Frontiers in Bioscience-Scholar (FBS) is published by IMR Press from Volume 13 Issue 1 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Sciences, Faculty of Natural and Applied Sciences, Notre Dame University - Louaize, Zouk Mosbeh, Lebanon Zouk Mikael
Abstract
RNA interference is currently one of the most advanced genomic tools providing promising insight to manage countless pathophysiological conditions that seemed unapproachable few years ago. Researchers across the globe have devised numerous methods in which small RNA molecules can be administered into target cells to manipulate cellular functions at the molecular level and eradicate defects that lead to pronounced phenotypes, which might sometimes be as fatal as malignant tumors. The present review provides an overview of the non-viral delivery approaches for siRNA therapeutics with an emphasis on the PLGA polymeric strategy and its use in different biological applications. Due to its versatile surface, PLGA is not only used in tissue engineering such as remodeling of bone and cartilage, but it indeed plays a crucial role as chemical carrier for a broad range of therapeutic agents. It is greatly implicated in gene therapies and inherited genetic defects.
Keywords
- RNA interference
- siRNA
- gene therapy
- Non-Viral Delivery
- PLGA nanoparticles
- Review
RNA interference (RNAi) is a conserved mechanism from yeast to humans that uses small regulatory non-coding RNAs to control the expression of genes. Over more than 1000 small RNAs are encoded in the human genome regulating approximately 60% of our genes (1, 2). The first hint to a role for RNA species in gene silencing came from the work of Napoli and Jorgensen who described in 1990 a revolutionary experiment done on Petunia flowers, which laid the foundations for the later discovery of RNAi as a mechanism of gene silencing. Initially, the aim of their study was to overexpress chalcone synthase (CHS) to test whether or not this enzyme is rate-limiting in the biosynthesis of anthocyanin, a purple plant pigment. Unexpectedly, a portion of the transgenic flowers ended up with a white instead of a violet color. Napoli and Jorgensen hypothesized that the dual transcription of the endogenous and introduced CHS genes coordinately suppressed their expression, thus resulting in the white phenotype (3). This phenomenon, known as post-transcriptional gene silencing (PTGS), was reported shortly afterwards in Caenorhabditis elegans. Two simultaneous reports at that time revealed that the gene lin-4 encodes small regulatory RNA which silence post-transcriptionally the lin-14 gene involved in the temporal regulation of postembryonic developmental events (4, 5). These initial studies left a dilemma on the nature of this RNA-RNA interaction phenomenon and the structure of the RNA species involved. It was not until 1998 that Craig C. Mello and Andrew Z. Fire, who shared the 2006 Nobel Prize in Medicine for their discovery, were able to solve this scientific dilemma presented by the earlier studies. They showed that the double-stranded RNA (dsRNA) manifests a potent gene silencing effect that is more pronounced than that of purified single-stranded RNAs (ssRNA) of either sense, by noting a synergistic effect of sense and antisense RNA mixture (6). Later, studies in plants and Drosophila identified the stable RNA intermediate generated from dsRNA that was involved in silencing the target messenger RNA (mRNA). It appeared to be an interfering antisense RNA of small size. Since its discovery, RNAi proved to be a potent tool for the large scale systematic analysis of gene function in many organisms including Arabidopsis (7), C. elegans (8), Drosophila (9, 10), mosquitoes (11, 12), Zebrafish (13), and human cells (14) as well as several others.
RNA interference has been observed in several living organisms, from plants to vertebrates. It has been the most extensively used reverse genetics tool to study gene function since the last decade. Its success is attributed to the evolutionary conserved pathways that regulate gene expression via small non-coding RNAs (15), the relatively simple way to generate siRNAs or dsRNA in vitro and their ease of use to silence genes in several organisms. Despite its success as a gene silencing tool, RNAi has also its limitations including; incomplete phenotypes due to the lack of complete silencing, off-target effects with the 3´ untranslated region of genes which may be even more problematic if the design of small RNAs is based on genomic sequences of low quality (16, 17). Additionally, some non-specific RNAi effects like activation of the interferon system by siRNA have been reported in the literature (18). Despite these drawbacks, RNAi remains the most powerful functional genomic tool recently developed.
