1 Department of Medicine, Lincoln Medical Center, New York, NY 10451, USA
2 Department of Internal Medicine, Danbury Hospital, Danbury, CT 06810, USA
3 Ateneo de Manila School of Medicine and Public Health, 1604 Pasig, Philippines
4 Department of Medicine, Houston Methodist, Houston, TX 77030, USA
5 King George Hospital, IG3 8YB Ilford, UK
6 Department of Medicine, San Beda University College of Medicine, 1005 Manila, Philippines
7 Cebu Institute of Medicine, 6000 Cebu, Philippines
8 Southern Philippines Medical Center, 8000 Davao, Philippines
9 Department of Cardiology, Keio University School of Medicine, 160-8582 Tokyo, Japan
10 University of Arizona, Tucson, AZ 85721, USA
11 Department of Cardiology, Rush University Medical Center, Chicago, IL 60612, USA
12 Section of Interventional Cardiology-Structural Heart, Montefiore Medical Center, Albert Einstein College of Medicine, New York, NY 10461, USA
Abstract
Background: Aortic stenosis (AS) is the world’s most prevalent heart
valve disease. Transcatheter aortic valve replacement (TAVR) or Implantation
(TAVI) is widely available yet adopting this procedure in Asia has been slow due
to high device cost, the need for specific training programs, and the lack of
specialized heart teams and dedicated infrastructures. The limited number of
randomized controlled trials describing TAVI outcomes among the Asian population
hampered the approval for medical reimbursements as well as acceptance among
surgeons and operators in some Asian countries. Methods: A comprehensive
medical literature search on TAVI and/or TAVR performed in Asian countries
published between January 2015 and June 2022 was done through MEDLINE and manual
searches of bibliographies. The full text of eligible articles was obtained and
evaluated for final analysis. The event rates for key efficacy and safety
outcomes were calculated using the data from the registries and randomized
controlled trials. Results: A total of 15,297 patients were included
from 20 eligible studies. The mean patient age was 82.88
Keywords
- transcatheter
- aortic valve
- aortic stenosis
- TAVR
- TAVI
- outcomes
- Asia
Aortic stenosis (AS) is the most prevalent heart valve disease worldwide [1, 2]. In Western countries, transcatheter aortic valve replacement (TAVR) or implantation (TAVI) has become a widely available and standardized procedure, such that the number of patients undergoing TAVI has surpassed the number of patients undergoing surgical AV replacement (SAVR) for AS each year over the last few years [3]. Since the birth of TAVI, the advancement of technology has paved the way for its rapid expansion and will most likely attain an “all-risk” indication [4, 5, 6]. TAVI procedures were done in Asia two years after it was introduced in Europe and the United States [7]. The first TAVI procedure was done in Singapore and since then it has been embraced across the rest of the Asian region [7, 8]. TAVI was also introduced later in China, with its use increasing rapidly due to the rising evidence of efficacy and safety from observational studies and randomized trials [9]. Although TAVI is expanding in western countries, implementation of this modality in some regions in Asia has been slow [7]. This is mainly driven by factors such as cost, paucity of centers that offer advanced training, and inaction from the government sectors [7]. Furthermore, it is difficult to lobby for procedural reimbursements resulting in patients using their own money to pay for the procedure.
This meta-analysis aims to evaluate the efficacy and safety outcomes of TAVI in Asia. For this purpose, we provide information about the key findings generated from Asian TAVI registries and clinical trials. Finally, we compare TAVI outcomes in Asia to the recent data from the US and other Western countries.
This study was first registered in the International Prospective Register of
Systematic Reviews (PROSPERO), with the ID number CRD42022359895 [10]. Two
independent investigators did a comprehensive search of the medical literature
using the MEDLINE database to identify all studies on TAVI was conducted.
Articles from inception to July 2022 were included. Search terms include but are
not limited to “TAVI”, “TAVR”, “transcatheter”, “transfemoral”,
“percutaneous”, “aortic valve”, “replacement”, “Implantation”, “Asia”,
“Japan”, “Korea”, “China”, “Vietnam”, “Thailand”, “India”, and other
Asian countries. Relevant keywords and their combinations were applied in the
search strategy and limited to results in the English language. Manual searches
of the bibliography of relevant papers supplemented the search strategy. The
multistage was used to determine inclusion for analysis. The eligibility criteria
for inclusion of studies are the following: randomized controlled trials or
observational cohort studies (both retrospective and prospective) of adults aged
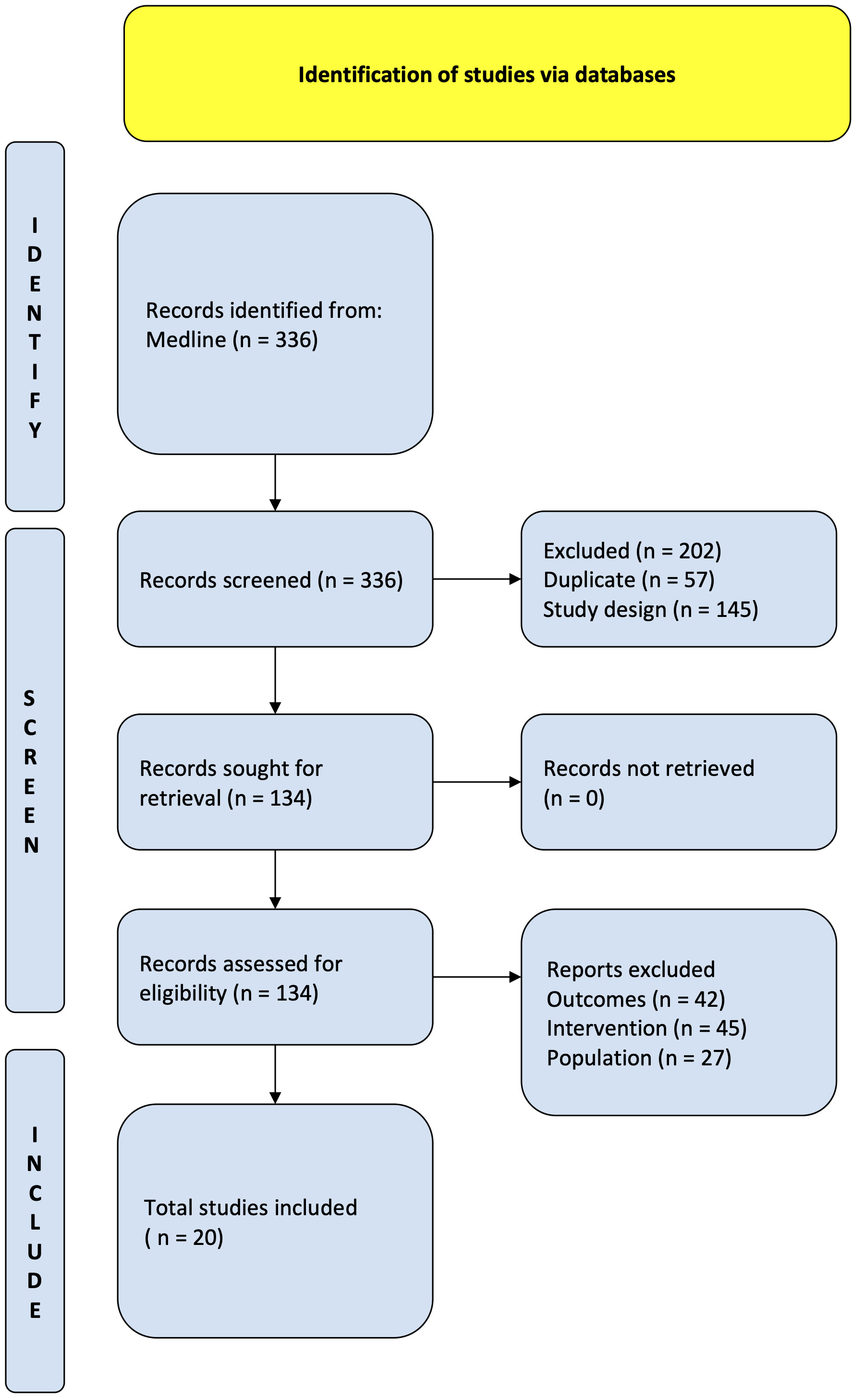
 Fig. 1.
Fig. 1.PRISMA flow diagram. Study flow based on the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P).
