1 Yenepoya Research Centre, Yenepoya (Deemed to be University), University Road, Deralakatte, Mangalore, 575018 Karnataka, India
2 Department of Pulmonary Medicine, K S Hegde Medical Academy, Nitte (Deemed to be University), Deralakatte, Mangaluru, 575018 Karnataka, India
3 Department of Pulmonary Medicine, Yenepoya (Deemed to be University), University Road, Deralakatte, Mangalore, 575018 Karnataka, India
Abstract
Lung cancer is a prominent global health issue responsible for the highest fraction of cancer-related mortality. The disease burden has incited the investigation of associated molecular pathways, to explore better therapeutic possibilities. MicroRNAs are extensively studied in recent years for their pivotal role in the regulation of several tumorigenic pathways. MicroRNA-30 (miR-30) family is primarily investigated in case of non-small cell lung cancer (NSCLC) and has been found to play the role of a tumour suppressor. There are six members of miR-30 family: miR-30a, miR-30b, miR-30c-1, miR-30c-2, miR-30d and miR-30e. They regulate several imperative signalling pathways like p53, PI3K/AKT, resulting in the modulation of key carcinogenic events involving cell proliferation, apoptosis, metastasis, epithelial-mesenchymal transition, and drug resistance. Their altered levels are documented in NSCLC tissue and blood samples. They are suggested as biomarkers of disease progression and therapeutic outcomes in lung cancer. They possess immense therapeutic potential in the treatment of lung cancer and combat the emerging problem of drug resistance by modulating prime regulatory axes. However, there are many limitations in the existing studies, and additional research is required for the comprehensive understanding of pathways so that the tumour suppressive potential of miR-30 can be translated into clinical benefits. In this review, we present a deeper understanding of the regulatory role and clinical significance of miR-30 and have emphasized the emerging roles in lung cancer.
Keywords
- Lung cancer
- miR-30 family
- Biomarker
- Therapeutic potential
- Signalling pathway
- Tumour-suppressor
Lung cancer is the most frequently diagnosed neoplasm, with 2.09 million new cases diagnosed in the year 2018 [1]. Even though the recent statistics by Siegel et al. [2] suggests that the death rates associated with lung cancer have decreased, mortality has increased over the years, from 1.59 million deaths in 2012 [3] to 1.76 million in 2018 [1]. Tobacco cigarette smoking (active and passive) is the principal risk factor, with exposure to air pollution, occupational exposure to asbestos, radon, metals, radiation therapy, human immunodeficiency virus (HIV) infection, alcohol consumption, and genetic susceptibility including epigenetics being other causal factors [4, 5]. Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) are the two main types of lung cancer with NSCLC being the most common. Histologically, squamous cell carcinoma, adenocarcinoma, and large cell carcinoma are the major subtypes of NSCLC. Additionally, the presence of specific DNA mutations (EGFR, ALK and ROS1) allow further molecular classification.
Early-stage detection of NSCLC offers surgical resection as a treatment with most favourable prognosis, and survival rates can reach up to 90% [6]. However, the disease is asymptomatic and is often diagnosed at later stages (stage III/IV) making it difficult to offer any kind of curative treatments with survival rates sinking up to 15% [7]. Treatment modules include surgery, radiation therapy, interventional pulmonology and chemotherapy or a combination of the same. Besides these, immunotherapy seems promising, such as PD-1/PD-L1 inhibitors which include Nivolumab. Pembrolizumab (Keytruda) targeting PD-1 and Atezolizumab (Tecentriq) targeting PD-L1 [6]. The IV stage of lung carcinoma metastasizes to distant organs that include brain, bones, and adrenal glands. High-grade squamous cell and adenocarcinomas have exhibited overexpression of certain genes that include high smooth muscle actin (ACTA), c-MET and focal adhesion kinase (FAK) that regulate type 1 matrix metalloprotease (MMP14) [8].
MicroRNAs (miRNAs) are small RNA molecules which can regulate several target genes, thus regulating complex regulatory pathways. Role of miRNAs is evident in health and disease as observed by growing number of reports [9, 10]. Many miRNAs like miR-21, miR-30, miR-34, miR-210 etc. are studied for their role in carcinogenesis, diagnosis, prognosis, and therapeutic potential in lung cancer [11]. Most importantly, several studies have shown a significant downregulation and tumour suppressive potential of miR-30 family members in lung cancer [12, 13, 14]. This review discusses an extensive overview of the current research on the role of miRNAs in lung cancer with a major focus on miR-30 family.
MicroRNAs (miRNAs) are endogenous, highly conserved, small non-coding RNA molecules involved in the regulation of gene expression [15, 16]. Mature miRNAs are single-stranded RNAs containing 18–25 nucleotides, which are the end products of processed primary and precursor miRNAs [17]. Approximately 1000 nucleotide long primary miRNA (Pri-miRNA) is processed using the micro-processor complex to produce 70 nucleotide long stem-looped precursor miRNA (pre-miRNA) in the nucleus. This pre-miRNA is transported to the cytoplasm using Exportin-5 and processed to mature single stranded miRNA using DICER [18]. With the advent of high throughput techniques, an increasing number of miRNAs are being sequenced. The latest miRBase registers 48,860 mature miRNA sequences from 271 organisms, and human genome encodes for 2,654 of them [19].
Duplex miRNA is loaded on to Argonaute along with other associated proteins comprising the RNA induced silencing complex (RISC). Argonaute proteins form the fundamental component of RISC performing endo-nucleolytic cleavage of target mRNA based on the guide (miRNA) and target strand (3’ UTR of mRNA) complementarity, resulting in gene silencing. Repression of gene expression can also result from translational inhibition if miRNA-mRNA possesses partial complementarity [15, 20]. The canonical pathway for miRNA biogenesis [18] and its mechanism of target inhibition is described in Fig. 1.
