1 Department of Mental Health, The Second Hospital of Lanzhou University, 730000 Lanzhou, Gansu, China
2 Department of Mental Health, Mental Health Institute of Central South University, 410000 Changsha, Hunan, China
Abstract
Obesity and depressive disorders are significant public health concerns, and their association is well-documented. This study investigates the role of inflammatory markers, specifically C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR), in mediating the relationship between obesity and depressive symptoms.
We utilized data from 37,538 adults from the National Health and Nutrition Examination Survey (NHANES), covering the period from 2005 to March, 2020, pre-pandemic. Depressive symptoms were measured using the Patient Health Questionnaire-9 (PHQ-9), while inflammatory markers were assessed via NLR and CRP levels.
Results indicated a positive correlation between obesity, NLR, and CRP levels, and depressive symptoms. Notably, CRP exhibited a significant mediating effect in the obesity and depressive symptoms link, whereas NLR did not. (NLR: 0.0926%, p = 0.740; CRP: 32%, p < 0.001). Furthermore, the mediating effect of CRP in the male group was significantly higher than in the female group (Men: 57%, p < 0.001; Women: 16%, p = 0.046).
These findings provide new insights into the mechanisms linking obesity and depressive symptoms, especially in men, and may guide future therapeutic strategies.
Keywords
- depressive symptom
- inflammatory mediators
- obesity
- CRP
- NLR
- NHANES
• Obesity and Depressive Symptoms: A significant association was found between obesity and depressive symptoms, which was stronger in women. • Inflammatory Markers (CRP and NLR): Obesity was linked to higher levels of C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR), both of which are associated with depressive symptoms. • Mediation by CRP: CRP played a key mediating role between obesity and depressive symptoms, with a stronger effect in men. • No Significant Mediation by NLR: While NLR was associated with both obesity and depressive symptoms, it did not show significant mediation between them. • Future Research and Treatment: Future longitudinal research to explore causal relationships and personalized treatments based on inflammatory markers is suggested.
Obesity and depressive disorders are prevalent conditions with substantial public health implications. They often co-occur, leading to significant morbidity. Previous research has shown a bidirectional relationship between obesity and depressive disorders, with one increasing the risk of the other. The association between obesity and depressive symptoms is well established, with evidence suggesting a bidirectional interaction between the two [1]. Recent systematic reviews and meta-analyses further emphasize this relationship, reporting that the prevalence of obesity among individuals with major depressive disorder (MDD) ranges from 10.1% to 26.7%, suggesting that obesity significantly contributes to the development of depressive symptoms, particularly in high-risk populations such as children, adolescents, and women [2]. The study has observed varying obesity–depression associations across different racial and ethnic groups, highlighting the importance of considering these differences when exploring potential interventions [3]. The relationship between obesity and depression is thought to be mediated by inflammation. Both human and animal study has shown that individuals with obesity often have elevated levels of inflammatory markers [4]. Obesity is commonly associated with a mild, chronic systemic inflammation, as evidenced by elevated levels of inflammatory markers in individuals who are obese [5]. Adipose tissue plays a significant role as an endocrine organ, secreting pro-inflammatory adipokines such as leptin and tumor necrosis factor-alpha (TNF-
Furthermore, sex differences in the obesity–depression relationship well documented, with women generally being at higher risk for depression than men. These differences are thought to be influenced by hormonal factors, as well as social and behavioral factors. For instance, inflammation and its effects on depression appear to be higher in women, potentially due to differences in fat distribution and immune system functioning between sexes [12]. Additionally, disparities in inflammatory responses in the obesity–depression relationship between different racial groups have been noted, potentially contributing to varying outcomes among different ethnic groups [13].
Although previous studies have shown a relationship between obesity and depressive symptoms, the specific mechanisms through which inflammatory markers mediate this relationship and which markers play the most significant role remain unclear. This knowledge gap has prompted us to explore the mediating role of two inflammatory markers—C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR)—in this context. Both CRP and NLR are key indicators of systemic inflammation, which plays a crucial role in the pathway linking obesity and depression. CRP, a well-known acute-phase protein, is elevated during inflammatory responses and has been directly associated with the pro-inflammatory state observed in both obesity and depression [1]. On the other hand, NLR, derived from routine blood tests, reflects the balance between neutrophils and lymphocytes and is indicative of ongoing inflammation and stress, factors that have been linked to both obesity and depressive symptoms [14]. While other inflammatory markers, such as IL-6 and chemokines, are also important, CRP and NLR were chosen for this study due to their greater clinical accessibility, cost-effectiveness, and well-documented associations with both obesity and depression. These markers provide a solid foundation for investigating their mediating effects in the relationship between obesity and depressive symptoms.
Previous systematic reviews suggest that sex hormones influence the relationship between obesity and depressive symptoms, with women being at higher risk of developing depression [12]. Considering these potential sex differences, we examined the associations between inflammation, obesity, and depressive symptoms separately for men and women. Given this, we hypothesized that inflammatory markers such as CRP and NLR play a different mediating role in the relationship between obesity and depression in women compared with men.
In our research, which analyzes data from the National Health and Nutrition Examination Survey (NHANES) from 2005 to March, 2020, pre-pandemic (n = 76,496), we also explored these relationships across diverse racial groups.
This study aimed to address three main questions: (1) Is there a connection between obesity, CRP, and NLR, and depressive symptoms? (2) Do CRP and NLR act as mediators between obesity and depressive symptoms? (3) Do sex differences influence the mediating effects of CRP and NLR in this relationship? Our hypotheses were: (1) There is a positive correlation between obesity, CRP, and NLR, and depressive symptoms; (2) CRP and NLR may partly mediate the effect of obesity on depressive symptoms; (3) Sex differences may influence the mediating role of CRP and NLR in this relationship.
