1 Department of Public Health, University of Chester, CH1 4BJ Chester, UK
2 Department of Medical Biochemistry, Alex-Ekwueme Federal University Ndufu Alike, 482131 Ikwo, Nigeria
3 World Health Organization, United Nations House, 900211 Abuja, Nigeria
Abstract
Hypertension (HTN) is a global disease of public health concern. It is considered a major cause of morbidity and mortality worldwide. The global and regional recommendations for the management of high blood pressure are complicated, with an increasing call for several adequate measures to commence treatment, increase the dosage, or introduce a new class of medication. Evidence suggests that most people with HTN require more than one drug regime to achieve blood pressure goals, with a greater percentage of patients reporting only having access to monotherapy. This work evaluated the combined effectiveness of angiotensin receptor blockers (ARBs) and hydrochlorothiazide (HCTZ) compared to ARB monotherapy in an uncontrolled hypertensive patients.
The search involved screening through databases such as Cochrane Library, PubMed, CINAHL, Embase, Medline, and the Web of Science, medical journals, and international registry on clinicals from the WHO were searched for primary studies not older than 13 years. Randomized control trials (RCTs) comparing the effectiveness of ARB/HCTZ versus ARB monotherapy in hypertensive patients were selected. Care was taken to include only studies that lasted at least four weeks. Meta-analysis was conducted on RevMan 5.3 statistical application software, following data extraction. Data quality and risk of bias assessment were also all assessed. A total of seven RCTs were considered for this study involving 4814 participants.
The result from the intervention arm revealed that ARB/HCTZ combination resulted in a higher rate of target blood pressure achievement when compared to ARB monotherapy relative risk (RR) = 1.53, 95% confidence interval (CI) (1.42, 1.65), with p < 0.00007. The adverse effects observed in the intervention arm were not significant.
The ARB/HCTZ combination therapy was more effective in lowering and controlling blood pressure when compared to ARB monotherapy without significant adverse drug effects reported by the participants. Health workers should therefore recommend ARB/HCTZ combination therapy for patients with uncontrolled hypertension.
Keywords
- high blood pressure
- ARB
- HCTZ
- hypertensive patients
- antihypertensive drugs
- combination
- monotherapy
Hypertension (HTN) is characterized by blood pressure (BP) values of
The cutoff point at which a person is considered to have hypertension is the condition’s defining characteristic. The threshold is still a matter of debate and conjecture since hypertension-related cardiovascular risk is evident at lower blood pressure levels [6].
Physicians often prescribe combined antihypertensive medication against monotherapy in individuals with essential hypertension, in addition to changes in lifestyle aimed at lowering the risk of cardiovascular disease [7]. To buttress this point, results from a big meta-analysis that aggregated data from Randomized control trials (RCTs), or observational the study revealed that, reducing blood pressure with dual antihypertensive drug significantly lowers the risk of vascular disease [8]. However, most of these clinical trials so far conducted focused mostly on calcium-channel blockers (CCBs) and beta-blockers. Diuretic and Angiotensin II receptor blockers are as effective, yet more tolerated than other classes in the management and controlling of myocardial infarction [9, 10].
Therefore, this was aimed to evaluate the effectiveness of combined angiotensin receptor blocker (ARB)/hydrochlorothiazide (HCTZ) in the control of hypertension amongst adult patients. The findings from this systematic review will go a long way in guiding Governments and parastatals when making informed choices on the most effective antihypertensive drugs.
This research was a systematic review. The following databases were used for the search—Cochrane Library, PubMed, CINAHL, Embase, Medline, and the Web of Science, medical journals and international registry on clinicals from the WHO were searched for primary studies not older than 13 years from the time of the search. Abstracts obtained from international conferences on high blood pressure and the Centre for Disease and Prevention (CDC) were also used. To drastically reduce publication bias, unbiased articles were searched with the help of the Health Technology Assessments Database (HTA) such as the dDgital Common Network and the WHO. The “text word searching” approach was adopted to get only relevant articles [11]. The text word searching approach entails searching and choosing phrases that precisely characterize the topic of interest best. Here, the papers or articles are searched using keywords, titles, abstracts and subheadings Randomized-control articles (RCTs) comparing the effectiveness of ARB/HCTZ versus ARB monotherapy in hypertensive patients were selected. Care was taken to include only studies that lasted up to four weeks. Meta-analysis was conducted on RevMan 5.3 statistical application software (Cochrane Collaboration, Vienna, Austria), following data extraction. Data quality and risk of bias assessment were also all assessed. Seven RCTs were considere d for this study involving 4814 participants (Fig. 1).