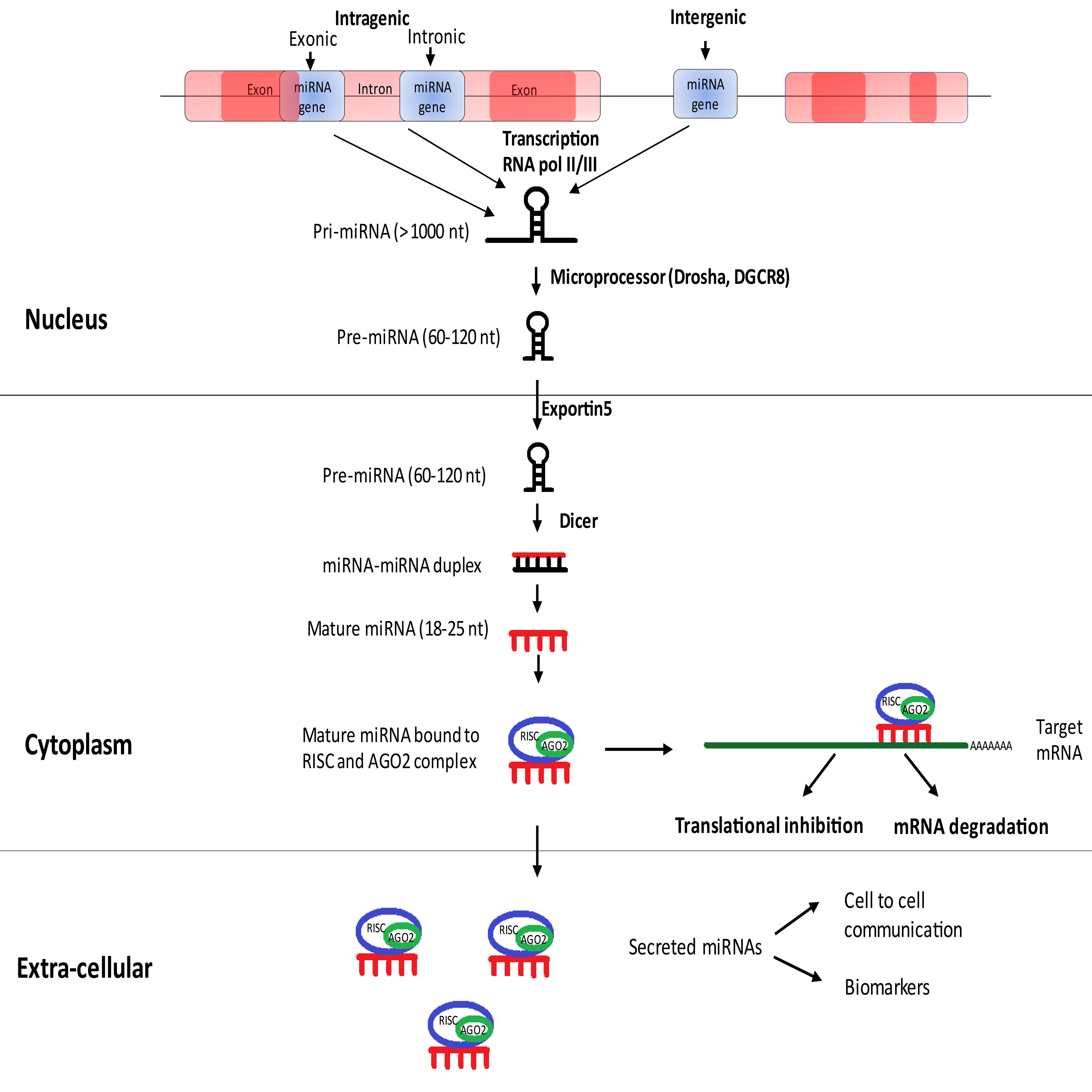 Fig. 1.
Fig. 1.Canonical pathway for miRNA biogenesis.
Different miRNAs are assembled into families indicating a derivation from a common ancestor. The miRNA family also hint towards similar sequence, secondary structure, and/or shared biological function [16]. Sister miRNAs of the family mostly share conserved seed sequence resulting in the silence of common target genes and hence shared regulatory role. Nevertheless, recent evidence suggests that complementarity may extend beyond seed region (3’ end of miRNA) resulting in diverse target profiles for the miRNAs of the same family [21].
Post-transcriptional crosstalk between miRNA and mRNA results in the complex regulation network leading to minimization of transcriptional noise, maintenance of threshold protein or mRNA levels, and/or acting as switch-like repression [22, 23]. These attributes of miRNA have made it relevant in most physiological and pathological conditions, including cancer [24]. The genomic locations of more than half of the miRNA population are at fragile sites signifying its relevance in cancer [25]. The deregulated expression levels of various miRNAs in several cancers have been documented, and they may act as either oncogenes or tumour suppressors depending upon their targets [26]. Also, some miRNAs do possess tissue specificity substantiating their regulatory role in differentiation status and tissue identity, as well as its therapeutic potential [27] as described in Fig. 2. Additionally, many miRNAs are investigated for their specific role in drug resistance [28] and metastasis [29].
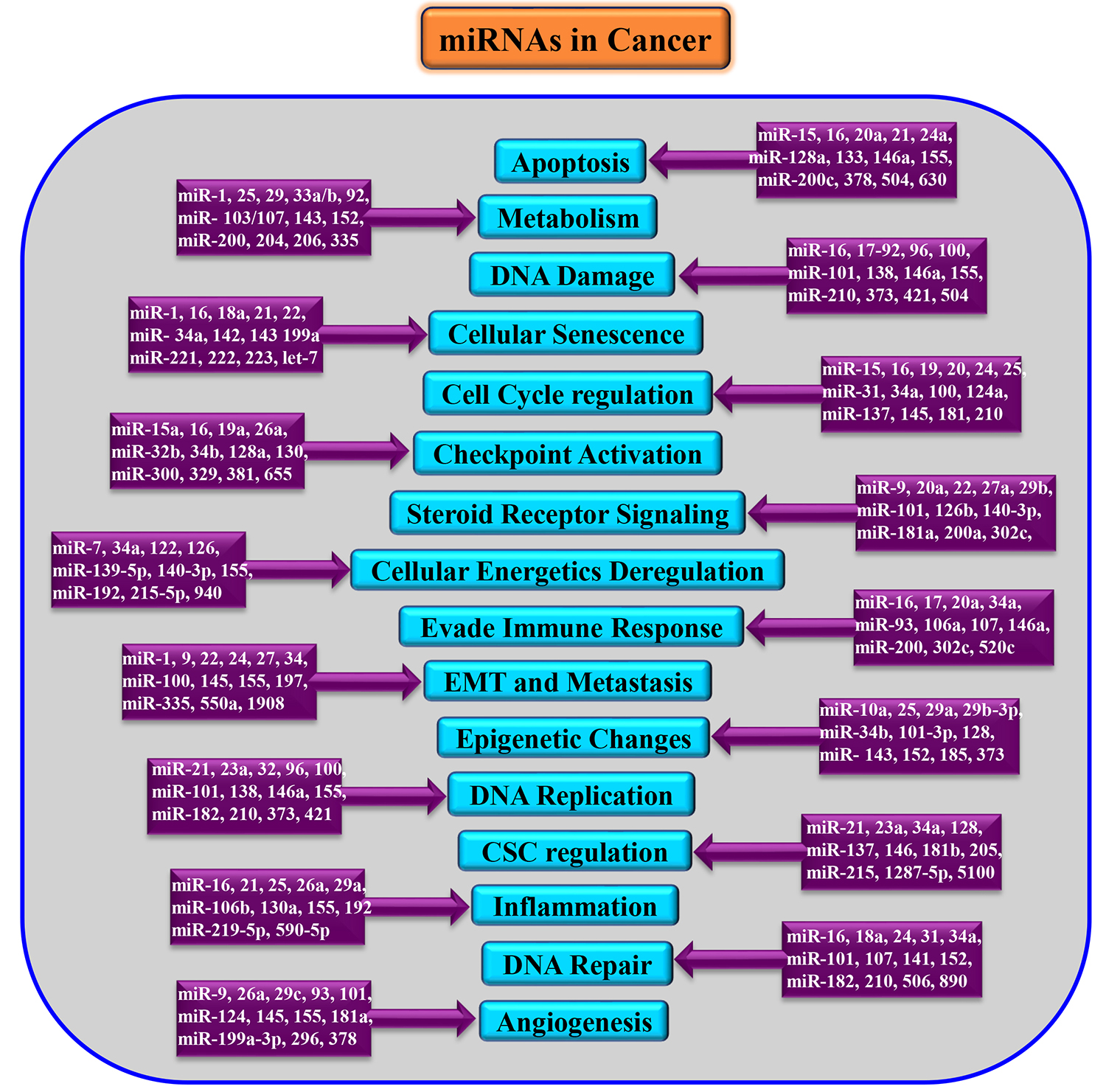 Fig. 2.
