1 IRCCS Ospedale Galeazzi – Sant’Ambrogio, Laboratorio di Biotecnologie Applicate all’Ortopedia, 20157 Milano, Italy
Abstract
Extracellular vesicles (EVs) are nanoscale, membrane-enclosed structures that are secreted by nearly all cell types. EVs include small EVs (exosomes), large EVs (microvesicles), and apoptotic bodies, which are distinguished by their biogenesis and size. EV biogenesis involves endosomal pathways or direct budding from the plasma membrane, influenced by cellular states and external stimuli. The complex composition of EVs, proteins, lipids, RNA, DNA, and metabolites reflects their cell of origin, enabling EVs to mediate intercellular communication. EV uptake by recipient cells occurs via endocytosis, membrane fusion, or receptor–ligand interactions, influencing diverse physiological and pathological processes. Indeed, the biological roles of EVs range from immune modulation to tissue repair and contributions to cancer, neurodegeneration, musculoskeletal pathologies, and other disorders. Advances in isolation methods, including ultracentrifugation, size exclusion chromatography, and immunoaffinity techniques, have improved the purity and yield of EVs. Characterization technologies, such as nanoparticle tracking analysis, electron microscopy, and omics approaches, provide insights into their heterogeneity and functional cargo. Thus, EVs hold promise as non-invasive biomarkers for disease diagnosis and prognosis, offering high specificity and stability. Furthermore, the natural biocompatibility, ability to cross biological barriers, and capacity for functional cargo delivery of EVs position them as therapeutic tools and drug-delivery vehicles. Some of the most promising fields of application for EVs include cancer, neurodegeneration, and joint diseases; however, challenges remain in scaling production, achieving targeted delivery, and ensuring regulatory compliance. This review highlights recent advances in EV biology, isolation, and applications, emphasizing their crucial potential in precision medicine while identifying knowledge gaps and future research directions.
Keywords
- extracellular vesicles
- intercellular communication
- biomarker
- regenerative medicine
- cancer
- joint diseases
- precision medicine
Extracellular vesicles (EVs) have emerged as pivotal players in intercellular communication, influencing various physiological and pathological processes. EVs are membrane-bound particles secreted by almost all cell types into the extracellular space, and they are categorized based on their size, origin, and composition. The three main types of EVs include small EVs (also called “exosomes”), large EVs (“microvesicles”), and apoptotic bodies, with small EVs being the most extensively studied. The role of EVs in cellular signaling and their potential as diagnostic biomarkers and therapeutic agents have garnered considerable attention in recent years [1].
A growing body of research has revealed the functional significance of EVs in a wide array of biological processes. EVs have been shown to play crucial roles in immune response modulation, tissue repair, and the transfer of cellular signaling molecules [2]. In addition to their roles in disease, EVs hold promise as diagnostic tools and therapeutic agents [3]. EVs are stable in biofluids such as blood, urine, saliva, and synovial fluid making them attractive candidates for non-invasive biomarker discovery [4]. Recent studies have demonstrated that EVs can be used to monitor disease progression, identify therapeutic targets, and predict treatment outcomes [5]. Furthermore, EVs are being explored as drug delivery vehicles due to their biocompatibility, ability to cross biological barriers, and natural targeting properties [6]. Various strategies, including genetic modification of donor cells and chemical conjugation of therapeutic molecules to EVs, have been developed to enhance their therapeutic potential.
Despite these advancements, several challenges remain in fully understanding and exploiting the potential of EVs. The isolation, characterization, and standardization of EVs are complex, as the heterogeneity of EV populations can vary based on cell type, condition, and method of isolation. Furthermore, the safety and efficacy of EV-based therapies require rigorous evaluation through clinical trials. As research continues to uncover the multifaceted roles of EVs, future studies will likely focus on refining isolation techniques, elucidating their functional cargo, and overcoming barriers to their clinical application.
This review explores recent advances in the understanding of EVs biology, their clinical applications, and the challenges that remain in fully harnessing their potential.