This study adopted the Valve Academic Research Consortium 2 (VARC-2) Scale to evaluate post-TAVI outcomes [15]. It includes perioperative complications including, but not limited to, stroke, acute kidney injury, and bleeding. In this paper, TAVI will refer to both TAVR and TAVI. Stroke is the sudden onset, localized or widespread neurological impairment due to damage from vascular infarct or hemorrhage. Closely related is transient ischemic attack (TIA), defined as any reversible neurological deficit lasting less than 24 hours. For this review, acute kidney injury (AKI) is characterized by changes in serum creatinine and urine output and following the diagnostic criteria of AKI in the VARC-2, it was extended from 72 hours to 7 days as a follow-up renal function assessment is done after seven days for patients until stabilization of the condition. Despite being rare, periprocedural myocardial infarction (MI) is also assessed. Bleeding is defined by the VARC-2 using the Bleeding Academic Research Consortium (BARC) criteria and staging. Procedural success, or device success, is defined by VARC-2 as the correct positioning of the prosthetic valve in its proper location, performing as it is intended, and without procedural mortality. Paravalvular leaks (PVL) is one of the most common complications of TAVI and have been associated with poor short-term and long-term outcomes. Atrioventricular blocks (AVB), which may require permanent pacemaker implantation, may occur from mechanical trauma or inflammation caused by the TAVI valve on the conduction system.
Event rates were calculated as the total number of events/occurrences in the
studies divided by the total number of patients in the studies with available
data. The approach to calculating individual rates for different studies and
combining them yields identical results if the weights are defined as the
proportion of patients in a study. Results were tabulated as weighted mean
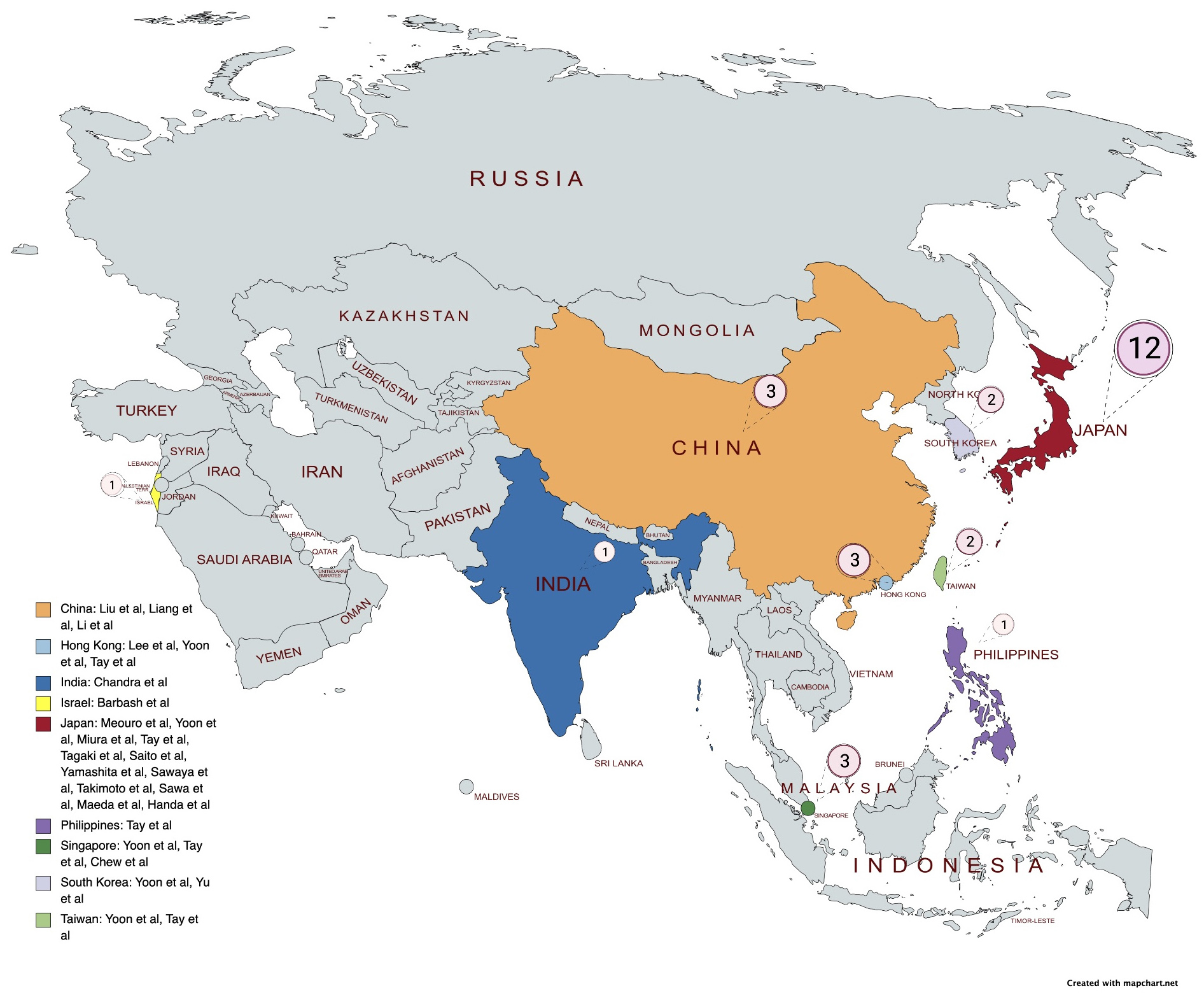
A comprehensive literature search identified 336 citations published within the predetermined time span of the search from January 2010–August 2022. After careful review, twenty studies comprising 15,295 patients undergoing TAVI met the study criteria and were selected for the current analysis. The registries and studies included data from Hong Kong, Japan, the Philippines, Singapore, Taiwan, South Korea, India, Israel, and China (see Fig. 2). An overview of the studies is provided in Table 1 (Ref. [8, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34]). Most of these studies had a moderate risk of bias in accordance with the Newcastle-Ottawa Scale, as shown in Supplementary Table 1.
 Fig. 2.
Fig. 2.Distribution of studies.
| Author (Year) | Study type | Study period | Country | N | Mean age | Male (%) | Female (%) | Logistic EuroSCORE | Mean STS score (%) |
| Lee (2017) [16] | Prospective | 2010–2015 | Hongkong | 56 | 81.9 |
64.3 | 36.7 | 22.6% |
7.0 |
| Meguro (2021) [17] | Prospective | 2013–2017 | Japan | 5870 | 85 (82–88) | 6.8 | 93.2 | 12.8 (9.3–21.6) | 6.7 (4.9–9.3) |
| Yoon (2016) [18] | Prospective | 2010–2016 | Singapore, Hong Kong, Taiwan, Japan, Korea | 848 | 81.8 |
46.7 | 53.3 | 16.5 |
5.2 |
| Miura (2017) [32] | Prospective | 2013–2015 | Japan | 112 | 84.5 |
33.9 | 66.1 | 16.0 (11.0–23.0) | 6.0 (4.0–9.0) |
| Liu (2018) [19] | Prospective | 2014–2015 | China | 43 | 73.9 |
69.8 | 30.2 | 25.5% |
- |
| Tay (2021) [8] | Prospective | 2009–2017 | Hong Kong, Japan, Philippines, Singapore, Taiwan | 1125 | 79.9 |
48.5 | 51.5 | 20.4 |
7.1 |
| Chandra (2021) [33] | Prospective | 2016–2018 | India | 40 | 74.5 |
60 | 40 | - | 5.6 |
| Takagi (2020) [34] | Prospective | 2013–2016 | Japan | 1613 | 84.4 |
29.6 | 70.4 | 17.0 |
8.3 |
| Saito (2021) [20] | Prospective | 2015–2016 | Japan | 50 | 84.0 |
40 | 60 | 4.6 |
6.4 |
| Yu (2018) [21] | Prospective | 2015–2017 | Korea | 576 | 79 (75–83) | 48.6 | 51.4 | 5.0 (2.0–15.0) | 5.2 (3.0–9.0) |
| Yamashita (2019) [22] | Prospective | 2016–2017 | Japan | 11 | 83 (80–86) | 27.3 | 72.7 | 7.2 (5.4–9.8) | |
| Sawa(2017) [23] | Prospective | 2010–2011 | Japan | 64 | 84.3 |
34.4 | 65.6 | - | 9.0 |
| Takimoto (2016) [25] | Prospective | 2013–2015 | Japan | 302 | 85.0 |
34.1 | 65.9 | - | 7.4 |
| Chew (2017) [26] | Prospective | 2010–2015 | Singapore | 59 | 76.8 |
61 | 39 | 18.7 |
6.9 |
| Liang (2021) [27] | Prospective | 2012–2018 | China | 175 | 76.6 |
59.4 | 40.6 | - | 2.67 (1.76, 3.8) |
| Sawa (2014) [24] | RCT | 2011–2012 | Japan | 55 | 82.5 |
30.9 | 69.1 | 21.5 |
8.0 |
| Maeda (2015) [28] | Prospective | 2013–2014 | Japan | 15 | 83.3 |
26.7 | 73.3 | 21.9% |
7.5 |
| Barbash (2015) [29] | Prospective | 2008–2014 | Israel | 1327 | 83 (79–86) | 43 | 57 | 14.24 (9.2-23.6) | 4.4 (3.1–6.6) |
| Li (2021) [30] | Prospective | 2012–2020 | China | 1202 | 73.8 |
57.2 | 42.8 | - | 6.0 (3.7–8.9) |
| Handa (2018) [31] | Prospective | 2013–2015 | Japan | 1752 | 85 (81–88) | 30.5 | 69.5 | - | 6.5 (4.5–9.3) |
| Abbreviations: EuroSCORE, European Systems for Cardiac Operations Risk Evaluation; STS Score, Society of Thoracic Surgeons Score; N, population. | |||||||||