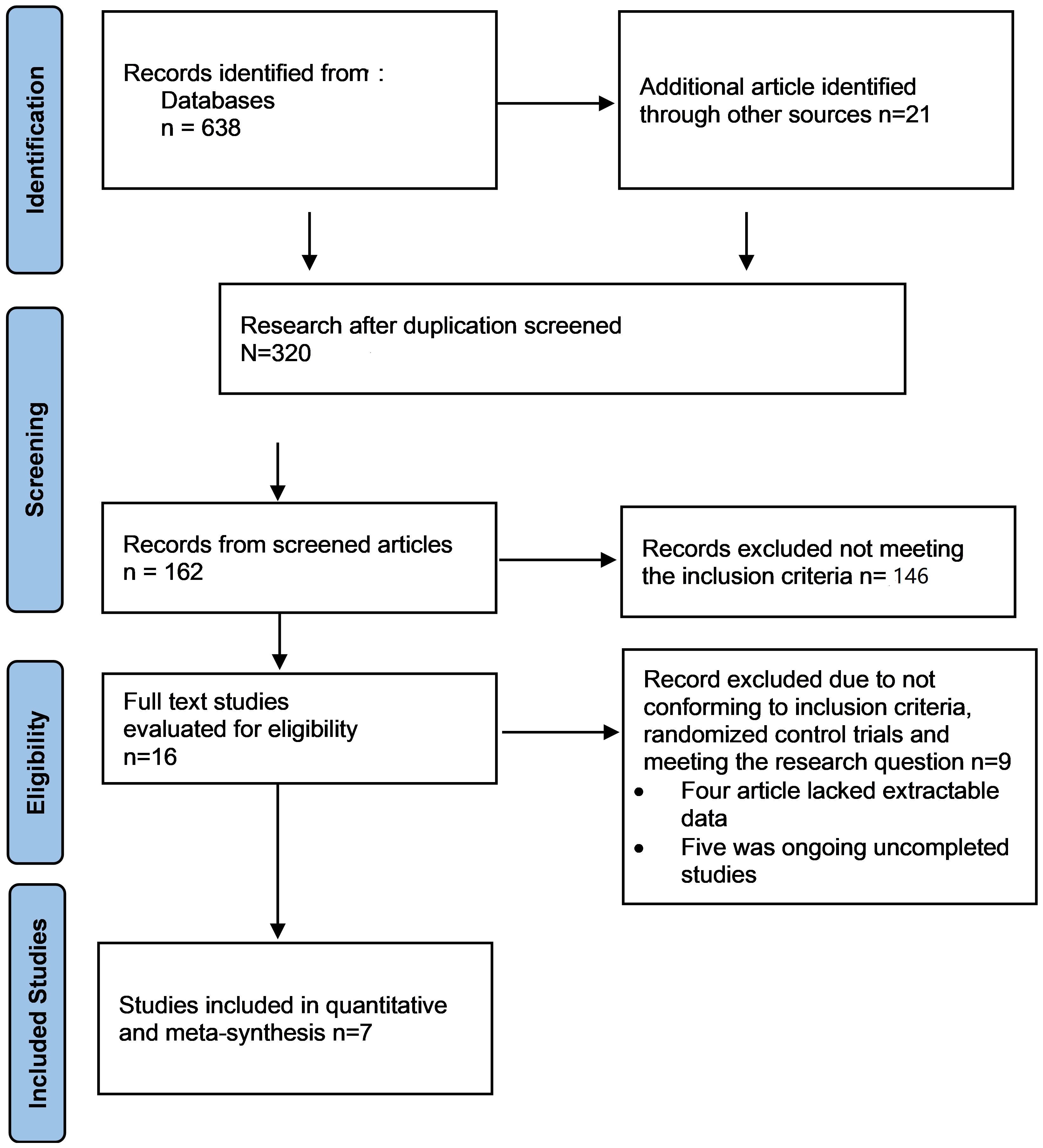 Fig. 1.
