1 Department of Dermatology, and Institute of Regenerative Medicine, Affiliated Hospital of Jiangsu University, 212001 Zhenjiang, Jiangsu, China
2 Department of Dermatology, The First People’s Hospital of Changzhou, 213003 Changzhou, Jiangsu, China
3 Haihe Laboratory of Cell Ecosystem, Institute of Hematology, Chinese Academy of Medical Sciences Institute of Hematology and Blood Diseases Hospital, 300020 Tianjin, China
4 Guangdong Provincial Key Laboratory of Large Animal Models for Biomedicine, and South China Institute of Large Animal Models for Biomedicine, School of Pharmacy and Food Engineering, Wuyi University, 529000 Jiangmen, Guangdong, China
5 Department of Medicinal and Life Sciences, Faculty of Pharmaceutical Sciences, Tokyo University of Science, 125-8585 Tokyo, Japan
Abstract
Aging is an inevitable reality that every individual has to face. People look forward to intervene and slow down this process, for example, skin anti-aging cosmetic and therapeutic treatments are commercially available in a variety of methods, such as skin tightening and dermal fillers, but these approaches do not fundamentally change the aging state of senescent cells. Fortunately, macrophages possess the capability to promote tissue repair and regeneration, induce angiogenesis, and improve the tissue microenvironment, making their application in the field of skin anti-aging potentially possible. In this review article, we unveiled the features of aged skin, including a reduction in the extracellular matrix, a decrease in vascular density, diminished defense capabilities, and increased inflammation. We then summarized the possible anti-aging functions of macrophages in this field, such as anti-inflammation, immunoregulation, promotion of angiogenesis, and regeneration. We also suggested potential strategies for utilizing macrophages in anti-aging therapies, including recruiting macrophages to the skin, supplying induced macrophages, and regulating macrophage activity. In conclusion, macrophages may play a role in cell therapy for skin anti-aging, though their potential efficacy and mechanisms need to be further explored.
Keywords
- skin
- macrophages
- anti-aging
- angiogenesis
In contemporary society, the rapid development of material production and swift advancements in technology have led to an elevated standard of living, where individuals’ demands are no longer confined to basic necessities such as food and shelter. Instead, there has been a significant increase in the emphasis on quality of life, which includes concerns related to skin aging and anti-aging measures. In recent years, the cosmetic surgery industry has experienced rapid growth, reflecting society’s increasing pursuit of beauty. More concerning is the accumulation of senescent cells in the skin, which may have distant effects on other organs and even impact the nervous system, potentially leading to a decline in cognitive abilities [1]. As material development in society becomes increasingly abundant, individuals are placing greater emphasis on skin anti-aging. The methods for combating aging have also diversified, including techniques such as intense pulsed light therapy [2], injectable fillers for shaping [3], and chemical antioxidants [4]. In addition to natural aging, certain treatment modalities can also contribute to skin aging. Macrophages, as inherent immune cells of the body, play a dual role; they not only engulf foreign substances and senescent cells but also secrete growth factors that promote tissue repair and angiogenesis [5]. From this perspective, macrophages possess significant potential for cosmetic anti-aging applications [6, 7].
Through proteomic analysis of fibroblasts cultured from skin biopsy samples ranging in age from 22 to 89 years, Tsitsipatis et al. [8] identified key pathways associated with the senescence of skin fibroblasts. These pathways include autophagy, reactive oxygen species (ROS) clearance, ribosome biogenesis, Deoxyribonucleic Acid (DNA) replication, and DNA repair. Although multiple proteins are involved, no single protein exhibited a significant change across all individuals within a specific age group. Therefore, it is likely that the aging process is the result of the collaborative actions of various proteins within specific pathways [8]. Moreover, in another research, the number of senescent cells did not show a significant correlation with age [9]. Therefore, it can be inferred that aging may not be an inevitable consequence of increasing age; rather, advancing age simply increases the likelihood of experiencing aging-related changes.
In previous rejuvenation procedures, physicians have utilized fillers such as hyaluronic acid, polynucleotides, calcium hydroxyapatite, and poly-L-lactic acid to promote skin regeneration. These fillers can induce a granulomatous inflammatory response, stimulating collagen synthesis in the skin and increasing skin volume [3]. Macrophages mediate this response, with classically activated macrophages (M1) secreting pro-inflammatory cytokines like IL-1
Researching skin anti-aging requires a thorough understanding of the differences between youthful and aging skin. To maintain the youthful state of the skin, it is essential to adopt a targeted approach. Clearly defining the distinctions between youthful and aging skin, identifying the factors that contribute to aging, and determining whether these factors can be controlled or reversed is crucial.
