTA) anticoagulant (111030049, IMPROVE Medical Corp, Zhuhai, Guangdong, China). The sample was gently mixed by inversion and then centrifuged at 1000
Five mL of venous blood was collected from each patient in a fasting state and placed in a plain tube without anticoagulant (111150147, IMPROVE Medical Corp). The sample was centrifuged at 3000
Three mL of venous blood was collected from each patient in a fasting state and transferred to a tube containing 10% EDTA anticoagulant. Immediate testing of the sample was performed following collection. The quantification of EOS was performed using a semiconductor laser flow cytometry method. A Sysmex XN-3000 hematology analyzer (SYSMEX Corp, Kobe, Japan) was employed as the analytical instrument, with reagents provided by Sysmex. The sample was gently mixed and then tested on the instrument. EOS were quantitatively analyzed by detecting the intensity of side-scattered light and side fluorescence.
On the day of blood collection, the severity of patients’ clinical symptoms was quantitatively assessed using the Positive and Negative Syndrome Scale (PANSS). The PANSS comprises three subscales totaling 30 items: the Positive Symptom Subscale (7 items), the Negative Symptom Subscale (7 items), and the General Psychopathology Subscale (16 items). Each item is rated on a 7-point scale ranging from 1 to 7, with an overall score range of 30 to 210. Higher scores correspond to more severe clinical symptoms.
Statistical analyses were performed using SPSS software (version 29, IBM Corp, Armonk, NY, USA). For comparisons between groups, continuous variables were first assessed for normality. If normally distributed, they were presented as
A total of 397 patients with schizophrenia were enrolled in this study, comprising 200 males (50.38%) and 197 females (49.62%). No significant differences in the gender ratio or age distribution were observed between the schizophrenia and the healthy control groups. The results showed that IL-5 and EOS levels were significantly higher in the schizophrenia group (p
| Patients with schizophrenia | Healthy individuals | p | ||||
| N | N | |||||
| IL-5 (pg/mL) | 397 | 2.88 | 72 | 2.26 | ||
| Age | p = 0.373 | p = 0.435 | ||||
| 77 | 2.88 | 19 | 2.24 | 0.039* | ||
| 116 | 2.79 | 20 | 2.28 | 0.041* | ||
| 138 | 3.05 | 19 | 2.27 | 0.048* | ||
| 66 | 2.69 | 14 | 2.23 | 0.284 | ||
| Gender | p = 0.498 | p = 0.523 | ||||
| Male | 200 | 2.83 | 34 | 2.17 | 0.004* | |
| Female | 197 | 2.93 | 38 | 2.34 | 0.009* | |
| Disease severity | p = 0.505 | |||||
| PANSS score ( | 231 | 2.84 | 72 | 2.26 | ||
| PANSS score ( | 166 | 2.94 | 72 | 2.26 | ||
| EOS (×108/L) | 397 | 1.50 | 72 | 1.23 | 0.013* | |
| IgA (mg/dL) | 397 | 274.46 | 72 | 259.56 | 0.119 | |
Inter-group comparisons were made using ANOVA, and the remaining comparisons using the t-test. “*” indicates a significant difference of p