Fig. 1.
Study selection diagram Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA flow chart). The figure shows the study selection processes, including the study selection from the main databases.
The primary outcome in the study was the percentage of antihypertensive patients’ baseline blood pressure that was successfully decreased by combined ARB/HCTZ intervention while the secondary outcome was the adverse effects experienced by the participants during or after the intervention.
Only RCTs published within the past 13 years, comparing ARB/HCTZ with ARB
monotherapy in the control of high blood pressure among adult patients (
After selecting the search strategy for each database, all studies obtained from different databases were added to EndNote X8 (Clarivate, Philadelphia, PA, USA), while the Preferred Reporting items for systematic review (PRISMA) were useful in explaining how information is transcribed at various stages of a Systematic Review as reported by Page et al. (PRISMA_2020_checklist) [12]. The articles were chosen from removing 320 duplicates leaving only 162 articles. Exactly 16 articles were evaluated in full for the eligibility criteria, from where 7 were finally selected for the meta-analysis [11] (Fig. 1), which ended up producing 7 papers that were used for the systematic review. In order to deliver the highest standard of evidence, only high-quality Randomized control trial (RCT) studies were employed during data extraction synthesis and meta-analysis because it is the most reliable evidence on the effectiveness of intervention and is often referred to as the gold standard [13].
In appraising the research papers, the Critical Appraisal Skills Programme (CASP) (https://casp-uk.net/) tool was employed, and the quality of each featured paper was evaluated separately by a different initial author. Challenges concerning initial quality rating choices were discussed together, to solve the problems noted, we chose to modify the tool. Following this, the whole articles were re-appraised. Clearly defined questions were asked when appraising the articles, among them were “Was the aim and purpose of the research clear”, “Were all the papers being considered randomized”, “Were ethical principles duly observed”, “Was the sample used a true representation of the study group” and finally, “Was all the number of participants during the intervention accounted for at the end of the day”.
To extract data, a ready-made checklist was used, which enquired about the first author’s name, year of publication, nature of study/duration, participants, intervention vs. comparison, and primary and secondary outcomes. The extracted data from this review were analysed with the help of Revman 5.3 software (Cochrane Collaboration, Vienna, Austria) which was recommended by Cochrane Collaboration. Considering this review basically compared the effectiveness of dual antihypertensive drugs versus monotherapy in adults, only studies with the primary outcome were selected. The summary of the data extraction can be found in Supplementary Table 1.
Risk of bias assessment was performed on each selected data using the Cochrane collaboration tool of accessing bias to minimize the risk of bias (Fig. 2).
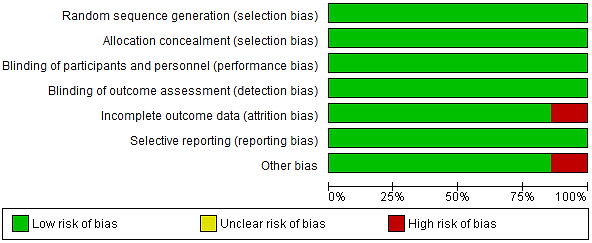 Fig. 2.
Fig. 2.
Risk of bias graph.
To reduce potential errors, inaccuracies, and publication bias, all the steps of searching, evaluating, identifying/selecting the studies, and extracting data were performed.
Comprehensive meta-analysis software was used to analyse the data. RevMan
version 5.3 statistical tool (Cochrane Collaboration, Vienna, Austria) was
employed for data extraction with a graphical representation of the result in a
funnel plot. The Chi-square and the I2 statistics were used to determine the
level of significance and heterogeneity respectively, while Z was used to
determine the overall effect. Finally, the p-value which was set at
This work evaluated the effectiveness of ARB/HCTZ compared with ARB monotherapy in the control of hypertension among hypertensive patients. Relevant RCTs that extensively examined the efficacy of the intervention of interest were assessed both manually and electronically with the help of different databases. After this, the exclusion and inclusion criteria for the article selection were considered with the assistance of the PRISMA flow chart as represented in Fig. 1. This was to allow transparency by reporting and analysing the undertaken steps involved during the article selection process.