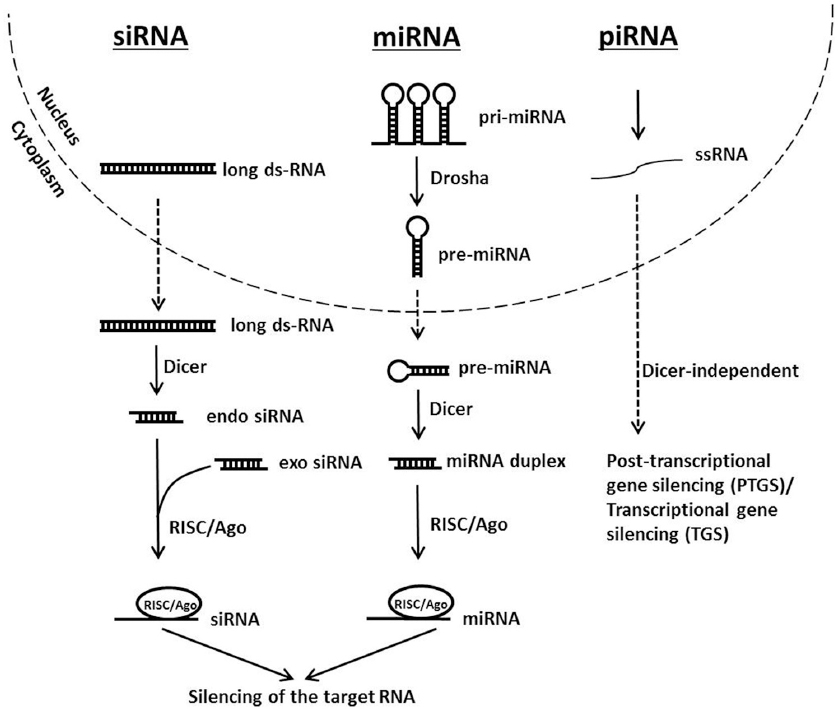
There are three species of small non-coding RNAs involved in gene regulation which have disparate origins but their pathways converge downstream when they assemble into the RNA-induced silencing complex (RISC). These are the microRNA (miRNA), the short interfering RNA (siRNA) and PIWI-interacting RNA (piRNA). The miRNAs originate from the genome as long primary transcripts (pri-miRNA) with single or clustered double-stranded hairpins (19) that are processed by a protein complex containing the RNase III family enzyme Drosha into short ~ 65-70 nucleotide pre-miRNAs (20-22) that are transported to the cytoplasm for further processing. On the other hand, siRNAs may originate from exogenously introduced dsRNA molecules, from viral genomes or from endogenous genomic loci such as centromeres, transposons or other repetitive sequences (23). The piRNA pathway however silences transposable elements in the gonads of metazoans through PTGS and transcriptional gene silencing (TGS). However the biogenesis and processing of piRNAs remain not fully understood but shows clear differences with the miRNA and siRNA pathways notably in being independent of Dicer (24, 25).
In the cytoplasm, pri-mRNA and long dsRNA, whether endogenous or exogenous, are diced by the endoribonuclease Dicer that contains two RNase III domains each involved in cleaving one strand of the duplex RNA (26). The resulting dsRNAs are 21-25 nucleotides in length containing 2 nucleotides overhangs at each 3´ terminal end, and a phosphate group at each 5´ end (27). Small dsRNAs are then loaded into the RNA-induced silencing complex (RISC) constituted of a tri-molecular assembly of dicer, TAR-RNA binding protein (TRBP), and Argonaute 2 (Ago2). In Drosophila, there are two Ago proteins with clearly distinct functions. Typically, miRNAs are loaded on Ago1 that lacks slicing activity and functions by repressing mRNA translation. While siRNAs are loaded on Ago2 which harbors slicing activity that results in target mRNA cleavage. In humans, however all four Ago proteins seem to participate in miRNA-mediated silencing (24). RISC assembly requires two steps: loading and unwinding (28, 29). Loading to Ago2 necessitates help from Dicer and TRBP in addition to ATP. Strand selection is not random; the strand harboring the thermodynamically less stable base pairing at its 5´ end will constitute the guide strand. In Drosophila, this thermodynamic instability is initially detected by dicer and the dsRNA-binding protein R2D2 (30). In the unwinding step Ago2 cleaves the passenger strand in a similar manner it does a target RNA. The cleaved strand is released from Ago2 to liberate the guide strand (31, 32). In case of miRNA, the strand that is loaded to the RISC is known as the miRNA and the opposing strand is the miRNA*. Finally, silencing of the target RNA may occur by endonucleolytic cleavage or by translation repression depending on the type of Ago present in the RISC and the extent of base-pairing between the guide strand (or miRNA) and the target RNA (33). Figure 1 shows a schematic representation of the siRNA, miRNA and piRNA silencing pathways.