We performed a cross-sectional analysis utilizing data from the NHANES program, managed by the Centers for Disease Control and Prevention (CDC), covering the period from 2005 to March, 2020, pre-pandemic. The NHANES survey is designed to evaluate the health and nutritional conditions of the US population. The study protocol was approved by the Ethics Review Board of the National Center for Health Statistics (NCHS), ensuring compliance with the ethical standards outlined in the updated Declaration of Helsinki. All participants gave written informed consent before being included in the study. For more detailed information about the NHANES program, please refer to the CDC website, https://www.cdc.gov/nchs/nhanes/?CDC_AAref_Val=https://www.cdc.gov/nchs/nhanes/index.htm (Centers for Disease Control and Prevention, 2024).
Participants were chosen based on these inclusion criteria: (1) aged 18 years or older, and (2) having at least one available inflammatory marker (NLR or CRP) for analysis. The exclusion criteria included: (1) incomplete Patient Health Questionnaire-9 (PHQ-9) data, (2) missing body mass index (BMI) information, and (3) reported use of anti-infective drugs, immunosuppressants, or immunostimulants.
Depressive symptom assessment: Depressive symptoms were assessed using the nine-item PHQ-9. The scale demonstrated good reliability in the original study (Cronbach’s
BMI: BMI is a measurement derived from a person’s weight and height, typically used to classify individuals into categories such as underweight, normal weight, overweight, and obese. It is calculated by dividing the weight (in kilograms) by the square of the height (in meters). A BMI value of 30 or higher is considered indicative of obesity [18].
To simplify the analysis and improve interpretability, participants were categorized into four groups based on PHQ-9 scores and BMI: “Depression with Obesity”, “Obesity without Depression”, “Depression without Obesity”, and “Neither Depression nor Obesity”. A PHQ-9 score greater than 9 was used to define depression, as this threshold has been widely validated in epidemiological studies as indicative of clinically significant depressive symptoms. This classification simplifies the continuous variables of the PHQ-9 scores and BMI, enabling a more straightforward examination of the co-occurrence and interactions between obesity and depressive symptoms in large populations. While we acknowledge that this approach may overlook more nuanced variations within these continuous measures, it provides a clear and interpretable framework for understanding the broad relationship between obesity and depression. Future studies could benefit from more refined analyses, such as examining the full distribution of PHQ-9 and BMI scores, to capture subtler associations between these variables.
The neutrophil-to-lymphocyte ratio (NLR) is calculated by dividing the neutrophil count by the lymphocyte count. Neutrophil and lymphocyte counts, expressed as cells per liter (109), were analyzed using the Beckman Coulter MAXM instrument (Beckman Coulter, Inc., Brea, CA, USA).
NLR was selected as an inflammation marker due to its ability to reflect systemic inflammation through a simple and inexpensive calculation derived from routine blood counts. Elevated NLR has been associated with various inflammatory diseases and has gained attention as a potential marker for assessing low-grade chronic inflammation in obesity [19]. The study has demonstrated that an elevated NLR is linked to both obesity and depressive symptoms, suggesting that it may serve as a useful tool for exploring the inflammatory mechanisms underlying these conditions [20]. NLR has been proposed as a reliable marker in large cohort studies due to its simplicity and cost-effectiveness, further supporting its use in this investigation.
C-reactive protein (CRP) levels were measured using different methods depending on the NHANES cycle. In the 2005–2010 cycle, CRP levels were measured using the latex-enhanced rate nephelometry method on Behring instruments (Dade Behring Diagnostics, Inc., Newark, DE, USA), with a lower limit of detection (LLOD) of 0.02 mg/dL. During the 2015 to March, 2020, pre-pandemic period, CRP levels were measured using Beckman UniCel analyzers (Beckman Coulter, Inc.), with LLOD values of 0.011 mg/dL for 2015–2016 and 0.015 mg/dL for 2017–March, 2020, pre-pandemic. For values below the LLOD, the LLOD was divided by the square root of 2 (LLOD/
CRP was chosen as an inflammation marker due to its well-established role as an acute-phase reactant. CRP levels rise in response to systemic inflammation, making it an ideal marker for evaluating inflammatory states associated with obesity and depression. Elevated CRP has been linked to both obesity and depressive symptoms in numerous studies, highlighting its potential as a biomarker for these conditions [9]. Recent research has shown that CRP is significantly elevated in individuals with obesity, and elevated levels of CRP have been associated with an increased risk of depression [1]. These findings support CRP’s utility in studies examining the relationship between inflammation, obesity, and depression.
Continuous covariates included age. Categorical variables, used for classification, encompassed race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other races), educational attainment (less than high school, high school graduate, more than high school), marital status (married/living with a partner, or never married/widowed/divorced/separated), alcohol consumption (yes/no), smoking status (never, former, current), physical activity (inactive, moderate, vigorous, or both moderate and vigorous), and the presence of comorbid conditions (yes/no).
Alcohol consumption was evaluated using two 24-hour dietary recalls, with participants categorized as alcohol consumers if they reported drinking in at least one of the recalls. Smoking status was classified as never smoked (fewer than 100 cigarettes), former smoker (smoked
All statistical analyses were conducted using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) (https://cran.r-project.org/) and employed the following related packages for analyzing interaction effects and mediation: “foreign”, “dplyr”, “magrittr”, “tidyr”, “compareGroups”, “mediation”, “lpSolve”, “missforest”, and “broom” (https://cran.r-project.org/web/packages/index.html). For all analyses, two-tailed p-values
As shown in Table 1 and Fig. 1, the study included a total of 76,496 participants. Following the screening process, a total of 37,538 participants met the inclusion criteria, which required them to be 18 years or older (n = 45,980), have available data for at least one inflammatory marker (n = 41,671), have complete PHQ-9 data (n = 37,918), and have BMI information (n = 37,538). Participants were categorized into four groups: “Depression with obesity”, “Obesity without depression”, “Depression without obesity”, and “Without obesity and depression”. A PHQ-9 score greater than 9 was used to indicate depression.