Multiple studies have found that aging skin becomes thinner, although the underlying mechanisms remain unclear [10, 11, 12]. Xiang et al. [12] proposed that the reduction in fibroblast size is associated with increased expression of hepatocyte growth factor (HGF). Zou et al. [10] found that during the process of skin aging, both epidermal thickness and dermal collagen density consistently decline. Further analysis found that genes upregulated with age are primarily related to inflammatory cytokines, apoptosis, and photoaging-related signaling pathways, while genes downregulated with age are mainly associated with epithelial cell proliferation and extracellular matrix (ECM) organization [10]. However, with aging, the TGF-
Skin aging is not merely a result of cellular senescence, the microenvironment also undergoes changes that interact with cellular processes. One study demonstrated that aging hair follicle stem cells (HFSCs) can regenerate hair follicles (HF) when supported by young dermis, whereas young HFSCs fail to regenerate HF when associated with aged dermis [14]. Additionally, the increase in tissue stiffness, a key mechanical property of tissues, can trigger mechanical signaling [15]. During skin aging, the depletion of hemidesmosomes leads to a loss of their supportive role in maintaining epidermal stem cells [15].
Senescent cells can trigger inflammatory responses, which in turn may promote apoptotic reactions [16]. However, why do organs and tissues continue to age with advancing age? This may indeed be one of the mechanisms by which senescent cells evade immune clearance and apoptosis [17, 18]. Specifically, caspase-3 activity, a key enzyme associated with apoptosis, is deficient in fibroblasts, and senescent fibroblasts maintain elevated levels of the pro-survival factor Bcl-2. This allows them to permanently exit the cell cycle and become resistant to ultraviolet (UV)- or staurosporine-induced apoptosis [17]. Research indicates that senescent dermal fibroblasts express the non-classical major histocompatibility complex molecule human leukocyte antigen E (HLA-E), which inhibits natural killer (NK) cell activity and cluster of differentiation 8 (CD8+) T cell activation, thereby preventing targeted elimination of senescent cells [18]. Concurrently, immunosenescence, or the decline in immune function, may also contribute to the inability to completely clear senescent cells as individuals age. For instance, the dysfunction of NK cells has been confirmed during human aging, which further exacerbates the accumulation of senescent cells over time [19].