No significant difference in the gender ratio was observed between the first-episode group, the recurrent/chronic group, and the healthy control group. However, the mean age of patients in the first-episode group was significantly younger than that of the other two groups (mean age: 23.5 years for the first-episode group, 49.5 years for the recurrent/chronic group, and 41.46 years for the healthy control group). Multi-group variance analysis showed that IL-5, EOS and IgA levels were significantly different between the groups (p
| Healthy individuals | First-episode group | Recurrent/chronic group | p | |
| Male [n (%)] | 34 (47.22) | 31 (54.39) | 169 (49.71) | 0.715 |
| Female [n (%)] | 38 (52.78) | 26 (45.61) | 171 (50.29) | |
| Age [M (QR) (Year)] | 41.46 (25.12) | 23.50 (8.50) | 49.50 (27.50) | |
| IL-5 (pg/mL) | 2.26 | 2.73 | 2.91 | 0.003* |
| EOS (×108/L) | 1.23 | 1.41 | 1.51 | 0.032* |
| IgA (mg/dL) | 259.56 | 257.79 | 279.14 | 0.022* |
Gender data were compared using the chi-square test, age data were compared using the Kruskal-Wallis test, and inter-group comparisons were analyzed using ANOVA. “*” indicates a significant difference of p
After two months of drug treatment, the PANSS scores and IL-5 levels all showed significant reductions in first-episode schizophrenia patients. No statistically significant differences were observed in the EOS and IgA levels before and after treatment (Table 3). Of the 57 patients, 37 showed a decrease in IL-5 level after treatment, 18 showed an increase and 2 showed no change, with a statistically significant difference (p
| Before treatment | After treatment | p | |
| PANSS Positive | 23.73 | 14.71 | |
| PANSS Negative | 19.23 | 13.73 | |
| PANSS General | 43.73 | 29.73 | |
| IL-5 (pg/mL) | 2.73 | 2.18 | |
| EOS (×108/L) | 1.41 | 1.37 | 0.349 |
| IgA (mg/dL) | 257.79 | 247.02 | 0.105 |
The paired t-test was used for comparisons; “**” indicates a significant difference of p
| Decreased IL-5 (n = 37) | Increased IL-5 (n = 18) | ||
| Gender | Male [n (%)] | 22 (59.46%) | 8 (44.44%) |
| Female [n (%)] | 15 (40.54%) | 10 (55.56%) | |
| p | 0.294 | ||
| IL-5 (pg/mL) | Before treatment | 2.95 | 2.33 |
| After treatment | 1.94 | 2.66 | |
| Reduced 34.24% | Elevated 14.16% | ||
| p | |||
Gender data were compared with the chi-square test, and other comparisons were made using the paired t-test. “**” indicates a significant difference of p
Correlation analysis revealed that, after adjusting for potential confounding factors such as age, gender, and disease status, IL-5 levels were significantly positively correlated with EOS levels (r = 0.338, p
| PANSS Positive | PANSS Negative | PANSS General | IL-5 | EOS | IgA | Disease duration | |
| PANSS Positive | 1 | –0.042 | 0.145 | 0.167 | 0.131 | 0.166 | 0.178 |