EVs encompasses different types of particles (Table 1 (Ref. [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17]) and Fig. 1). Small EVs, typically ranging from 30 to 150 nm in diameter, originate from the inward budding of multivesicular bodies (MVBs) within the endosomal compartment. Upon fusion of MVBs with the plasma membrane, small EVs are released into the extracellular space [18]. Large EVs, which range in size from 100 nm to 1 µm, are formed by the outward budding of the plasma membrane [7]. Apoptotic bodies, released during cell death, are larger in size (1–5 µm) and are involved in the clearance of cellular debris [19]. EVs carry various biomolecules, including proteins, lipids, RNAs, and DNA, reflecting the cellular state and environment from which they originate [20]. This cargo is protected by the lipid bilayer, making EVs resistant to degradation and enabling them to mediate long-distance communication [21]. While small and large EVs serve similar functions in mediating cell-to-cell communication, their biogenesis mechanisms differ, with the tetraspanin protein family playing a central role in both pathways [22].
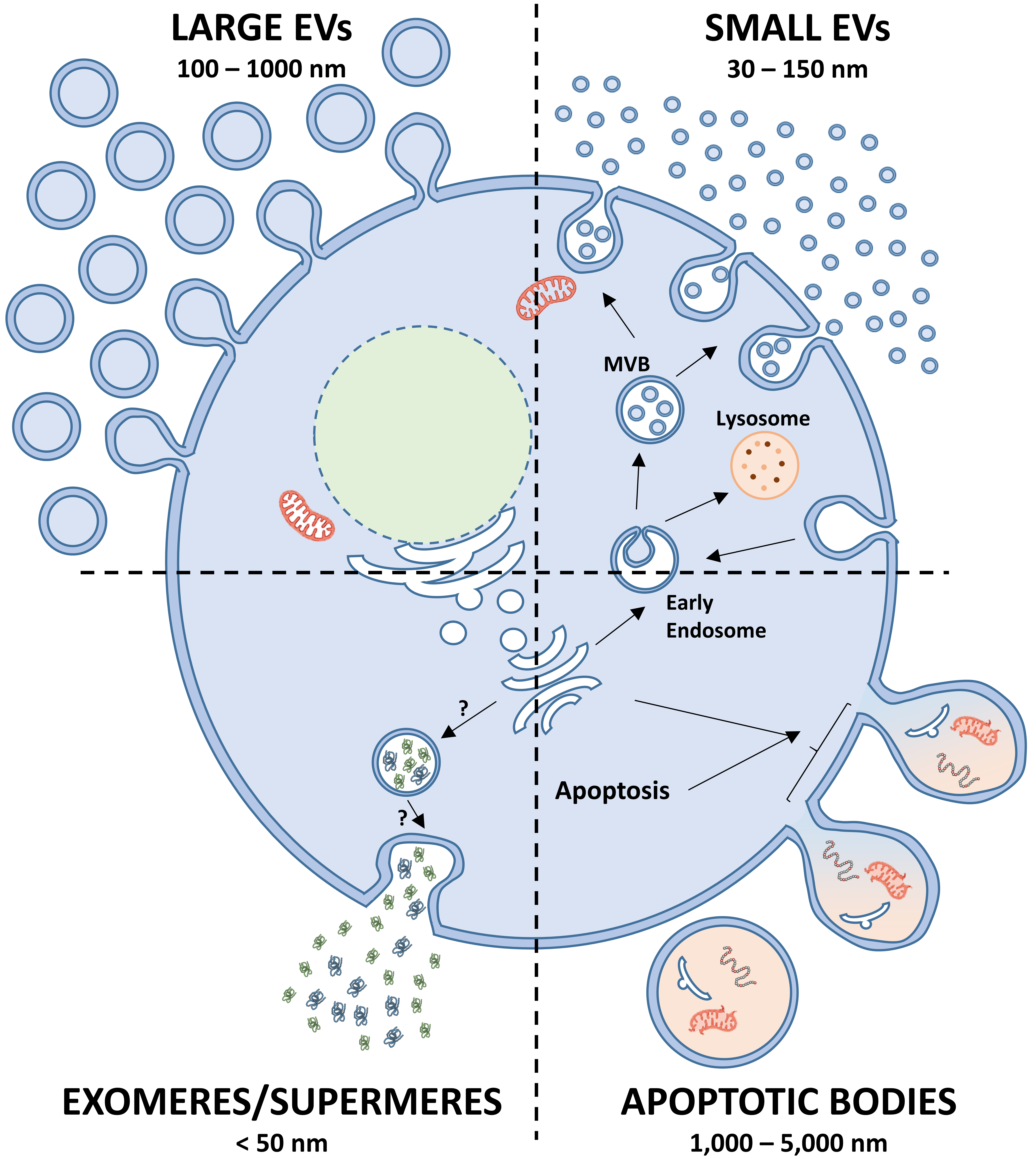 Fig. 1.