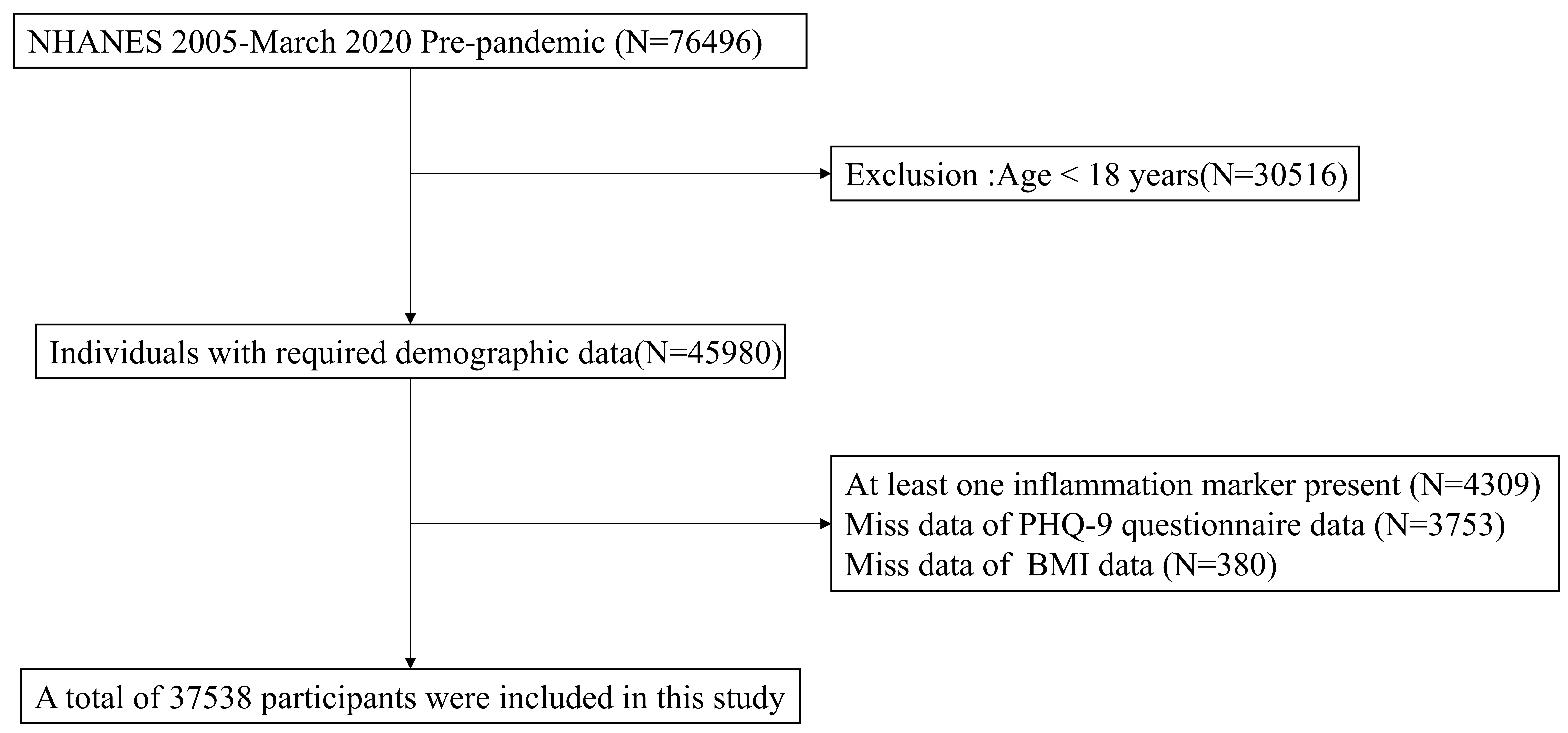 Fig. 1.
Fig. 1. Participant selection process. NHANES, National Health and Nutrition Examination Survey; PHQ-9, Patient Health Questionnaire-9; BMI, body mass index.