| PANSS Negative | 1 | –0.021 | 0.139 | –0.097 | 0.093 | 0.146 | |
| PANSS General | 1 | 0.156 | 0.141 | 0.144 | 0.159 | ||
| IL-5 | 1 | 0.338** | 0.183 | 0.141 | |||
| EOS | 1 | 0.098 | 0.171* | ||||
| IgA | 1 | 0.197 | |||||
| Disease duration | 1 |
Correlation analysis was conducted using partial correlation analysis, p values were corrected by Bonferroni correction. “*” indicates a significant difference of p
ROC curve analysis revealed that IL-5 and EOS exhibited area under the curve (AUC values) for schizophrenia of 0.641 and 0.596, respectively, with sensitivities of 52.9% and 86.9%, and specificities of 69.4% and 31.9%. The optimal cut-off points were determined to be 2.445 pg/mL for IL-5, and 0.75
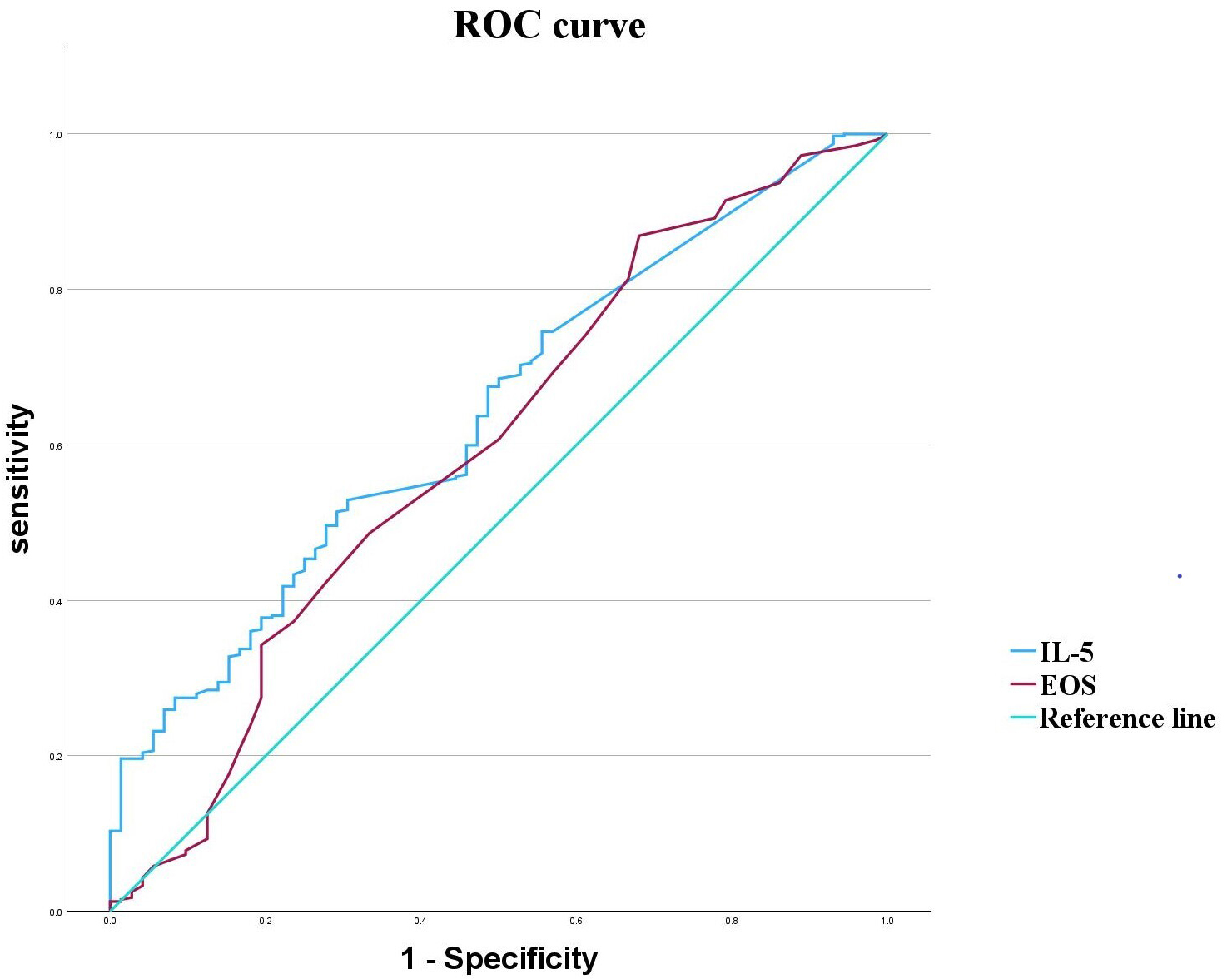 Fig. 1.
Fig. 1. ROC curves for IL-5 and EOS in schizophrenia.
| AUC | 95% CI | Sensitivity (%) | Specificity (%) | Cut-off value | Youden’s index | p | |
| IL-5 | 0.641 | 0.575–0.706 | 52.90 | 69.40 | 2.445 (pg/mL) | 0.223 | |
| EOS | 0.596 | 0.521–0.672 | 86.90 | 31.90 | 0.750 (×108/L) | 0.188 | 0.013* |
“*” indicates a significant difference of p
An increasing number of studies have demonstrated that immune inflammation plays a significant role in the pathogenesis of schizophrenia. Inflammatory mediators can activate the tryptophan-kynurenine metabolic pathway to promote the synthesis of quinolinic acid and 3-hydroxykynurenine. This causes neurotoxicity in the central nervous system, thereby affecting adjacent neurons and nerve cells [29, 30]. A comparative review of serum/plasma biomarkers for differentiating schizophrenia from healthy individuals found that
In the present study, IL-5 levels in patients with schizophrenia were significantly higher than those in healthy individuals, consistent with previous findings [32, 33]. However, no significant difference in IL-5 was observed among patients over 60 years old. This may be attributed to a decline in immune function in elderly individuals or to the use of medications such as non-steroidal anti-inflammatory drugs (NSAIDs) for other chronic diseases [17]. Both male and female patients exhibited significantly higher IL-5 levels than healthy controls, with no significant gender difference. However, female patients showed greater variability in the IL-5 level and a higher prevalence of abnormal levels than male patients. Other studies have also reported similar gender-related effects on cytokine levels in patients with schizophrenia, possibly due to the influence of menstrual cycles and sex hormones on cytokine concentrations in women [34, 35, 36], or it could be attributed to the differences in male and female genes, such as Female individuals carrying the homozygous T allele of IL-8 gene rs1126647 polymorphism exhibit an increased susceptibility to paranoid schizophrenia [37].