Characteristics of the patient population are summarized in Table 2a. The mean
patient age was 82.88
| Characteristics | No. of publications with data | Overall no. of patients | No. of events | Weighted mean |
|---|---|---|---|---|
| Age (years) | 20 | 15,295 | N/A | 82.88 |
| Male gender (%) | 20 | 15,295 | 5666 | 37.04 |
| Female gender (%) | 20 | 15,295 | 9484 | 62.01 |
| STS score, % | 19 | 15,252 | N/A | 6.28 |
| Logistic EuroSCORE, % | 12 | 11,187 | N/A | 14.85 |
| Logistic EuroSCORE II, % | 4 | 3991 | N/A | 4.80 |
| NYHA 1 and 2 (%) | 19 | 13,968 | 8417 | 60.26 |
| NYHA 3 and 4 (%) | 19 | 13,968 | 5548 | 39.72 |
| CAD (%) | 19 | 15,120 | 5424 | 35.87 |
| Previous CABG (%) | 16 | 13,914 | 1335 | 9.33 |
| Prior PCI (%) | 15 | 14,819 | 3770 | 25.44 |
| Previous valve surgery (%) | 4 | 7097 | 729 | 10.27 |
| CVA (%) | 19 | 15,231 | 1636 | 10.74 |
| PAD (%) | 15 | 7459 | 1306 | 17.51 |
| COPD (%) | 19 | 15,231 | 2617 | 17.18 |
| DM (%) | 19 | 15,231 | 4133 | 27.14 |
| Hypertension (%) | 18 | 15,181 | 11,523 | 75.90 |
| Dyslipidemia (%) | 11 | 12,269 | 6368 | 51.90 |
| CKD (%) | 16 | 14,725 | 2544 | 17.28 |
| Total Body Surface Area | 4 | 7855 | N/A | 1.42 |
| Abbreviations: CABG, Coronary artery bypass graft; CAD, Coronary Artery Disease; CKD, chronic kidney disease; COPD, Chronic obstructive pulmonary disease; DM, Diabetes Mellitus; EuroSCORE, European System for Cardiac Operative Risk Evaluation; NYHA, New York Heart Association; PAD, Peripheral artery disease; STS score, Society of Thoracic Surgeon scores. | ||||
Different types of transcatheter heart valves were used as cited by the twenty studies (Table 2b, Ref. [8, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34]). Most of these studies used SAPIEN and CoreValve which are largely imported from western countries, while a minority used heart valves produced in Asia like the J-Valve and LOTUS. The most commonly used valve sizes among Asians were the 26-mm (38.5%) and 23-mm variants (37.9%). Most of these valves (81.8%) were installed via the transfemoral route. Prosthesis-patient mismatch occurred in 826 out of 6108 (13.5%) patients.
| Study/Year | N | Valve type | Valve sizes (Most prevalent) | Access | PPM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brand | 23 mm | 26 mm | 29 mm | Other | TF | TAo | TAp | Others | |||
| Lee (2017) [16] | 56 | (NS) | 4 | 25 | 22 | 5 | 54 | 1 | 0 | 1 | 9 |
| Meguro (2021) [17] | 5870 | SAPIEN XT | 2388 | 2260 | 975 | 247 | 4694 | 0 | 0 | 1176 | Severe |
| SAPIEN 3 | 124 | ||||||||||
| CoreValve | Moderate | ||||||||||
| Evolut R | 691 | ||||||||||
| Yoon (2016) [18] | 848 | SAPIEN | 549 | 299 | 0 | 0 | 731 | 0 | 0 | 117 | |
| CoreValve | (NS) | ||||||||||
| Miura (2017) [32] | 112 | SAPIEN XT | 77 | 32 | 3 | 0 | 69 | 0 | 34 | 9 | 3 |
| Liu (2018) [19] | 43 | J-Valve | (NS) | (NS) | (NS) | (NS) | (NS) | (NS) | (NS) | (NS) | (NS) |
| Tay (2021) [8] | 1125 | SAPIEN | 343 | 379 | 222 | 181 | 910 | 11 | 72 | 132 | |
| SAPIEN 3 | |||||||||||
| SAPIEN XT | |||||||||||
| CoreValve | |||||||||||
| Evolut R | (NS) | ||||||||||
| Chandra (2021) [33] | 40 | Hydra | 0 | 18 | 0 | 22 | 40 | 0 | 0 | 0 | (NS) |
| Takagi (2020) [34] | 1613 | SAPIEN | (NS) | (NS) | (NS) | (NS) | 1283 | 0 | 0 | 330 | |
| CoreValve | (NS) | ||||||||||
| Saito (2021) [20] | 50 | LOTUS | 24 | 0 | 0 | 26 | 40 | 10 | 0 | 0 | (NS) |
| Yu (2018) [21] | 576 | SAPIEN | 159 | 229 | 155 | 33 | 586 | 10 | 0 | 0 | |
| CoreValve | |||||||||||
| LOTUS | (NS) | ||||||||||
| Yamashita (2019) [22] | 11 | SAPIEN | 8 | 2 | 0 | 1 | 9 | 2 | 0 | 0 | |
| CoreValve | 1 | ||||||||||
| Sawa (2017) [23] | 64 | SAPIEN XT | (NS) | (NS) | (NS) | (NS) | 37 | 0 | 27 | 0 | (NS) |
| Takimoto (2016) [25] | 302 | SAPIEN XT | 193 | 96 | 10 | 3 | 200 | 0 | 99 | 3 | (NS) |
| Chew (2017) [26] | 59 | SAPIEN | 21 | 30 | 6 | 2 | 40 | 1 | 18 | 0 | |
| CoreValve | |||||||||||
| Evolut R | 8 | ||||||||||
| Liang (2021) [27] | 175 | SAPIEN XT | (NS) | (NS) | (NS) | (NS) | 134 | 13 | 26 | 2 | |
| Venus A | |||||||||||
| TaurusOne | |||||||||||
| VitaFlow | |||||||||||
| J-Valve | (NS) | ||||||||||
| Sawa (2014) [24] | 55 | CoreValve | 0 | 29 | 14 | 12 | 43 | 6 | 0 | 6 | (NS) |
| Maeda (2015) [28] | 15 | ACURATE | (NS) | (NS) | (NS) | (NS) | 10 | 0 | 0 | 5 | |
| Neo/TF | (NS) | ||||||||||
| Barbash (2015) [29] | 1327 | SAPIEN | 200 | 637 | 413 | 77 | 1160 | 101 | 0 | 66 | |
| CoreValve | (NS) | ||||||||||
| Li (2021) [30] | 1202 | (NS) | (NS) | (NS) | (NS) | (NS) | 1193 | 0 | 5 | 4 | (NS) |
| Handa (2018) [31] | 1752 | SAPIEN XT | (NS) | (NS) | (NS) | (NS) | 1237 | 0 | 449 | 66 | (NS) |
| Abbreviations: NS, Not Specified; PPM, prosthesis-patient mismatch; TF, transfemoral; TAo, transaortic; TAp, transapical. | |||||||||||
Seventeen of the twenty studies reported their mean baseline transaortic
gradient and aortic valve area as shown in Supplementary Table 2
[8, 16, 17, 18, 20, 21, 22, 23, 24, 25, 26, 28, 29, 30, 32, 33, 34]. The mean baseline transaortic gradient
was 50.93
In summary, the following are the outcomes that our study have looked into in Asian studies involving TAVR. These are reflected in Table 3. Further details are given below.