This systematic review was carried out using 7 RCTs which evaluated the efficacy of combined antihypertensive drugs against monotherapy as a common outcome of interest. Forest and funnel plots were employed to graphically represent the combined scientific pooled results at the end of the study (Figs. 2,3,4,5). More so, important statistical parameters motioned included p-values, relative risk (RR), confidence interval (CI), and test for heterogeneity. In this review, the effect measure utilized in the analysis of elevated high blood pressure as a primary outcome in this review was the RR at a confidence interval (CI) of 95%. While there were two meta-analyses performed in this review, the first meta-analysis involved all the 7 RCTs studies included to generate a pooled effect, and the second meta-analysis would depict four RCTs studies representing the adverse events and tolerability of the studies. The adverse events focussed on were headache, nasopharyngitis, respiratory tract infection, dizziness, vertigo and diarrhoea.
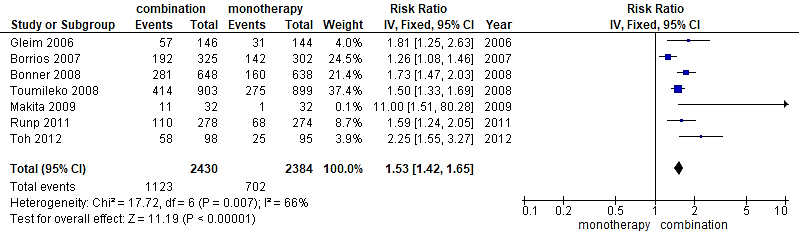 Fig. 3.
Fig. 3.
A forest plot displaying comparison angiotensin receptor blocker (ARB)/hydrochlorothiazide (HCTZ) combination against ARB monotherapy in elevated blood pressure reduction. CI, confidence interval.
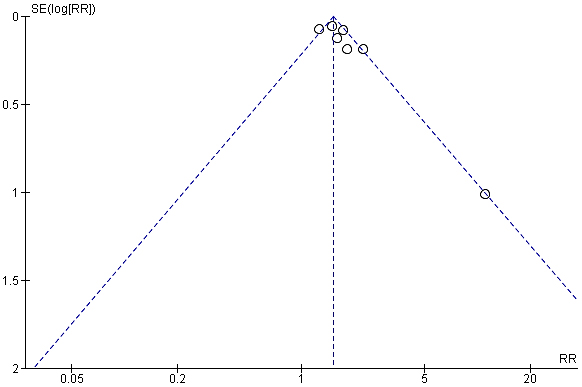 Fig. 4.
Fig. 4.
Funnel plot showing comparison in reduction rate of high blood pressure between ARB/HCTZ and ARB monotherapy. SE, Standard Error; RR, Relative Risk.
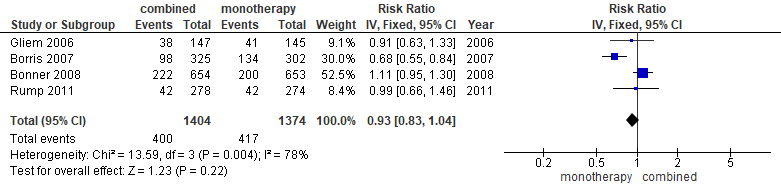 Fig. 5.
Fig. 5.
Forest plot displaying total adverse events of ARB/HCTZ compared to ARB monotherapy.
The intervention arm of the first meta-analysis as represented in Fig. 3 consisted of a total of 2430 participants involving ARB/HCTZ combined therapy while the control arm was made up of 2384 participants treated with only ARB monotherapy. Also, the experimental group displayed 1123 total events while 702 events were recorded in the control group. The relationship between the two variables was ascertained using a chi-square test and I2 was employed to test for heterogeneity with results showing Chi2 = 17.72 and I2 = 66% respectively. Furthermore, the Z-value for the overall effect was Z = 11.19 at a p-value of 0.0001. RR = 1.53 (1.42, 1.65), and at a confidence interval 95% CI. The publication bias or level of homogeneity in the meta-analysis is represented in Fig. 4.