Therefore, the primary changes observed in aging skin include thinning of the skin, reduction of the ECM, decreased vascular density, diminished immune defense capabilities, and increased inflammation. In addition, changes in the microenvironment and immune compromise are also critical reasons for skin aging.
As early as 2008, researchers classified macrophages into three subtypes based on their functions: host defense, wound healing, and immune regulation [5]. These subtypes do not represent distinct functions; rather, they can overlap and transition from one to another (Fig. 1). It was emphasized that macrophages exhibit significant plasticity, enabling them to spontaneously alter their phenotype in response to environmental signals [5]. The understanding of macrophages is commonly described in terms of two subpopulations: classically activated M1 macrophages and alternatively activated M2 macrophages [20]. M1 macrophages exhibit pro-inflammatory characteristics and can be polarized by lipopolysaccharides, Th1 cytokine interferon-gamma (IFN-
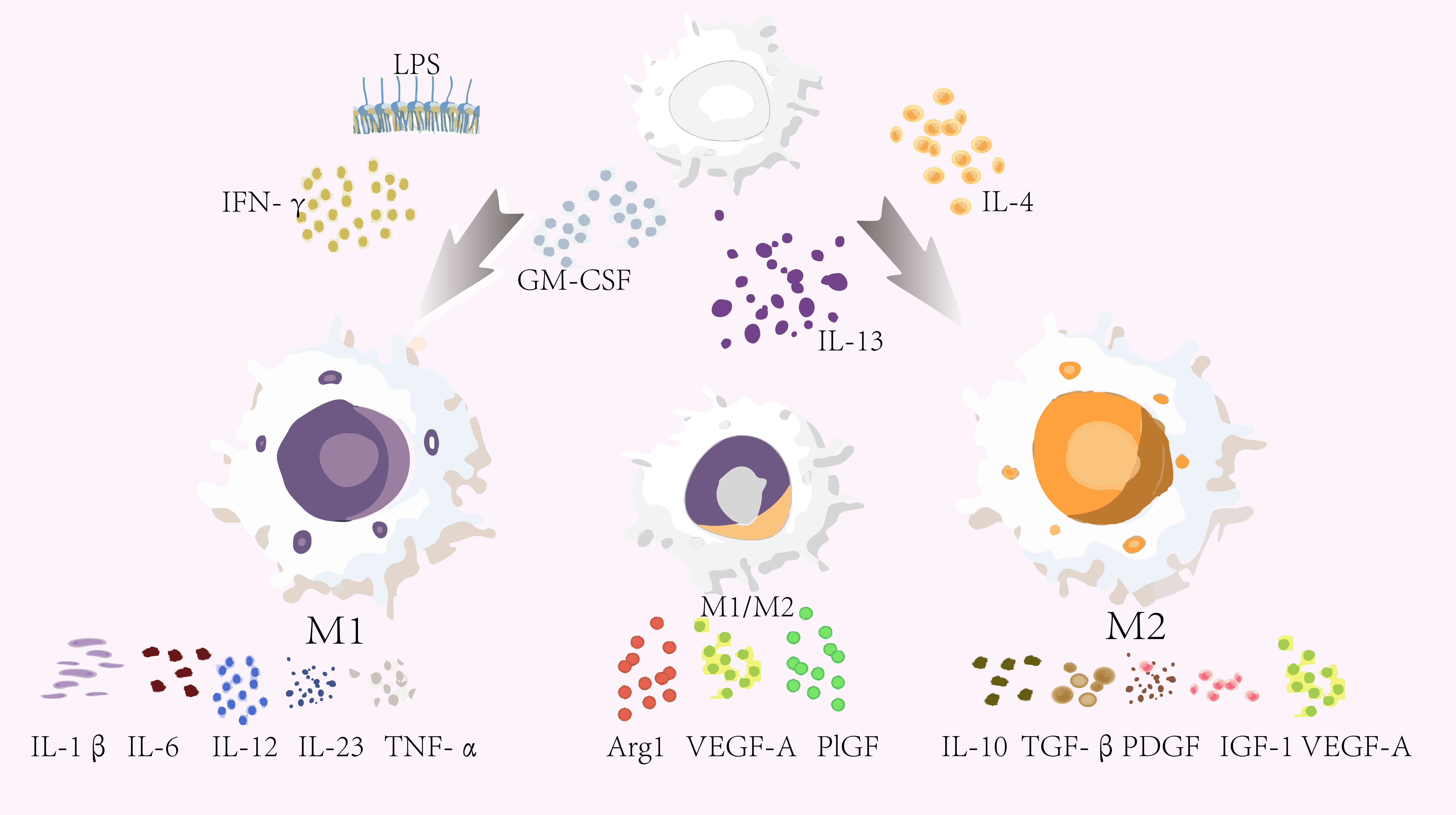 Fig. 1.
Fig. 1. Macrophage subpopulations and their functions. Inactive macrophages can be polarized into M1 macrophages upon exposure to lipopolysaccharides (LPS) or Th1 cytokines (such as LPS, IFN-
The reparative function of macrophages in tissue damage is widely recognized. Interestingly, some researchers have found that during the process of fracture healing, there is an elevation in markers associated with the senescence-associated secretory phenotype (SASP). This suggests that aging may share similarities with the tissue repair process; however, the specific molecular mechanisms remain unclear [23]. Saul et al. [23] utilized dasatinib and quercetin, a combination that can enhance the recognition and clearance of senescent cells by immune cells such as macrophages, to promote the process of fracture healing. Ogata et al. [24] suggest that SASP can inhibit macrophage function, while senescent fibroblasts may lead to the accumulation of senescent cells. Dasatinib and quercetin treatment could potentially activate CD11b-positive cells [25], which include macrophages. Therefore, we infer that macrophage activation may clear senescent cells, thereby improving the SASP phenotype. Selective elimination of senescent skin cells can alleviate and improve the aging phenotype of the skin, and anti-aging drugs may hold potential as new therapeutic agents for the treatment of skin aging [26].
