1 Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI 53226, USA
2 Department of Radiology, Medical College of Wisconsin, Milwaukee, WI 53226, USA
Abstract
Lesions of the central nervous system (CNS) can present with numerous and overlapping radiographical and clinical features that make diagnosis difficult based exclusively on history, physical examination, and traditional imaging modalities. Given that there are significant differences in optimal treatment protocols for these various CNS lesions, rapid and non-invasive diagnosis could lead to improved patient care. Recently, various advanced magnetic resonance imaging (MRI) techniques showed promising methods to differentiate between various tumors and lesions that conventional MRI cannot define by comparing their physiologic characteristics, such as vascularity, permeability, oxygenation, and metabolism. These advanced MRI techniques include dynamic susceptibility contrast MRI (DSC), diffusion-weighted imaging (DWI), dynamic contrast-enhanced (DCE) MRI, Golden-Angle Radial Sparse Parallel imaging (GRASP), Blood oxygen level-dependent functional MRI (BOLD fMRI), and arterial spin labeling (ASL) MRI. In this article, a narrative review is used to discuss the current trends in advanced MRI techniques and potential future applications in identifying difficult-to-distinguish CNS lesions. Advanced MRI techniques were found to be promising non-invasive modalities to differentiate between paraganglioma, schwannoma, and meningioma. They are also considered promising methods to differentiate gliomas from lymphoma, post-radiation changes, pseudoprogression, demyelination, and metastasis. Advanced MRI techniques allow clinicians to take advantage of intrinsic biological differences in CNS lesions to better identify the etiology of these lesions, potentially leading to more effective patient care and a decrease in unnecessary invasive procedures. More clinical studies with larger sample sizes should be encouraged to assess the significance of each advanced MRI technique and the specificity and sensitivity of each radiologic parameter.
Keywords
- BOLD
- fMRI
- perfusion-MRI
- DWI
- ASL
- (DCE) MRI
- MRI (DSC)
- paraganglioma
- schwannoma
- neurofibromatosis type 2 (NF2)
- meningioma
- glioblastoma multiforme (GBM)
- lymphoma
- metastases
- glioma
- pseudoprogression
Neurological tumors differ significantly in their microstructure, molecular constructs, common anatomic locations, and classic features on conventional imaging [1]. However, their imaging features and anatomic locations can overlap and are not specific enough for diagnosis [2, 3]. For these reasons, a definitive diagnosis can often only be achieved using biopsy for histopathological confirmation, which is invasive and challenging in some locations [1]. Recently, many advanced magnetic resonance imaging (MRI) techniques have been used for tumor differentiation by assessing the internal biological features of different tumors, such as their vascularity, cellularity, oxygenation, and microstructure [4, 5, 6]. This provides non-invasive, rapid novel techniques to differentiate between neurologic tumors, guide treatment plans, and avoid unnecessary surgeries.
This article aims to provide a review of the use of advanced MRI techniques in neurologic tumor differentiation and outline advances underlying current evidence. To our knowledge, this is the first review of the potential of advanced MRI techniques in assisting diagnosis of neurologic tumors.
In contrast to conventional MRI, which delineates the anatomical structures and shows gross changes in the structure of the tumor, advanced MRI techniques represent dynamic physiological properties of tissue, which may be helpful in differentiating undiagnosed lesions based on properties including vascularity, cellularity, and metabolism [7, 8, 9]. The term advanced MRI techniques is used throughout this article to refer to dynamic contrast-enhanced (DCE) MRI, dynamic susceptibility contrast (DSC) MRI, diffusion-weighted imaging (DWI), arterial spin labeling (ASL) MRI, and blood oxygen level-dependent functional MRI (BOLD fMRI). In Figs. 1,2, we present different MRI images of two cases with different brain lesions including right frontal lobe oligodendroglioma (WHO grade II) and left lateral ventricle cavernous malformation. These two figures demonstrate the main differences between conventional MRI which delineates the anatomical borders of the brain lesions and advanced MRI techniques which demonstrate the internal physiologic features of different tumors, such as their vascularity, cellularity, perfusion, oxygenation, and microstructure.
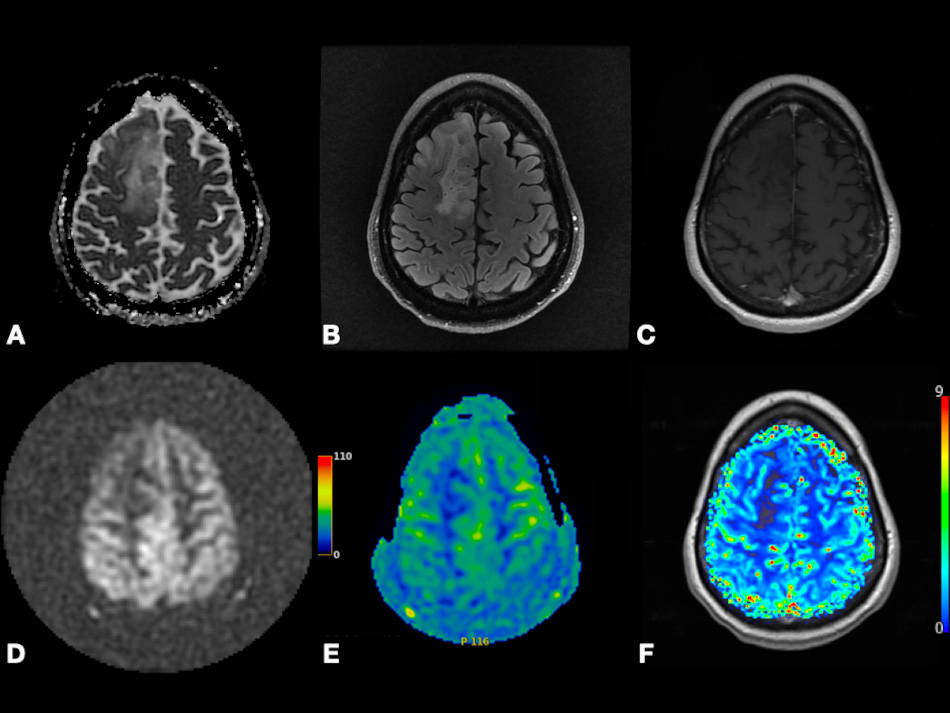 Fig. 1.
Fig. 1.Right frontal lobe oligodendroglioma (WHO Grade 2). Axial ADC map (A), FLAIR (B), and post-contrast T1-weighted (C) images depicting non-enhancing tumor with T2 prolongation in gray and white matter and increased diffusivity (i.e., shine-through artifact). Axial pcASL source data (D), color-coded CBF map (E) from pcASL data, and color-coded rCBV map (F) from DSC data depicting no hyperperfusion. Abbreviation: ADC, Apparent diffusion coefficient; FLAIR, Fluid attenuated inversion recovery; pcASL, Pseudo-Continuous Arterial Spin Labeling; CBF, cerebral blood flow; rCBV, relative cerebral blood volume; DSC, Dynamic susceptibility contrast MR imaging.