Fig. 1. Types of nanoscale particles released by cells. (i) Small extracellular vesicles (EVs) are formed through endocytosis, where early endosomes merge with endocytic vesicles. Intraluminal vesicles (ILVs) develop via inward budding of the endosomal membrane during the maturation of early endosomes into multivesicular bodies (MVBs). ILVs are either degraded when MVBs fuse with lysosomes or secreted into the extracellular space upon MVB fusion with the plasma membrane. Once released, ILVs are referred to as small EVs. (ii) Large EVs originate directly from the plasma membrane, which undergoes outward protrusion and subsequent release into the extracellular environment. (iii) Apoptotic bodies are formed from plasma membrane blebs during apoptosis and are released into the extracellular space. Their biogenesis is regulated by several pathways, with caspase-3 activation and increased calcium levels playing critical roles. (iv) Exomeres and supermeres are recently discovered nanoparticles found in the extracellular space, though their mechanisms of biogenesis remain unclear. The Figure was created with PowerPoint.
| Type of EV | Size range | Biogenesis pathway | Formation mechanism | Key proteins involved | Cargo | Role |
| Small EVs [7, 8, 9, 10] | 30–150 nm | Inward budding of MVBs within the endosomal compartment; fusion with plasma membrane to release EVs. | ESCRT-dependent (ESCRT-0, -I, -II, -III, ALIX, TSG101, VPS4) and ESCRT-independent (tetraspanin-enriched microdomains) | Tetraspanins (CD9, CD81, CD63), ALIX, TSG101, small GTPases, SNARE proteins | Proteins, lipids, RNAs, DNA | Cell-to-cell communication, biomolecule transport, long-distance signaling. |
| Large EVs [9, 11, 12, 13] | 100 nm to 1 µm | Outward budding of the plasma membrane. | Membrane and cytoskeletal rearrangement, accumulation of lipid-anchored proteins to induce curvature | Tetraspanins (CD9, CD81, CD63), ESCRT-I subunits, small GTPases (e.g., ARF) | Proteins, lipids, RNAs, cellular components | Cell communication, signaling, membrane remodeling. |
| Apoptotic bodies [14, 15] | 1–5 µm | Released during apoptosis. | Fragmentation of dying cells, regulated process of apoptotic cell disassembly. | Histones, DNA, glyco-epitopes | DNA, histones, cellular fragments | Clearance of cellular debris, anti-inflammatory responses. |
| Exomeres & supermeres [16, 17] | Non-vesicular nanoparticles, distinct from traditional EVs. | Not clearly defined, but they are smaller than traditional EVs and likely formed from specific cellular processes. | Distinct proteomic profiles, but specific proteins not fully defined | Proteins, lipids, non-vesicular components | Cell communication, tissue accumulation, distinct from EVs. |
EVs, extracellular vesicles; MVBs, multivesicular bodies; ESCRT, endosomal sorting complex required for transport; ALIX, ALG-2-interacting protein X; TSG101, Tumor susceptibility gene 101; VPS4, Vacuolar Sorting Protein 4; SNAREs, SNAP Receptors; ARF, ADP Ribosylation Factor.
Small EVs are formed through the inward budding of the endosomal membrane, which leads to the creation of MVBs. These MVBs can either fuse with lysosomes for degradation or merge with the plasma membrane to release small EVs [8]. Two main pathways are involved: the endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent pathways. The ESCRT-dependent pathway involves the recruitment of several protein complexes, such as ESCRT-0, -I, -II, and -III, as well as ALG-2-interacting protein X (ALIX), Tumor susceptibility gene 101 (TSG101), and Vacuolar Sorting Protein 4 (VPS4), which facilitate the formation of intraluminal vesicles (ILVs) within MVBs [9]. On the other hand, the ESCRT-independent pathway relies on the formation of ILVs by tetraspanin-enriched microdomains (TEMs) and lipid rafts, with proteins like CD9, CD81, and CD63 playing a significant role in cargo sorting and vesicle release [7]. Additionally, small GTPases and SNAP Receptors (SNAREs) regulate the movement, maturation, and fusion of MVBs with the plasma membrane, ensuring efficient small EVs release [10].