Fig. 2.Dysregulation of miRNAs in various cancers. EMT, Epithelial-Mesenchymal Transition; CSC, Cancer Stem cells.
miR-21 is a putative oncogene that functions as an anti-apoptotic factor which is the most studied miRNA in the context of cancer. It is found to be overexpressed in breast cancer, colorectal cancer, neuroblastoma, lung cancer, hepatocellular carcinoma (HCC), pancreatic cancer, glioblastoma, leukaemia and lymphoma [30] which can serve as a therapeutic target. Clinical trials using anti-miR-21 oligonucleotides have shown to reduce reactive oxygen species (ROS) thus decreased tumour size [24]. Similarly, miR-17-92 cluster is often studied for its oncogenic role in several cancers, and it is said to influence, cell proliferation, angiogenesis, and metastasis. Moreover, the targets of this cluster include phosphatase and tensin homologue deleted on chromosome 10 (PTEN) and E2 family of transcription factors. Overexpression of this cluster inhibits PTEN, thereby phosphorylation of AKT leading to, cell proliferation, angiogenesis and metastasis [31]. Further, miR-122 is frequently studied liver-specific miRNA, in case of HCC by targeting ADAM10, SRF, IGF1R [32], and FOX family genes besides its regular role in homeostasis and metabolism [33]. Additionally, miR-34 is a well-known tumour suppressor miRNA and is shown to inhibit carcinogenesis via regulating TP53. MRX34, the miR-34a mimic, is a first tumour-targeting miRNA to get into clinical trials for the treatment of several solid tumours including, HCC, renal cell carcinoma (RCC), lung cancer, melanoma etc. [34, 35]. Similarly, several circulating miRNAs individually and as a signature profile are explored for their biomarker potential in the diagnosis and prognosis of cancer [36].
In lung cancer, many miRNAs are investigated to understand their role in the process of tumorigenesis and to evaluate their biomarker potential [37]. miR-150 is demonstrated to promote metastasis in primary lung cancer by targeting FOXO4. It is also proved to be involved in the proliferation of A549 cell lines through p53 mediated mechanism as it harbours miR-150 binding site [38]. Meanwhile, a decreased expression of miR-145 is observed in NSCLC tissues. Grouped under a tumour suppressor, it inhibits cell migration and invasion by silencing RIOK2 and NOB1. It is usually downregulated in many cancers and is shown to be involved in cell cycle regulation. This miRNA is considered to be a therapeutic target for the management of lung cancer [31]. miR-486 may act as a potential therapeutic target in NSCLC by regulating PI3K/AKT pathway [39]. Another study has shown its high diagnostic and prognostic accuracy from the meta-analysis of different cancers as described in Fig. 3. Moreover, the circulating miR-486 had higher diagnostic value compared to the direct specimen that substantiates its value as a biomarker [40].
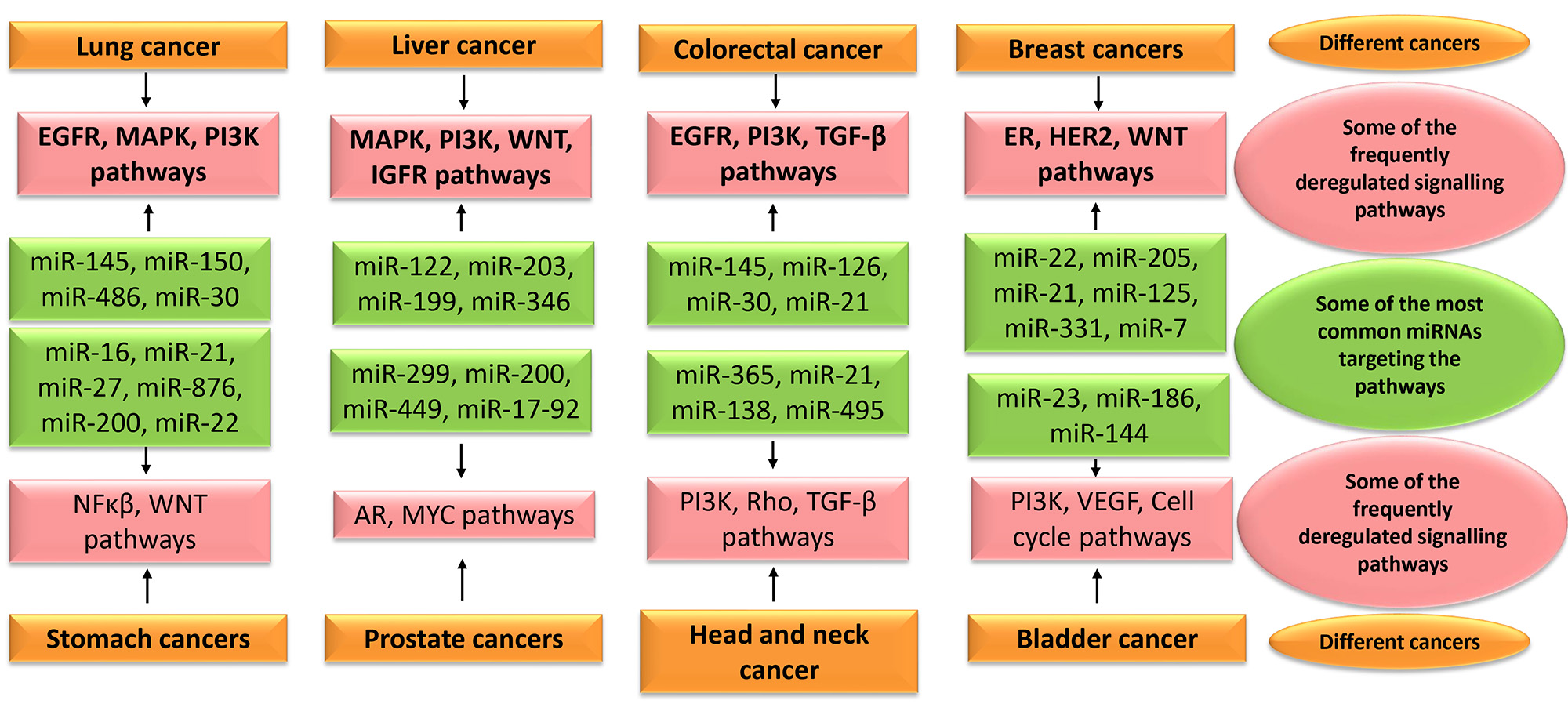 Fig. 3.