Hasegawa et al. [9] indicated that human cytomegalovirus is reactivated in senescent cells. Certainly, if it were possible to clear senescent cells by targeting viral infections, this would facilitate rejuvenation efforts, making them more accessible and targeted. Macrophages are rich in sensor proteins such as Toll-like receptors (TLRs). Upon activation by IFN-
Macrophages are essential for wound repair. Compared to young skin, aged skin wounds exhibit more pronounced inflammatory characteristics, and there is an increased abundance of the arginase-1 (Arg1) high expression macrophage subpopulation [28]. This Arg1 high expression macrophage subpopulation represents an intermediate state with both M1 and M2 characteristics [28]. They express inflammatory genes while also displaying molecular features indicative of high hypoxia and glycolysis, but low oxidative phosphorylation [28]. These findings suggest that in aged skin, macrophages play a crucial role in both anti-inflammatory responses and tissue repair. In healing response, macrophages are playing an integral role in synthesizing numerous potent growth factors, that promote angiogenesis, cell proliferation and the synthesis of ECM molecules by resident skin cells [29]. These growth factors include TGF-
Exposure to environmental factors such as UV radiation or errors in the mitochondrial respiratory chain can lead to oxidative stress, which in turn results in aging [30]. During the process of photoaging, UV radiation induces oxidative stress in epidermal cells and stimulates keratinocytes to release inflammatory cytokines such as IL-1
Angiogenesis plays a crucial role in anti-aging [32]. Numerous studies have demonstrated that macrophages can mediate tissue vascularization [33, 34, 35, 36]. Keren et al. [32] found that transplanting aged human skin onto young severe combined immunodeficiency (SCID) mice improved human skin angiogenesis, epidermal pigmentation, and aging-related biomarkers. In this process, mouse-derived VEGF-A initiated a cascade reaction, leading to the upregulation of VEGF-A expression and secretion in the aged human skin, thereby promoting angiogenesis and improving the aging condition [32]. It is known that aging is associated with skin hardening, and Ichijo et al. [37] suggest that this is due to the degeneration of skin vasculature. By inducing dermal vascular softening, it may be possible to improve the dysregulation of interfollicular epidermal stem cells in aged skin, potentially improving the aging condition of the skin [37]. During the wound healing process, macrophages from different sources can produce numerous growth factors that promote cell proliferation and blood vessel development, including PDGF, insulin-like growth factor (IGF-1), and VEGF-A [21].
VEGF-A has been shown to be critical for angiogenesis [38, 39], and blood-derived inflammatory C-C chemokine receptor type 2 (CCR2+) lymphocyte antigen 6 complex, locus C (Ly6C+) high monocytes/macrophages have been identified as a key source of VEGF-A in skin wounds [35]. In the early stages of skin injury, inflammatory CCR2+ Ly6C+ macrophages rapidly accumulate, and these macrophage populations express pro-angiogenic factors including VEGF-A, placental growth factor (PlGF), and Arg1. The dynamics of CCR2+ cell recruitment are associated with the strong induction of the CCR2 ligand C-C motif chemokine ligand 2 (CCL2) at the wound site. Macrophages also play a crucial role in muscle angiogenesis. The deficiency of folliculin interacting protein 1 (FNIP1) in myofibers induces the transcription of chemokine genes that activate peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1
M2 macrophage-derived exosomes regulate induced angiogenesis and increase flap survival through the hypoxia-inducible factor 1 alpha inhibitor (HIF1AN)/ hypoxia-inducible factor 1-alpha (HIF-1
In addition to inducing angiogenesis, recent studies have identified clonogenic endothelial macrophage (EndoMac) progenitor cells in the adventitia of the mouse aorta. These bipotent progenitor cells possess proliferative and vasculogenic properties, contributing to the formation of new vessels and perfusion in ischemic tissues when transplanted [43]. EndoMac progenitor cells can differentiate into endothelial cells and macrophage lineages at the single-cell level. This study broadens our understanding of the origins of macrophages, suggesting that their homology with endothelial cells may help explain the phenomenon of macrophages, as immune cells, promoting angiogenesis. However, the underlying mechanisms still require further exploration by scientists.
Macrophages were first discovered over a century ago and have since been shown to be important participants in tissue-specific environments, playing various roles under both homeostatic and pathological conditions [44]. Tissue-resident macrophages exhibit effective communication and can modify their phenotypes in response to microenvironmental cues, thereby influencing their local niches during development and tissue homeostasis. The characteristics of the tumor microenvironment are known to be associated with prognosis. In the case of diffuse large B-cell lymphoma, Croci et al. [45] found that ecreted protein acidic and rich in cysteine (SPARC)-positive macrophages and stromal cells were directly correlated with improved progression-free survival and overall survival. This suggests that macrophages may regulate disease progression by enhancing the microenvironment.
Aged skin macrophages aid wound healing, but hypoxia and ischemia slow their repair compared to younger skin [28]. This highlights the importance of the microenvironment in the repair process. Although healing is delayed in aged skin, it can still slowly repair under the influence of macrophages, suggesting that macrophages retain their reparative functions in aging cells. The observed decline in efficiency may be attributed to an overly strong inflammatory response [28]. Therefore, we can consider that macrophages can influence the microenvironment while also being influenced by it.