| Characteristic | Total Participants | Depression with Obesity (I) | Depression without Obesity (II) | Obesity without Depression (III) | Without Obesity and Depression (IV) | p-value | |
| n | 37,538 | 1610 | 1673 | 12,704 | 21,551 | ||
| Age y, mean (SD) | 47.9 (18.6) | 49.1 (15.8) | 46.3 (18.1) | 49.2 (17.3) | 47.2 (19.5) | ||
| Sex n (%): | |||||||
| Male | 18,455 (49.2%) | 503 (31.2%) | 695 (41.5%) | 5850 (46.0%) | 11,407 (52.9%) | ||
| Female | 19,083 (50.8%) | 1107 (68.8%) | 978 (58.5%) | 6854 (54.0%) | 10,144 (47.1%) | ||
| Education n (%): | |||||||
| 8295 (23.4%) | 510 (32.5%) | 564 (36.1%) | 2824 (23.0%) | 4397 (21.9%) | |||
| Completed high school | 8208 (23.2%) | 389 (24.8%) | 389 (24.9%) | 2987 (24.3%) | 4443 (22.2%) | ||
| 18,941 (53.4%) | 670 (42.7%) | 608 (38.9%) | 6462 (52.7%) | 11,201 (55.9%) | |||
| Race n (%): | |||||||
| Mexican American | 5924 (15.8%) | 265 (16.5%) | 239 (14.3%) | 2242 (17.6%) | 3178 (14.7%) | ||
| Other Hispanic | 3669 (9.77%) | 213 (13.2%) | 226 (13.5%) | 1196 (9.41%) | 2034 (9.44%) | ||
| Non-Hispanic White | 15,720 (41.9%) | 640 (39.8%) | 690 (41.2%) | 5047 (39.7%) | 9343 (43.4%) | ||
| Non-Hispanic Black | 8220 (21.9%) | 391 (24.3%) | 358 (21.4%) | 3472 (27.3%) | 3999 (18.6%) | ||
| Other Race | 4005 (10.7%) | 101 (6.27%) | 160 (9.56%) | 747 (5.88%) | 2997 (13.9%) | ||
| Marital n (%): | |||||||
| Married/Living with partner | 21,270 (59.2%) | 742 (47.1%) | 695 (43.9%) | 7511 (60.6%) | 12,322 (60.4%) | ||
| Widowed/Divorced/Separated/Never married | 14,663 (40.8%) | 832 (52.9%) | 888 (56.1%) | 4877 (39.4%) | 8066 (39.6%) | ||
| Alcohol n (%): | |||||||
| No | 31,647 (90.0%) | 1436 (94.7%) | 1401 (89.9%) | 11,193 (92.8%) | 17,617 (88.0%) | ||
| Yes | 3516 (10.00%) | 80 (5.28%) | 157 (10.1%) | 868 (7.20%) | 2411 (12.0%) | ||
| Smoke n (%): | |||||||
| Never | 20,314 (56.0%) | 711 (44.6%) | 636 (39.5%) | 7201 (57.9%) | 11,766 (57.0%) | ||
| Former | 8688 (23.9%) | 412 (25.8%) | 303 (18.8%) | 3272 (26.3%) | 4701 (22.8%) | ||
| Current | 7296 (20.1%) | 471 (29.5%) | 671 (41.7%) | 1974 (15.9%) | 4180 (20.2%) | ||
| Exercise level n (%): | |||||||
| Inactive | 18,903 (52.7%) | 915 (59.4%) | 852 (53.9%) | 6273 (51.6%) | 10,863 (52.7%) | ||
| Moderate | 8563 (23.9%) | 305 (19.8%) | 369 (23.4%) | 3003 (24.7%) | 4886 (23.7%) | ||
| Vigorous | 1773 (4.94%) | 87 (5.65%) | 71 (4.49%) | 563 (4.63%) | 1052 (5.10%) | ||
| Both moderate and vigorous | 6660 (18.6%) | 234 (15.2%) | 288 (18.2%) | 2319 (19.1%) | 3819 (18.5%) | ||
| Comorbidity n (%): | |||||||
| No | 25,599 (68.2%) | 733 (45.5%) | 1027 (61.4%) | 7749 (61.0%) | 16,090 (74.7%) | ||
| Yes | 11,939 (31.8%) | 877 (54.5%) | 646 (38.6%) | 4955 (39.0%) | 5461 (25.3%) | ||
| PHQ-9 score, mean (SD) | 2.59 (3.66) | 12.1 (3.75) | 11.8 (3.58) | 1.91 (2.14) | 1.56 (1.98) | 0.000 | |
| NLR, median (Q1–Q3) | 1.94 (1.44–2.58) | 2.07 (1.50–2.79) | 1.95 (1.43–2.66) | 1.96 (1.47–2.59) | 1.90 (1.42–2.55) | ||
| CRP mg/dL, median (Q1–Q3) | 1.91(0.80–4.56) | 4.54 (2.30–8.91) | 1.40 (0.60–3.70) | 3.50 (1.70–7.09) | 1.20 (0.51–2.81) | ||
NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein.
The average age of participants was 47.9 years, with 49.2% being male and 50.8% female. Racial/ethnic distribution was as follows: 15.8% Mexican American, 9.77% other Hispanic, 41.9% non-Hispanic White, 21.9% non-Hispanic Black, and 10.7% from other racial groups. The prevalence data indicated that 8.74% of the population had depressive symptoms, while 38.13% were classified as obese. Among those with depressive symptoms, 49.04% also had obesity, and 11.24% of the obese population had depressive symptoms. Median CRP levels and NLR were 4.20 mg/dL and 2.17, respectively.