Similarly, Fig. 5 depicted the second meta-analysis representing the adverse events of the study—the recorded effect of the drug on both the intervention arm and the control groups respectively. The meta-analysis involving four RCT studies recorded that, a total of 1404 individuals participated in the ARB/HCTZ intervention group, with a corresponding 1374 participants taking part in the control arm of the meta-analysis. The experimental group however recorded 400 events leaving the control with 417 events. Other corresponding statistical data observed as recorded are I2 = 78%, Chi2 = 13.59 and, Z = 1.23 with a p-value of 0.004. The publication bias or level of homogeneity in the meta-analysis is represented in Fig. 6.
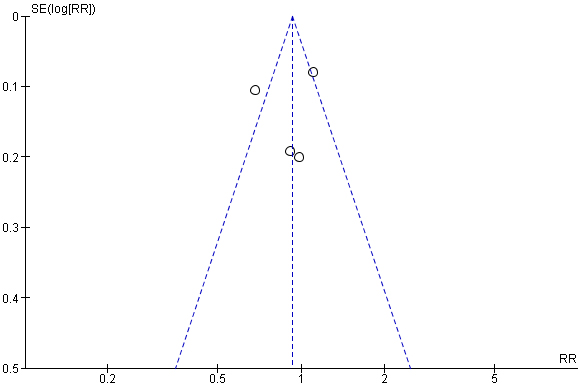 Fig. 6.
Fig. 6.
Forest plot representing total adverse events of ARB/HCTZ compared to ARB monotherapy arm.
The characteristics of the meta-analysis is presented in Table 1 (Ref. [14, 15, 16, 17, 18, 19, 20]).
| Study/title | Nature of study/duration | Participants | Intervention vs. comparison | Primary Outcomes | Secondary outcome |
| Efficacy and tolerability of Olmesartan Medoxomil in patients with mild to moderate essential hypertension. [14] | Prospective intervention study, double-blinded | 627 Adult patients, 302 into control group and 325 into intervention. | OLM group (20–40 mg) vs. OLM+HCTZ (20/12.5 mg) | Achieved target BP goal | Although ARB treated group was associated with a reduced frequency of adverse events compared to ARB/HCTZ combined therapy with 21% and 28.3% respectively, each intervention was generally tolerated with (p |
| 12 weeks | 18–75 years | Reduction in SBP/DBP from baseline | |||
| OLM group: 15.5/9.9 | |||||
| OLM+HCTZ: 20.1/11.8 mmHg | |||||
| Antihypertensive efficacy and tolerability of Candesartan –hydrochlorothiazide 32/12.5 mg and 32/25 mg in patients not optimally controlled with Candesartan monotherapy. [15] | Prospective double-blinded trial that lasted for 16 weeks | 1975 Adult patients, 654 into control group and 1321 into intervention group. | CASTN group vs CASTN+HCTZ (32/12.5 mg and 32/25 mg) | Achieved target BP goal | All study treatments were generally well tolerated. |
| Mean age of 55 years | Reduction in SBP/DBP from baseline | ||||
| CASTN: 6.1/5.6 mmHg | |||||
| CASTN+HCTZ | |||||
| 32/12/5 mg: 13/8.8 mmHg | |||||
| 32/25 mg: 16/10 mmHg | |||||
| A multi-centre, randomized, double-blind, parallel group trail of the antihypertensive efficacy and tolerability of a combination of once-daily Losartan 100 mg/hydrochlorothiazide 12.5 mg compared with Losartan 100 mg monotherapy in the treatment of mild to severe essential hypertension. [16] | RCT, double-blinded trial | 292 Adult patients, 147 into control group and 145 into the intervention group. | LSTN group (100 mg) vs. LZT+HCTZ (100/12.5 mg) | Reduction in baseline BP | Similar adverse effect was recorded in both groups of patients with respiratory tract infection (6.2% vs. 3.4%, respectively), dizziness (2.1% vs. 0.7%), and headache (0.7% vs. 3.4%). |
| 6 weeks | Mean age of 53.8 years | Reduction in sitting DBP | |||
| LZT: 5.2 mmHg | |||||
| LZT+HCTZ: 8.3 mmHg | |||||