Considering the biological functions of macrophages, which include anti-inflammatory repair and regeneration, promotion of angiogenesis, and microenvironment regulation, they have been found to be associated with anti-aging functions in multiple tissues (Table 1, Ref. [2, 6, 7, 46, 47, 48]). Thus, they have the potential to serve as a promising cellular tool for anti-aging interventions in the skin (Fig. 2).
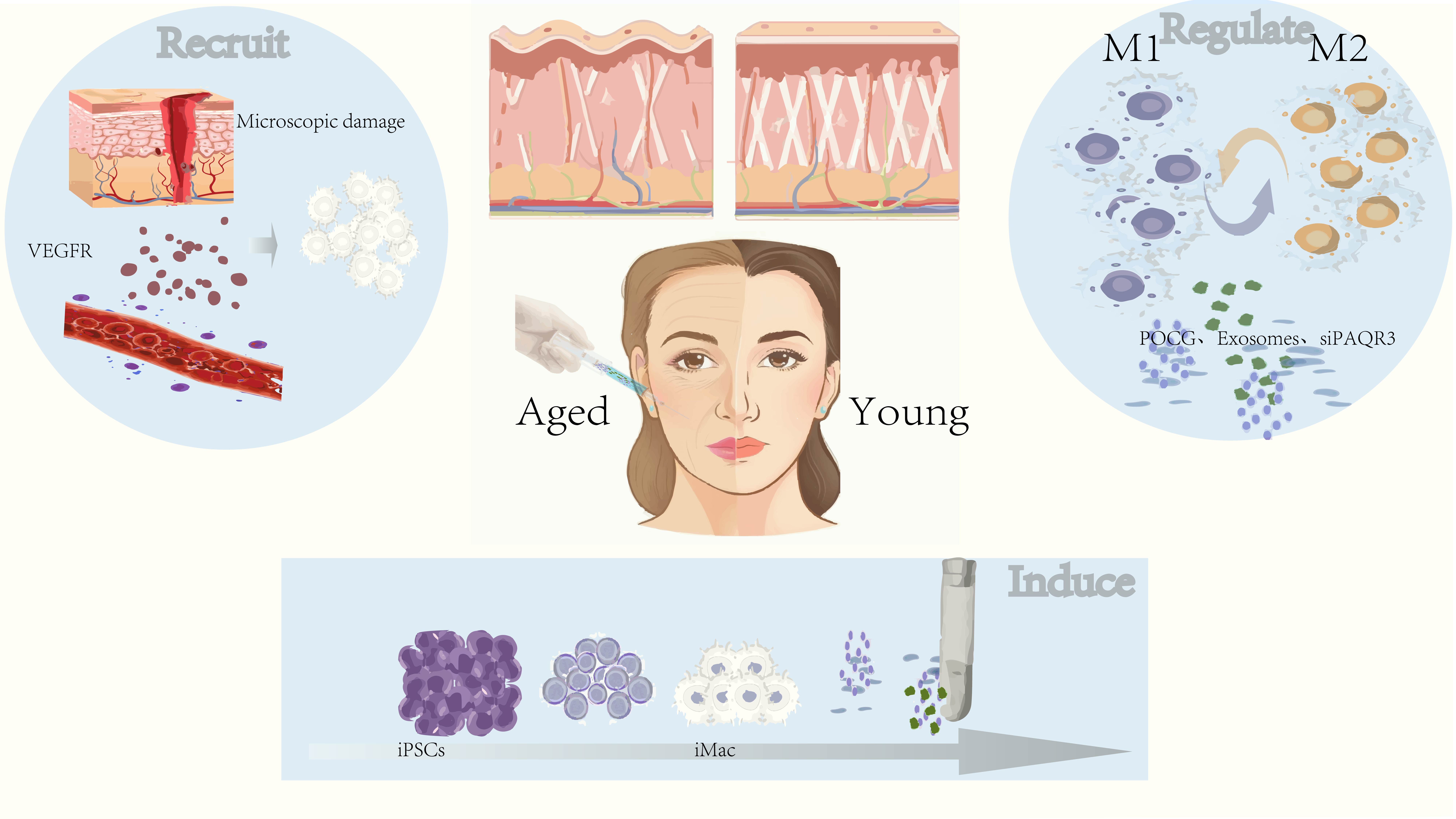 Fig. 2.