Fig. 3.Some of the most common miRNAs targeting the frequently deregulated signalling pathways in various cancers. EGFR, epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; PI3K, Phosphoinositide 3-kinase; IGFR, Insulin-like growth factor receptor; TGF, transforming growth factor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; AR, Androgen receptor; VEGF, Vascular endothelial growth factor.
Similarly, the miR-200 family and miR-183~96~182 cluster are downregulated in lung cancer and are involved in suppressing metastasis. Two of the targets for the cluster is FOX2 and ZEB1 whose expression triggers EMT, migration and metastasis [41, 42]. Furthermore, single nucleotide polymorphism (SNP) variant in miR-196a2 and miR-146a exhibited enhanced risk for lung cancer in North Indian population [43]. miR-30 family is one of the widely studied miRNAs in lung cancer, and here we highlight their physiological role, upstream regulation, downstream targets, functional role and clinical significance in lung cancer.
The miR-30 is a broadly conserved miRNA family containing twelve mature miRNA members including miR-30a, miR-30b, miR-30c-1, miR-30c-2, miR-30d, and miR-30e, generated from both 5p and 3p arms of respective pre-miRNA [19, 44]. All mature miRNAs generated from the 5p arm of miR-30 family pre-miRNAs are active, and that from 3p are rare or inactive (3p*) [45]. miR-30c-1 and miR-30e are encoded by the genes located in the positive strand of Chromosome 1, while miR-30a and miR-30c-2, as well as miR-30d and miR-30b, are encoded by genes located in the negative strand of Chromosome 6 and 8 respectively [19] (Table 1). We found that only miR-30c-1 and miR-30e are intragenic miRNAs located in the intron region of Nuclear Transcription Factor Y Subunit Gamma (NFYC) gene using miRIAD database [46]. However, the association of miR-30 and NFYC in lung cancer not explored so far. The 5p mature miRNAs have a conserved 8mer seed sequence of “GUAAACA”; however, 3p mature miRNAs have either UUUCAGU /UGGGAGG(A) [47].
| S. No. | miRNA | Chromosome location of the gene | Mature miRNA sequence (with underlined seed sequence) |
| 1 | hsa-miR-30a-5p | 6q13 (71403551..71403621, ‘–’ve strand) | UGUAAACAUCCUCGACUGGAAG |
| 2 | hsa-miR-30b-5p | 8q24.22 (134800520..134800607, ‘–’ve strand) | UGUAAACAUCCUACACUCAGCU |
| 3 | hsa-miR-30c-1-5p | 1p34.2 (40757284..40757372, ‘+’ve strand) | UGUAAACAUCCUACACUCUCAGC |
| 4 | hsa-miR-30c-2-5p | 6q13 (71376960..71377031, ‘–’ve strand) | UGUAAACAUCCUACACUCUCAGC |
| 5 | hsa-miR-30d-5p | 8q24.22 (134804876..134804945, ‘–’ve strand) | UGUAAACAUCCCCGACUGGAAG |
| 6 | hsa-miR-30e-5p | 1p34.2 (40754355..40754446, ‘+’ve strand) | UGUAAACAUCCUUGACUGGAAG |
| 7 | hsa-miR-30a-3p* | 6q13 (71403551..71403621, ‘–’ve strand) | CUUUCAGUCGGAUGUUUGCAGC |
| 8 | hsa-miR-30b-3p* | 8q24.22 (134800520..134800607, ‘–’ve strand) | CUGGGAGGUGGAUGUUUACUUC |
| 9 | hsa-miR-30c-1-3p* | 1p34.2 (40757284..40757372, ‘+’ve strand) | CUGGGAGAGGGUUGUUUACUCC |
| 10 | hsa-miR-30c-2-3p* | 6q13 (71376960..71377031, ‘–’ve strand) | CUGGGAGAAGGCUGUUUACUCU |
| 11 | hsa-miR-30d-3p* | 8q24.22 (134804876..134804945, ‘–’ve strand) | CUUUCAGUCAGAUGUUUGCUGC |
| 12 | hsa-miR-30e-3p* | 1p34.2 (40754355..40754446, ‘+’ve strand) | CUUUCAGUCGGAUGUUUACAGC |
| *Rare or inactive. | |||
There are limited studies related to the upstream regulation of miRNAs in
physiology and diseases. Hypermethylation of the promoter region of miR-30 gene
was observed in pancreatic cancer [48], breast cancer [49] and head and neck
squamous cell carcinoma (HNSCC) [50] resulting in the downregulation of mature
miRNA. Moreover, in HNSCC, copy number loss was also responsible for the
decreased expression of miR-30 [50]. SP1, a transcription factor
involved in energy metabolism, acts as an activator of miR-30c expression by
binding to the promoter region [51]. Similarly,
The role of miR-30 has been observed in various aspects of human physiology. Its expression in mammalian cells was first discovered in the HeLa cell line by Lagos-Quintana et al. [54], along with 21 other novel miRNAs. The sister miRNAs are involved in the regulation of various physiological signalling pathways, including osteogenic differentiation and development [55, 56], as well as animal reproductive development [57]. Besides, miR-30 was also shown to play a crucial role in lipogenesis and adipose differentiation [58]. miR-30b and miR-30c are involved in vascular development and angiogenesis [59]. They favour epithelial phenotype by silencing genes involved in mesenchymal transition [60]. These studies suggest the significance of miR-30 family in development and differentiation of various cell types. The prominent physiological involvement of miR-30 shows that their deregulation is involved in several pathological conditions, including cancer [61].
Although miR-30 has a prominent tumour repressing role in many cancers, few studies also have documented its oncogenic role in some of the cancers indicating its diversity of targets. Overexpression of miR-30 has been reported in many cancers by inhibiting p53 and p16INK4A disrupting senescence in cells [62]. In breast cancer, it plays the role of tumour suppressor [63] by regulating several key signalling pathways like MAPK/KRAS [64], p53 etc. [65]. miR-30 acts as a tumour suppressor in thyroid cancer and also helps in distinguishing anaplastic thyroid cancer and differentiated thyroid cancer [66]. Saleh et al. [50] showed that miR-30a/e could inhibit growth and tumorigenicity in HNSCC; they also revealed the therapeutic potential of miR-30a. However, it was reported by Saad et al. [67] that miR-30a is upregulated in case of alcohol-induced HNSCC resulting in pathogenesis and progression of cancer. In HCC, downregulated levels of miR-30e have been observed, and it is said to target JAK1/STAT3 pathway to restrain tumorigenesis. Similarly, miR-30a can inhibit proliferation, migration, and invasion in HCC by directly inhibiting FOXA1 [68, 69, 70]. Xiong et al. [71] showed that miR-30b impedes epithelial-mesenchymal transition (EMT) and hinders oncogenicity in pancreatic cancer. However, an oncogenic role of miR-30a/b/c was also observed in pancreatic cancer and are involved in the regulation of drug resistance, migration, and invasion [72]. In gastric cancer, miR-30a is involved in decreasing multidrug resistance [73] and inhibits cell growth by regulating RAB31/GLI1 pathway [74]. In RCC, miR-30a and miR-30b are documented to inhibit tumorigenesis, metastasis, and EMT by targeting GRP78, a crucial regulator of endoplasmic reticulum (ER) stress response, and GNA13, a member of G-protein family [75]. miR-30a acts as a tumour suppressor in melanoma by downregulating metastasis through regulating SOX4 and ZEB2 [76, 77]. miR-30d and miR-30e were associated with failure-free survival (FFS) in classical Hodgkin lymphoma (cHL). Additionally, miR-30d acts as an oncomiR and may also be involved in doxorubicin related chemoresistance in cHL [78]. Further, miR-30d serves as one of the prognostic markers for primary central nervous system lymphoma [79]. Hence, the literature suggests the diverse role of miR-30 family in various cancers by regulating an array of targets (Table 2, Ref. [59, 60, 62, 64, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 80]).