Analysis of inflammatory markers revealed significant differences in NLR and CRP levels across the four groups (“Depression with obesity”, “Obesity without depression”, “Depression without obesity”, and “Without obesity and depression”) (p
As shown in Table 2 and Fig. 2, the unadjusted model revealed significant positive correlations between obesity, depressive symptoms, and NLR and CRP levels. However, after adjusting for confounding markers such as age, sex, race, alcohol consumption, smoking status, education level, marital status, exercise level, and presence of a comorbidity, the association between obesity and NLR was no longer significant (NLR:
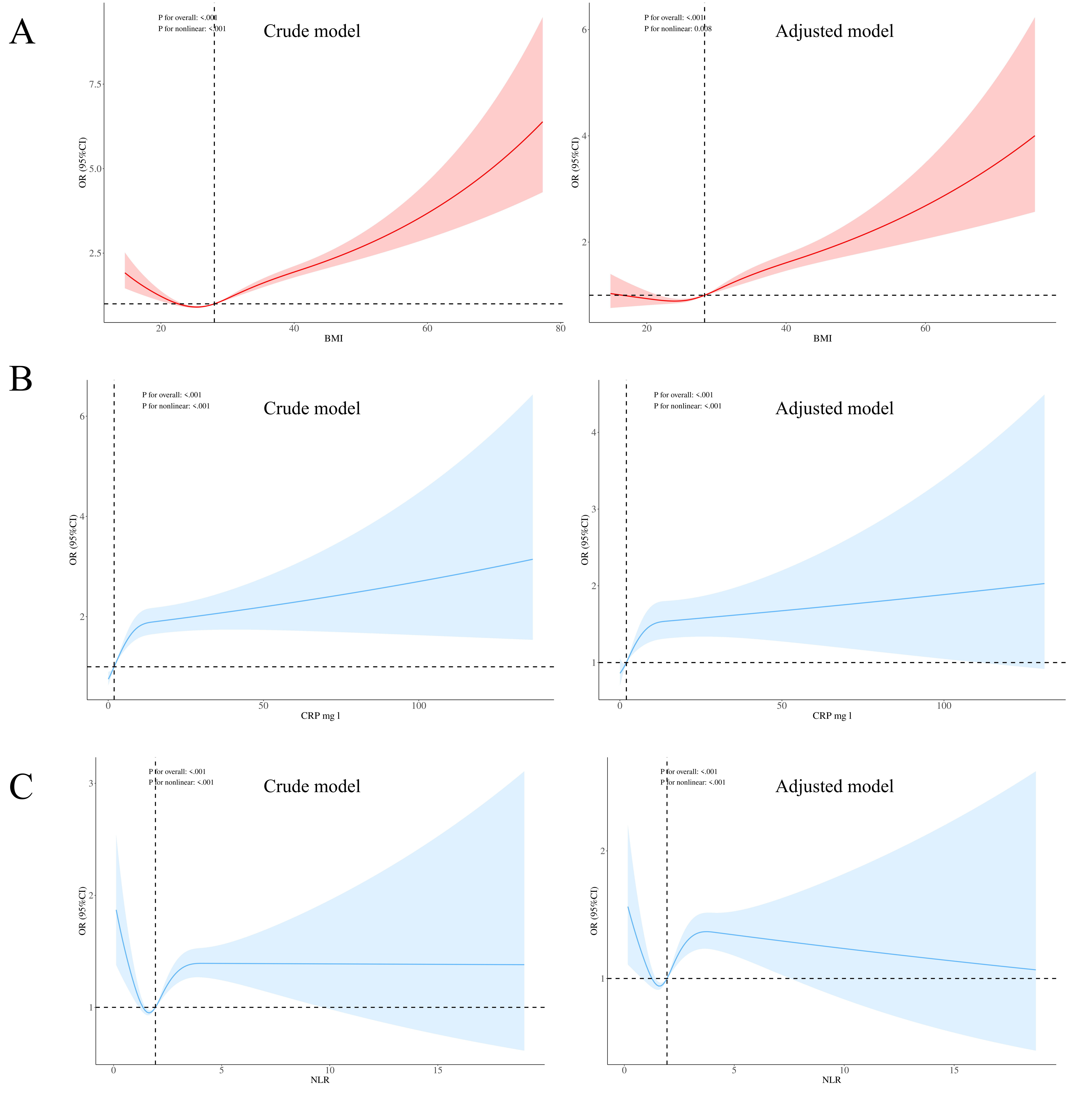 Fig. 2.
Fig. 2. Correlations between Obesity, Depressive Symptoms, and NLR and CRP Levels. (A) The association between BMI and depressive symptoms. (B) The association between CRP levels and depressive symptoms. (C) The association between NLR and depressive symptoms. CRP is expressed in mg/dL and NLR is calculated as the ratio of neutrophil count to lymphocyte count. Both CRP and NLR were transformed using natural logarithms for the analysis. Crude models were not adjusted, while adjusted models accounted for variables such as age, sex, race, alcohol consumption, smoking status, education, marital status, exercise level, and comorbidities.
| Variable | Crude | p | Adjusted | p | Effect Size (Cohen’s d/Partial η2) | |
| Association of obesity with depression | ||||||
| Total | 0.040440 (0.0346, 0.0463) | 0.031806 (0.0254, 0.0382) | Cohen’s d = 0.23 | |||
| Male | 0.021747 (0.0143, 0.0292) | 0.019103 (0.011, 0.0273) | Cohen’s d = 0.28 | |||
| Female | 0.051119 (0.0422, 0.0601) | 0.041151 (0.0314, 0.0509) | Cohen’s d = 0.29 | |||
| Association of obesity with NLR | ||||||
| Total | 0.022040 (0.0122, 0.0319) | 0.001737 (–0.0089, 0.0123) | 0.748 | Cohen’s d = 0.10 | ||
| Male | 0.030345 (0.0157, 0.0449) | 0.005281 (–0.0102, 0.0208) | 0.504 | Cohen’s d = 0.15 | ||
| Female | 0.018271 (0.0050, 0.0316) | 0.005345 (–0.0091, 0.0198) | 0.467 | Cohen’s d = 0.08 | ||
| Association of obesity with CRP | ||||||
| Total | 1.020641 (0.9916, 1.0497) | 0.922152 (0.8911, 0.9532) | Partial η2 = 0.05 | |||
| Male | 0.82064 (0.7790, 0.8630) | 0.737754 (0.6930, 0.7830) | Partial η2 = 0.06 | |||
| Female | 1.16352 (1.1240, 1.2030) | 1.097530 (1.0550, 1.1400) | Partial η2 = 0.04 | |||
| Association of NLR with depression | ||||||
| Total | 0.01254 (0.0065, 0.0186) | 0.014480 (0.0078, 0.0211) | Cohen’s d = 0.30 | |||
| Male | 0.019183 (0.0118, 0.0266) | 0.017340 (0.0090, 0.0257) | Cohen’s d = 0.35 | |||
| Female | 0.00797 (–0.0016, 0.0176) | 0.104 | 0.011250 (0.0009, 0.0216) | 0.033 | Cohen’s d = 0.28 | |
| Association of CRP with depression | ||||||
| Total | 0.014478 (0.01192, 0.01704) | 0.009853 (0.0070, 0.0128) | Cohen’s d = 0.28 | |||
| Male | 0.011638 (0.0083, 0.0150) | 0.010400 (0.0066, 0.0142) | Cohen’s d = 0.30 | |||
| Female | 0.013437 (0.0095, 0.0173) | 0.008693 (0.0044, 0.0130) | Cohen’s d = 0.26 | |||
CI, confidence interval.