Large EVs are generated through the outward budding of the plasma membrane, a process that does not rely on endosomal trafficking pathways [11]. Their biogenesis involves the rearrangement of membrane and cytoskeletal elements, with proteins having lipid anchors (e.g., myristoylation or palmitoylation) accumulating at the membrane to induce curvature [12]. ESCRT-I subunits are also recruited to the plasma membrane during the formation of ectosomes, and small GTPases like ADP Ribosylation Factor (ARF) regulate cargo assembly and secretion [9]. The final pinching off of ectosomes from the membrane requires cytoskeletal disassembly, which is facilitated by calcium and the activity of ESCRT-III complexes [13].
Apoptotic bodies are released during the final stages of apoptosis and contain cellular fragments, including DNA, histones, and glyco-epitopes. While traditionally considered remnants of dying cells, apoptotic bodies may also trigger anti-inflammatory or tolerogenic responses in recipient cells [14]. Despite some controversy regarding their classification as EVs, emerging evidence suggests that apoptotic bodies are formed through a regulated process called apoptotic cell disassembly [15].
Other types of EVs are exomeres and supermeres [16], which are smaller than 50 nm and have been identified as non-vesicular nanoparticles with distinct proteomic profiles and bio-distribution patterns compared to traditional EVs. These particles are thought to play significant roles in cellular communication and tissue accumulation [17].
EVs molecules are catalogued in databases like Exocarta (http://www.exocarta.org/) [23] and Vesiclepedia (http://www.microvesicles.org) [24], describing DNA, RNA, proteins, lipids and metabolites that are detected or associated with EVs (Table 1).
Proteins play a crucial role in receptor-ligand recognition through interactions with other proteins, lipids, and glycans. Key protein groups involved in these processes include: (i) Tetraspanins, such as CD9, CD63, and CD81, are abundant on extracellular vesicles (EVs) and act as markers. They form tetraspanin-enriched microdomains (TEMs), facilitating EV docking and selective uptake by target cells. These interactions are vital for processes like disease progression or drug delivery [25]. (ii) Lectins recognize and bind glycans, aiding cell communication and immune regulation. They are categorized into C-type lectins, Siglecs, and galectins [26]. (iii) Integrins are membrane proteins that regulate cell proliferation, migration, and survival [27]. (iv) Scaffold proteins, such as ALIX and Syntenin, are essential for EV biogenesis and cargo loading. ALIX, for example, helps load Programmed death-ligand 1 (PD-L1) onto EVs, impacting immune evasion in cancer. These proteins collectively influence cellular communication, immune responses, and therapeutic strategies [28].
The family of EVs outer proteins also encompasses the so called “corona”. Two layers can be found: an inner hard layer and an outer soft layer [29]. The hard corona forms through the irreversible adsorption of high-affinity proteins, while the soft corona consists of low-affinity proteins interacting reversibly. The hard corona’s composition is relatively stable and can provide insights into the identity of biomolecules in both healthy and diseased states. The composition of the soft corona is dynamic and influenced by environmental factors. The evolving nature of the soft corona, however, reflects ongoing changes in the biological environment. A recent literature suggested how protein corona may influence EVs half-life and uptake, thus representing a camouflage mechanism driving particles biological role and distribution [30].
Extracellular vesicles (EVs) also carry diverse inner protein cargo, with the composition largely reflecting their parental cells and EV subtypes. Key proteins such as Alix, TSG101, and heat shock proteins are commonly found in EVs and play roles in their biogenesis [7]. Alix, for example, is associated with EV formation, while Y box binding protein 1 (YBX1), an RNA-binding protein, may regulate RNA sorting into EVs. Cytoplasmic proteins like actin and tubulin are also passively incorporated during EV formation [31]. Of note, the protein mass in EVs is proportional to their size relative to the parent cell. As an example, a HeLa cell with ~150 picograms of protein translates to ~1.5
RNA-binding proteins (RBPs) also contribute to EV cargo, with RBPs making up around 25% of EV proteins. These proteins mediate the loading of microRNAs (miRNAs) and other RNA species into EVs. Specific RNA motifs, such as CGGGAG, can be recognized by RBPs like Fus and Alyref to promote the enrichment of miRNAs in EVs [33]. In this frame, RNA content in EVs differs significantly from the RNA composition of their parent cells, confirming the presence of selective mechanisms for RNA sorting into EVs [34]. Factors such as the source of EVs, sample processing, and analytical methods significantly influence the types of RNAs detected, leading to variability across studies. The RNA species commonly found within EVs include miRNAs, messenger RNAs (mRNAs), long non-coding RNAs, and transfer RNAs (tRNAs). Small RNAs, particularly tRNAs, are the most abundant in EVs, with tRNAs comprising over 50% of the small RNA content in mesenchymal stromal cells [35]. Regulatory RNAs like miRNAs, however, make up only a small fraction (up to 10%) of total small RNAs in EVs [36]. Thus, despite the prominence of miRNAs in EV-related research, often less than one copy is present per vesicle. This suggests that most EVs may lack functional regulatory RNAs, raising questions about whether only specific subsets of EVs carry functional RNAs or whether abundant miRNA encapsulation is rare. Further investigation is needed to understand the mechanisms underlying RNA sorting and the functional significance of RNA cargo in EV-mediated communication.