Fig. 2. Utilizing macrophages to combat skin aging. Macrophages can be recruited to skin tissue to promote tissue regeneration either by inducing skin damage or by administering exogenous pro-macrophage factors such as VEGFR. Additionally, regenerative medicine techniques can be employed to differentiate effective macrophages in vitro, which can then be supplemented into aged skin tissue to stimulate inflammatory apoptosis of senescent cells and promote skin rejuvenation. Furthermore, substances that modulate macrophage states, such as POCG, PAQR3 inhibitors, or exosomes from young fibroblasts, can be used to appropriately adjust the physiological status of macrophages according to specific needs. This approach not only facilitates the clearance of senescent cells from aging skin but also promotes angiogenesis and improves the microenvironment of the skin tissue. VEGFR, Vascular Endothelial Growth Factor Receptor; iPSCs, Induced Pluripotent Stem Cells; iMac, induced Macrophages; POCG, Poly(octanediol-citrate-polyglycol); siPAQR3, Small Interfering RNA targeting PAQR3; M1, Classically Activated Macrophages (or M1 Macrophages); M2, Alternatively Activated Macrophages (or M2 Macrophages).
| Tissues or organs | Mechanism of action | Macrophages origin | Year |
| Skin | Enhancing action potentials of macrophages. | Recruitment | 2006, [2] |
| Skeletal muscle | In situ phagocytosis and pro-proliferative effect. | Recruitment | 2018, [47, 48] |
| Cardiac tissue | Inhibiting TLR4/MyD88/NF- | Recruitment | 2022, [7] |
| Aorta, kidney, liver, lung and spleen | C1qb macrophages affect the Sirt1 pathway, regulating NAD+ metabolism. | Tissue-resident | 2024, [6] |
| Articular cartilage and the synovial tissue | Suppressed IL-1 | Exogenous supplementation | 2024, [46] |
Note: IL-1
Macrophages at the wound site are recruited from the blood besides few resident macrophages [29] (Table 2, Ref. [11, 20, 21, 29, 38, 46, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60]). Macrophages may promote vascular development by inducing the expression of insulin-like growth factor binding protein 7 (IGFBP7) and HGF [34]. Additionally, Transwell experiments revealed that the positive effect of non-contact co-culture on angiogenesis was reduced, and the vascular density was lower compared to mixed culture with macrophages, suggesting that cell contact is necessary for promoting angiogenesis [34]. Therefore, the recruitment of macrophages to aging skin is essential for skin regeneration and rejuvenation.
| Origins of Macrophage | Induced by | Effectiveness | Year, Ref |
| In vivo recruiting | Wounds | Gathering in the wounds | 2007, [29] |
| VEGFR-3 | Inducing neovascularization | 2009, [38] | |
| In vitro generation from iPSCs | BMP4, bFGF, VEGF, SCF, IGF-1, IL-3, M-CSF and GM-CSF | Orthogonal phagocytosis and polarization and antitumor functions | 2024, 2020 [56, 57] |
| SCF, VEGF, BMP4, Y27632, M-CSF and IL-3 | M1 or M2 polarization when exposed to LPS + IFN- | 2021, [51] | |
| BMP4, VEGF, SCF, TPO, IL-3, IL-6, Flt-3L, M-CSF | LPS triggered IL-8 | 2022, [58] | |
| BMP4, Y-27632, bFGF, Cytokine Mix E, VEGF, IL-6, IL-11, M-CSF, IL-3 | Modeling and therapeutic targeting of inflammation-induced hepatic insulin resistance | 2023, [59] | |
| BMP4, CHIR99021, VEGF, bFGF, SCF, IL-3, IL-6, DKK1, CSF-1 | Modulating NPC differentiation | 2023, [49] | |
| SCF, VEGF, BMP4, bFGF, Y27632, IGF1, IL-3, M-CSF, GM-CSF | Tumor-suppressive | 2023, [50] | |
| BMP4, GlutaMAX, VEGF, bFGF, SCF, Flt3L, IL-3, M-CSF, TPO, GM-CSF, M-CSF used in an incorporating organoid culture model | Improving drug sensitivity and selectivity | 2024, [60] | |
| Phenotype transition | LPS/IFN- | M1 polarization | 2018, 2017 [20, 21] |
| IL-4/IL-13 | M2 polarization | 2018, 2017 [20, 21] | |
| Damaged cells | Clear damaged cells and release matrix metalloproteinases to degrade the ECM | 2020, [11] | |
| POCG | Downregulating pro-inflammatory cytokines, polarizing M2 phenotype, facilitating the vascularization of endothelial cells | 2022, [52] | |
| Silencing of PAQR3 | M2 polarization, facilitating angiogenesis, and accelerates wound healing | 2022, [53] | |
| Macrophage-specific nanoparticles that inhibiting JMJD3 | Enhance the healing process of diabetic wounds | 2022, [54] | |
| Spermidine | Transition of M1 to M2; Improved the degeneration of articular cartilage | 2024, [46] | |
| Fibroblast-derived exosomes | Transition from M1 to M2 phenotype | 2025, [55] |
Note: BMP4, Bone Morphogenetic Protein 4; bFGF, Basic Fibroblast Growth Factor; CHIR99021, CHIR99021 (GSK3 Inhibitor); CSF-1, Colony Stimulating Factor 1; Cytokine Mix E, Cytokine Mixture E; DKK1, Dickkopf Wnt Signaling Pathway Inhibitor 1; ECM, Extracellular Matrix; Flt-3L, Fms-like Tyrosine Kinase 3 Ligand; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; GlutaMAX, Glutamine Supplement; IFN-