Model1, Crude.
Model2, Adjusted: Age, Sex, Race, Alcohol, Smoke, Education, Marital, Exercise level, Comorbid.
As shown in Table 3 and Fig. 3, both NLR and CRP exhibited mediation effects in the unadjusted model. For NLR, the proportion mediated (PM) was 0.693%, with average causal mediation effects (ACME) of 2.56
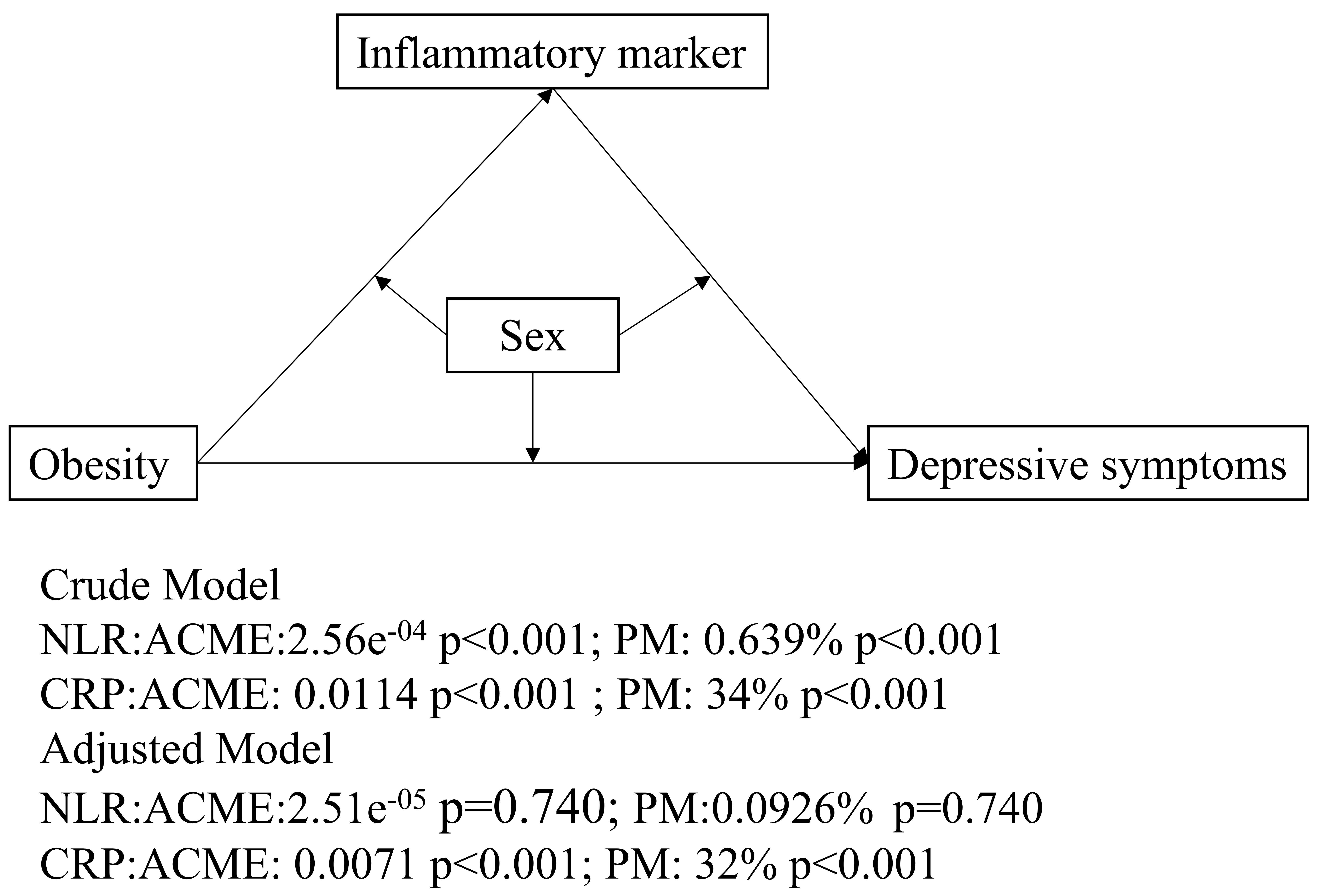 Fig. 3.
Fig. 3. Mediation models. The figure illustrates mediation models with obesity as the independent variable, inflammatory markers (NLR or CRP levels) as the mediators, and depressive symptoms as the dependent variable. ACME represents the average causal mediation effects (i.e., the indirect effect), while PM refers to the proportion of the total effect that is mediated by the inflammatory markers. Abbreviations: PM, proportion mediated.