Another molecule present in EVs is DNA. In the first studies, EVs were primarily known to carry single-stranded DNA (ssDNA), mitochondrial DNA (mtDNA), and repetitive transposons [37]. However, direct evidence of dsDNA in cancer-derived EVs was released [37, 38], with findings revealing that most genomic deoxyribonucleic acid (gDNA) in EVs is indeed dsDNA. Importantly, dsDNA in EVs has diagnostic potential, e.g., reflecting the molecular characteristics of tumours [39]. This discovery has led to broader applications of these methods to differentiate between ssDNA and dsDNA in EVs, ensuring accurate characterization of internally packaged DNA. Subsequent research has built on these techniques to explore the diagnostic and prognostic implications of EVs DNA [40].
Together with mtDNA, several studies have demonstrated the presence of intact mitochondria in extracellular vesicles (EVs), which retain their energy metabolism function and facilitate intercellular energy transfer [41]. Mitochondrial transfer via large EVs significantly increased Adenosine triphosphate (ATP) levels in brain endothelial cells, enhancing energy availability and antioxidant capacity in recipient cells. This adaptation helps cells cope with stressful environments [42]. Similarly, mitochondrial transfer to chondrocytes improved mitochondrial function, suggesting potential therapeutic applications for cartilage repair and ischemic or degenerative diseases [43]. Although mitochondrial transfer has been associated with inflammatory responses, studies indicate that intact mitochondrial membranes prevent damage-associated molecular pattern leakage, minimizing immune activation. In fact, mitochondrial transfer had an anti-inflammatory potential, as EV-delivered mitochondria restored mitochondrial dynamics and reduced pro-inflammatory markers in inflammatory cells [44]. Thus, mitochondrial supplementation via EVs not only enhances cellular energy supply but also supports tissue repair and inhibits inflammation [45]. This efficient delivery method makes mitochondria more accessible to recipient cells, offering promising strategies for managing acute and chronic inflammatory conditions.
Lipids are vital components of EVs membrane, playing a key role in their interaction with cells [46]. Lipids are categorized into glycerophospholipids, sphingolipids, and cholesterol, each varying in carbon chains and double bonds. Lipids organize into “mobile rafts” that facilitate cell signalling, contributing to processes such as oncogenesis and metastasis [47]. Phosphatidylserine, a glycerophospholipid, is essential in cellular communication, binding to receptors like T-cell membrane protein 4 (TIM4) through calcium coordination. Other receptors, such as Receptor for advanced glycation endproducts (RAGE), Brain-specific angiogenesis inhibitor 1 (BAI1), and Stabilin 2 (STAB2), also interact with phosphatidylserine to mediate cell clearance, particularly of apoptotic cells and aged red blood cells. Additionally, the phosphatidylserine-Gas6 complex activates Mer tyrosine kinases on macrophages, promoting an anti-inflammatory response [48]. Ceramide, a sphingolipid abundant on EV surfaces, influences mobile raft formation and cell signaling pathways [49], further emphasizing the importance of lipids in EV function and cellular communication. In fact, with a focus on therapy, studies showed that EVs, when produced under different conditions, alter their lipid and metabolite composition to influence biological functions. For example, PC3 cells co-cultured with hexadecylglycerol exhibited altered protein and lipid composition, highlighting the impact of external stimuli [50]. Similarly, EVs derived from mesenchymal stromal cells (MSC) cultured under priming conditions were found to modulate lipids and metabolites linked to MSCs immunomodulatory properties, including macrophage polarization [51]. These findings emphasize the dynamic nature of EVs composition.