In existing studies, dermal micro-punching has been considered a safe and effective anti-aging treatment method. It involves the removal of small cylindrical sections of skin, which subsequently leads to the production of new collagen, improving skin laxity, softening wrinkles, and remodeling scars [61]. Although the principles and mechanisms are not fully understood, it is clear that this process creates controlled micro-injuries that stimulate collagen regeneration [61]. Ultimately, its essence lies in creating new injuries, which then facilitate regeneration during the repair process. A research team utilized cytotoxic pyrrolobenzodiazepine (PBD) to ablate aging dermal fibroblasts expressing apolipoprotein D to eliminate senescent cells [62]. However, PBD may cause systemic adverse reactions in patients affecting multiple systems, including the circulatory, digestive, metabolic, nervous, musculoskeletal, respiratory, and urinary systems [62]. In contrast, creating micro-injuries to recruit macrophages and stimulate the onset and resolution of inflammation to eliminate senescent cells does not induce severe side effects and is relatively safe. Additionally, a study utilized particles containing VEGFR-3 specific ligands to recruit macrophages that secrete VEGF-A, thereby inducing neovascularization [38].
Therefore, we can utilize the creation of micro-injuries or VEGFR-3 specific ligands to recruit macrophages to aging skin, thereby promoting skin regeneration.
During the aging process, the oxidative stress responses and detoxification capacities of various cells, including keratinocytes, fibroblasts, macrophages, and endothelial cells, are significantly impaired [63]. When the macrophages recruited by the body are unable to express normal activity or when there is a dysregulation in self-regulation, exogenous macrophages may serve as a viable alternative. Research has demonstrated the potential to induce pluripotent stem cells to differentiate into functional macrophages [49, 50, 51, 64] (Table 2). Therefore, utilizing pluripotent stem cells to produce sufficient quantities of young, active macrophages for anti-aging interventions presents a highly attractive strategy for skin rejuvenation. Meanwhile, we can utilize in vitro sorting techniques to obtain more efficient macrophages for anti-aging treatments. It is known that macrophages are a major source of VEGF during the early stages of tissue repair; however, only a small subset of macrophages at the wound site expresses VEGF, and these VEGF-expressing macrophages exhibit a mixed M1/M2 phenotype [35]. Additionally, other studies have suggested that CD11c+ macrophages possess pro-angiogenic properties and are essential for experimental choroidal neovascularization [36].
Macrophages exhibit plasticity, characterized by various types and the ability to change their state in response to metabolic cues (Table 2). Therefore, to enhance the anti-aging effects of macrophages, appropriate adjustments to their states may be necessary.
Research has indicated that the transition of synovial macrophages from M1 to M2, modulated by spermidine, can improve the degeneration of articular cartilage [46]. In skin tissue engineering research, Xie et al. [52] proposed that Poly (octanediol-citrate-polyglycol) (POCG) downregulates pro-inflammatory cytokines, thereby polarizing macrophages to an anti-inflammatory (M2) phenotype. This polarization promotes the secretion of angiogenic factors such as VEGF as well as the expression of CD31, which facilitate the vascularization of endothelial cells. Silencing of progestin and adipoQ receptor family member 3 (PAQR3) promotes M2 macrophage polarization by inhibiting stress-induced phosphoprotein 1 homology and U-box containing protein 1 (STUB1)-mediated ubiquitination and degradation of peroxisome proliferator-activated receptor gamma (PPAR
Fibroblast-derived exosomes can regulate the polarization state of macrophages and accelerate the transition from the M1 to M2 phenotype [55]. Under IL-4 stimulation, fibroblasts can enhance the M2 polarization response of macrophages by upregulating the expression of CD206 and ARG1. In addition, fibroblast-derived exosomes can also facilitate the timely conversion from the M1 to M2 phenotype. Fibroblast-derived exosomes serve as remote modulators of macrophage polarization, calibrating the immune transitions necessary for tissue repair. Utilizing exosomes represents a previously unreported approach to guide effective macrophage activation states, holding immense therapeutic potential for promoting healing in chronic inflammatory diseases. Cell microtransplantation can be employed to achieve in vivo skin regeneration and wound healing [65]. Macrophage reprogramming offers a favorable immune microenvironment, contributing positively to tissue repair processes [66].