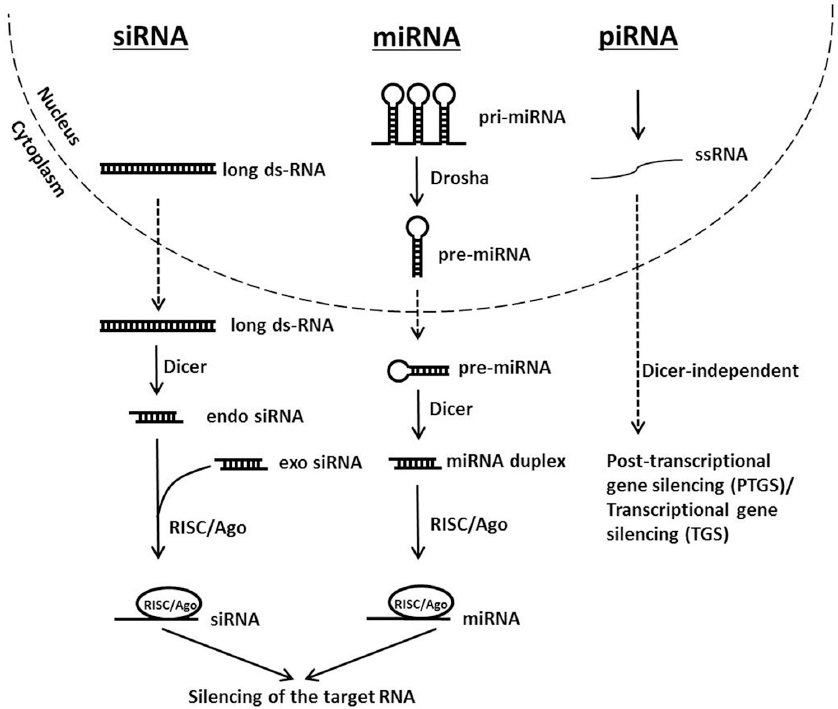 Figure 1
Figure 1Schematic representation of siRNA, miRNA and piRNA silencing pathways.
Gene therapy uses a set of approaches to deliver genetic information to a target cell in order to prevent or cure the incidence or progression of a disease. It has recently gained enormous attention as an option for therapeutic treatment of genetic or acquired diseases. siRNA-based gene therapy showed promising potential applications in many research studies. However, the development of naked siRNA-based treatment is hindered by the difficulty of its delivery into the desired cells, tissues or organs. Due to their negative charge and molecular size, siRNAs are unable to cross the lipophilic cellular membranes. In addition, they are subject to enzymatic degradation and are rapidly eliminated by the renal system. To extend their circulation time, facilitate their transfection efficiency, and enable their selective and efficient delivery, siRNAs can be carried through viral and non-viral carriers. These carriers have been extensively researched in many ex vivo, in vivo and in vitro applications. Both types of carriers have their advantages and disadvantages and hence, the use of one instead of the other depends on the experimental conditions and applications (34, 35). In this review, we will focus on non-viral carriers. For the targeted delivery of siRNAs using viral vectors the readers are referred to the following excellent reviews (34, 36-38).
Non-viral gene delivery was developed to avoid the mentioned serious limitations related to viral gene carriers. It involves the use of physical or chemical techniques to transfer nucleic acids into cells (34, 39, 40). Although non-viral vectors have low transfection efficiency, they are much safer than viral vectors. Physical methods, such as electroporation (41, 42), microinjection (43), sonoporation (44), gene gun (45), magnetofection (46), and electric field-induced molecular vibrations (47) rely on the application of physical forces to facilitate the entrance of the desired nucleic acids into the target cell across the phospholipid bilayer. Chemical methods, on the other hand, include direct modification of the siRNA backbone, its conjugation with various molecules such as lipids, polymers, peptides, and aptamers, and siRNA encapsulation in different nanoparticles (NPs) formulations (48). All these modifications decrease the likelihood of triggering an unwanted innate immune response upon the introduction of the siRNA, lower the incidence of undesirable off-target effects, and hence improve the treatment outcomes.
Direct chemical and structural modifications conducted on the siRNAs terminals, backbone, nucleobases and sugars were found to be effective at improving their pharmacodynamics and pharmacokinetics properties. Given that the 2’ position of pentose sugars is not required for siRNA recognition as well as for RISC activity, incorporation of 2’-fluoro, 2’-O-methyl, 2’-halogen, 2’-amine or 2’-deoxy at this site showed a remarkable enhanced stability compared to wild-type siRNA (49). Moreover, locked nucleic acids (LNA) and unlocked nucleic acids (UNA) proved to be beneficial for siRNA delivery. LNA are rigid structures characterized by the presence of a methylene bridge between the 2’ oxygen and the 4’ carbon however UNA are highly flexible structures obtained following the cleavage of the C2’-C3’ bond of the sugar ring. Although they have differing physical and chemical properties, they were able to protect the siRNA duplex from degradation, increase its stability and reduce the off-target effects (49, 50). Substitution of the phosphodiester bonds by methylphosphonate, phosphorothioate, phosphorodiothioate, and triazole linkages was also investigated in many research studies and was able to improve siRNAs target affinity and plasma half-life (51, 52).