| S. No. | Type of cancer | miR-30 family members | Role | Reference |
| 1 | Breast Cancer | miR-30a/c/e | Tumor suppressor | [59, 60, 62] |
| 2 | Thyroid cancer | miR-30a/b/c/d/e | Tumor suppressor | [64] |
| 3 | Head and neck cancer | miR-30a/b/c/d/e | Tumor suppressor | [43] |
| miR-30a | Oncogenic | [66] | ||
| 4 | Hepatocellular carcinoma | miR-30a/e | Tumor Suppressor | [67, 68, 69] |
| 5 | Pancreatic cancer | miR-30a/b/c | Oncogenic | [71] |
| miR-30b | Tumor suppressor | [70] | ||
| 6 | Gastric cancer | miR-30a/c-2 | Tumor suppressor | [72, 73, 80] |
| 7 | Renal cell carcinoma | miR-30a/b | Tumor suppressor | [74, 75] |
| 8 | Melanoma | miR-30a | Tumor suppressor | [76, 77] |
| 9 | Lymphoma | miR-30d/e | Oncogenic | [78] |
miR-30 family is one of the top five highest expressed miRNAs in normal adult human and mouse lung, suggesting the evolutionary conservation [81]. It is also one of the families found to be airway specific with higher expression in alveolar macrophages [82]. The role of miR-30 family is mostly studied in case of NSCLC (adenocarcinoma and squamous cell carcinoma), and only limited reports are available in case of large cell carcinoma, SCLC, and mesothelioma of lung. miR-30a is the most studied sister miRNA of miR-30 family, and maximum studies have observed lower expression of miR-30 family members in case of lung cancer tissue, blood, cell lines as well as in animal models, signifying its tumour suppressive role. Various studies have elucidated several targets and pathways through which these sister miRNAs regulate different steps of carcinogenesis (Fig. 4).
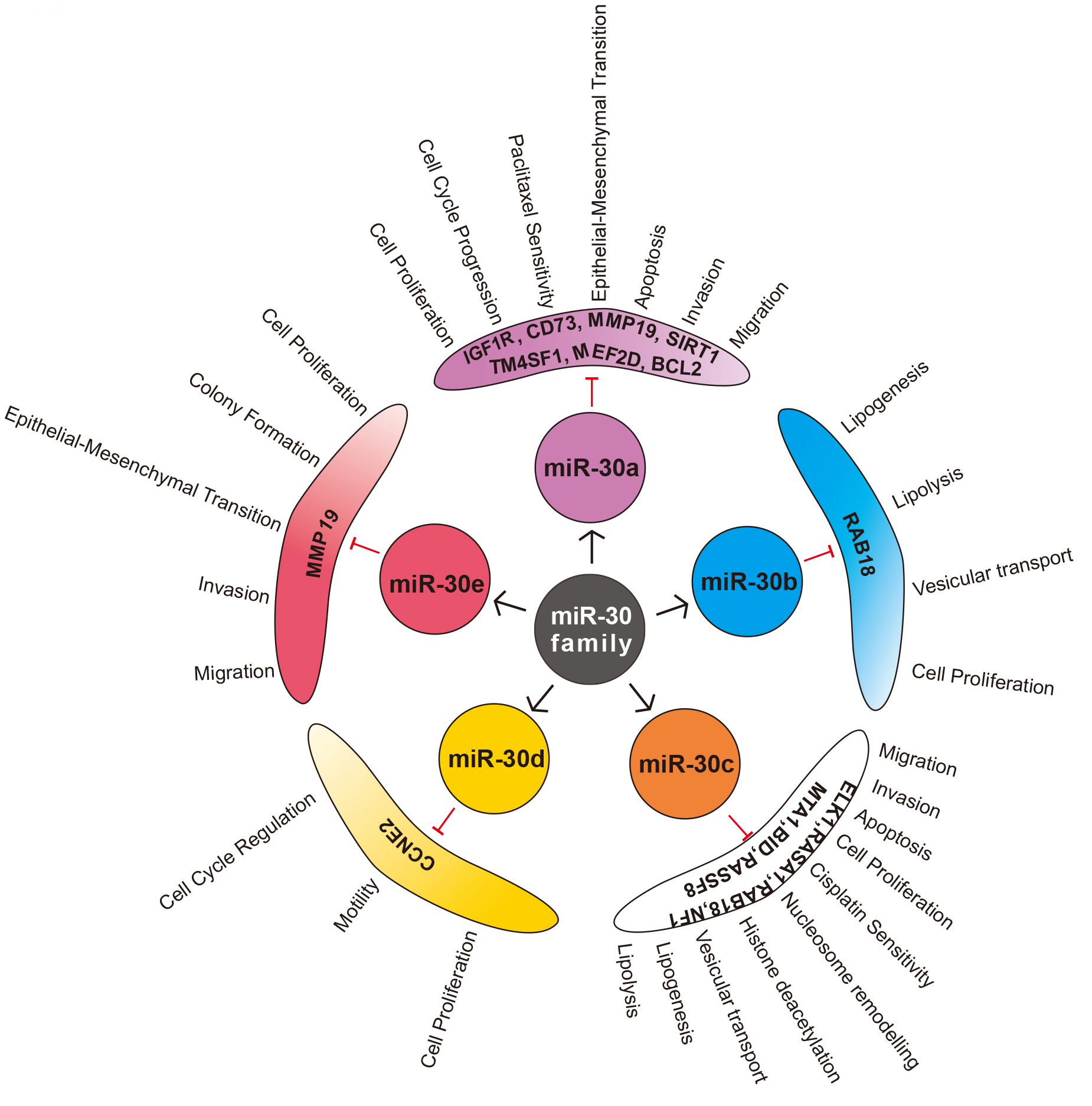 Fig. 4.
Fig. 4.Sister miRNAs of miR-30 family, its validated targets and functional significance in Non-small cell lung cancer (NSCLC).