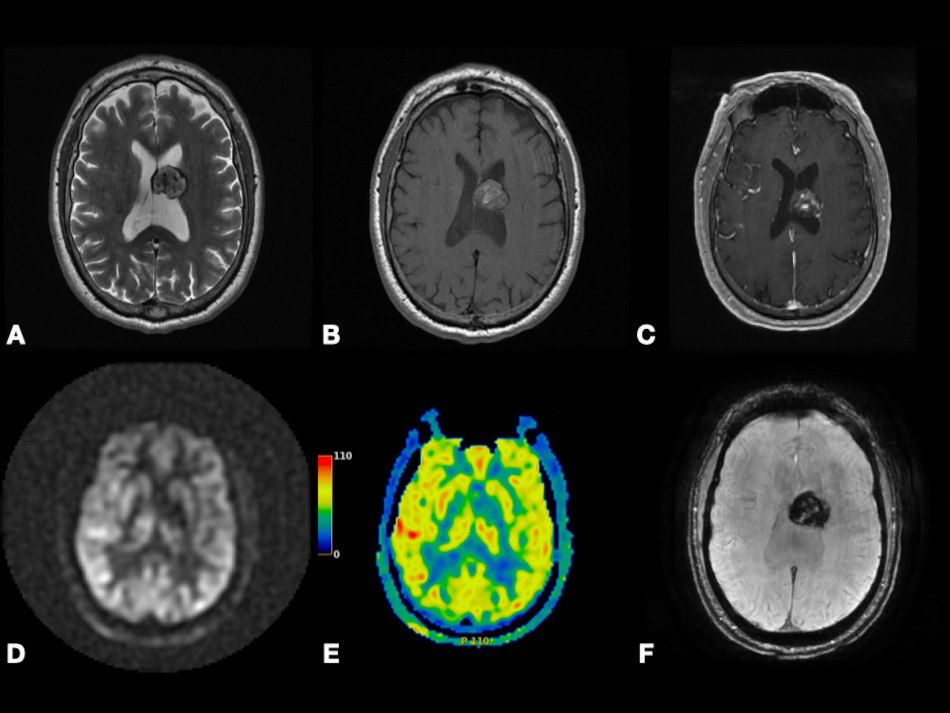 Fig. 2.
Fig. 2.Left lateral ventricle cavernous malformation. Axial T2-weighted (A), pre- and post-contrast T1-weighted (B,C), and SWI (F) images depicting lesion with heterogenous enhancement, heterogenous predominantly hyperintense signal, peripheral hemosiderin rim, and extensive blooming. Axial pcASL source data (D) and color-coded CBF map (E) depicting no hyperperfusion. Abbreviations: SWI, Susceptibility weighted imaging; pcASL, Pseudo-Continuous Arterial Spin Labeling; CBF, cerebral blood flow.
Perfusion MRI or perfusion-weighted imaging (PWI) techniques provide information about hemodynamic parameters such as cerebral blood volume, cerebral blood flow, and transit time by following the temporal passage of specific particles through the microvascular bed of the lesion of interest. Perfusion MRI sequences are considered relatively advanced tools that have been used recently for differentiation between various neurological and non-neurological tumors and include 3 main techniques: DCE-MRI, DSC-MRI, and ASL [8, 10, 11].
In contrast to DCE and DSC techniques that use gadolinium particles as an exogenous tracer, ASL quantifies cerebral blood perfusion by labeling arterial blood water molecules magnetically as endogenous tracers [10, 12]. By acquiring a control image before the arrival of the labeled water protons and the subtracted difference between the tagged and control images, ASL visualizes cerebral perfusion with avoidance of cerebral blood flow (CBF) overestimation [13, 14].
DSC-MRI relies on the temporal measure of signal changes during the injected paramagnetic gadolinium particles’ passage [15, 16]. The qualitative nature of DSC results is considered a severe limitation [17]. For obtaining quantitative results, the technique is extended by Rempp et al. [18]. Gadolinium particles decrease the signal intensity of T2 images so that the changes in gadolinium concentration can infer three important parameters including cerebral blood flow (CBF), cerebral blood volume (CBV), and the mean transit time (MTT) by applying tracer kinetic theory [19, 20]. CBF represents the blood volume passing through a specific region of brain tissue per unit of time, while CBV represents the blood volume that occupies a specific brain region [15]. It is usually termed relative cerebral blood volume (rCBV) as the arterial input function is not measured in most cases [21]. In the case of brain tumors, rCBV is considered the ratio between CBV in the tumor and CBV in the contralateral white matter [22]. MTT equals CBV divided by CBF and represents the average time for a gadolinium particle to pass through the vascular bed of the lesion or region of interest [15].
DCE-MRI quantifies the pharmacokinetics of the injected gadolinium particles as
illustrated in Fig. 3 [23]. Gadolinium passage increases the signal intensity of
T1, so T1 signal intensity changes before, during, and after administration can
indicate permeability and vascularity of the lesion of interest [24, 25]. By
applying the pharmacokinetic model described by Tofts et al. [26],
quantitative parameters can be measured like fractional plasma volume
(V
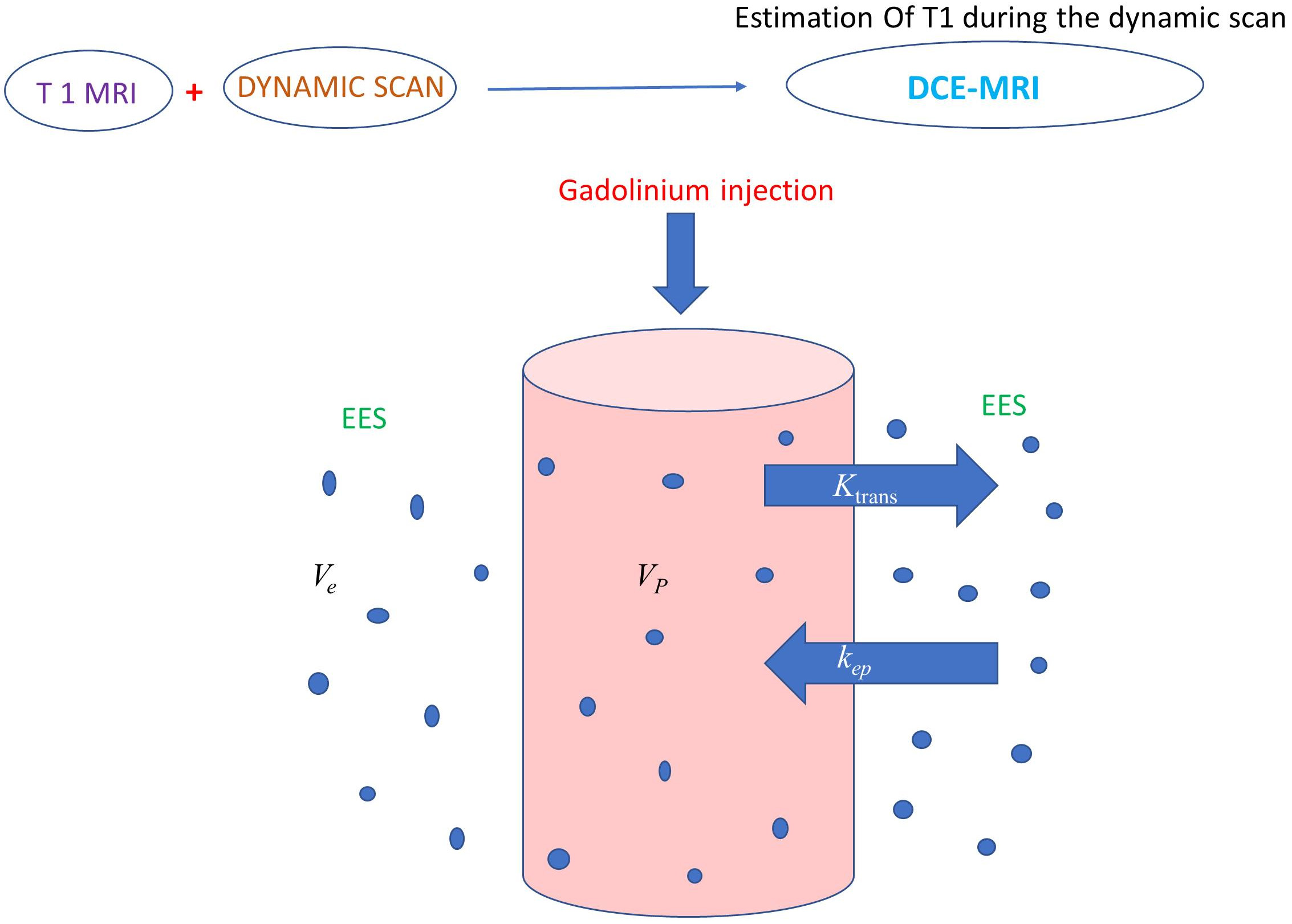 Fig. 3.