Furthermore, tagging siRNAs with moieties such as cholesterol, peptides, polymers and aptamers lead to beneficial impact on transfection efficiency and allow targeting specific cells and organs. For example, complexation of epidermal growth factor-type 2 siRNA with polyethylenimine (PEI) increased siRNA stability and reduced subcutaneous tumor growth in a mice model (53). Likewise, Soutschek and coworkers developed chemically modified siRNAs towards apolipoprotein B (apoB) for the treatment of hypercholesterolemia. Their intravenous injection in mice exhibited a silencing effect in liver and jejunum denoted by decreased plasma levels of apoB and total cholesterol (54). Cell penetrating peptides (CPP) are a class of short peptides able to translocate across cell membranes. They are used to deliver DNA, RNA, protein molecules, nanomaterials, or pharmaceutical drugs into cells primarily via endocytosis. The bond between cell penetrating peptides and the cargo might be covalent or non-covalent (55). CPP-mediated siRNA delivery has been used to treat diseases such as cancer, infectious diseases, and some genetic disorders and was proved to have high transfection efficiency and low cytotoxicity (56).
Although chemical modifications and siRNA bioconjugation can somehow avoid some problems related to siRNA delivery, nanoparticles that encapsulate siRNA are better at protecting it from nucleases and immune recognition (57). Thus, delivery materials are needed, among them lipid-based and polymer-based vectors hold promising potentials for siRNA transport.
Liposomes, globular vesicles with an aqueous core surrounded by a phospholipid layer, are by far the most widely used lipid-based vectors. They are biodegradable, biocompatible, and suitable for the delivery of hydrophilic as well as hydrophobic drugs (56). They can be cationic, anionic, or neutral. However, due to the polyanionic nature of RNAi, cationic and neutral liposomes are usually preferred as carriers. The promising in vitro results obtained by Halder et al. (2006) with focal adhesion kinase (FAK) silencing using neutral liposomes pointed out that in vivo delivery of FAK siRNA might be a potential novel therapeutic approach to ovarian cancer (58). Cationic liposomes, on the other hand, are slightly different. They comprise three basic components: a lipophilic tail group, a connecting linker, and a cationic hydrophilic head with one or more amine groups. The positively charged head interacts with the negative phosphate backbone of nucleic acids allowing their condensation (59-61). The structure and the physicochemical properties of each liposome component, as well as the nature of the head group can affect the transfection efficiency (62, 63). Indeed, it has been shown that variables such as the size of lipoplexes and the lipid-to-DNA ratio alter significantly the level of gene expression, in experimental set-ups as well as in the lung, heart, spleen, liver and kidneys (64, 65).
Polymer-based carriers, termed polymeric nanoparticles, have been extensively used as drug vesicles. Many are biodegradable, biocompatible and non-toxic which make them good candidates for biomedical applications. They are characterized by their high-drug loading capacity, and their ability to incorporate a broad range of therapeutic agents. The polymeric shell provides protection against enzymatic degradation and rapid renal filtration. The type and molecular weight of the polymer used as well as the drug-to-polymer ratio can affect the drug loading efficiency and release profile. They also exhibit a great potential for surface functionalization that allows improving pharmacokinetic and pharmacodynamics properties and permits targeting drug delivery and accumulation of nanoparticles at the desired site of action (66-68). Cyclodextrin, poly-L-lysine (PLL), PEI, chitosan, dendrimers and poly (D,L-lactide-co-glycolide) (PLGA) have received a lot of attention as delivery carriers.
Dendrimers are spherical, highly symmetrical, and usually monodisperse macromolecules with hyperbranched arrays. They have a central core surrounded by concentric layers called generations. Among the many different available dendrimers, polyamidoamine (PAMAM) has gained a particular interest due to its versatile nature that allows the biological and physiochemical modifications of its end groups. It has unique features such as a nanosized, easily modifiable, cationic surface. The primary amine groups on the surfaces of the PAMAM molecules facilitate their electrostatic interaction with nucleic acids, thus forming nanoscale complexes known as “dendriplexes” (69). A recent study screened a library of surface-engineered dendrimers for structure-activity relationships regarding siRNA delivery. It was demonstrated that dendrimers modified with hydrophobic ligands such as aliphatic chains and aromatic rings, as well as functional ligands such as fluorine, bromine, boronic acid, and guanidine groups improve the efficacy of the knockdown with minimal cytotoxicity (70).