| Efficacy of low-dose hydrochlorothiazide in combination with telmisartan on early morning blood pressure in uncontrolled hypertensive patients. [17] | RCT | 64 Adult patients, 32 into control group vs. 32 into intervention group | ARB group (candesartan 8 mg/day or valsartan 80 mg/day) vs. TMS+HCTZ (40/12.5 mg) | Significant reduction observed in BP | No apparent metabolic deterioration with (p |
| 12 weeks | Reduction in SBP/DBP | ||||
| ARBs (Control): 4.2/3.7 mmHg | |||||
| TMS+HCTZ: 15.3/6 mmHg | |||||
| Effect of high dose olmesartan medoxomil plus hydrochlorothiazide on blood pressure control in patients with grade 2 and grade 3 hypertension. [18] | A two-phase randomized double blinded trial | 698 Adult patients, 140 into control group vs. 558 into intervention group. | OLM group (40 mg) vs. OLM+HCTZ (40/12.5 mg or 40/25 mg) | Achieved target BP goal | Despite eleven serious emergent adverse effects in the open label period and twelve in the double-blinding treatment period, overall treatments were well tolerated. |
| 16 weeks | Reduction in SBP/DBP | ||||
| OLM: –8.9/5.7 mmHg | |||||
| OLM+HCTZ | |||||
| 40/12.5: 13.5/9.1 mmHg | |||||
| 40/25: 16.2/11.2 mmHg | |||||
| Comparison of medium-dose Losartan/hydrochlorothiazide and maximal-dose angiotensin II receptor blockers in the treatment of Japanese patients with uncontrolled hypertension the Kobe-connect study. [19] | RCT, Patients randomly assign to ARB/HCTZ combination and monotherapy respectively. | 200 Adult patients, 100 into control group vs. 100 into intervention group. | ARB (LSTN-100 mg, VST-1660 mg, CSNT-12 mg, OLM-40 mg, ISTN-200 mg or TMS-80 mg) (Max dose) vs. LSTN +HCTZ (50/12.5 mg) | Achieved target BP goal | Adverse changes noticed in the study were highest among the ARB/HCTZ treated group than ARB monotherapy group. |
| 52 weeks | Reduction in SBP/DBP: | ||||
| ARBs (Control): 11.7/4.5 mmHg | |||||
| 154.6/85.4 to 142.9/80.9 | |||||
| LSTN+HCTZ: 22.6/9.6 mmHg | |||||
| Combination therapy with Valsartan/hydrochlorothiazide at doses up to 320/25 mg improves blood pressure levels in patients with hypertensive inadequately controlled by Valsartan 320 mg monotherapy. [20] | 3802 Patients, 1100 into control group vs. 2702 into intervention group. | 3802 Patients, 1100 into control group vs. 2702 into intervention group. | VST group (320 mg) vs. VST +HCTZ group (320/12.5 mg or 320/25 mg). | Achieved target BP goal. | Similar adverse effects were observed in the two groups which was comparable in us as the ones recorded with other RCTs; all treatments were generally well tolerated. |
| 12 weeks | Reduction in SBP/DBP | Regardless of the interaction between the study drugs, the most common adverse event across treatment groups was headache with a frequency of 3.9–4.9%. Nasopharyngitis, dizziness, vertigo and diarrhoea. | |||
| VST: 12.8/9.7 mmHg | |||||
| VST+HCTZ: | |||||
| 320/12.5 mg: 20.2/13.6 mmHg | |||||
| 320/25 mg: 21.9/14.2 mmHg |
Note: OLM, Olmesartan; VST, Valsartan; CASTN, Candesartan; TMS, Telmisartan; LSTN, Losartan; ISTN, Irbesartan; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; BP, blood pressure; RCT, Randomized control trial; HCTZ, Hydrochlorothiazide; CSNT, Candesartan; LZT, Lercanidipine.