Smoking is the primary cause of lung cancer and cigarette smoke is also
implicated in miRNA dysregulation. Although the exact mechanisms behind the
abrogation of miRNA expression is unclear, the highly mutagenic oxidative
compounds and free radicals generated by smoking are said to interact with the
nucleotides. As most of the miRNA genes are located in fragile sites of the
genome, they are more vulnerable [83, 84]. Even though the miR-30 family has not
been investigated independently in relation to smoking, several studies have
observed its association, along with other miRNAs. miR-30 family is one of the
most prominently downregulated miRNA families in lung tissues of Sprague-Dawley
rats exposed to environmental cigarette smoke for four weeks (miR-30a/c;
Many studies have suggested its association with several clinical parameters of lung cancer by observing its varied levels in human serum, plasma and/or tissue (Table 3, Ref. [88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105]). Functionally, miR-30 family regulates chief pathways involved in NSCLC which are discussed below in detail.
| Parameters | miRNA | Levels | Reference | |
| NSCLC sample (cancer vs normal) | Tissue | miR-30a-3p/5p, miR-30b, miR-30c, miR-30d, miR-30e-3p/5p | ↓ | [88, 89, 90, 91, 92, 93, 94, 95, 96, 98, 100, 101, 102, 103, 104] |
| Plasma | miR-30e-3p/5p | ↓ | [102] | |
| Serum | miR-30a and miR-30e-3p | ↑ | [94] | |
| Age (elderly vs young) | miR-30a | ↑ | [93] | |
| Gender (female vs male) | miR-30d | ↑ | [103] | |
| Stage (advanced vs initial) | miR-30a | ↓ | [88, 93] | |
| miR-30e-3p | ↑ | [105] | ||
| ↓ | [94] | |||
| Metastasis (yes vs no) | miR-30a/d/e-3p | ↓ | [93, 103, 105] | |
| Dedifferentiation (yes vs no) | miR-30e | ↓ | [94] | |
| Tumor Size (high vs low) | miR-30a and miR-30e-3p | ↓ | [93, 94] | |
| Survival | OS (↑) | miR-30a, miR-30c, miR-30e | ↑ | [89, 99, 102] |
| PFS (↑) | miR-30a, miR-30c, miR-30e-3p/5p | ↑ | [89, 99, 102, 105] | |
| Prognosis (poor) | miR-30d/a | ↓ | [88, 103] | |
| Drug Response | Erlotinib (PFS ↑) | miR-30b/c | ↓ | [99] |
| Paclitaxel (DFS ↓ and No RR) | miR-30a | ↓ | [97] | |
| OS, Overall survival; PFS, Progression free survival; DFS, Disease free
survival; | ||||
miR-30 family can regulate proliferation, cell cycle and apoptosis through various pathways to inhibit tumorigenesis in NSCLC. Guan et al. [14] showed that miR-30a could directly target SIRT1, a NAD-dependent deacetylase to suppress lung cancer. miR-30a mimics inhibited the expression of SIRT1, while miR-30a inhibitors produced the opposite effect. SIRT1 can deacetylate p53, preventing its downstream transcriptional activity and apoptosis [89]. Interestingly, p53 can bind to miR-30a promoter and transcriptionally activate the same [65]. Clinical samples of NSCLC show low p53 acetylation, lower levels of miR-30a and upregulation of SIRT1 expression [14, 90] suggesting an active loop of miR-30a/SIRT1/p53 in tumorigenesis. Further, miR-30 mimics can also target MEF2D, a prominent transcription factor regulating cell proliferation and apoptosis [12]. Evidence suggests that MEF2D is highest expressed in NSCLC patients with COPD compared to NSCLC patients without COPD and COPD patients without NSCLC [91]. These observations indicate MEF2D may act as a mechanistic link between chronic inflammation and cancer development. As miR-30a regulates MEF2D, it would be interesting to understand its expression in COPD, which forms a significant risk factor for lung cancer. Additionally, miR-30b/c can inhibit Rab18, a Ras superfamily G protein to impede the cell proliferation in NSCLC [92]. CCNE2, a cyclin regulating cell cycle, driving the G1/S transition is a target of miR-30d [13]. The expression of CCNE2 is profoundly increased in NSCLC patients suggesting that it acts as an oncogene [93]. Further, miR-30a can also directly target Insulin-like growth factor 1 receptor (IGF1R) and regulate PI3K/AKT pathway by inhibiting several cell cycle regulators like CDK2, CDK4, Cyclin A2, Cyclin D1. The authors observed inhibition of cell proliferation, G1/S and S/G2 transition in vitro [94]. miR-30a also found to target CCNE2 in prostate cancer [95] indicating that miR-30 family members may act as a regulator of the cell cycle to render its tumour suppressive functions.
Conversion of stationary epithelial cells to migratory mesenchymal cells by the process of epithelial-mesenchymal transition (EMT) is the hallmark of cancer metastasis [96]. miR-30 is demonstrated to regulate EMT in NSCLC under many studies. Matrix metalloproteases (MMP) are one of the main class of proteolytic enzymes involved in tumour invasion by enabling basement membrane penetration. Several members of MMPs (MMP-1, MMP-2, MMP-7, MMP-9, MMP-12, MMP-13, MMP-19, MMP-26) are found to be upregulated in lung cancer [97, 98]. Yu et al. [98] found a negative association between miR-30 and MMP-19, hinting that it might be a direct target. Moreover, miR-30 also found to downregulate the expression of MMP-2 and MMP-9 in prostate cancer [99]. Further, CD73 is a cell surface enzyme which dephosphorylates extracellular AMP to adenosine, is significantly overexpressed in NSCLC cell lines and tissues [106]. It is said to modulate tumour immune response and promote growth and metastasis [107]. miR-30a mimics have been shown to inhibit proliferation and migration by directly regulate CD73 in NSCLC [106] as well as colorectal cancer [100]. Metastasis associated protein 1 (MTA1), a promoter of EMT and metastasis is overexpressed in NSCLC as evidenced in various studies [101, 108, 109]. miR-30 can directly inhibit MTA1 by binding to 3’-UTR, in hepatocellular carcinoma [102], gastric cancer [103] and NSCLC [108]. NFIB is another oncogenic transcription factor regulated by miR-30d to modulate migration and invasion in lung cancer [110]. miR-30a can also target predominant pro-metastatic protein of Eya family called EYA2, and inhibit migration as well as invasion [80]. miR-30a/c can regulate TM4SF1, a glycoprotein involved in the regulation of metastasis, cell proliferation and stemness [111]. In contrast, Kawaguchi et al. (2017) observed only slight increase in migration capacity of A549 cells transfected with miR-30a compared to controls and no change in any cellular properties like proliferation, cell cycle, drug sensitivity [104].
Drug resistance is a prominent reason for therapeutic failure resulting in
disease progression or recurrence in lung cancer. Treatment with paclitaxel
(alone and in combination) is an approved therapy for advanced NSCLC [105].
Multiple studies have found the enhanced expression of anti-apoptotic protein
BCL-2 in chemoresistance, including paclitaxel [112, 113]. Xu et al.