Furthermore, cationic polymers such as PLL, PEI, and chitosan have attracted the attention of many scientists due to their ability to form complexes with nucleic acids by means of stable electrostatic interactions (71, 72). Their properties such as size, surface charge, and structure are dependent on the ratio of the positive charges on cationic polymers to the number of negatively charged phosphate groups in nucleic acids. Polyethyleneimine has been examined as a potential non-viral delivery vehicle for oligonucleotides, siRNA and plasmid DNA in vitro and in vivo (73). PEI exists in different molecular weights and with degrees of branching (74). Its amino groups confer two important properties: a superior buffering capacity over a wide range of pHs and an ability to condense nucleic acids to form positively charged complexes. The toxicity and the transfection efficiency using this compound depend on the properties of the molecule used. When PEI is more branched and has a higher molecular weight, it condenses siRNA more efficiently thus increasing the delivery efficiency but at the expense of being more toxic (75). Hence, the toxicity associated with PEI has been regulated by using low molecular weight molecules, by incorporating them into other polymeric constructs, or by modifying them to either shield the charge or transform the backbone into its biodegradable analogue (71). The complexation of PEI and siRNA demonstrated a reduction in target gene in different cellular models. Kim et al. demonstrated the delivery of siRNA against vascular endothelial growth factor (VEGF) through polyelectrolyte complex micelles formed by the conjugation of PEGylated siRNAs (siRNA-PEG) with PEI. These micelles showed enhanced stability compared to naked VEGF siRNA. Furthermore, they were able to silence VEGF gene expression in prostate carcinoma cells up to 96.5%. They also demonstrated a greater VEGF gene silencing effect than VEGF siRNA/PEI complexes (76).
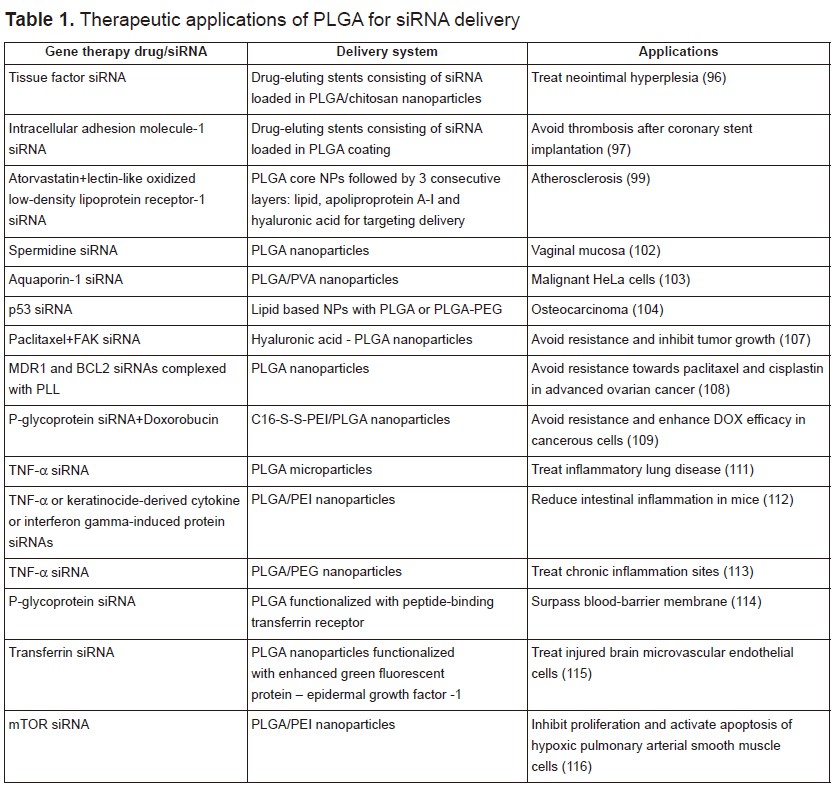
Among all, PLGA has shown immense potential as a good delivery material for a variety of drugs including anticancer and antihypertensive agents, hormones, vaccines, as well as for macromolecules such as nucleic acids, proteins, peptides, and antibodies. PLGA, is a copolymer of poly(D,L-lactic acid) and poly(glycolic acid). It is biocompatible, biodegradable, and non-toxic. Most significantly, it has been approved for human use by the US Food and Drug administration (77). In the next section, we will mainly focus on the in vitro and in vivo biological applications of PLGA vectors for gene silencing. Some of the selected examples listed below are summarized in table 1.