Fig. 3.Illustration showing a two-compartment model (plasma space and
extravascular and extracellular space) to calculate gadolinium pharmacokinetic
parameters. DCE-MRI involves rapid multiple T1 images before, during, and after
gadolinium administration to quantify microvascular permeability.
V
High temporal and spatial resolution can be provided by continuous 3D data acquisition through a new DCE technique called Golden-Angle Radial Sparse Parallel (GRASP) MR imaging [29, 30, 31]. GRASP has the same qualitative, semiquantitative, and quantitative parameters as DCE. Using DCE and GRASP, there are three types of time-intensity curve (TIC) patterns representing qualitative parameters as illustrated in Fig. 4. TIC type (1) represents rapid wash-in followed by continuous persistent enhancement representing slower wash-in without washout, while TIC type (2) demonstrates rapid wash-in followed by a plateau, and TIC type (3) shows rapid wash-in followed by slow washout [29, 30].
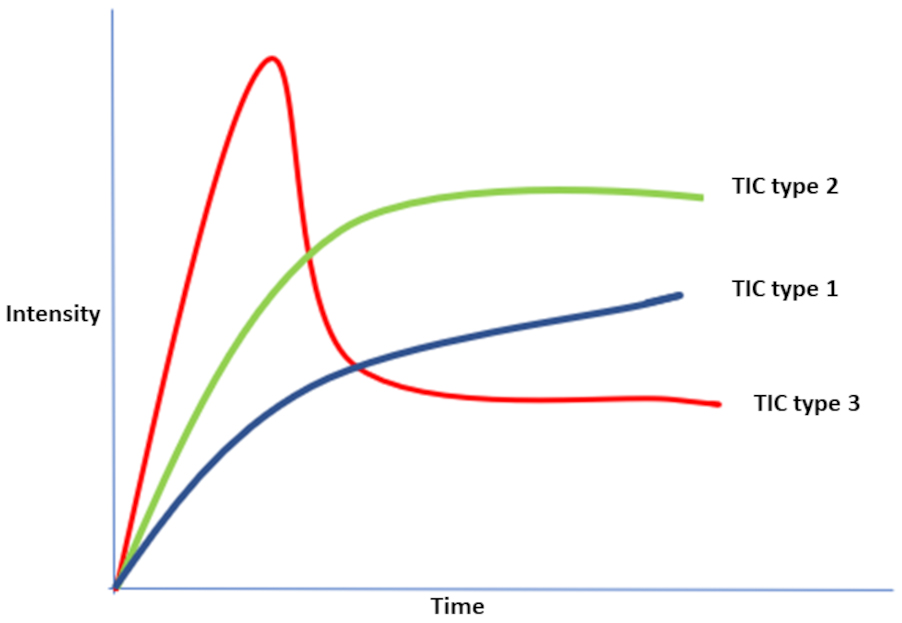 Fig. 4.
Fig. 4.Illustration showing the three possible types of TIC patterns representing qualitative parameters produced by DCE or GRASP. TIC type (1) represents rapid wash-in followed by continuous persistent enhancement representing slower wash-in without washout, while TIC type (2) demonstrates rapid wash-in followed by a plateau, and TIC type (3) shows rapid wash-in followed by slow washout. Abbreviations: DCE-MRI, dynamic-contrast enhanced MRI; TIC, time intensity curve; GRASP, Golden-Angle Radial Sparse Parallel MR imaging.
DWI depicts the water molecules’ diffusion in biological tissues, thus allowing diffusion process mapping [32]. It can reflect cellular membrane integrity and tissue cellularity [27]. DWI should be performed at two or more b values (b-value is a factor reflecting the gradients’ time and strength to generate DWI images). Apparent diffusion coefficient (ADC) is a quantitative parameter reflecting water molecules’ diffusion ability [33]. It is derived from the gradient of the signal intensity (SI) log between at least two b-values as illustrated in Fig. 5. In the case of presence of factors restricting water diffusion, the ADC value is decreased [9].
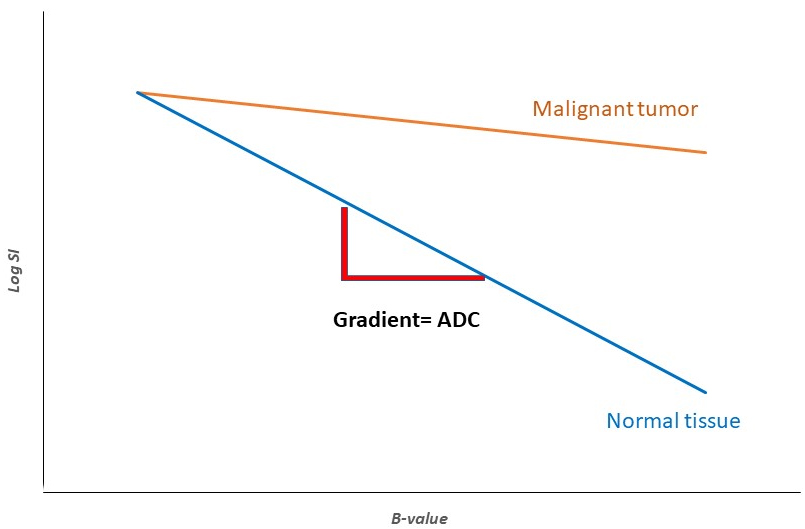 Fig. 5.