In the conventional understanding Macrophages are rich in sensor of M1/M2 macrophages, M1 primarily drives the exacerbation of inflammation, while M2 dominates tissue repair. During aging, due to dysregulation of immune mechanisms, the normal clearance function declines. As a result, M1 can stimulate inflammation, while M2 promotes repair. The interplay between these two processes may contribute to anti-aging effects. The issue of off-target effects in vivo also needs consideration. We can leverage the chemotactic properties of macrophages to attract their aggregation at specific sites, minimizing their activity in other areas.
Although macrophages possess significant potential for anti-aging therapies, they also present certain risks. Classically activated macrophages are essential components of host defense; however, their activation must be strictly regulated, as the cytokines and mediators they produce can lead to host tissue damage. In fact, classically activated macrophages are key mediators in the immunopathology of certain autoimmune diseases, such as rheumatoid arthritis and inflammatory bowel disease [5]. Macrophages are implicated in the pathogenesis of systemic sclerosis (SSc), as they can secrete fibrosis-promoting factors under conditions of immune dysregulation, leading to associated clinical manifestations, including vascular lesions, inflammation, and fibrosis [67]. In SSc, the expression of the macrophage-recruiting chemokine CCL2 is significantly elevated, suggesting that macrophage phenotypes contribute to the development of SSc skin lesions. The underlying mechanisms may include the ability of macrophages to promote fibroblast-to-myofibroblast transition. Currently, there is no mature and systematic methodology for the precise regulation of macrophages, which means that improper use could result in unpredictable side effects.
In contemporary society, the demand for anti-aging solutions has expanded. This paper analyzes the differences between aged and youthful skin, highlighting the role of macrophages in mediating anti-inflammatory responses, phagocytosing senescent cells, promoting angiogenesis, facilitating tissue repair, and regulating the microenvironment. It proposes that macrophages could serve as a promising candidate for anti-aging cellular therapies. In addition to recruiting endogenous macrophages to aged skin, exogenous macrophages can be supplemented using pluripotent stem cells. Furthermore, the functional states of macrophages can be adjusted according to the activating factors that correspond to their different states of transition. In summary, macrophages hold significant potential in anti-aging interventions, and their therapeutic efficacy warrants further investigation.
Arg1, Arginase 1; CD31, Cluster of Differentiation 31; DNA, Deoxyribonucleic Acid; ECM, Extracellular Matrix; EndoMac, Endothelial Macrophage; ERK1/2, extracellular signal-regulated kinase 1/2; bFGF, Basic Fibroblast Growth Factor; HGF, Hepatocyte Growth Factor; HF, Hair Follicles; HFSCs, Hair Follicle Stem Cells; HLA-E, Human Leukocyte Antigen E; IGF-1, Insulin-like Growth Factor; IGFBP7, Insulin-like Growth Factor Binding Protein 7; IL, Interleukin; JAK1, Janus Kinase 1; JMJD3, Jumonji Domain Containing 3; NK, Natural Killer; PDGF, Platelet-Derived Growth Factor; PBD, Pyrrolobenzodiazepine; POCG, Poly (octanediol-citrate-polyglycol); PPAR
HZ, YWZ and YML conceived and designed the study. HZ and YW wrote the original draft and made figures and tables. YWZ and HZ reviewed and discussed the manuscript. YML and YWZ supervised the study and provided resources. YWZ, YML, and HZ acquired funding. All authors contributed to manuscript editing and approved the final version. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research was partially funded by the National Natural Science Foundation of China (82270697), Jiangsu Provincial Key Discipline Cultivation Unit (JSDW202229), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_3717), Haihe Laboratory of Cell Ecosystem Innovation Fund (HH24KYZX0008).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