|
Different mechanisms are used to synthesize PLGA. Ajioka et al. prepared high molecular weight PLGA by azeotropic dehydration, using a solution of diphenylether with L-lactic acid and glycolic acid in the presence of tin powder as a catalyst at 130°C. It typically requires 20-40 hours to obtain PLGA using this protocol. However, this reaction is hard to control and the desired product has to be purified from many other by-products (78). To prepare PLGA in a shorter time, the cationic ring-opening polymerization of cyclic dimers, glycolide and lactide, is performed. Since lactide exists in two enantiomeric isomers, the synthesized PLGA polymer can be under the form of D-, L-, or D, L- stereoisomer. This reaction requires 2-6 hours and is commonly done in the presence of stannous octoate as catalyst at 175°C. During its synthesis, fine-tuning of relevant parameters such as molecular weight, lactide to glycolide ratio, and lactide stereoisomeric composition (L- or D, L-lactide) is one of the key components for acquiring the appropriate and specific properties needed for various applications. Therefore, PLGA solubility, crystallinity, thermal stability, mechanical properties, and degradation can be controlled widening its performance spectrum (79, 80). Due to these characteristics, PLGA have found a broad range of applications in tissue engineering including blood vessel replacement, bone, cartilage and ligament restoration as well as skin healing (81-88). Furthermore, PLGA nanoparticles have been demonstrated as a promising drug delivery platform owing to the aforementioned properties, along with their ability to sustain optimal therapeutic doses for a prolonged time. They have been used for diagnosis, vaccine delivery, and for the treatment of many pathophysiological diseases carrying a variety of drugs, as well as, antibiotics, proteins, and nucleic acids (89-91). PLGA nanoparticles’ cells uptake involves an endocytic pathway. However, the major drawback is their low intracellular retention due the fast exocytosis. Surface functionalization and complexation with ligands can reduce this occurrence, enhance cellular uptake and permit targeted drug-delivery (92). For instance, nanoparticles conjugated with transferrin ligands showed increased cellular uptake and accumulation in cell populations filled with transferrin receptors. It was demonstrated that only 50% of transferrin-conjugated nanoparticles exhibit exocytosis vs. 75% of the unconjugated nanoparticles. This is mainly related to different endocytic pathways involved in their internalization (93).
A recent report from the American Heart Association estimated that 92.1 million American adults are living with some form of cardiovascular disease. Coronary heart disease is the leading cause of deaths in the US (43.8%), followed by stroke (16.8%), high blood pressure (9.4%) and heart failure (9%) (94). PLGA have found many applications in cardiology, in particular as scaffolds and coating matrices for drug-eluting stents, which have been proven to be highly effective in reducing stent thrombosis and restenosis (95). Qiu et al. prepared a novel external stent by deposition of hybrid fibrous membranes consisting of tissue factor siRNA loaded in PLGA/chitosan nanoparticles. These siRNA eluting stents were able to attenuate neointima hyperplasia in rat vein grafts (96). In another study, siRNAs against intracellular adhesion molecule-1 (ICAM-1) were incorporated into different PLGA resomers. These coatings proved to have no cytotoxic effect towards human umbilical vein cell line and a good hemocompatibility since no reduction in the number of platelets, leukocytes, lymphocytes, monocytes, and granulocytes was noticed after 1 h incubation. ICAM-1 siRNAs loaded in the PLGA Resomer® RG 752 H (75:25, acid terminated, 4,000–15,000 Da) exhibited optimal results with 95% transfection efficiency and 36% knockdown effect. These findings prove the potential incorporation of siICAM-1 in PLGA coatings for the elaboration of novel drug eluting stents (97). Cohen-Sacks and coworkers showed that platelet-derived growth factor beta-receptor antisense loaded PLGA nanoparticles could serve as effective gene delivery system for the treatment of restenosis. The synthesized nanoparticles displayed a homogenous size, high encapsulation efficiency and a sustained release (98). Dual targeting approach has been greatly reported for enhanced drug delivery and synergistic effect. In a recent study, Zhao et al. prepared dual-targeting bifunctional core-shell nanoparticles to deliver atorvastatin and siRNA against lectin-like oxidized low-density lipoprotein receptor-1 to endothelial cells and macrophages in the atherosclerotic lesions. The drugs were encapsulated within the first PLGA core shell followed by three consecutives layers made up from lipid for cholesterol receiving, apoliprotein A-I for macrophage targeting and hyaluronic acid for endothelial cell targeting. These nanoparticles showed enhanced delivery and improved anti-atherosclerotic efficacy (99).