Fig. 5.Illustration showing ADC, a quantitative parameter reflecting water molecules’ diffusion ability using DWI. The ADC is derived from the gradient of the MRI signal intensity and at least two b-values. It is influenced by cellular membrane integrity and tissue cellularity. In the case of the presence of factors that can restrict water diffusion, such as fluid viscosity and high cellularity, DWI demonstrates higher levels of SI and subsequently, lower levels of ADC. Abbreviations: SI, signal intensity; ADC, apparent diffusion coefficient; DWI, diffusion-weighted MRI.
BOLD-MRI is a non-invasive, widespread, low-cost MRI technique demonstrating the
temporal regional changes in brain metabolism. It has commonly been used in
cognitive neuroscience, psychiatry, and psychology research [34, 35, 36, 37]. Increased
metabolism in a specific region in the brain following increased activity in this
area induces production of many chemicals (CO
Paragangliomas (PGs) are rare, slowly growing neuroendocrine tumors arising from neural crest cells that are present in any autonomic ganglia with the carotid body, middle ear, and jugular foramen as the most common sites [42, 43, 44]. Carotid body and cervical sympathetic tumors present with a painless neck mass, and also possibly Horner’s syndrome. Jugulotympanic PG, also known as glomus tympanicum and glomus jugulare, can present with hearing loss, tinnitus, and compression on the jugular foramen contents including the glossopharyngeal nerve, vagus nerve, and accessory nerve [45, 46]. In a few cases of PGs, there is catecholamine secretion that can cause hypertension, palpitations, tachycardia, and headache, although all PGs can theoretically secrete catecholamines [47]. On MRI, PGs are visualized as heterogeneously enhancing tumors with salt and pepper appearance and with necrotic or cystic changes [48].
Combination of avid heterogenous enhancement, markedly hyperintense T2-weighted signal with scattered flow voids (salt and pepper appearance), and affecting specific locations including cochlear promontory in middle ear cavity, jugular fossa, and carotid bifurcation favors PG [45, 46, 47, 48]. Splaying of carotid bifurcation favors glomus caroticum (carotid body tumor). However, anterior displacement of carotid bifurcation can be seen with glomus vagale or schwannoma (SC) [45, 46, 47, 48].
Meningiomas (MGs) are the most common extra-axial brain tumors. They arise from the arachnoid cells and are more common in middle-aged women [49]. Their clinical picture varies according to the compression effect on different locations. The most commonly reported symptom is headache [50, 51, 52]. Their most common location is parasagittal and parafalcine areas, followed by convexity, tuberculum sellae, sphenoid ridge, and olfactory groove, respectively [53].
MGs show isointensity to hypointensity on T1, hyperintensity on T2, and post-contrast enhancement. Rarely, it can show cystic changes and internal hemorrhage. Calcifications may also be seen inside the tumor [50, 51, 54, 55]. The cerebrospinal fluid (CSF) cleft sign surrounding extra-axial tumors like MGs, PGs, and SCs is hypointense on T1 because of the CSF accumulation between the lesion and the brain parenchyma, and the blood vessels trapped between the tumor and the brain [50, 55, 56]. This sign is not specific and can be also found in many other lesions, such as glioma, metastasis (MT), and lymphoma [57, 58, 59]. Dural tail on MRI, hyperostosis and dilatation of a paranasal sinus (pneumosinus dilatans) favor MG. MGs are infrequently calcified. Also, bone erosion, invasion, and/or remodeling may be seen with MGs [50, 51, 52, 53, 54, 55, 56, 57, 58, 59].
SCs are benign tumors originating from Schwann cells wrapping the nerve sheath. They are considered the second most common extra-axial brain tumor after MGs. Theoretically, SCs can originate from any peripheral and cranial nerve except the optic nerve as it is myelinated by oligodendrocytes, not Schwann cells. Vestibular and trigeminal nerves are the most affected cranial nerves respectively [50, 60, 61, 62, 63, 64, 65].
Vestibular SCs can grow initially inside the internal auditory canal and then extend into the cerebellopontine angle; it is characterized by absence of CSF signal in the internal auditory canal on MRI imaging. SCs show isointensity to hypointensity on T1, hyperintensity on T2, and post-contrast enhancement. It can show heterogeneous post-contrast enhancement and signal intensity which is suggestive of cystic changes and internal hemorrhage [50, 66, 67]. The main differential diagnosis of SC in the cerebellopontine angle is MG. The common differences between MG and SC on conventional MRI are summarized in Table 1 [50, 66, 67]. SCs occupying the foramen magnum usually originate from the glossopharyngeal nerve (CN IX) [50]. Additionally, SCs located in the carotid sheath can only originate from the vagus nerve (CN X) [50]. At both locations, it is difficult to differentiate between SCs versus PGs. Combination of location along an expected course of a cranial nerve and widening or remodeling of adjacent bones, such as porus acousticus of internal auditory canal (IAC) and foramen oval, favors SCs [50, 66, 67].
| Feature | Meningioma | Schwannoma |
|---|---|---|
| Internal auditory canal involvement | Rare | Almost always |
| Centered around internal auditory canal | No, eccentric | Yes |
| Hemorrhage | Rare | More common |
| Cystic/necrotic changes | Rare | More common |
| Calcifications | Possible | Less common |
| Dural tail | Frequent | Rare |
| Bony reaction | Osteolysis or hyperostosis | Rare |
| Angle made with dura | Obtuse | Acute |
Although on MRI, PGs are visualized as heterogeneously enhancing tumors with salt and pepper appearance with necrotic or cystic changes, SCs can share similar features [48, 68, 69]. Subsequently, PGs can be misdiagnosed and cause unwanted surprises during and after surgery. The final diagnosis usually relies on their histopathologic features, although the risk of excessive hemorrhage and adrenergic crisis associated with biopsy of highly vascularized PGs and the risk of injury to neurovascular structures in the jugular foramen and carotid sheath make biopsy challenging [70, 71]. Therefore, finding a rapid, non-invasive, reliable way to differentiate between these two tumors is ideal.
Recently, advanced MRI techniques have been utilized to differentiate between
various tumors. Using DSC, Ota et al. [72] found a significant increase
in normalized rCBV and normalized rCBF in intracranial PGs compared to SCs. On
DCE for neck mass imaging, Malla et al. [73] found that wash-in rate,
wash-out rate, V
Using GRASP, Pires et al. [75] and Demerath et al. [76] found
that all PGs showed TIC type 3 (rapid wash-in followed by slow washout) which can
be explained by PGs’ hypervascularity and arteriovenous shunting [77]. On the
other hand, SCs showed TIC type 1 (rapid wash-in followed by continuous slower
wash-in without washout) which can be explained by the increased permeability to
the EES caused by the small thin-walled blood vessels [75, 76, 78]. Pires
et al. [75] found that V
On DWI, two retrospective studies of intracranial tumors did not find a significant difference in ADCmean between PGs and SCs [72, 74]. Only one study showed significantly lower ADCmean values in PGs compared to SCs; this prospective study included only neck masses [73]. The insignificant difference in ADC values in intracranial tumors is likely because SCs have various microstructures like Antoni A and Antoni B histologic patterns [79]. Antoni A areas are cellular and have high mitotic activity, while Antoni B areas are hypocellular and contain deformed blood vessels with hyalinized walls [80]. SCs size may also play a role as cystic changes are more likely to develop in larger SCs and these cystic changes might increase ADC values [74, 81]. Based on these studies, ADC values may not be a reliable radiologic biomarker to differentiate between PGs and SCs.