| Outcomes | No. of studies | Overall No. of patients | No. of events | Weighted mean |
|---|---|---|---|---|
| Success | 15 | 10,989 | 10,470 | 95.28% |
| In-hospital mortality (%) | 5 | 3174 | 76 | 2.39 |
| 30-day mortality (%) | 18 | 13,509 | 224 | 1.66 |
| 1-year mortality (%) | 14 | 7515 | 655 | 8.79 |
| 30-day stroke | 15 | 12,704 | 224 | 1.75 |
| 1-year stroke | 7 | 2019 | 63 | 3.22 |
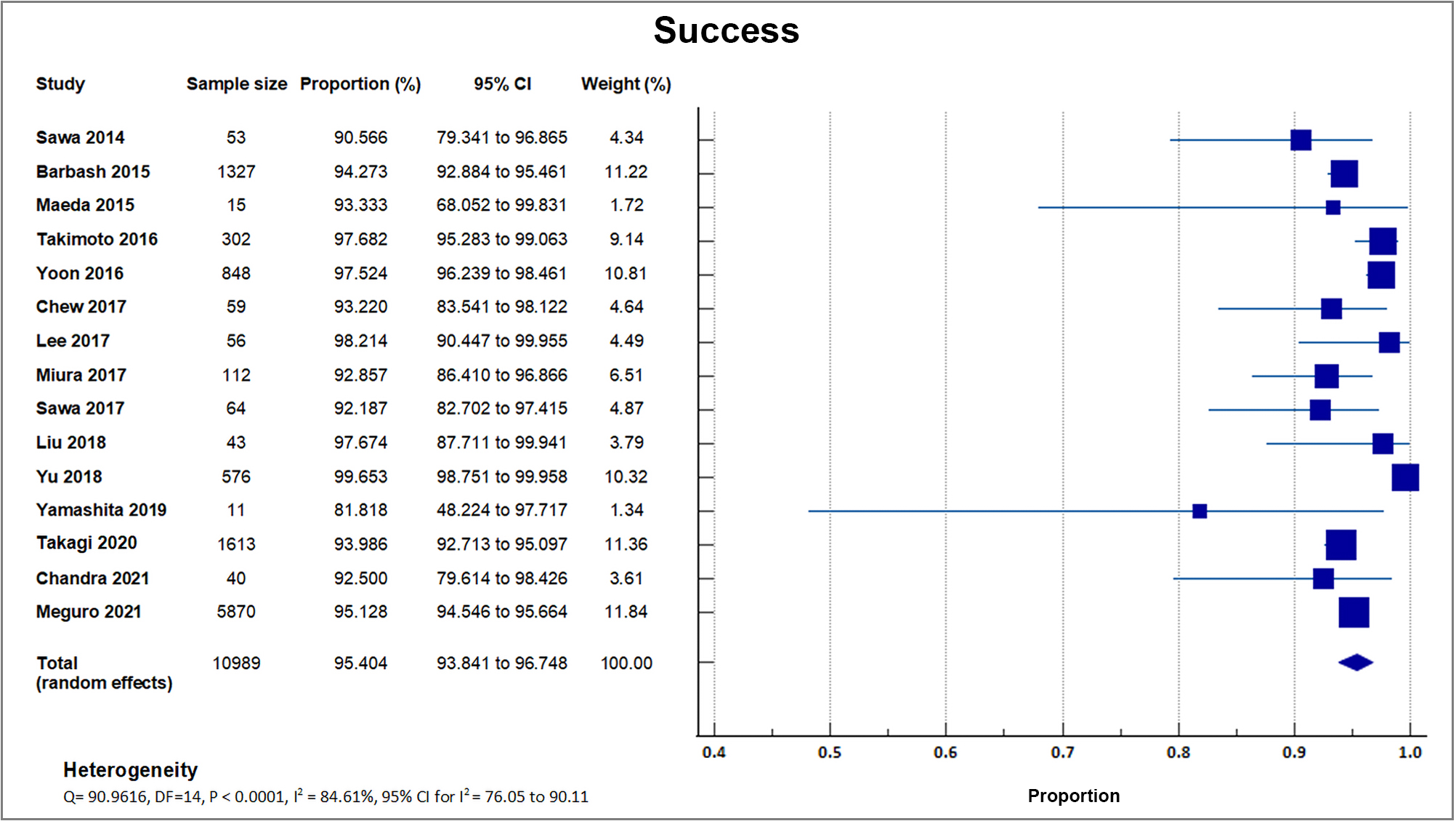
Fifteen registries and studies reported procedural success [16, 17, 18, 19, 21, 22, 23, 24, 25, 26, 28, 29, 32, 33, 34]. The mean success rate was 95.40% with a weighted standard deviation (SD) of 1.5% [16, 17, 18, 19, 21, 22, 23, 24, 25, 26, 28, 29, 32, 33, 34]. The highest success rate was reported in a study done by Yu in 2018 [21], with a procedural success rate of 99.7% (574/576), followed by Lee in 2017 [16], with a procedural success rate of 98.2% (55/56). A forest plot presenting all the reporting studies is shown in Fig. 3.
 Fig. 3.
Fig. 3.Procedural success. Forest plot on the rates of procedural success as an outcome of TAVR performed in Asian patients.
Only five studies reported procedural and in-hospital mortality, with a total sample size of 3174 [16, 21, 22, 29, 30]. The mean in-hospital mortality rate was 2.28% [16, 21, 22, 29, 30]. Among the five studies, Barbash et al. [29] (2015) reported the highest in-hospital mortality (3.17%, 42/1327) in Israel. A forest plot presenting all the reporting studies is shown in Fig. 4.
 Fig. 4.
Fig. 4.In-hospital mortality. Forest plot showing rates of in-hospital mortality as an outcome of TAVR performed in Asian patients.
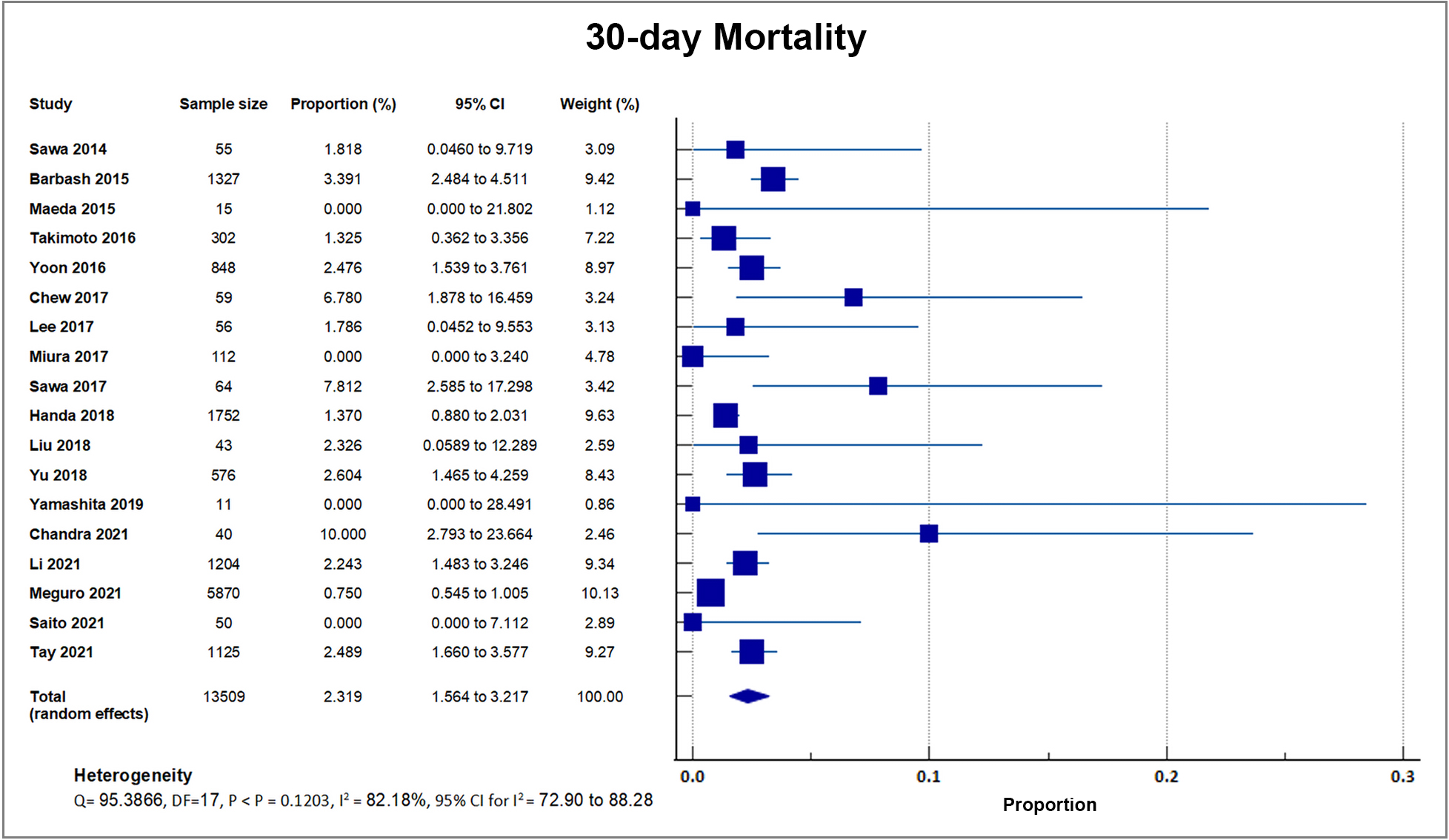
Eighteen studies reported that a total of 224 out of 13,509 patients died by 30 days after TAVI, giving a weighted mean for 30-day mortality rate of 1.66%, with a weighted standard deviation of 1.2% [8, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 28, 29, 30, 31, 32, 33]. Four small center trials and registries with sample sizes ranging from only 15–50 recorded a 0% mortality. These four studies had a weight ranging from 0.08% to 0.37%. A forest plot presenting all the reporting studies is shown in Fig. 5 [20, 22, 28, 32].