To put it into perspective, the study conducted by Bönner et al. (2008) [15] involved a total of 3521 patients with treated or untreated hypertension and sitting diastolic blood pressure (DBP) 90–114 mmHg (Table 1). These patients entered a single-blind run-in phase with candesartan (16 mg for 2 weeks, followed by 32 mg for 6 weeks). At the end of the run-in phase, 1975 patients who still had DBP 90–114 mmHg were randomized to 8 weeks’ double-blind treatment with either candesartan. The efficacy of candesartan/HCTZ was higher than Candesartan monotherapy which increased with the dose of the HCTZ.
As recorded by research conducted by Barrios et al. (2007) [14], the
intervention study was a prospective, parallel group, partially randomized,
double-blind study in 463 centres in nine European countries (Table 1). It
involved 2306 male and female adult patients aged 18–75 years with mild to
moderate essential hypertension. At the end of 12 weeks of open-label treatment,
patients displayed a reduction in sitting blood pressure of 62% amongst
participants treated with ARB/HCTZ against 47% witnessed in ARB treated groups.
Although ARB treated group was associated with a reduced frequency of adverse
events compared to ARB/HCTZ combined therapy with 21% and 28.3% respectively,
each intervention was generally tolerated with (p
Also, Toh et al. (2012) [19] enrolled over 30 year’s participants from
24 participating centres in and around Kobe, Japan. It was noticed that at the
end of the RCT trials the percentage of patients that responded to the
therapeutic target baseline blood pressure goal was greater in the ARB/HCTZ group
at 59.2% compared to the ARB monotherapy group at 26% with a p
Similarly, the RCT study conducted by Makita et al. (2009)
[17] produced much the same result. The research involves hypertensive patients
whose BPs were uncontrolled despite the use of antihypertensive agents. The study
lasted for 12 weeks (Table 1). It involved assigning patients randomly to
ARB/HCTZ combination and monotherapy respectively. The office and home BPs were
significantly reduced in the group treated with combination therapy at 63%
compared to those in the group that received only ARB with 34% reduction in
their baseline blood pressure. Sufficient and long-acting BP lowering effect, as
reflected in decreased early morning BP, was obtained with the combination of
low-dose HCTZ and ARB without apparent metabolic deterioration (p
Furthermore, Rump et al. (2011) [18], recorded a significant reduction
in BP achievement in patients treated with ARB/HCTZ of 40% compared to less than
25% recorded in patients administered with ARB monotherapy with a p
A study by Gleim et al. (2006) [16], evaluated the effectiveness of low
dose ARB/HCTZ in patients with an uncontrolled hypertension. The trial conducted
in Europe involves randomizing mostly white patients with a mean age of
53.8 years to a double-blind treatment period for 6 weeks. At week 6 after
randomization, the BP was significantly lowered in the ARB/HCTZ group with a
reported 63% reduction from baseline blood pressure while in the ARB monotherapy
group, the reduction was 44% (p
The last RCTs study conducted by Tuomilehto et al. (2008) [20] was a
double-blind, active-controlled, parallel-group, randomized trial. Patients were
recruited from 237 centres in Europe and North America with mean age of 50 years.
The percentage baseline reduction in the ARB/HCTZ was 74% while it was 52.7% in
the ARB monotherapy group (p
A meta-analysis was deployed to statistically summarize the results of these
studies. Two major analyses were carried out and their results are represented in
the Forest plot in Figs. 3,5 with their corresponding funnel plots in Figs. 4,6
respectively, explaining variation within the system. There was a total pooled
effect of 4814 randomized participants in both the experimental and control arms
of the study, with a RR value of 1.53 at 95% CI ((1.42, 1.65) and p =
0.0007). From the result of the meta-analysis and the direction of the effect
size relative to the line of no effect, it can be deduced that the meta-analysis
favours combination therapy against monotherapy. The significance of this result
can be explained further statistically by a p-value
Furthermore, since precision is largely driven by sample size, the study conducted by Tuomilehto et al. (2008) [20], with a weight of 899 and RR = 1.50 (1.33, 1.69) will generally have a larger sample size compared with smaller study by Makita et al. (2009) [17] with RR = 11.00 (1.51, 80.28). Thus, the weight of any study indicates how much influence an individual study exerts on the overall effects of the systematic analysis. On the other hand, the wider the confidence interval as demonstrated by the wider line, the lesser the precision of the study.