Alongside surface proteins and lipids, glycans play a key role in the binding of EVs to cells through interactions involving sugars and proteins. One important glycan structure is proteoglycan (PG), which consists of a protein core attached to glycosaminoglycan chains [52]. PGs, such as heparan sulfate proteoglycan (HSPG), are involved in numerous biological interactions, including binding to polyamines, nucleic acids, cationic lipids, and viral proteins. HSPG is particularly crucial in EV-mediated communication, especially in the tumor microenvironment, where it helps facilitate growth factor signaling and serves as an initial attachment site for macromolecular ligands [53]. Beyond tumors, changes in PGs also contribute to diseases like insulin resistance and dyslipidemia. Other glycan structures, such as glycolipids and glycoproteins containing sialic acid or mannose, also participate in EV binding and influence EV uptake. Given their central role in EV binding, glycans are promising targets for engineering EV-based therapies. Modifying glycosylation on EV surfaces can control their uptake by specific cells, enhancing drug delivery and targeting [54].
After binding to the surface of the recipient cells, several distinct pathways for EVs uptake have been identified: membrane fusion, clathrin-dependent endocytosis, caveolin-mediated endocytosis, macropinocytosis, phagocytosis, and lipid raft-mediated internalization (Table 2, Ref. [55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70]).
| Mechanism | Description | Key features | Key proteins/factors |
| Cell Membrane Fusion [55, 56, 57] | Direct fusion of EV membrane with target cell plasma membrane. Transfers luminal and membrane-associated cargo without involving endocytic or lysosomal pathways. | Simplest method; influenced by pH, temperature, lipid composition, and membrane alterations. | Annexin V, PSGL-1 |
| Clathrin-dependent Endocytosis [58, 59, 60] | EVs internalized via clathrin-coated pits, forming vesicles. These vesicles are cleaved by dynamin and fuse with endosomes for further processing. | Dependent on clathrin-coated vesicle formation; dynamin is essential for vesicle scission. | Clathrin, dynamin-2 |
| Caveolin-mediated Uptake [61, 62] | EVs internalized through caveolae, small invaginations enriched in cholesterol and sphingolipids. | Involves highly organized lipid microdomains; less understood compared to other pathways. | Caveolin-1, cavin proteins |
| Macropinocytosis [63, 64, 65] | Actin-driven process creating macropinosomes that engulf extracellular material. | Prominent in immune cells for clearing debris; requires phosphatidylinositol 3-kinase and dynamin-2. | Phosphatidylinositol 3-kinase, dynamin-2 |
| Phagocytosis [66, 67, 68] | Receptor-mediated engulfment of EVs by specialized phagocytes, such as macrophages. | Effective for large EVs; recognition often aided by phosphatidylserine (PS) on EV membranes. | PI3Ks, phosphatidylserine (PS) |
| Lipid Raft-mediated Internalization [69, 70] | Involves lipid rafts, independent of clathrin and caveolin. Requires proteins like flotillins and components like cholesterol and glycosphingolipids. | Inhibited by cholesterol and glycosphingolipid disruption; precise mechanisms unclear. | Flotillins, cholesterol, glycosphingolipids |
EVs, extracellular vesicles; PSGL-I, P-selectin glycoprotein ligand-1; PI3Ks, Phosphoinositide 3-kinases.
Cell Membrane Fusion: This is the simplest method of EVs uptake, involving the direct fusion of the EVs membrane with the plasma membrane of the target cell [55]. This process allows the transfer of both luminal and membrane-associated cargo without engaging endocytic or lysosomal pathways. Membrane fusion can be influenced by factors such as pH, temperature, lipid composition, and membrane alterations [56]. Proteins such as annexin V and PSGL-1 play a crucial role in the fusion process [57], although the exact regulatory mechanisms remain under investigation.
Clathrin-dependent Endocytosis: It involves the formation of clathrin-coated pits on the plasma membrane, which internalize EVs into clathrin-coated vesicles [58]. The process is driven by dynamin, a large GTPase that facilitates the scission of the vesicle from the membrane. Once internalized, the clathrin coat is shed, allowing fusion with endosomes for further processing [59]. Inhibiting dynamin-2 reduces EVs uptake, demonstrating its importance in the process [60].
Caveolin-mediated Uptake: In this pathway, EVs are internalized via small invaginations called caveolae, which are rich in cholesterol and sphingolipids [61]. Caveolin-1 is the main protein responsible for caveolae formation, and its dimers interact with cavin proteins to facilitate the process [62]. Caveolae are highly organized microdomains, playing an essential role in trafficking and internalization of EVs, although this process is less understood compared to other mechanisms.