 Fig. 5.
Fig. 5.30-day mortality. Forest plot showing rates of 30-day Mortality as an outcome of TAVR performed in Asian patients. Abbreviations: TAVR, Transcatheter aortic valve replacement.
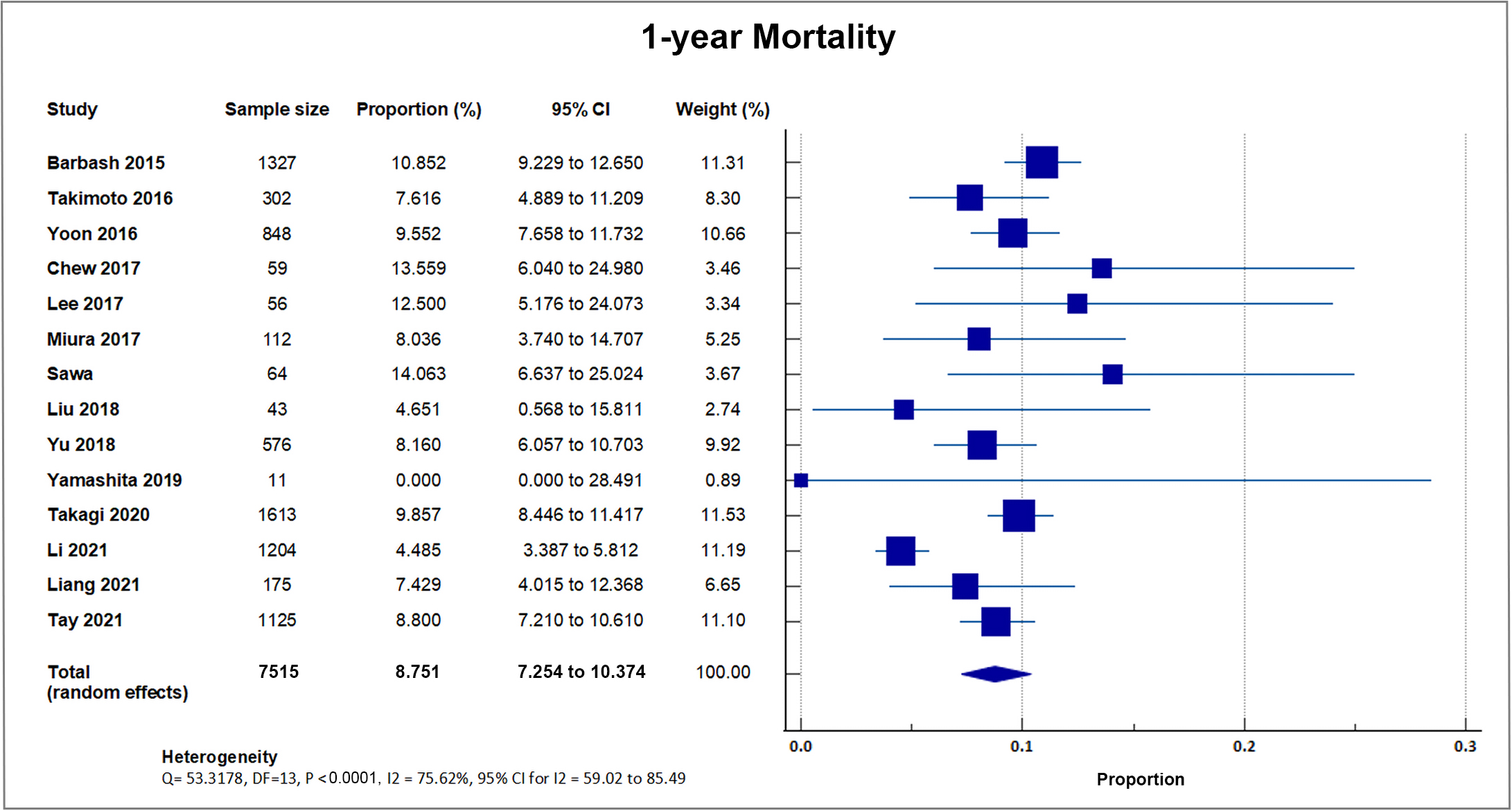
Fourteen studies reported one-year all-cause mortality with a total of 655 out of 7515 patients bringing the weighted mean at 8.79, SD 2.3% [8, 16, 18, 19, 21, 22, 24, 25, 26, 27, 29, 30, 32, 34]. A forest plot presenting all the reporting studies is shown in Fig. 6.
 Fig. 6.
Fig. 6.1-year mortality. Forest plot showing rates of one-year mortality as an outcome of TAVR performed in Asian patients. Abbreviations: TAVR, Transcatheter aortic valve replacement.
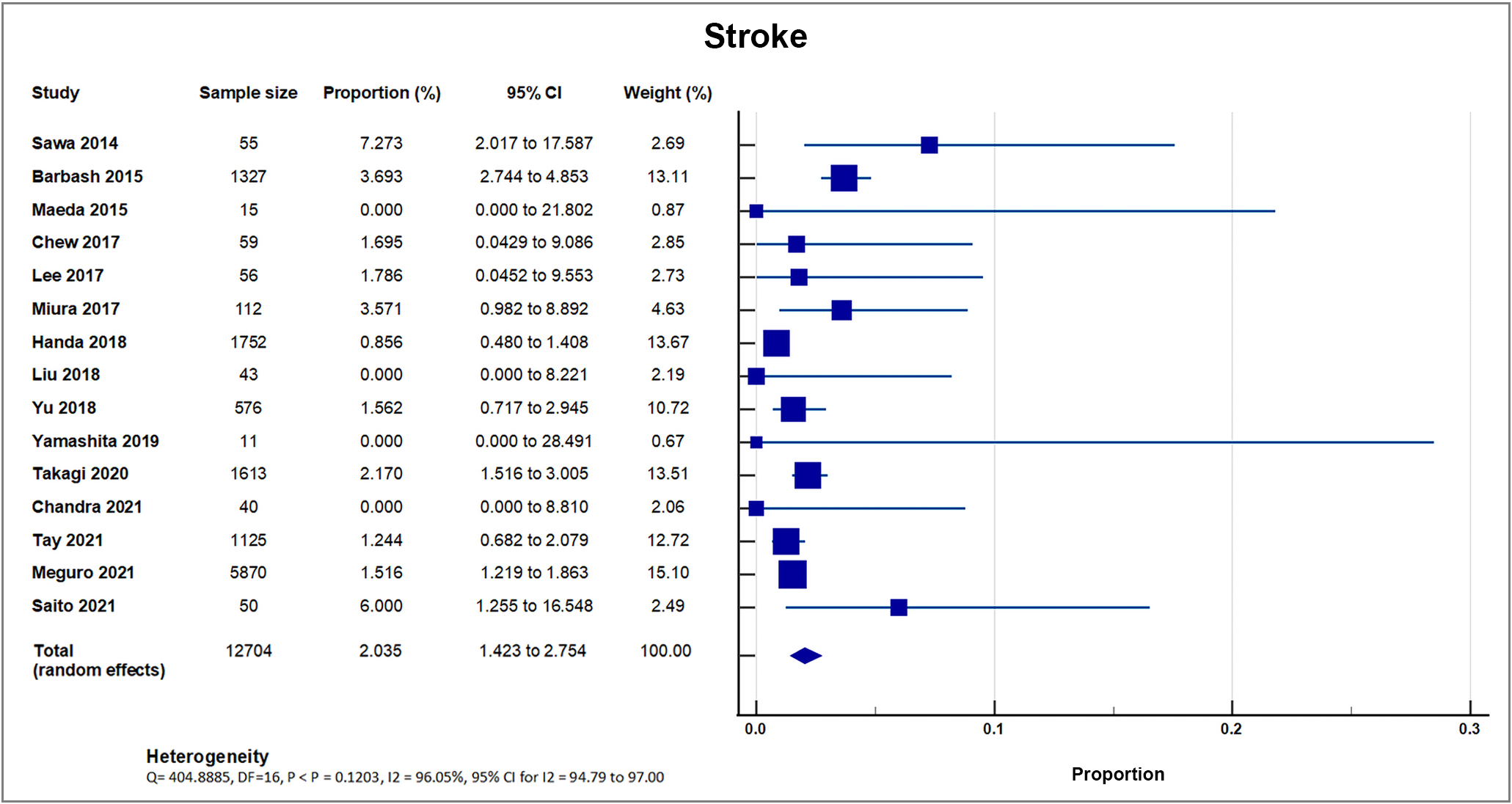
All included studies reported stroke events as outcomes. Out of 20, three small sample studies and registries with sample sizes ranging from only 15–50 reported no occurrence of stroke [19, 28, 33]. 17 studies reported occurrence of stroke, with a total of 303 out of 15,297 patients, with a weighted mean of 1.98%, SD 1.49% [8, 16, 17, 18, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 32, 34]. 15 studies reported the incidence of stroke within 30 days as an outcome, with a mean of 1.75% [8, 16, 17, 19, 20, 21, 22, 24, 26, 29, 30, 31, 32, 33, 34]; and seven studies reported the incidence of stroke in one year as an outcome, with a mean of 3.22%. A forest plot presenting these 15 studies is shown in Fig. 7 [8, 19, 21, 22, 23, 27, 30].