A mutation in the neurofibromatosis type 2 (NF2) gene on chromosome 22 causes an autosomal dominant multiple-tumor syndrome called NF2 [82]. It is associated with the development of many nervous system tumors including SCs, MGs, and gliomas [83, 84, 85, 86]. Although bilateral SC is considered a characteristic that is nearly diagnostic of NF2 and affects 95% of NF2 patients, NF2 can present with unilateral SC [87].
Using DSC, Ota et al. [72] also found a significant increase in
normalized rCBV and normalized rCBF in NF2-related SCs compared to
sporadic SCs. Using DCE, another study showed that K
Using DWI, a significant decrease in mean normalized ADC in NF2-related SCs compared to sporadic SCs is observed [72, 88]. This may be explained by the features of NF2-associated SCs including presence of hypercellular foci, whorl patterns, and lobular patterns, leading to restricted fluid diffusion in NF2- associated SCs; these features are not typically seen in sporadic SCs [91, 92].
Differential diagnosis of parasellar tumors includes PGs, MGs, and pituitary adenoma. Rarely, PGs can also occupy the parasellar region [2]. On conventional MRI, it is difficult to differentiate between these tumors [2]. Similarly, it is difficult to differentiate between MGs and vestibular SCs in the cerebellopontine angle and internal auditory canal [93]. Advanced MRI techniques show a promising non-invasive rapid modality to differentiate between MGs and SCs or PGs.
Using DCE, Ota et al. [74] revealed that V
On DWI, Pavlisa et al. [94] found that ADCmean in SCs is significantly higher than both typical and atypical MGs. However, Ota et al. [74] found that there is no significant difference in ADC values between SCs versus MGs, PGs versus MGs, or SCs versus PGs. Thus, more clinical studies containing a large sample size are needed to evaluate the significance of DWI to differentiate between MGs and other extra-axial tumors. In Fig. 6, we present a case of right cerebral convexity atypical MG. The axial ADC map shows reduced diffusivity, while axial Pseudo-Continuous Arterial Spin Labeling (pcASL) source data and grayscale CBF map show marked heterogenous hyperperfusion. This demonstrates that MGs have high permeability and low diffusivity as mentioned in the literature. All of the previously mentioned studies differentiating between PG, SC, and MG using DCE, DSC, GRASP, and DWI are mentioned in Table 2 (Ref. [72, 73, 74, 76, 75, 88, 94]).
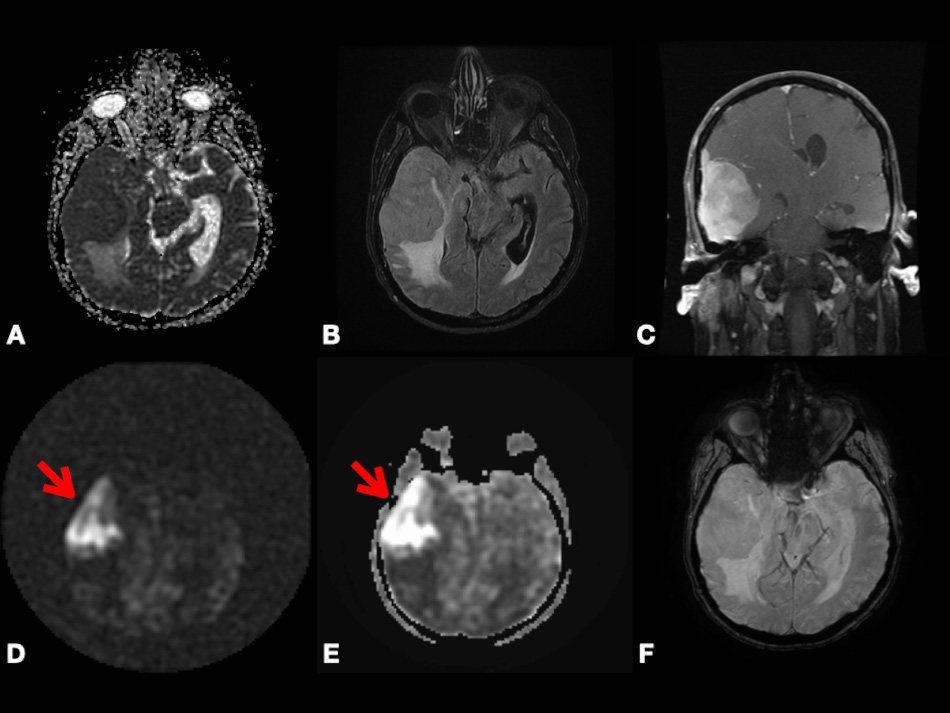 Fig. 6.
Fig. 6.Right cerebral convexity atypical meningioma (WHO Grade II). Axial ADC map (A), axial FLAIR (B), coronal fat-suppressed post-contrast T1-weighted (C), and axial SWI (F) images depicting large avidly enhancing plaque-like non-calcified mass with dural tail, reduced diffusivity, extensive vasogenic edema and mass effect with uncal herniation and ventricular trapping. Axial pcASL source data (D) and grayscale CBF map (E) depicting marked heterogenous hyperperfusion (red arrows). Abbreviations: ADC, Apparent diffusion coefficient; FLAIR, Fluid attenuated inversion recovery; SWI, Susceptibility weighted imaging; pcASL, Pseudo-Continuous Arterial Spin Labeling; CBF, cerebral blood flow.