| Variable | Crude | p | Adjusted | p | Effect Size (Cohen’s d) | |
| NLR | ||||||
| ACME | ||||||
| Total | 2.56 × 10-4 (1.05 × 10-4, 4.70 × 10-4) | 2.51 × 10-5 (–1.23 × 10-4, 1.90 × 10-4) | 0.740 | Cohen’s d = 0.02 | ||
| Male | 5.63 × 10-4 (2.44 × 10-4, 9.82 × 10-4) | 9.11 × 10-5 (–1.76 × 10-4, 3.84 × 10-4) | 0.510 | Cohen’s d = 0.08 | ||
| Female | 1.27 × 10-4 (–5.97 × 10-5, 3.77 × 10-4) | 0.160 | 5.90 × 10-5 (–1.01 × 10-4, 2.78 × 10-4) | 0.480 | Cohen’s d = 0.05 | |
| ADE | ||||||
| Total | 3.98 × 10-2 (3.42 × 10-2, 4.58 × 10-2) | 2.71 × 10-2 (2.02 × 10-2, 3.37 × 10-2) | Cohen’s d = 0.23 | |||
| Male | 2.12 × 10-2 (1.37 × 10-2, 2.84 × 10-2) | 1.56 × 10-2 (6.59 × 10-3, 2.44 × 10-2) | Cohen’s d = 0.20 | |||
| Female | 5.02 × 10-2 (4.06 × 10-2, 5.94 × 10-2) | 3.65 × 10-2 (2.63 × 10-2, 4.69 × 10-2) | Cohen’s d = 0.34 | |||
| Total Effect | ||||||
| Total | 4.00 × 10-2 (3.45 × 10-2, 4.60 × 10-2) | 2.71 × 10-2 (2.02 × 10-2, 3.36 × 10-2) | Cohen’s d = 0.25 | |||
| Male | 2.18 × 10-2 (1.42 × 10-2, 2.90 × 10-2) | 1.57 × 10-2 (6.72 × 10-3, 2.50 × 10-2) | Cohen’s d = 0.17 | |||
| Female | 5.04 × 10-2 (4.08 × 10-2, 5.95 × 10-2) | 3.65 × 10-2 (2.63 × 10-2, 4.69 × 10-2) | Cohen’s d = 0.34 | |||
| Prop. Mediated | ||||||
| Total | 6.39 × 10-3 (2.63 × 10-3, 1.18 × 10-2) | 9.26 × 10-4 (–4.80 × 10-3, 7.12 × 10-3) | 0.740 | Cohen’s d = 0.03 | ||
| Male | 2.59 × 10-2 (1.15 × 10-2, 4.88 × 10-2) | 5.79 × 10-3 (–1.34 × 10-2, 2.95 × 10-2) | 0.510 | Cohen’s d = 0.12 | ||
| Female | 2.51 × 10-3 (–1.17 × 10-3, 7.52 × 10-3) | 0.160 | 1.61 × 10-3 (–2.84 × 10-3, 7.86 × 10-3) | 0.480 | Cohen’s d = 0.08 | |
| CRP | ||||||
| ACME | ||||||
| Total | 1.14 × 10-2 (0.86 × 10-2, 1.41 × 10-2) | 0.71 × 10-2 (0.42 × 10-2, 0.98 × 10-2) | Cohen’s d = 0.20 | |||
| Male | 0.87 × 10-2 (0.56 × 10-2, 1.15 × 10-2) | 0.72 × 10-2 (0.39 × 10-2, 1.03 × 10-2) | Cohen’s d = 0.26 | |||
| Female | 0.90 × 10-2 (0.41 × 10-2, 1.47 × 10-2) | 0.50 × 10-2 (0.21 × 10-3, 1.02 × 10-2) | 0.046 | Cohen’s d = 0.18 | ||
| ADE | ||||||
| Total | 2.26 × 10-2 (1.51 × 10-2, 3.01 × 10-2) | 1.54 × 10-2 (0.72 × 10-2, 2.33 × 10-2) | Cohen’s d = 0.24 | |||
| Male | 0.89 × 10-2 (2.18 × 10-5, 1.82 × 10-2) | 0.050 | 0.54 × 10-2 (–0.49 × 10-2, 1.54 × 10-2) | 0.296 | Cohen’s d = 0.08 | |
| Female | 3.50 × 10-2 (2.31 × 10-2, 4.64 × 10-2) | 2.58 × 10-2 (1.36 × 10-2, 3.8 × 10-2) | Cohen’s d = 0.33 | |||
| Total Effect | ||||||
| Total | 3.40 × 10-2 (2.64 × 10-2, 4.08 × 10-2) | 2.25 × 10-2 (1.50 × 10-2, 3.00 × 10-2) | Cohen’s d = 0.22 | |||
| Male | 1.76 × 10-2 (0.90 × 10-2, 2.67 × 10-2) | 1.26 × 10-2 (0.27 × 10-2, 2.22 × 10-2) | Cohen’s d = 0.18 | |||
| Female | 4.37 × 10-2 (3.32 × 10-2, 5.48 × 10-2) | 3.08 × 10-2 (1.98 × 10-2, 3.80 × 10-2) | Cohen’s d = 0.34 | |||
| Prop. Mediated | 0.34 (0.24, 0.46) | 0.32 (0.18, 0.54) | Cohen’s d = 0.25 | |||
| Male | 0.49 (0.29, 0.98) | 0.57 (0.25, 2.12) | Cohen’s d = 0.28 | |||
| Female | 0.21 (0.10, 0.36) | 0.16 (0.01, 0.37) | 0.046 | Cohen’s d = 0.18 | ||
ACME, average causal mediation effects; ADE, average direct effect.
Model1: Crude.
Model2: Adjusted: Age, Sex, Race, Alcohol, Smoke, Education, Marital, Exercise level, Comorbid.
In the sex subgroup analysis, the mediating effect of CRP was found to be more pronounced in males and less significant in females, indicating that sex serves as an important moderating factor. This indicates that CRP serves as a more reliable mediator in the association between obesity and depressive symptoms, particularly in males (CRP: PM, 57%; ACME = 0.72
The aim of this study was to investigate the relationship between obesity and depressive symptoms in American adults, as well as to explore the potential mediating role of inflammatory markers in this association.