According to the national institute of cancer, cancer is a set of malignant diseases characterized by uncontrolled proliferation of mutated cells in a body tissue that has been exposed to mutagens. In 2012, there were 14.1 million new cases and this number is expected to rise to 23.6 million per year by 2030 (100). Several treatment options such as chemotherapy, radiotherapy, surgery and gene therapy have been used so far but each has its implications on patients. Targeting efficiently cancerous cells, while sparing normal cells from the adverse effects of the delivered chemicals and genes, remain a challenging goal. Numerous targeted nanoparticle approaches are currently being investigated to deliver the appropriate drugs or genes into the malignant cells by mean of ligand-based targeting mechanisms. For that purpose, nanoparticles must undergo some modifications to allow their efficient delivery into the abnormal cells. This can be done using hydrophilic surfaces, coatings with a positive charge carrier and/or with proper markers to improve cellular incorporation, increase their circulation duration, and permit the differentiation between normal and cancerous cells (101). PLGA nanoparticles have attracted extensive interest for applications in cancer therapy. Woodrow et al. reported the efficient and sustained gene silencing of siRNA-spermidine loaded PLGA nanoparticles for the treatment of vaginal mucosa (102). Another study reported the use of siRNA-chitosan complex, encapsulated into a multilayer shell of PLGA and poly (vinyl alcohol) (PVA) for silencing Aquaporin-1 in cancer cells. These nanoparticles demonstrated high stability under physiological conditions. They reduced the expression of Aquaporin-1 by 70%, and showed no cytotoxicity up to 72 hours of incubation (103). Kundu and coworkers reported the use of lipid based NPs with either PLGA or PLGA-PEG to encapsulate p53 siRNA against osteocarcinoma. Incorporation of 10% PLGA showed the highest p53 knockdown efficiency (104).
Despite all the efforts, drug resistance remains a major challenge in the treatment of cancer. It can occur by different mechanisms, including decreased drug uptake, increased drug efflux, activation of DNA repair mechanisms, suppression of drug-induced apoptosis, etc (105). It has been shown that overexpression of FAK plays a major key role in ovarian cancer and promotes chemoresistance (106). Byeon et al. suggested the development of hyaluronic acid-PLGA nanoparticles encapsulating both paclitaxel and FAK–siRNA to reverse chemoresistance. This combination therapy proved to be effective in inhibiting tumor growth in patient-derived xenograft model compared with paclitaxel alone (107). However, other researchers showed optimal suppression of chemoresistance when siRNAs loaded PLGA nanoparticles are administrated 1 day earlier than drugs. Therefore, they designed PLGA nanoparticles to co-deliver MDR1 and BCL2 siRNA aimed for the simultaneous inhibition of drug reflux and cell death defense pathways and to avoid resistance towards paclitaxel and cisplastin in recurrent or advanced ovarian cancer. To ensure high encapsulation efficiency, siRNAs were complexed with the cationic poly-L-lysine (108). Furthermore, Wu et al. reported the elaboration of programmable PLGA biomaterials for dynamic and consecutive release of siRNA followed by the drug. In this work, o-nitrobenzyl ester derivative caged doxorubicin (DOX) was encapsulated in polymeric NPs constructed by the self-assembly of C16-S-S-PEI and PLGA. siRNA of P-glycoprotein (P-gp) were adsorbed onto its cationic polymer shell and their release were triggered by the reductive cleavage of the disulfide bond in the presence of high levels of glutathione in cancer cells. DOX is afterwards released upon irradiation and its conversion DOX prodrug into hydrophilic active DOX. This sequential release strategy showed enhanced efficacy of DOX following P-gp silencing and can be used against multiple drug resistance (109).