| Re. | Year | Study design | Number of patients | Location | Age mean/range | M/F | Histopathology | Type of technique | Significant parameters |
| [72] | 2022 | R | 41 | Intracranial-Infratentorial | 7–74 | 18/23 | PG (12)/SC (29) | DSC | nrCBV, nrCBF |
| [72] | 2022 | R | 29 | Intracranial-Infratentorial | N/A | N/A | Sporadic SC (19)/NF2-related SC (10) | DSC, DWI | nrCBV, nrCBF, nADCmean |
| [73] | 2021 | P | 40 | Neck | 32.65 +/- 12.36 | 24/16 | PG (33)/SC (15) | DCE, DWI | Wash-in rate, wash-out rate, K |
| [74] | 2021 | R | 57 | Cerebellopontine angle, jugular foramen | 51.2 +/- 17.8 | 16/41 | MG (35)/PG (30) /SC (20) | DCE, DWI | K |
| [88] | 2021 | R | 28 | Vestibular | 11–67 | 19/9 | Sporadic SC (23)/NF2-related SC (5) | DCE, DWI | K |
| [94] | 2008 | P | 41 | N/A | 18–83 | 19/22 | SC (15)/MG (26) | DWI | ADC |
| [76] | 2020 | R | 11 | Head and neck | N/A | N/A | PG (6)/SC (5) | GRASP | TIC |
| [75] | 2021 | R | 30 | Jugular foramen | 49.5/26–79 | N/A | PG (22)/SC (8) | GRASP | TIC/V |
| Re., reference; R, retrospective; P, prospective; M, male; F, female; N/A, not
applicable; SC, schwannoma; PG, paraganglioma; NF2, neurofibromatosis type 2;
DSC, dynamic susceptibility contrast MRI; DWI, diffusion-weighted MRI; nrCBV,
normalized relative cerebral blood volume; nrCBF, normalized relative cerebral
blood flow; nADCmean, normalized mean apparent diffusion coefficient; MG,
meningioma; DCE, dynamic contrast-enhanced; V | |||||||||
Anywhere in the central nervous system, glial cells can develop into gliomas, which are considered the most common primary brain tumors in adults [95]. Finding non-invasive biomarkers, such as serum long non-coding RNAs and micro RNAs to diagnose gliomas, differentiate them from post-radiologic changes and MT, and identify glioma grading is a trend now [95]. Advanced MRI techniques are also promising non-invasive modalities to differentiate gliomas from lymphoma, post-radiation changes, pseudoprogression, demyelination, and MT.
Primary central nervous system lymphoma (PCNSL) is a non-Hodgkin’s lymphoma located in the central nervous system including the spinal cord, brain, leptomeninges, and eyes. It presents often as a single brain mass in both immunocompetent and immunocompromised patients [96, 97]. No survival benefit is observed after surgical resection, and surgery is associated with higher risks; thus, the role of surgery is limited to biopsy [98, 99]. High doses of intravenous methotrexate is an effective treatment. Additionally, corticosteroids can decrease tumor-surrounding edema [100, 101]. Periventricular location, hyperdensity on CT, and T2 shortening (hypointense T2-weighted signal) on MRI favor lymphoma. However, on conventional MRI, PCNSL differentiation from high-grade glioma is difficult and sometimes impossible due to its diffuse infiltrative nature and occasional presence of atypical features like necrosis, hemorrhage, or heterogeneous enhancement [102, 103]. Given the significant survival benefit with surgical resection of a glioma (compared to no benefit with lymphoma), as well as the desire to start corticosteroids as soon as possible, there would be a substantial benefit to better differentiate the two lesions rapidly and non-invasively [95, 101].
On DSC, many studies have shown that CBV is significantly lower in PCNSL
compared to gliomas [104, 105, 106, 107, 108, 109]. Additionally, other studies demonstrate that the
maximum rCBV ratio is significantly lower in PCNSL than in gliomas [110, 111].
Both corrected CBV ratio and uncorrected CBV ratio are significantly lower in
PCNSL [112, 113, 114]. Another study demonstrated the ability of CBF to differentiate
between PCNSL and gliomas [115]. Using DCE, Kickingereder et al. [116]
found that median K
Many vascular changes causing increased permeability are observed by electron
microscopy in PCNSLs, such as thinned endothelial cells, and increased
fenestrations between capillary endothelial cells [119]. Higher vascular
permeability in PCNSLs caused by blood brain barrier (BBB) disruption can be the
cause of increased permeability parameters like K
Some demyelinating lesions are exceedingly difficult to differentiate from tumors. Due to BBB disruption in some demyelinating lesions, these lesions can enhance with contrast and can be misdiagnosed as potential malignant lesions [122, 123]. Theoretically, advanced MRI techniques can differentiate between demyelinating lesions and gliomas by comparing vascularity and permeability.
Gliomas, especially high-grade ones, are expected to have higher CBV because of their hypercellularity, high metabolism, and neo-angiogenesis [124]. In 2008 Hourani et al. [122] demonstrated that rCBV is significantly higher in high-grade gliomas than in demyelinating lesions. However, in 2011 Blasel et al. [125] found that some autoimmune demyelinating lesions can share high levels of rCBV like high-grade gliomas, leading to misdiagnosis and affecting the diagnostic accuracy of DSC. In 2017 Hiremath et al. [126] found that combining DSC parameters with diffusion tensor metric parameters improved DSC diagnostic accuracy.
The most aggressive gliomas are GBM. Their standard treatment includes surgical removal, chemotherapy, and radiotherapy [95]. After completing radiotherapy, enhancing lesions on conventional MRI can be challenging as these changes can represent true progression, radiation-induced pseudoprogression, or radiation necrosis [127, 128, 129]. In case of tumor recurrence, reoperation or changing chemotherapy can be necessary. In contrast, radiation-induced pseudoprogression is self-limited and can be treated conservatively with serial imaging [130, 131]. Radiation-induced pseudoprogression results from liquefactive necrosis of the injured area, fibrinoid deposition, and vascular hyalinization [130, 132]. It is crucial to differentiate between true progression and radiation-induced changes to avoid any unnecessary surgery or biopsy. Based on the fact that GBM is highly vascular and the radiation-induced changes are not, using vascularity and permeability parameters measured by advanced MRI techniques can easily differentiate between them [133, 134]. Using DSC, many studies have shown that CBV is significantly higher in GBM compared to pseudoprogression [133, 134, 135, 136].
In Fig. 7, we present a case of left temporal lobe GBM (WHO Grade 4) with false-positive perfusion imaging for recurrence. Using DSC, baseline images showed peripheral hyperperfusion. Four months later after the surgery, images showed enhancement and hyperperfusion concerning for recurrence; the patient underwent subsequent resection with no tumor on pathology. This case shows that using color-coded rCBV maps can be misleading as both tumor recurrence and pseudoprogression may show relatively increased perfusion. However, as we discussed in the earlier paragraph, CBV is found to be significantly higher in GBM recurrence compared to pseudoprogression. This highlights the importance of having more studies comparing CBV between pseudoprogression versus recurrence to define a specific CBV value or threshold that can be associated with GBM recurrence. Calculating the specificity and sensitivity of this CBV value is also encouraged. This also highlights the importance of using quantitative parameters, such as tumoral and peritumoral CBV and CBF to compare between GBM and MTs as depending on observing hyperperfusion signals on the color-coded maps is subjective and can be misleading.