The heterogeneity test using I2 was another statistical tool used in a systematic review to show the variation in study outcomes between studies. At I2 = 66% (Fig. 3), it represents a moderate heterogeneity which signifies that the selected studies were homogenous. The summary of the studies represented by the diamond shape does not cross the line of no effect located within the direction of the intervention arm indicating statistical validity of the meta-analysis.
Fig. 5 depicts meta- analysis result performed to ascertain the adverse effect
of ARB/HCTZ versus ARB monotherapy after administration. The meta-analysis
included four studies that generated a total of 2778 participants pooled from
both the intervention and the control group. The result was not statistically
significant, with RR = 0.99 (0.66, 1.46) 95% CI with a p = 0.22. The
forest plot indicated a significantly high degree of heterogeneity at I2 =
78% which can again be attributed to sampling errors originating from selection
bias. While the direction of effect size from both studies of Gleim et
al. (2006) [16] and Barrios et al. (2007) [14] favoured the control arm
against the intervention; however, the two remaining studies conducted by
Bönner et al. (2008) [15] and Rump et al. (2011) [18] whose
confidence interval was crossing the line of no effect. Similarly, as observed
from the position of the summary of the effect size coming in contact with the
line of no effects, with a p-value
The commonest adverse events associated with ARB/HCTZ are orthostatic hypotension, dizziness, headache and fatigue which are comparable to those associated with ARB monotherapy [21, 22]. This therefore supports the use of ARB/HCTZ over ARB alone in the management of hypertension especially in uncontrolled cases.
Similar research conducted by Edes [23] on the effectiveness of candesartan combined with HCTZ compared to monotherapy revealed that the intervention was effective in lowering the participants’ baseline blood pressure which agreed with the outcome of this research. This finding was further corroborated by that of Ma et al. [24] who revealed that ARB/HCTZ combination therapy is more efficacious for controlling blood pressure, with a comparable safety outcome to ARB monotherapy when low-dose HCTZ is used. Similarly, Filipova et al. [25] carried out a systematic review of alternative drugs to combine with ARB and discovered that despite the existence of several diuretics, ARB is most frequently combined with hydrochlorothiazide which is reflected in the result of the meta-analysis of this study of treatment of hypertension, combining ARB with thiazide diuretics is both extremely effective and well tolerated. When necessary, this combination might be administered as the first therapy or later during treatment. Despite the availability of several diuretics, the ARB is most frequently paired with hydrochlorothiazide. According to the safety profile as discovered by Flack [26], the addition of HCTZ did not affect the rate of adverse events, which is consistent with the outcome of this study which found that the combination of ARB and HCTZ had a higher potency but a comparable safety profile.
The ARB/HCTZ combination therapy was effective in lowering and controlling blood pressure when compared to ARB monotherapy with comparable adverse drug effect reported by the participants. Health workers should therefore recommend ARB/HCTZ combination therapy for patients with uncontrolled hypertension to improve treatment outcomes especially as ARBs reduce cardiovascular risks associated with hypertension such as stroke, heart and renal diseases.
While this research stated some clinical importance of combined antihypertensive drug over monotherapy in the fight against elevated blood pressure, however in extreme conditions, a significant percentage of patients will require a triple therapy combination to achieve the BP goal. It will be a welcome idea in the foreseeable future for this grey area of study to be exploited. Additionally, all the RCTs employed in carrying out this study was all conducted in the western world, it will be interesting to repeat this research using participants from other parts of the world especially of Africa origin to understand how race, social class and age may affect the study.
The datasets were deposited in the archives of Chester University England.
AOA and COE designed the research study. AOA and COE performed the literature search. JC, IBO and AOA analysed the data. AOA and COE reviewed the work and evaluated the quality of each featured paper. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The review did not involve direct primary trials; however, an ethical application was filed and obtained from the research and ethical committee of the Faculty of Health and Social Care, University of Chester (FRC1222-580).
The authors wish to acknowledge the different databases used for this work such as Cochrane Library, PubMed, CINAHL, Embase, Medline, and the Web of Science.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.jmcm0701001.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