Firstly, the study identified a significant association between obesity and depressive symptoms, with the relationship being stronger in women. The bidirectional link between obesity and depressive symptoms is well documented, as obesity increases the risk of depressive symptoms [23], while individuals with depression have a 70% higher likelihood of developing obesity [13]. Furthermore, sex hormones including testosterone, estrogen, and progesterone could significantly influence the occurrence of depressive symptoms in women [12].
Secondly, the results showed a significant correlation between obesity and NLR and CRP levels. Obesity is commonly considered a condition characterized by low-grade chronic inflammation [5], in which macrophages in adipose tissue, especially in abdominal fat, are activated and release pro-inflammatory markers such as TNF-
This study found that NLR and CRP levels were positively associated with depressive symptoms and this association remained significant even after adjusting for covariates. Previous research has similarly demonstrated a bidirectional relationship between inflammation and depressive symptoms [9], as well as a nonlinear relationship between NLR, platelet-to-lymphocyte ratio (PLR), and depressive symptoms [20]. Chronic inflammatory disorders, including those associated with obesity, diabetes, and cardiovascular diseases, have been associated with a higher likelihood of experiencing depressive symptoms [19]. Inflammation may impact brain function through various mechanisms, including neurotransmitter imbalances, neuronal damage, and impaired neurogenesis, all of which may contribute to depressive symptoms [19]. Additionally, the study suggests that anti-inflammatory drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs), could benefit certain patients with depressive symptoms, further supporting the connection between inflammation and depression [32].
Inflammation is thought to play a crucial role in the comorbidity of obesity and depressive symptoms, as higher levels of inflammatory markers are commonly found in individuals with both conditions [33]. This study found that NLR and CRP levels significantly mediated the association between obesity and depressive symptoms in adults. After adjusting for covariates, the mediation effect of NLR was no longer significant, while the mediating effect of CRP increased in men and decreased in women. While the results indicated statistical significances in many comparisons, the large sample size necessitates careful interpretation. As such, effect sizes were provided for a more comprehensive understanding of the magnitude of the associations.
This study is the first to identify inflammatory markers in the relationship between obesity and depression in an adult population, with the advantage of having a large sample size containing diverse racial groups. We used NLR and CRP levels as markers of inflammation, with the strengths of these indicators being their low cost and ease of use in clinical settings. However, there are important limitations to consider. This study, which employed a cross-sectional design, could not establish causal relationships. Although our results revealed a significant link between obesity, inflammatory markers, and depressive symptoms, the direction of these associations remains uncertain. One key limitation of cross-sectional studies is their ability to capture data at a specific moment in time, which prevents the determination of causal effects. Although we observed that inflammatory markers were elevated in individuals with obesity and depression, it is possible that depression may contribute to the development or worsening of obesity and inflammation, rather than the reverse. Therefore, while these associations are noteworthy, future longitudinal studies are necessary to investigate the causal direction and mechanisms underlying these relationships. The use of the PHQ-9 to assess depressive symptoms has certain limitations. As a self-reported questionnaire, the PHQ-9 cannot fully substitute for professional diagnoses. It measures only the severity of depressive symptoms and does not differentiate between subtypes of depression, such as major depressive disorder or anxiety-related depression. Additionally, the study population consisted of community-based samples rather than clinically diagnosed patients with depressive symptoms, which may limit the generalizability of the results to clinical populations. The wide age range of participants introduces variability in physiological and psychological states across different age groups, which could potentially affect the findings. Moreover, this study utilized only two inflammatory markers (NLR and CRP), which may not fully capture the complexity of the inflammatory state. While we have accounted for a range of confounding factors, it is important to note that genetic factors and early life experiences could play a significant role in the observed associations. For instance, genetic variations may predispose individuals to both obesity and depression, making it difficult to disentangle their independent effects. Additionally, early life experiences such as childhood trauma or socioeconomic disadvantage have been shown to influence both mental and physical health outcomes, further complicating the interpretation of these associations. These factors, which were not directly assessed in this study, may affect the observed relationships between obesity, inflammation, and depression.
Future studies should focus on conducting longitudinal research to explore the causal relationships between obesity, inflammation, and depression. More accurate assessment tools, such as professional diagnostic scales for depressive symptoms, should be utilized. The study population should be expanded to include clinically diagnosed patients with depressive symptoms, ensuring greater generalizability of the findings. A broader range of inflammatory markers should also be incorporated to provide a more comprehensive assessment of the inflammatory state. Additionally, the influence of genetic and psychosocial markers should be carefully considered in future research.
The results of this study revealed significant pairwise associations between inflammatory markers (NLR and CRP levels), obesity, and depressive symptoms. Following the adjustment for covariates, CRP levels were identified as a significant mediator in the association between obesity and depressive symptoms. Subgroup analyses indicated that the mediating effect of CRP was weaker in women compared with men, suggesting that sex differences may influence the interactions between inflammatory markers, obesity, and depressive symptoms. Future longitudinal studies are necessary to determine whether obese individuals are more susceptible to inflammation-driven depressive symptoms, which could ultimately inform personalized treatment strategies for managing depressive symptoms.
The data used in this study are available on the National Health and Nutrition Examination Survey website: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Conception–PL; Design–PL, YZ; Supervision–YZ, QD; Fundings–YZ; Materials–PL, QD; Data Collection and/or Processing–PL, JL, QD; Analysis and/or Interpretation–PL, YZ, JL, JD, NY; Literature Review–PL, JL, JD, NY; Writing–PL, YZ, JL, JD, NY; Critical Review–QD. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Thanks to everyone who helped us.
This work was supported by Natural Science Foundation of Gansu Province (No. 21JR1RA125).
The authors declare no conflict of interest.
The authors confirm that they primarily wrote and reviewed the work. During the preparation of this work, they used AI tools to assist with translation and text polishing. After using this tool, they reviewed and edited the content as needed and took full responsibility for the publication’s content.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