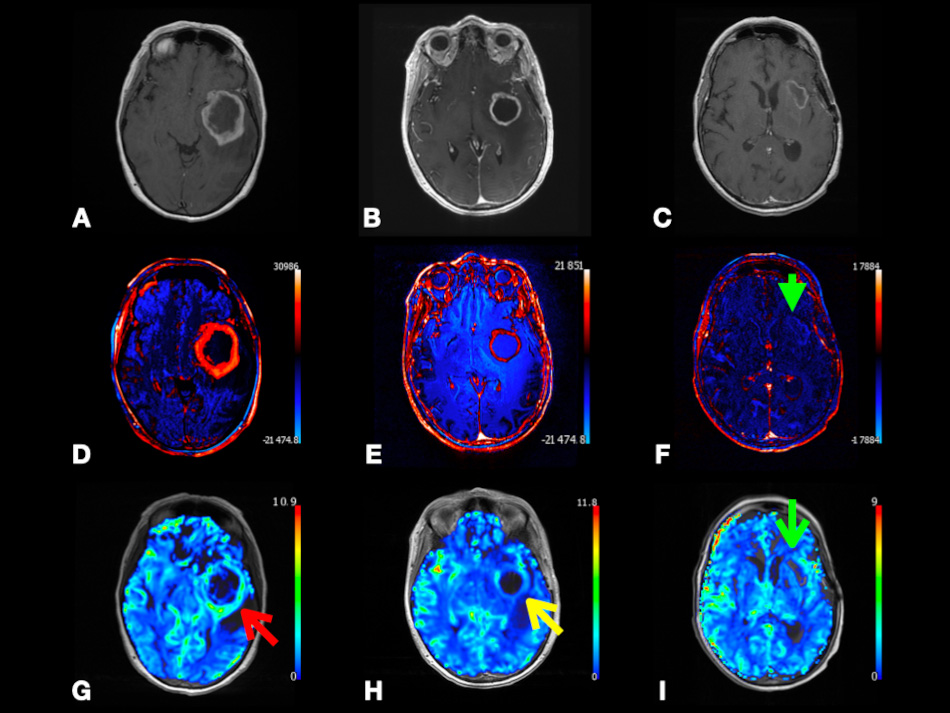 Fig. 7.
Fig. 7.Left temporal lobe glioblastoma (WHO Grade 4) with false-positive perfusion imaging for recurrence and subsequent treatment-mediated response. Axial post-contrast T1-weighted (A–C), color-coded subtraction maps (D–F), and color-coded rCBV maps (G–I) from DSC data. Baseline images (A,D,G) depicting enhancing mass with central necrosis and peripheral hyperperfusion (red arrow). Images obtained four months later (B,E,H) depicting enhancement and hyperperfusion (yellow arrow) concerning for recurrence; subsequent redo resection with treatment effect and no tumor on pathology. Images obtained eight months later (C,F,I) after temozolomide and Avastin depicting T1 shortening, resolved enhancement (green arrowhead), and resolved hyperperfusion (green arrow) at the superior margin of the expanded resection cavity compatible with treatment response. Abbreviations: rCBV, relative cerebral blood volume; DSC, Dynamic susceptibility contrast MRI.
Using BOLD fMRI, Muscas et al. [137] found that for both newly diagnosed GBM and radiation-induced necrosis lesions, the CVR values are impaired. Moreover, for the radiation-induced pseudoprogression lesions, the mean CVR values were significantly lower than those in the newly diagnosed GBM lesions. This can be explained by GBM’s higher vascularity, neo-angiogenesis, and disrupted BBB that leads to loss of regulative capacity and impaired CVR [137, 138, 139, 140]. Also, the nonreactive blood vessels surrounded by necrotic tissues and fibrinoid deposition can cause more impaired CVR [133, 136, 137]. They also found that for radiation-induced pseudoprogression, there is markedly dramatic CVR improvement in the immediate perilesional areas compared to the perilesional areas in the newly diagnosed GBM that show no significant improvement [137]. GBM’s hypermetabolism, neo-angiogenesis, and higher blood flow can cause perilesional vasodilation to bring more blood supply to the lesion. Moreover, the infiltrative and aggressive behavior of GBM can disrupt the existing perilesional blood vessels by the tumor cells surrounding the tumor. Thus, there is no apparent CVR improvement observed in the peritumoral area [137, 141, 142]. On the other hand, the radiation-induced lesions are focal inflammatory reactions with lower blood flow, so it causes rapid CVR normalization in the perilesional areas [133, 135, 137].
Of interest, Fierstra et al. [143] found that the altered intraoperative BOLD CVR in the peritumoral non-enhancing tissue predicted the exact location of the future tumor recurrence. This can help in achieving optimal tumor resection in the future to decrease the reoperation rate for patients with high-grade gliomas. In contrast to its widespread use in cognitive neuroscience research, very few studies discussed the potential of BOLD fMRI in tumor diagnosis and treatment. More clinical studies are encouraged to examine the BOLD fMRI ability to predict future tumor recurrence and prognosis and differentiate between different tumors.
It is easy to identify MTs from gliomas in the case of presence of systemic MTs or multiple cerebral lesions. However, when the cerebral MTs present as solitary lesions without systemic manifestations, it becomes difficult to differentiate between them [144]. Presence of multiple lesions, location at grey-white junction, and vasogenic edema favor metastases or infection over primary brain tumors. Additionally, large tumor size with disproportionately little vasogenic edema and mass effect favor a primary brain tumor over metastases. However, on conventional MRI, both GBM and MTs are sometimes similar and may show heterogenous appearance with ring enhancement surrounded by edema [144, 145]. As they have different management plans, accurate differentiation between GBM and MTs is mandatory.
On DSC, Calli et al. [146] found that maximum rCBV ratio is not
statistically significant between GBM and MT. On DCE, Lu et al. [117]
found that K
In Fig. 8, we present a case of right frontal lobe non-small cell lung cancer MT. Color-coded CBF map from pcASL data and color-coded rCBV map from DSC data show heterogenous hyperperfusion amongst nodules at super lesion margin and enhancing margins elsewhere. This demonstrates that MTs may show hyperperfusion using ASL or DSC. This highlights the importance of using quantitative parameters, such as tumoral and peritumoral CBV and CBF to compare between GBM and MTs as depending on observing hyperperfusion signals on the color-coded maps is subjective and can be misleading.
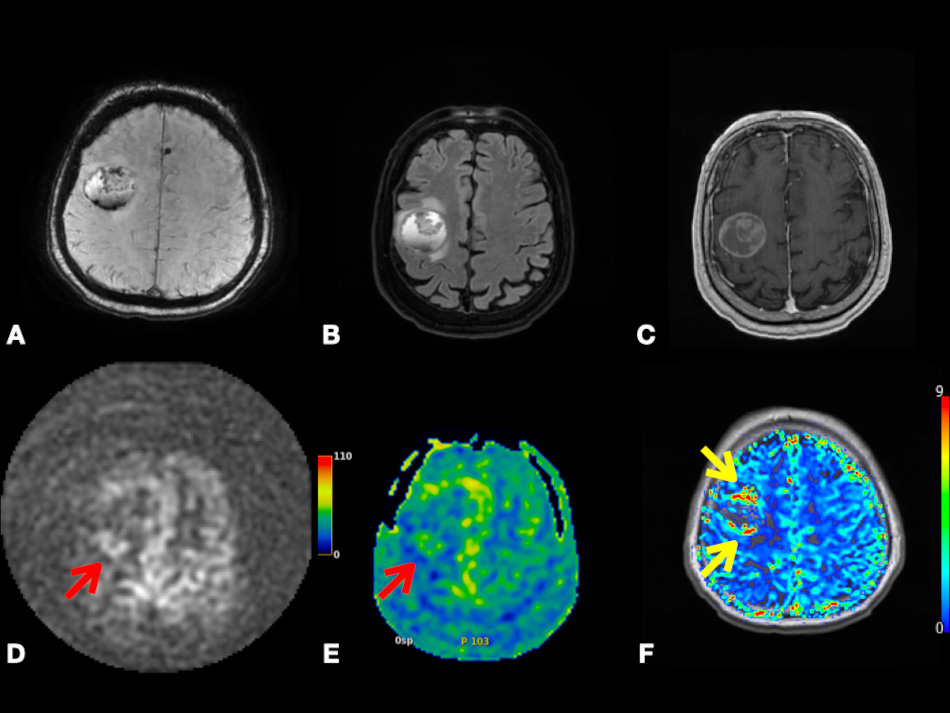 Fig. 8.