 Fig. 7.
Fig. 7.Stroke. Forest plot showing rates of stroke as an outcome of TAVR performed in Asian patients. Abbreviations: TAVR, Transcatheter aortic valve replacement.
Table 4 (Ref. [8, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34]) shows an overview of the acute procedural complications reported by the included studies as outcomes.
| Complication | No. of studies | Overall no. of patients | No. of events | Weighted mean |
|---|---|---|---|---|
| AKI (%) | [8, 16, 18, 19, 20, 23, 26, 28, 29, 31, 32, 33, 34] | 7104 | 489 | 6.88 |
| Major vascular complications (%) | [8, 16, 18, 19, 20, 21, 22, 24, 25, 26, 27, 30, 31, 32, 33] | 6408 | 167 | 2.58 |
| Major bleeding (%) | [8, 16, 18, 19, 20, 21, 22, 24, 25, 26, 28, 29, 30, 31, 32, 33, 34] | 9188 | 359 | 3.88 |
| Perivalvular Aortic Regurgitation, Moderate to Severe (%) | [8, 16, 17, 18, 19, 20, 22, 24, 25, 26, 28, 29, 33, 34] | 11,410 | 1720 | 15.07 |
| Permanent Pacemaker Insertion (%) | [8, 16, 17, 18, 19, 20, 21, 23, 24, 26, 27, 28, 29, 30, 31, 33, 34] | 15,110 | 1177 | 7.76 |
| New Onset Atrial Fibrillation (%) | [20, 26, 27, 30, 34] | 3099 | 75 | 2.42 |
| Abbreviations: AKI, Acute kidney injury. | ||||
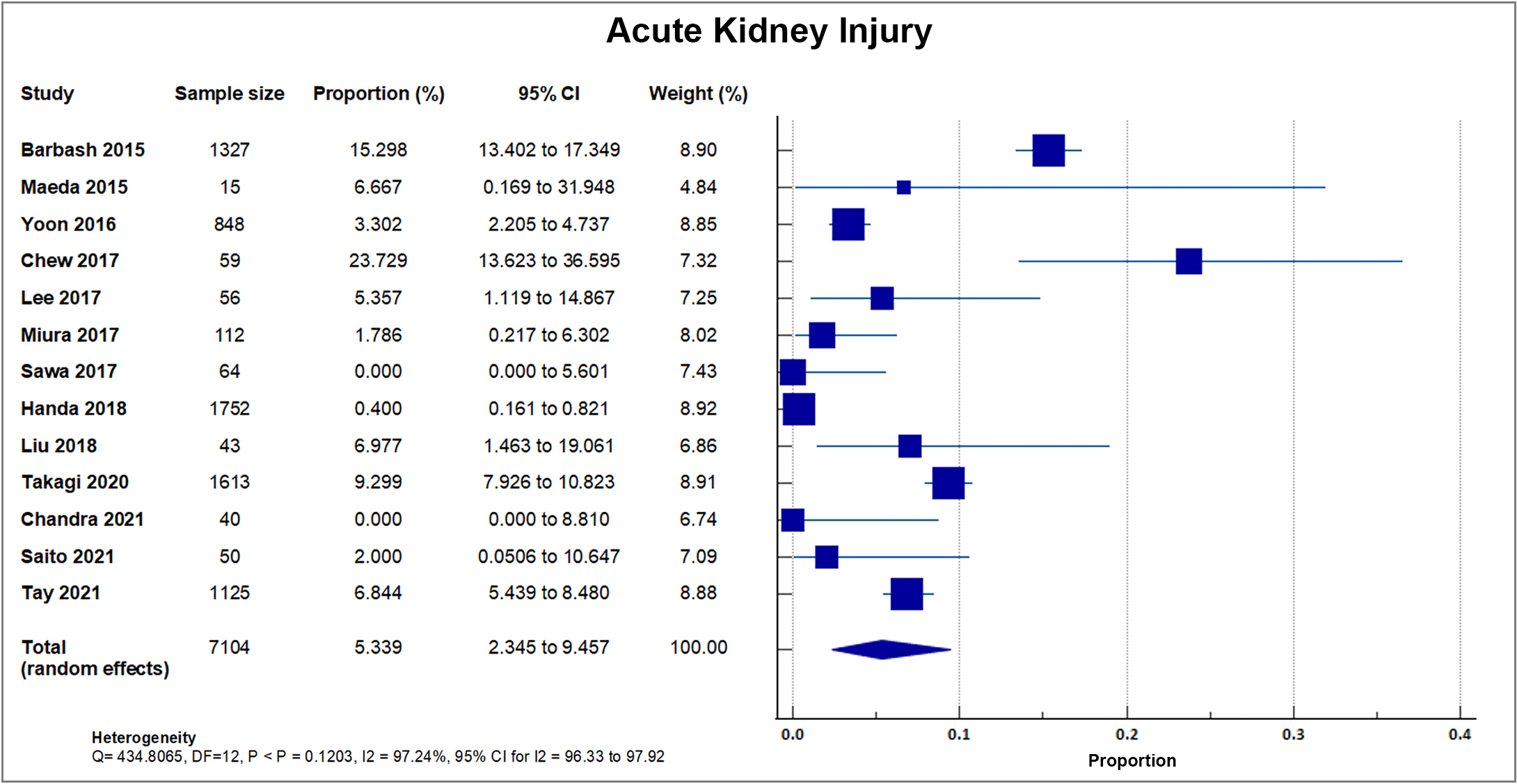
Thirteen studies reported Acute Kidney Injury (AKI) in 489 out of 7104 patients, with a mean AKI rate of 6.88, SD 5.71% [8, 16, 18, 19, 20, 23, 26, 28, 29, 31, 32, 33, 34]. The highest percentage was reported in a single center study in Singapore with 23.7% (14/59). Two studies reported 0% incidence of AKI [23, 33]. A forest plot presenting all the reporting studies is shown in Fig. 8.
 Fig. 8.
Fig. 8.Acute kidney injury (AKI). Forest plot showing rates of AKI as an outcome of transcatheter aortic valve replacement (TAVR) performed in Asian patients.
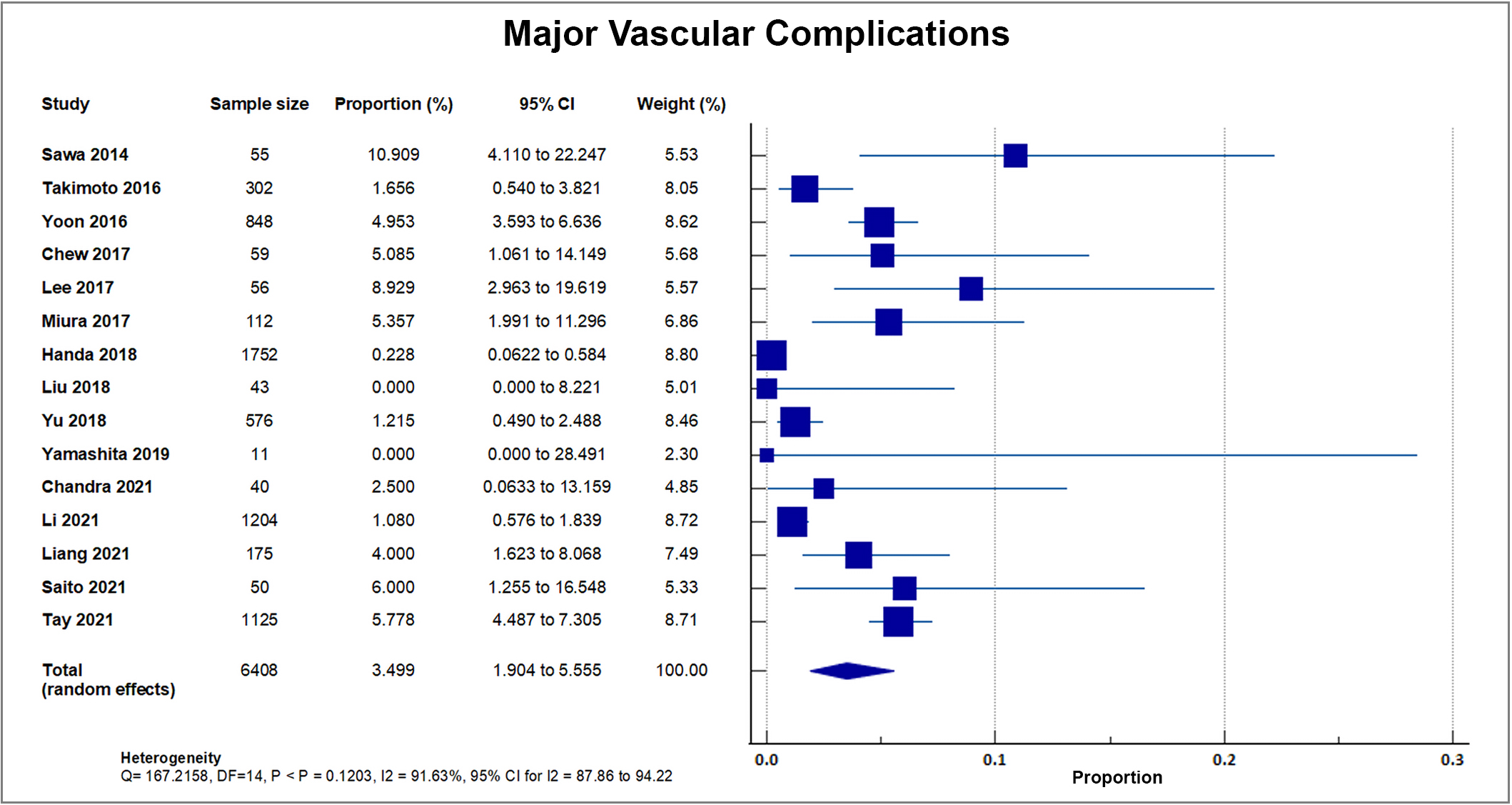
As reported by 15 out of 20 studies, a total of 167 out of 6408 patients suffered from major vascular complications, accounting for an overall rate of 2.48%, SD 2.54%. A forest plot presenting all the reporting studies is shown in Fig. 9 [8, 16, 18, 19, 20, 21, 22, 24, 25, 26, 27, 30, 31, 32, 33].