[114] proved that BCL2 is the direct target of miR-30a using mimics and
antimiRs. miR-30a can directly inhibit BCL2 and enhance paclitaxel
sensitivity by modulating apoptosis. A lower expression of miR-30a in cells
indicate paclitaxel resistance. Additionally, PI3K is involved in the
resistance mechanisms of several tyrosine kinase inhibitors (TKIs), including
gefitinib and erlotinib, are also regulated by miR-30 [115]. MET
(hepatocyte growth factor receptor tyrosine kinase) expression and
phosphorylation is observed with primary and acquired resistance to EGFR TKI
treatment. miR-30b/c, miR-221, and miR-222 were found to be regulated both by
EGFR and MET and may have therapeutic benefits in overcoming
drug resistance to TKIs [116]. miR-30c is also regulated in the upstream by wild
type and mutant KRAS (KRAS
Several studies have compared the expression of sister miRNAs belonging to the miR-30 family in tissue and blood samples of NSCLC patients. These studies have used as low as five [92] to as high as 160 paired tissue samples [111]. Most of the studies observed a downregulated expression of miR-30a [12, 82, 111, 118, 119], miR-30b [92], miR-30c-1 [92, 108, 120], miR-30c-2 [121], miR-30d [98, 122, 123, 124], miR-30e [92, 98, 125] in NSCLC tissues as compared to adjacent normal tissue samples, suggesting its major role as tumour suppressor miRNA. Only one study reported an elevated expression of miR-30c in tissue samples of NSCLC patients [117]. The paradoxical role of a single miRNA acting as both tumour suppressor and oncogene is observed in many studies as well [126, 127]. This observation may be attributed to the fact that a single miRNA can target many mRNAs which include both oncogenes and tumour suppressors. Additionally, single mRNA can be regulated by multiple miRNAs complicating the regulation process. Moreover, several other factors like the immune system, tumour microenvironment, infections, therapy sensitivity, and mutations may also affect the miRNA-mRNA interactions. The net effect of a miRNA as a tumour suppressor or oncogene depends on fine-tuning the balance between these interactions [128].
The altered miR-30 expression is correlated to several clinical parameters like the stage of the tumour [129], tumour size [111], lymph node metastasis and poor prognosis [123, 129], smoking status [122], and NSCLC dedifferentiation [118]. miR-30 downregulation is also associated with survival parameters like shorter overall survival (OS), progression-free survival (PFS) [111]. Interestingly, older patients showed higher expression of miR-30a [129]. The same miRNA is demonstrated as age-dependent miRNA that can impair keratinocyte differentiation and induce apoptosis in another study [130]. Also, expression of miR-30d was higher in females [123]. Its sister miRNA miR-30b is regulated by estrogen, indicating a hormonal control [131]. These observations highlight the importance of considering patient age and gender while comparing different studies. However, few studies found no correlation between altered miR-30 expression levels and any clinicopathological features of patients [82, 111, 122].
The first evidence of miRNAs as biomarkers in cancer was recognized during 2008 in the serum of diffuse large B-cell lymphoma patients [132]. Since then, many miRNAs are examined for their biomarker potential in cancer [133]. Most of the studies involving miR-30e in lung cancer are from the perspective of the biomarker. Its altered expression was detected in three studies based on different array technology platforms [14, 118, 134]. Silva et al. [134] examined the levels of miR-30e-3p in more than 100 NSCLC patient’s plasma vesicles and found it to be downregulated. The levels of the same were significantly upregulated in advanced stages of the tumour distinguishing the patients for their possibility of surgery. An enhanced level of miR-30e-3p was also associated with higher disease-free survival (DFS) rates. However, no association was observed with OS. Significant downregulation of miR-30e-5p was also observed in both tissue and plasma of 59 NSCLC patients. Although the tissue levels of miR-30e-5p did not correlate with any clinicopathological features, its circulatory levels were associated with OS [122]. Another study showed that miR-30e-5p was significantly downregulated in NSCLC tissues while miR-30e-3p was elevated in the serum of NSCLC patients as compared to healthy subjects. However, serum levels had no association with any clinical features or survival [118]. Further, miR-30b and miR-30c may act as a predictive biomarker for intrinsic erlotinib resistance; it might be useful in screening around 30% of non-responders to the drug despite EGFR mutation. Lower pre-treatment level of miR-30b was associated with enhanced PFS and OS after erlotinib treatment in NSCLC adenocarcinoma patients with activating EGFR mutation [125]. miR-30b/c also act as prognostic biomarkers for PFS and OS of first-line TKIs treatment [135]. A single study has checked the levels of miR-30a in circulation, and they found it to be elevated in NSCLC patients as compared to healthy subjects [118]. Besides, miR-30 has been checked along with other miRNAs for its potential as a part of a panel of signature miRNAs. A signature miRNA panel with upregulated miR-205-5p and miR-3917 and downregulated miR-27a-5p, miR-30a-3p, miR-30a-5p, miR-30c-2-3p, and miR-30d-5p indicates the enhanced risk of lung cancer [121]. Additionally, miR-30d was one of the four miRNAs along with miR-486, miR-1, and miR-499 associated with OS of NSCLC adenocarcinoma patients treated with surgery and adjuvant chemotherapies [136].
Further, Single nucleotide polymorphisms (SNPs) in miRNA seed region, genes, regulatory regions and its binding sites as observed in several studies are added determinants of its expression and activity [137]. Such SNPs can also increase the risk and aggressiveness of cancer by altering the function of miRNAs [138]. In a case-control study performed by Xie et al. [139], rs763354 in miR-30a was found to be significantly associated with NSCLC risk, but no association was observed in survival analysis. However, SNP in the flanking region of pre-miR-30c-1 (rs928508) was found to a predictor in survival analysis and may act as a prognostic biomarker [140]. Although the above biomarker studies seem promising for translation to bedside, none has reached the stage of clinical trials indicating the need for advanced research in this area.
To further understand the significance of miR-30 family in NSCLC, we performed target search under the database miRTarBase 80, (2020) [141]. We selected only the experimentally validated targets of miR-30 family by luciferase reporter assay (Table 4). We found a total of 33 target genes validated till now in different pathophysiological conditions. Next, using the database for annotation, visualization, and integrated discovery (DAVID), we performed functional annotation clustering and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis [142]. Prominently enriched gene ontology cluster was related to transcription regulation and DNA binding with the enrichment score of 3.26, suggesting that miR-30 family may mostly regulate other transcription factors. The other highly enriched clusters broadly include zinc finger, splicing, transcriptional regulation apoptosis and proliferation (Supplementary Table 1). The KEGG pathway analysis revealed that Neurotrophin signaling pathway was the most significantly ranked according to p value (0.0025). Other significant terms included prion diseases, transcriptional misregulation in cancer, chronic myeloid leukemia, miRNAs in cancer, thyroid hormone signaling pathway and Epstein-Barr virus infection (Table 5). Neurotrophins are growth factors, best characterized for their involvement in neuronal survival, differentiation and conduction. They are associated with an array of neuronal diseases like Alzheimer’s disease, Parkinson’s disease and Brain tumour. Neurotrophin signalling has been recently recognized in lung health, but its role is weakly characterized [143]. The role of miR-30 in the regulation of neurotrophin signalling in NSCLC is also mostly unknown. Hence, examining these unexplored areas in NSCLC may shed more light in understanding the complex molecular aspects of the disease.