Certain anti-psychotic drugs can cause eosinophilia in patients [38], and IL-5 promotes the growth, differentiation, and survival of EOS. Therefore, the potential impact of antipsychotic drugs on IL-5 levels cannot be excluded. To further investigate this effect, we compared IL-5 levels between first-episode and recurrent/chronic patient groups, as well as in first-episode schizophrenia patients before and after treatment. The findings indicate that elevated IL-5 and EOS levels are more likely associated with the disease itself rather than being induced by medication. Previous in vitro and in vivo studies have also demonstrated the anti-inflammatory effects of anti-psychotic drugs, with clozapine, chlorpromazine, haloperidol, aripiprazole and risperidone all shown to reduce the production of inflammatory cytokines [17, 18, 39, 40]. Notably, the inconsistent trend of IL-5 changes observed before and after treatment may be attributed to the type of drugs administered and individual variability among patients.
In the current study, no significant differences in serum IgA levels were observed between the different groups. However, animal studies have demonstrated that IL-5 can activate B cells in the large intestine of mice, leading to the induction of IgA+ B cells and the recruitment of EOS around the activated B cell areas, thereby enhancing IgA secretion in the large intestine [41]. Similarly, studies on schizophrenia have reported elevated gut IgA levels in patients with schizophrenia, which are negatively correlated with the richness of the gut microbiota [42]. The difference in IgA levels between blood and mucosa suggests that the effect of IL-5 on IgA may mainly be manifested in mucosal sites such as the intestine in schizophrenia.
In the analysis of EOS levels, it was observed that the EOS levels in the relapse/chronic group were significantly higher compared to the healthy controls, a mild positive correlation was observed between EOS levels and the duration of the disease. It has been reported that the level of the EOS chemotactic factor Eotaxin-1/CCL11, which selectively recruits EOS to inflammatory sites, is significantly increased in patients with schizophrenia, and the severity of negative symptoms exhibits a positive correlation with the concentration of Eotaxin-1/CCL11 [25]. In addition, during the acute phase of schizophrenia, EOS levels are observed to temporarily decrease; following 6 weeks of treatment, their levels gradually return to normal; during the remission phase of schizophrenia, EOS levels may exhibit an increase. Changes in EOS counts are found to be negatively correlated with both the total score and the positive symptom subscore of the PANSS [43]. This is largely consistent with our observation results. However, we did not detect the transient decrease of EOS in the acute phase or its correlation with PANSS scores, which may be attributable to the grouping strategy and sample size. These findings indicate firstly that short-term drug treatment does not significantly affect EOS levels, although the potential influence of long-term medication remains uncertain; secondly fluctuations in EOS levels may correlate with the progression stage of schizophrenia.
Based on the known characteristics of EOS, it is hypothesized that they may contribute to two distinct roles in the pathogenesis and progression of schizophrenia. First, in patients with schizophrenia, overactivation of the 5-hydroxytryptamine 2A receptor (5-HT2A) triggers upregulation of the eosinophil chemotactic factor Eotaxin-1/CCL11. This upregulation facilitates the migration of eosinophils across the compromised blood-brain barrier to inflammatory sites within the brain, thereby induces degranulation, leading to the release of eosinophil peroxidase (EPO), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and various cytokines, including IL-5, thereby exacerbating neuroinflammation and causing neuronal damage [25, 44, 45, 46, 47]. Additionally, EOS are involved in tissue repair and remodeling processes. T cells can regulate the expression of IL-4 in the decellularized nerve or acellular nerve allograft (ANA) environment by influencing EOS, thereby promoting the repair and regeneration of peripheral nerves [47, 48]. However, considering the irreversible nature of central neuronal damage in schizophrenia, this potential repair function may have limited clinical relevance.