Inflammation occurs when tissues are injured by bacteria, trauma, toxins, heat, or any other cause. It is a defense mechanism in the body and an essential component of many illnesses including rheumatoid arthritis, sepsis, lung and gastrointestinal diseases, etc. Tumor necrosis factor-α (TNF-α) might be considered as potential therapeutic target due to its high levels in many acute and chronic inflammations. It was shown that PLGA nanoparticles loaded with TNF-α siRNA have a significant effect in decreasing the paw scores and joints effusion in a murine arthritis model (110). Furthermore, kelly et al. used anti-TNF-α siRNA encapsulated in PLGA microparticles for the treatment of inflammatory lung disease. This work revealed a sustained siRNA knockdown of TNF-α in primary monocytes for up to 72 hours (111). In another study, siRNA against TNF-α, keratinocide-derived cytokine or interferon gamma-induced protein were adsorbed to the surface of a calcium phosphate core, followed by their encapsulation into PLGA nanoparticles coated with an outer layer of PEI to enhance cellular uptake and subsequent endosomal escape. Intrarectal application of these nanoparticles demonstrated a significant decrease of the target gene with a subsequent reduction of intestinal inflammation in mice (112). Aldayel and coworkers reported the elaboration of TNF-α siRNA loaded PLGA NPs by the double-emulsion method using a special emulsifying agent which renders the nanoparticles PEGylated and the PEGylation readily sheddable in low pH environment such as that in chronic inflammation sites. This technique showed a higher accumulation of nanoparticles in inflamed mouse foot compared to nanoparticles prepared by an acid-insensitive emulsifying agent (113).
siRNA loaded PLGA nanoparticles have attracted a lot of interest in many other biomedical applications. They have been used to enhance drug delivery to human brain endothelial cells for future medical therapeutic prospects aiming to surpass the blood-brain-barrier and overcome central nervous system disorders. PLGA nanoparticles were loaded with siRNA against P-gp, a drug efflux transporter, and were functionalized with a peptide-binding transferrin receptor. They demonstrated no cytotoxicity and a 4-fold increased efficiency compared to non-functionalized NPs (114). In another study, Chen et al. reported the delivery of transferrin-siRNA loaded PLGA NPs to injured brain microvascular endothelial cells. These nanoparticles showed very low cytotoxic effect and good transfection efficiency mainly due to their functionalization with enhanced green fluorescent protein – epidermal growth factor -1 that targets the overexpressed tissue factor in the injured cells (115). Moreover, mTOR siRNA was loaded into hybrid NPs made from PEI and PLGA. They inhibited proliferation and activated apoptosis of hypoxic pulmonary arterial smooth muscle cells (116).
Due to the rapid progress in the applications of RNAi, it is considered as one of the most powerful tools for gene therapy. First described in 1990, it now opens endless possibilities and provides a promising insight to manage countless pathophysiological conditions that seemed inaccessible few years ago. Scientists across the globe have devised numerous methods in which small RNA molecules can be administered into target cells to manipulate cellular functions at the molecular level and eradicate defects that lead to pronounced phenotypes, which might sometimes be as fatal as malignant tumors. Targeted delivery of these RNA molecules, the action of which relies on the RNAi and subsequent gene silencing, can be done using viral or non-viral vectors. PLGA is one of the chemicals that have been approved by the FDA for use in humans. This huge medical leap is extended beyond its initial aim. Hence, it is no longer only used in tissue engineering such as remodeling of bone and cartilage, but it indeed plays a crucial role now as chemical carrier for a broad range of therapeutic agents. It is greatly implicated in gene therapies and inherited genetic defects. This field, as interesting and promising as it seems, is still in its neonatal stages. Investigations are being done in collaboration with physiologists, biomedical engineers, medical doctors, biochemists, and specialists from all scientific disciplines to adopt this modern approach and improve it, invest it optimally to yield the best results, and subsequently achieve what sounded once far-fetched.
This work is supported by the National Council for Scientific Research (CNRS) of Lebanon.
Abbreviations: apoB: apolipoprotein B, Ago2: Argonaute 2, CHS: chalcone synthase, CPP: cell-penetrating peptides, dsRNA: double-stranded RNA, DOX: doxorubicin, FAK: focal adhesion kinase, ICAM-1: intracellular adhesion molecule 1, LNA: locked nucleic acids, mRNA: messenger RNA, miRNA: micro RNA, NPs: nanoparticles, piRNA: PIWI-interacting RNA, pre-miRNA: precursor-microRNA, pri-miRNA: primary-microRNA, PTGS: post-transcriptional gene silencing, RISC: RNA-induced silencing, RNAi: RNA interference, siRNA: small interfering RNA, ssRNA: single-stranded RNA, TRBP: TAR-RNA binding protein, PAMAM: polyamidoamine, PEG: polyethylene glycol, PEI: complex polyethyleneimine, P-gp: P-glycoprotein, PLGA: poly(lactide-co-glycolide), PLL: poly-L-lysine, PVA: poly (vinyl alcohol), TGS: transcriptional gene silencing, UNA: unlocked nucleic acids, TNF-α: Tumor necrosis factor-α, VEGF: vascular endothelial growth factor.