| S. No. | Target gene (Official Gene Symbol) | Gene function |
| 1 | BDNF | Brain-derived neurotrophic factor; growth factor |
| 2 | NOTCH1 | NOTCH homolog 1; transmembrane receptor |
| 3 | BECN1 | Autophagy related gene |
| 4 | TNRC6A | Trinucleotide repeat containing adaptor 6A; involved in post-transcriptional gene silencing |
| 5 | MBNL1 | Muscleblind like splicing regulator 1; Splicing regulator |
| 6 | SMAD1 | Mothers against decapentaplegic homolog; signal transducers for receptors of the transforming growth factor beta |
| 7 | DTL | Denticleless E3 Ubiquitin Protein Ligase Homolog; polyubiquitination |
| 8 | SNAI1 | Zinc finger protein; transcription factor |
| 9 | PIK3CD | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase; phosporylation |
| 10 | PRDM1 | PR domain zinc finger protein 1; repressor of beta-interferon gene expression |
| 11 | 7-Sep | Septin 7 |
| 12 | AVEN | Apoptosis and caspase activation inhibitor |
| 13 | FOXD1 | Forkhead box D1; transcription factors |
| 14 | ABL1 | ABL proto-oncogene 1; non-receptor tyrosine kinase |
| 15 | MTDH | Metadherin; involved in RNA-induced silencing complex |
| 16 | VIM | Vimentin; type III intermediate filament protein |
| 17 | RUNX2 | RUNX family transcription factor 2 |
| 18 | ERG | ETS-related gene; transcription factor oncogene |
| 19 | ESR2 | Estrogen Receptor 2; nuclear receptor transcription factors |
| 20 | BCL11A | B-cell lymphoma/leukemia 11A; zinc-finger protein |
| 21 | BCL9 | B-cell CLL/lymphoma 9; transcriptional co activator |
| 22 | HSPA5 | Heat shock protein family A (Hsp70) member 5; folding and assembly of proteins in the ER |
| 23 | EYA2 | EYA Transcriptional Coactivator And Phosphatase 2 |
| 24 | SOX4 | SRY-box transcription factor 4 |
| 25 | IL21R | Interleukin 21 receptor; type I cytokine receptors |
| 26 | UBE3C | Ubiquitin protein ligase E3C |
| 27 | CBX3 | Chromobox 3; chromobox 3 component of heterochromatin |
| 28 | TP53 | Tumor protein P53; tumor suppressor protein containing transcriptional activation |
| 29 | CD99 | CD99 Molecule (Xg Blood Group); cell surface glycoprotein |
| 30 | LOX | Lysyl oxidase |
| 31 | MBNL3 | Muscleblind like splicing regulator; egulation of alternative splicing |
| 32 | NCAM1 | Neural cell adhesion molecule 1; cell adhesion protein |
| 33 | MBNL2 | Muscleblind like splicing regulator 2; zinc finger protein |
| Category | Term | Count | % | p value | Genes | List total | Pop hits | Pop total | Fold enrichment | Bonferroni | Benjamini | FDR |
| KEGG_PATHWAY | hsa04722: Neurotrophin signaling pathway | 4 | 12.5 | 0.002456001 | BDNF, ABL1, PIK3CD, TP53 | 17 | 120 | 6879 | 13.48823529 | 0.218001303 | 0.136238747 | 0.13487636 |
| KEGG_PATHWAY | hsa05020: Prion diseases | 3 | 9.375 | 0.002724775 | NOTCH1, HSPA5, NCAM1 | 17 | 34 | 6879 | 35.70415225 | 0.238792576 | 0.136238747 | 0.13487636 |
| KEGG_PATHWAY | hsa05202: Transcriptional misregulation in cancer | 4 | 12.5 | 0.006235795 | SMAD1, ERG, TP53, RUNX2 | 17 | 167 | 6879 | 9.692145122 | 0.465023211 | 0.207859847 | 0.205781249 |
| KEGG_PATHWAY | hsa05220: Chronic myeloid leukemia | 3 | 9.375 | 0.011792009 | ABL1, PIK3CD, TP53 | 17 | 72 | 6879 | 16.86029412 | 0.694623124 | 0.294800222 | 0.29185222 |
| KEGG_PATHWAY | hsa05206: MicroRNAs in cancer | 4 | 12.5 | 0.026640176 | NOTCH1, ABL1, VIM, TP53 | 17 | 286 | 6879 | 5.659399424 | 0.932804271 | 0.454713559 | 0.450166423 |
| KEGG_PATHWAY | hsa04919: Thyroid hormone signaling pathway | 3 | 9.375 | 0.02853579 | NOTCH1, PIK3CD, TP53 | 17 | 115 | 6879 | 10.55601023 | 0.94470568 | 0.454713559 | 0.450166423 |
| KEGG_PATHWAY | hsa05169: Epstein-Barr virus infection | 3 | 9.375 | 0.031829949 | PIK3CD, VIM, TP53 | 17 | 122 | 6879 | 9.950337512 | 0.96063009 | 0.454713559 | 0.450166423 |
Accumulating studies have revealed that miR-30 family is abnormally expressed in several tumours, including lung cancer particularly, NSCLC. All sister miRNAs of miR-30 family have been studied to understand their role in NSCLC. Functionally, it can regulate cell proliferation, metastasis, EMT, cell cycle, and other prominent tumorigenic pathways. Even though its potential role has been observed in relation to smoking, the studies are minimal, and its exact function is unclear.
Moreover, its role has not been substantiated in chronic obstructive pulmonary disease (COPD), the most prominent associated illness along with lung cancer, suggesting that its absence may have a transforming potential and it might possess a disease-specific role in carcinogenesis. The review of the literature also indicates only limited in vivo studies in NSCLC. Studies reveal its enormous potential to be a diagnostic and prognostic biomarker, highlighting its clinical significance and translational value. Although advanced research is required to validate its role, the miR-30 family may be a ray of hope for better diagnosis and treatment of lung cancer.
SK participated in literature review, interpretation, drafting of the manuscript; NB and IK participated in reviewing of the manuscript; RCK participated in interpretation and critical review of the manuscript.
Not applicable.
We acknowledge Rekha P.D., Deputy Director, Yenepoya Research Centre, Yenepoya (Deemed to be University), Mangalore, Karnataka, India, for support, guidance, and critical discussion on the manuscript.
R. C. Koumar acknowledges the receipt of Seed grant (YU/Seed Grant/090-2020) from Yenepoya (Deemed to be University). Shruthi Kanthaje acknowledges Yenepoya (Deemed to be University), Mangalore, Karnataka, India for post-doctoral fellowship.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://www.fbscience.com/Scholar/articles/10.52586/S558.