Macropinocytosis: Macropinocytosis is an actin-driven process that involves the formation of large membrane protrusions called macropinosomes. These protrusions engulf extracellular material, which is then internalized and processed by the cell [63]. This pathway is particularly utilized by immune cells like macrophages and microglia for clearing debris and pathogens [64]. Macropinocytosis requires the activity of phosphatidylinositol 3-kinase and dynamin-2, and disruption of these pathways can inhibit EVs uptake [65].
Phagocytosis: In phagocytosis, large particles such as EVs are internalized by specialized phagocytic cells through receptor-mediated mechanisms [66]. Phagocytes, including macrophages, are highly efficient at engulfing EVs, particularly when they express phosphatidylserine (PS) on their membranes [67]. PS is often found on the outer membrane of EVs, aiding their recognition and internalization [68]. Inhibition of pathways like phosphoinositide 3-kinases can reduce EVs uptake, suggesting their role in this process.
Lipid Raft-mediated Internalization: EVs uptake via lipid rafts is independent of clathrin and caveolin, although it requires proteins like flotillins [69], which regulate raft-mediated endocytosis. Inhibition of lipid raft components such as cholesterol and glycosphingolipids reduces EVs uptake, confirming their involvement in this internalization pathway [70]. However, the precise mechanisms and scope of lipid raft-mediated uptake remain unclear.
EVs, once considered simply a means for cells to dispose of unnecessary materials [71], have now been recognized for their broader roles in cellular communication, maintenance and disease. As an example, EVs are involved in immune response activation [72], acting as antigen-presenting vesicles, or are integral to the joint system by supporting tissue formation, cartilage survival, and damage regeneration [73]. However, in joint diseases, EVs may also contain pathogenic miRNAs or circRNAs, which could contribute to disease progression [74].
The first step in EVs function starts after their internalization through the endocytic pathway, where they may be degraded in lysosomes, a process associated with waste management [75]. Alternatively, EVs can be released without degradation [76] or, less commonly, escape digestion and release their contents into the cytoplasm of recipient cells [7]. This release can influence the recipient cell’s function and phenotype by providing mitochondria, proteins, lipids, DNA, and RNA, with miRNAs playing a pivotal role in cell communication. A typical example is miRNAs and long non coding ribonucleic acid (lncRNA) functional roles in cartilage homeostasis and osteoarthritis pathogenesis [77]. Also, protein and lipid cargoes can further regulate immune responses and bioactive lipid species in recipient cells. For example, proteins like CC chemokine receptor type 5 (CCR5), released by EVs, influence immune responses against human immunodeficiency virus (HIV) [78]. These functions highlight the significant role of EVs in both health and disease, suggesting their therapeutic promise.
Under these premises, in the recent years, a new paradigm emerged: the Stoichiometric speculation. Stoichiometric and quantitative considerations are often overlooked in most EV research. Additionally, the EV fraction contains a heterogeneous mix of EVs with diverse miRNAs, making it nearly impossible to pinpoint a single miRNA as the sole driver of any phenotypic change in recipient cells [79]. This challenge is compounded by the fact that a combination of miRNAs, or even other molecules, can produce the same phenotypic effect. In fact, as previously mentioned, miRNAs are present at very low levels in EVs and, according to stoichiometric analyses, EVs contain approximately 0.01 copies of miRNA per particle [80]. For this reason, when the function of a given miRNA is studied, cells are engineered to overexpress the molecule that may be found in a 102–104 higher amount. A similar issue exists for mRNAs that, similar to miRNAs, were reported to be transferred in recipient cells [81, 82], as well as for proteins that in raw materials (culture supernatant or body fluid) were detected in a quite low abundance (0.5 µg of protein/mL). Due to this evidence, it is now proposed that EVs may influence target cells or tissue also through non-cargo molecules. One very recent example is the protein corona that may direct tissue uptake [30]. Again, a study highlighted the role of surface proteins on EVs in influencing recipient cells. For instance, cancer-derived EVs carry proteins that promote phenotypic changes, including a Vascular endothelial growth factor (VEGF) variant that binds EVs and evades neutralizing antibodies [83]. Overall, the characteristics of both the EVs and the recipient cell type collectively contribute to regulating EVs target specificity, underscoring the importance of surface molecules in EV-mediated communication.
Under these premises, what is the true biological function of EVs? Alongside their role in intercellular communication, EVs may serve additional biological purposes. One potential function is their involvement in cellular homeostasis. EVs secretion could act as a mechanism to remove unwanted or harmful materials from cells. For example, research has shown that EVs can expel fragmented DNA from the cytoplasm into the extracellular space during cellular senescence [84]. The secretion of EVs may offer a complementary method for maintaining cellular balance and preventing the accumulation of damaging materials. This suggests that EVs might serve as a waste disposal system to help preserve cellular integrity and function.