IL-5, EOS, and IgA are not isolated entities but integral components of the body’s immune system. Studies have shown that patients with schizophrenia exhibit elevated levels of monocytes, neutrophils, and C-reactive protein (CRP). Additionally, various cytokines (e.g., monocyte chemoattractant protein-1, IL-1
IL-5 and EOS are significantly elevated in patients with schizophrenia and exhibit a certain degree of correlation. Although the ROC curve analysis demonstrated that IL-5 exhibited superior predictive ability for schizophrenia compared to EOS, when IL-5 is assessed as a standalone biomarker without integration with other cytokines, its diagnostic utility remains relatively restricted. Future studies should explore integrated analyses that incorporate cytokine profiles, cell surface markers, and broader functional outcome measures, or conduct more refined stratified analyses based on the optimal cut-off value of IL-5. Such approaches would contribute to a deeper understanding of the immune mechanisms underlying schizophrenia and improve the evaluation of the diagnostic significance of immune-related indicators.
The differences observed before and after treatment indicate that antipsychotic drugs exert a clear anti-inflammatory effect, suggesting that the elevated levels of IL-5 and EOS are associated with the disease rather than being caused by the medication. However, first-episode schizophrenia patients in our study primarily received medications such as risperidone, aripiprazole, olanzapine, and paliperidone upon admission. Due to poor patient compliance or adverse drug effects, some patients switched medications during treatment. There were instances of combined use of antidepressants, anxiolytics, and sedatives; therefore complicating the medication regimen and hence the interpretation of results. Moreover, differences may exist between first-line and second-line treatments for schizophrenia, and the effects of various drugs on IL-5, EOS, and IgA levels may not be entirely consistent. For example, patients taking clozapine exhibit lower IgA levels compared to those using other medications [54]. Furthermore, clozapine has a more pronounced inhibitory effect on neutrophils while also significantly increasing the level of EOS [38]. Therefore, assessing data from first-episode schizophrenia patients two months post-treatment presents certain limitations and the potential influence of adverse reactions resulting from long-term medication on the outcome cannot be excluded. Previous studies have demonstrated that conducting long-term follow-up research on patients with first-episode schizophrenia holds substantial significance [55]. Such research can not only identify risk factors associated with readmission and systematically evaluate the effects of medication changes on immune markers, but also offer an in-depth analysis of the potential roles that immune-related indicators, such as IL-5, EOS, and IgA, play in the pathogenesis and progression of schizophrenia.
Our findings indicate that in patients with schizophrenia, the levels of IL-5 and EOS were significantly elevated. The increase in EOS counts may be associated with elevated IL-5 levels, and patients with longer disease durations tended to exhibit higher EOS counts. Compared to EOS, IL-5 demonstrated greater predictive accuracy for schizophrenia; however, its diagnostic value as a standalone marker remains limited. IL-5 can be considered a key driver regulating the growth and differentiation of EOS, while EOS may function as effector cells in the body, exhibiting both pathogenic and reparative properties. Currently, the roles of IL-5, EOS, and IgA in the pathogenesis and progression of schizophrenia remain unclear. Further mechanistic studies are warranted. Potential approaches include incorporating additional relevant biomarkers and genetic analyses, conducting animal experiments, or performing long-term cohort studies to gain deeper insights into these mechanisms.
The data supporting the results of this study are available from the corresponding author upon reasonable request.
XL: experimental design, article writing; XYW: data organization; QQZ: statistical analysis; QSZ: detection of IL-5, EOS, IgA; SJP: study design; research guidance, funding support. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Ethics Committee of Beijing Huilongguan Hospital (Approval No.: 2023-92-科, Approval Date: October 2023). The study was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants prior to their involvement in this study.
We thank the colleagues in our department for their guidance and strong support during this research collaboration.
This study was supported by the National Natural Science Foundation of China for Young Scientists (Grant No. 82301691).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