 Fig. 9.
Fig. 9.Major vascular complication. Forest plot showing rates of major vascular complications as an outcome of TAVR performed in Asian patients. Abbreviations: TAVR, Transcatheter aortic valve replacement.
Seventeen studies reported that during the first 30 days after TAVI, 359 out of
the total of 9188 patients suffered from major bleeding as defined in VARC-2,
accounting for an overall bleeding rate of 3.88
 Fig. 10.
Fig. 10.Major bleeding. Forest plot showing rates of major bleeding as an outcome of TAVR performed in Asian patients. Abbreviations: TAVR, Transcatheter aortic valve replacement.
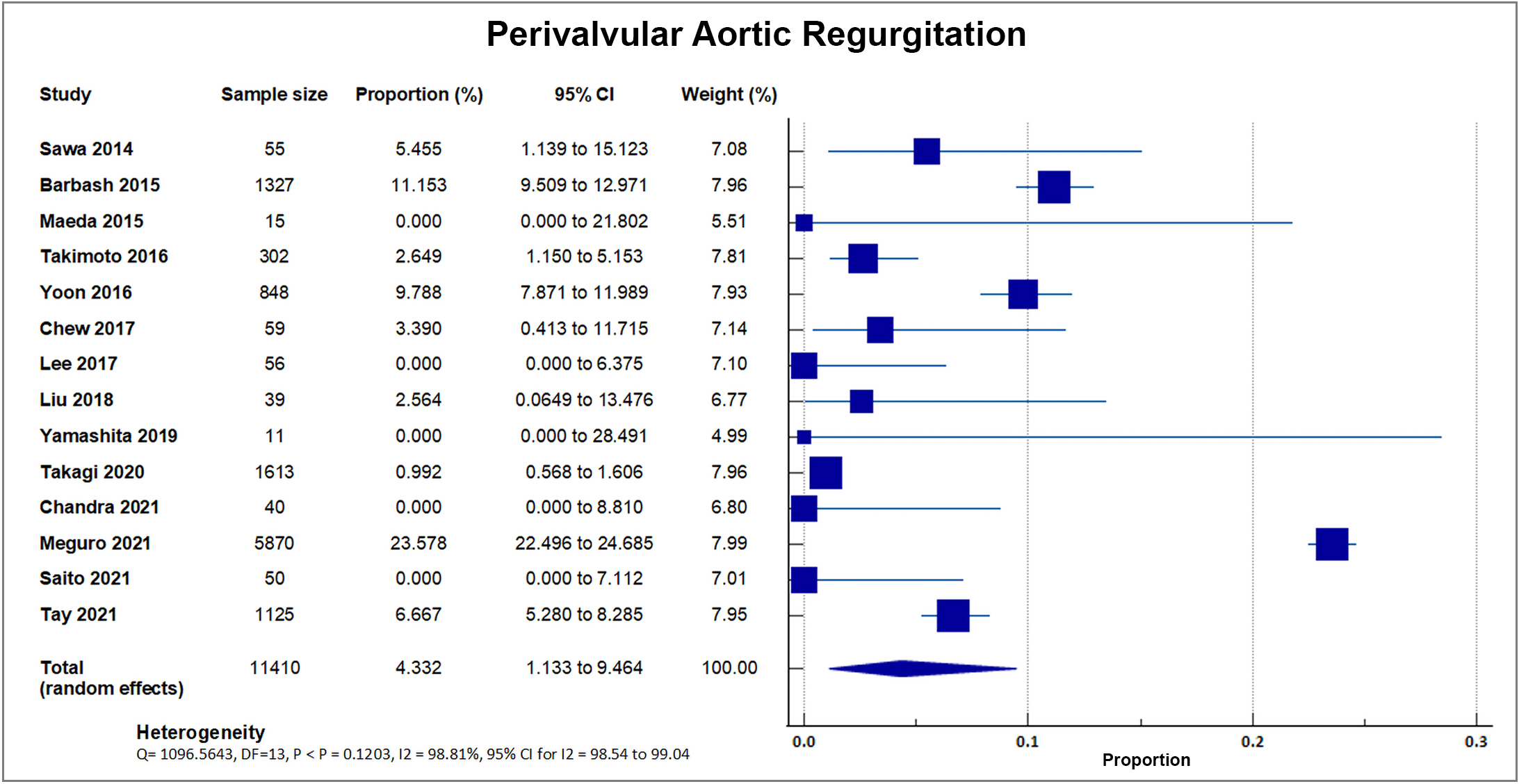
Fourteen out of 20 studies reported moderate to severe perivalvular aortic regurgitation. A total of 1720 out of 11,410 patients experienced postprocedural aortic regurgitation, accounting for a weighted rate of 15.07, SD 9.58% [8, 16, 17, 18, 19, 20, 22, 24, 25, 26, 28, 29, 33, 34]. J-TVT, a large registry developed by 4 Japanese academic societies with 5870 enrolled patients, reported the highest percentage of paravalvular leakage at 23.58% with a weight of 51.43%. A forest plot presenting all the reporting studies is shown in Fig. 11.
 Fig. 11.
Fig. 11.Perivalvular aortic regurgitation. Forest plot showing rates of perivalvular aortic regurgitation as an outcome of transcatheter aortic valve replacement (TAVR) performed in Asian patients.