Fig. 8.Right frontal lobe non-small cell lung cancer metastasis. Axial SWI (A), FLAIR (B), and post-contrast T1-weighted (C) images depicting lesion with heterogenous enhancement, perilesional vasogenic edema, central necrosis, and intralesional hemorrhage. Axial pcASL source data (D), color-coded CBF map (E) from pcASL data, and color-coded rCBV map (F) from DSC data depicting heterogenous hyperperfusion amongst nodules at super lesion margin (red arrowheads) and enhancing margins elsewhere (yellow arrowheads). Abbreviations: SWI, Susceptibility weighted imaging; FLAIR, Fluid attenuated inversion recovery; pcASL, Pseudo-Continuous Arterial Spin Labeling; CBF, cerebral blood flow; rCBV, relative cerebral blood volume; DSC, Dynamic susceptibility contrast MR imaging.
Using DWI, both tumoral and peritumoral ADC are found to be significantly higher in MTs compared to gliomas [149]. This finding suggests that MTs cause more edema and fluid production compared to gliomas [150]. The higher degree of edema in MTs can be explained by the differences in the BBB characteristics between gliomas and MTs. MTs completely lack a BBB and subsequently, cause prominent capillary fenestrations, while the BBB disruption in GBM varies and as a result, the degree of permeability can vary from normal to increased [151, 152]. This can explain why ADC values are found to be higher in MTs. This increased fluid production and edema produced by MTs can also cause more compression on the microcirculation [153]. Eventually, the peritumoral rCBV decreases. This explains why rCBV is lower in MTs compared to gliomas. Table 3 (Ref. [14, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 149]) shows a summary of studies differentiating between glioma, lymphoma, and MTs using DCE, DSC, ASL, and DWI.
| Re. | Year | Study design | Number of patients | Age | M/F | Histopathology | Type of technique | Significant parameters |
|---|---|---|---|---|---|---|---|---|
| [104] | 2002 | R | 37 | N/A | N/A | HGG (21), LGG (8), LM (8) | DSC | rCBV ratio |
| [105] | 2019 | R | 145 | M = 53.3 | 75/70 | GBM (89), LM (56) | DSC | nCBV |
| Ra = 28–86 | ||||||||
| [106] | 2011 | R | 67 | N/A | 24/33 | GBM (26), MT (25), LM (16) | DSC | rCBV |
| [107] | 2014 | R | 60 | M = 54 | 33/27 | GBM (41), LM (19) | DSC, DWI | Maximum nCBV, and minimum ADC |
| Ra = 25.0–83.0 | ||||||||
| [108] | 2014 | R | 38 | N/A | 21/17 | HGG (26), LM (12) | DSC | rCBV |
| [109] | 2010 | R | 62 | M = 46 | 33/29 | GBM (28), MT (22), LM (12) | DSC | nCBV |
| Ra = 15–73 | ||||||||
| [110] | 2003 | N/A | 24 | N/A | N/A | GBM (12), LM (12) | DSC | rrCBV ratio |
| [111] | 2009 | R | 20 | Ra = 14–72 | 13/7 | HGG (11), LM (9) | DSC | maximum rCBV ratio |
| [112] | 2015 | R | 28 | N/A | 13/15 | GBM (18), LM (10) | DSC | cCBV ratio |
| [113] | 2013 | R | 35 | N/A | 27/8 | GBM (20), LM (15) | DSC | CBV ratio, cCBV ratio |
| [114] | 2018 | R | 22 | mean = 59.8 | 11/11 | HGG (14), LM (8) | DSC, DCE | cCBV, K |
| Ra = 7–86 | ||||||||
| [115] | 2006 | P | 79 | 57 |
43/36 | HHG, LGG, MT, LM | DSC | CBF, Peritumoral CBF |
| [116] | 2014 | R | 71 | N/A | N/A | GBM (60), LM (11) | DCE | Median K |
| [117] | 2016 | R | 75 | N/A | 40/35 | GBM (38), LM (16), MT (21) | DCE | Mean K |
| [118] | 2019 | P | 35 | N/A | 20/15 | HGG (21), LM (8), MT (6) | DSC, DCE | rCBF, V |
| [149] | 2004 | P | 26 | 25–76 | 12/14 | HGG (14), LM (12) | DWI, DSC | Peritumoral rCBV, peritumoral ADC |
| [14] | 2016 | R | 128 | 19–84 | 76/52 | GBM (89)/MT (39) | ASL | nCBV, nCBF |
| Re., reference; R, retrospective; P, prospective; M, male; F, female; N/A, not
applicable; GBM, glioblastoma; LM, lymphoma; MT, metastases; HGG, high grade
glioma; LGG, low grade glioma; DSC, dynamic susceptibility contrast MRI; DWI,
diffusion-weighted MRI; nCBV, normalized cerebral blood volume; nCBF, normalized
cerebral blood flow; rCBV, relative cerebral blood volume; rrCBV, regional
relative cerebral blood volume; cCBV, corrected cerebral blood volume; DCE,
dynamic contrast-enhanced; V | ||||||||
Advanced MRI techniques, such as DCE, DWI, ASL, DSC, and BOLD show promising methods to differentiate between various tumors and lesions that conventional MRI cannot define by comparing their physiologic characteristics, such as vascularity, permeability, oxygenation, and metabolism. Hopefully, these imaging modalities will help avoid invasive procedures, such as biopsy or traditional surgeries. More clinical studies with larger sample sizes should be encouraged to assess the significance of each advanced MRI technique and the specificity and sensitivity of each radiologic parameter.
CNS, Central Nervous System; FMRI, Functional MR Imaging; DCE, Dynamic
Contrast-Enhanced MR Imaging; DSC, Dynamic Susceptibility Contrast MR Imaging;
DWI, Diffusion-Weighted Imaging; ASL, Arterial Spin Labeling MR Imaging; BOLD,
Blood Oxygen Level-Dependent MR Imaging; CBF, Cerebral Blood Flow; CBV, Cerebral
Blood Volume; MTT, Mean Transient Time; rCBV, Relative Cerebral Blood Volume;
V
AME took the responsibility for conceptualization, designing the study, and interpreting the data. AME prepared the figures. RTB prepared radiological figures and their descriptions. RWT, DMA, and HH provided help and advice on presenting the data. AME wrote the initial draft. RWT, DMA, and HH participated in writing the final draft. HH provided supervision and advice. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.