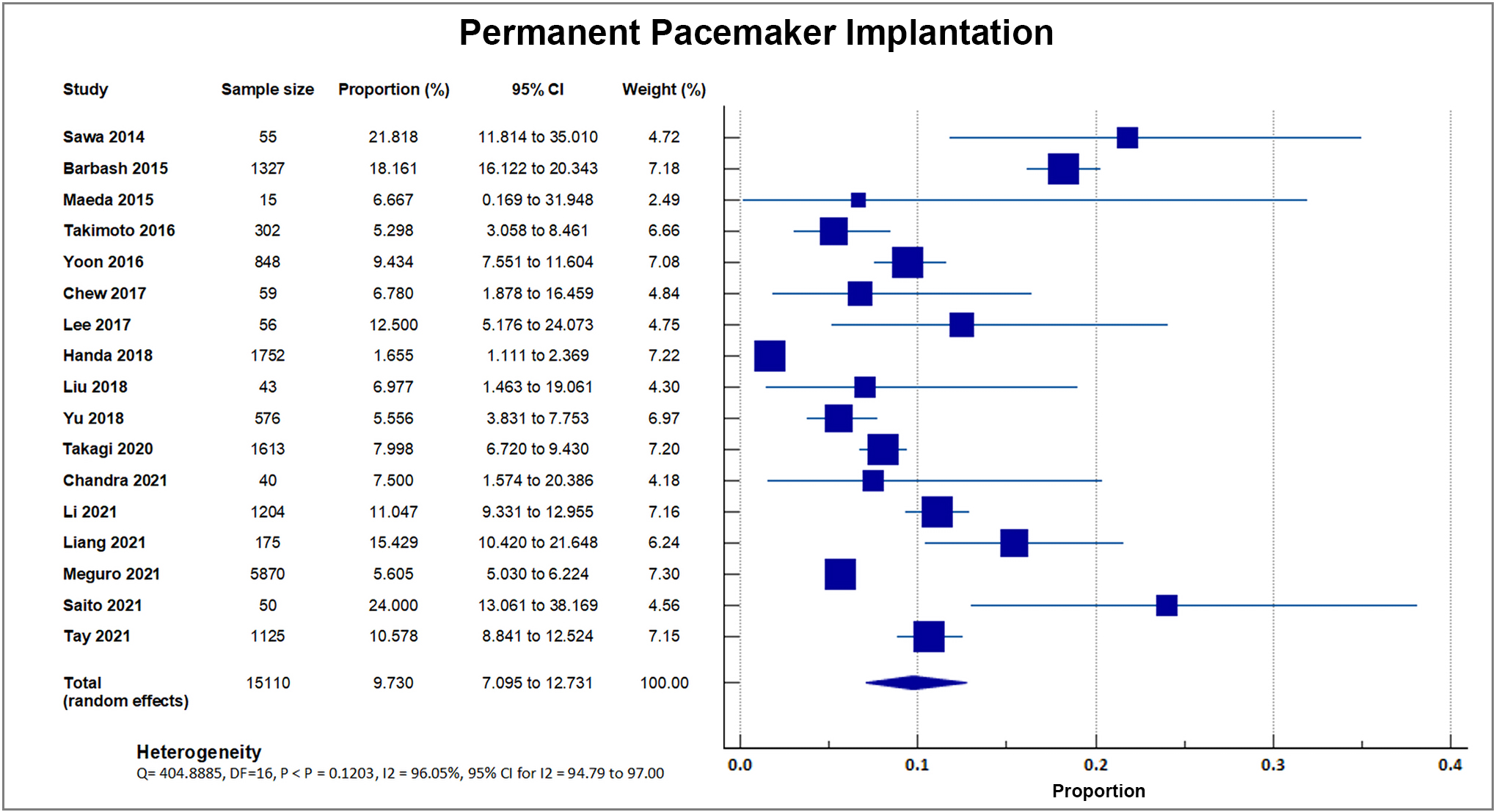
Seventeen studies reported a post-procedural need for permanent pacemaker implantation. A total of 1177 out of 15,110 patients required permanent pacemaker implantation, accounting for an overall rate of 7.76%, SD 4.6% [8, 16, 17, 18, 19, 20, 21, 23, 24, 26, 27, 28, 29, 30, 31, 33, 34]. A forest plot presenting all the reporting studies is shown in Fig. 12.
 Fig. 12.
Fig. 12.Permanent pacemaker insertion. Forest plot showing rates of PPI as an outcome of TAVR performed in Asian patients. Abbreviations: PPI, permanent pacemaker implantation; TAVR, Transcatheter aortic valve replacement.
It was only after the publication of randomized trials demonstrating TAVI as a
solid and unquestionable treatment modality for aortic stenosis that case numbers
begin to rise in Asia [7]. Demographics play a role in the rapid expansion of
TAVI centers in Asia [35]. The elderly population, usually those aged
 Fig. 13.
Fig. 13.Percentage of the population ages 65 and older.
Compared to their American and European counterparts, Asian patients who underwent TAVI experienced less 1-year all-cause mortality, bleeding, and vascular complications; but had more postprocedural aortic regurgitation. 30-day mortality and incidence of stroke, AKI, and the need for a permanent pacemaker were similar. Detailed comparisons of Asian figures for the key outcomes compared to those found in America and Europe are found below.
Our analysis of eighteen registries that reported the 30-day mortality revealed
a weighted average of 1.66
Our analysis of the fourteen reports which included 1-year mortality following
TAVR yielded a weighted mean of 8.79
There have been no large-scale studies directly comparing outcome differences in
TAVI recipients between Asian and Western populations; however, a recent report
on racial disparities in outcomes from the TVT registry showed that the adjusted
1-year mortality rate was significantly lower among patients of Asian/Native
American/Pacific Islander descent than when compared to White patients [45].
However, of the 70,221 patients included in the report, Asian patients only
comprised
Ischemic stroke is a feared complication associated with TAVI. TAVI is
associated with a significantly higher ischemic cerebrovascular events [CVE] risk
in the early phase (hazard ratio (HR) 5.35 [95% CI 3.50–8.17]; p
In the thirteen publications that reported AKI with 7104 patients, the event
occurred in 489 patients within the first 30 days from the procedure resulting in
a weighted mean of 6.88
Post-TAVI bleeding, major or life-threatening, increases 30-day postoperative
mortality [54]. Seventeen publications reported major bleeding, and out of 9188
patients, the event occurred in 359 with a mean of 3.88
Patients undergoing TAVI between 2011 to 2016 showed a vascular complication
rate of 9.3% (n = 3257) and an in-hospital bleeding event rate of 7.6% (n =
2651). Rates of vascular complications and bleeding events decreased over time
(p for trend test
Vascular complications are one of the major concerns during TAVR, primarily due
to using large bore sheaths to establish adequate access [56]. In the PARTNER
(Placement of AoRTic TraNscathetER Valve) Trial, sixty-four patients (15.3%) had
major vascular complications, and 50 patients (11.9%) had minor vascular
complications within 30 days of the procedure [1]. Most TAVI procedures performed
in Asia used the transfemoral approach [8]. Transfemoral access use was similar
in the US (US-TVT) and Japan (Japan-TVT) at rates of 90.9% and 88.7%,
respectively [38, 57]. However, transapical access was more commonly done in Japan
than in the US (20.1% versus 42.5%; p
Aortic regurgitation after TAVI is linked to adverse outcomes, and the most common cause is PVL. PVL occurs in undersized valves, markedly elliptical annulus geometry, and if the prosthetic valve is not apposed properly to the native valve due to extensive calcification or malposition [59]. The study reveals comparable rates of moderate to severe paravalvular aortic regurgitation compared to the reported incidence in Western Countries, i.e., PRAGMATIC 2015 (2.3%) and Swiss-TAVI (5.0%) [42, 43]. The use of similar types of valves between Asian and Western groups may explain the similar rates of PVL, however, the differences in anatomy (i.e., incidence of bicuspid valve and smaller valve diameters) and center and surgeon experience have to be considered [60].
Since its inception, TAVI has grown tremendously, and various registries report constantly declining mortality and complication rates with the procedure. The demand for TAVI in Asia is expected to rise due to its aging population. Our research suggests similar post-TAVI mortality and complications in Asian countries compared to the US and Europe. One-year mortality, bleeding, and vascular complications occurred less frequently, but postprocedural aortic regurgitation was more common. Anatomical differences and disparities in access to technical expertise and health resources may play a major role in these differences. More studies with a greater sample size focusing on the clinical outcomes and anatomic differences among Asians are needed to make a more robust comparison between Asian and Western populations. The significant socioeconomic barriers to TAVI access must be addressed for broader implementation of the procedure in Asia.
This meta-analysis explored TAVI outcomes and complications in Asia, which features cohorts from Hong Kong, Japan, the Philippines, Singapore, Taiwan, South Korea, and Israel. To our knowledge, this paper is the largest aggregated report available at this time of writing. However, the total of 15,297 patients this study described still does not compare to the sample size reported by Western registries in the US and Europe; as such, making direct comparisons with this disparity in sample size is challenging. Direct comparison using meta-analysis with other randomized western registries is limited. Furthermore, data gathered from registries and trials concentrated on high-income Asian economies in the region and may not accurately represent the entire Asian population.
Adoption of TAVI in Asia has been slow, particularly among developing countries with a significant infrastructural gap that hinders more widespread use of the procedure [2, 41]. A closer look at these disparities is highly recommended for future research.
TAVR, Transcatheter Aortic Valve Replacement; TAVI, Transcatheter Aortic Valve Implantation; EuroSCORE, European Systems for Cardiac Operations Risk Evaluation; STS Score, Society of Thoracic Surgeons Score; NYHA, New York Heart Association; CAD, Coronary Artery Disease; CABG, Coronary artery bypass graft; PAD, Peripheral artery disease; COPD, Chronic obstructive pulmonary disease; DM, Diabetes Mellitus; CKD, chronic kidney disease.
FBR is the main author of this report. DVDL assisted FBR in conceptualizing the work. MFMA, RTN, GPF, JVM, SWC, JPA, JSTG, WFCS and GFEM all contributed in the literature review, collection of data and its analysis and writing of the final report. MLPM, TI, KV, FMSC and AL gave expert opinion to further refine the paper. All the authors had concurred with the final version of this report.
Not applicable.
Not applicable.
This research received no external funding.
Krishnaswami Vijayaraghavan is serving as one of the Editorial Board members and Guest Editors of this journal, Azeem Latib is serving as Guest Editor of this journal. We declare that Krishnaswami Vijayaraghavan and Azeem Latib had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Gianluca Rigatelli.
The rest of the authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.