Thus, due to their interconnected biological roles of shuttling material of originating cells and influencing target cells, EVs may serve in the clinical field as either therapeutic agents or biomarker for diagnosis and prognosis.
EVs isolation is a crucial process for understanding their biological activity due to their nanoscale size and low buoyant density, making their separation from biological samples, both from body fluids and from cell culture approaches, challenging. Efficient and reproducible isolation is critical, given the complexity of starting samples and the presence of various vesicle populations and cellular debris. Multiple methodologies exist to achieve this, typically leveraging differences in size, density, charge, or molecular composition. Common approaches include ultracentrifugation, ultrafiltration, size-based chromatography, immunoaffinity capture, polymer-based precipitation, and microfluidics, each offering distinct advantages and limitations (Table 3, Ref. [85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98]).
| Method | Advantages | Limitations | Applications |
| Ultracentrifugation (UC) [85, 86, 87] | Cost-effective, scalable | Time-intensive, potential EVs damage | Large sample volumes |
| Ultrafiltration (UF) [88, 89] | Fast, low cost | Membrane clogging, yield loss | Initial concentration of large samples |
| Size exclusion chromatography (SEC) [90, 91, 92] | Maintains integrity, reproducible | Requires pre-treatment, lower purity | Clinical and research applications |
| Immunoaffinity capture (IAC) [93, 94] | High specificity, allows subtype isolation | Expensive, low yield, labour-intensive | Functional and subtype-specific studies |
| Precipitation (PEG) [95, 96] | Simple, rapid, cost-efficient | Lower purity | Clinical and therapeutic research |
| Microfluidics (MF) [97, 98] | High precision, small sample compatibility | Specialized equipment, limited scalability | High-throughput research and diagnostics |
Ultracentrifugation (UC), the most widely used method, is considered the gold standard for EVs isolation due to its high efficiency and cost-effectiveness for large sample volumes [85]. It separates EVs based on sedimentation properties through high-speed spinning. There are two main UC types: differential ultracentrifugation (DUC) and density gradient ultracentrifugation (DGUC). DUC involves sequential centrifugation to progressively isolate particles by size and density [86]. While accessible and economical, it can lead to EVs loss due to handling, co-isolation of contaminants, and structural damage from high shear forces. DGUC, on the other hand, employs density layers to achieve better purity but requires more time and effort [87]. This approach is less suitable for large-scale isolation due to its labour-intensive nature and lower yields, despite producing purer EVs.
Ultrafiltration (UF) uses membrane filters to separate EVs based on size and molecular weight, offering a quicker alternative to ultracentrifugation [88]. It employs filters with pore sizes or molecular weight cutoffs to concentrate EVs while removing larger contaminants and soluble proteins. Though effective for volume reduction, UF can suffer from drawbacks like shear stress-induced damage, loss from membrane adhesion, and potential blockage from particle aggregation. Despite these challenges, UF methods such as tangential flow filtration and sequential filtration remain valuable for rapid isolation [89].
Size exclusion chromatography (SEC) is another gentle technique that isolates EVs based on size [90]. The method involves passing samples through porous stationary phases, with larger particles eluting slower than smaller contaminants [91]. SEC preserves vesicle integrity and function, providing high yields and repeatable results. However, it requires pretreatment steps like ultracentrifugation or ultrafiltration to reduce impurities. While it is cost-effective and accessible, achieving pure EVs preparations remains a challenge, and its efficiency can be limited compared to precipitation-based methods [92].
Immunoaffinity-based capture (IAC) isolates EVs by targeting specific surface proteins with antibodies or ligands attached to magnetic beads [93]. This approach allows for the isolation of distinct EVs subpopulations and maintains vesicle functionality post-isolation. The high specificity makes IAC suitable for studying particular EVs subtypes, but it is limited by sample volume, cost, and time requirements. Additionally, not all EVs may express the targeted markers, resulting in lower yields [94]. IAC often requires complementary methods like SEC or ultracentrifugation to improve purity.
Precipitation techniques, especially those using polyethylene glycol (PEG), offer a rapid and simple way to isolate EVs by precipitating them with hydrophilic polymers [95]. PEG disrupts the solubility of EVs in solution, encouraging their aggregation and precipitation. These methods are user-friendly, require minimal equipment, and are ideal for clinical applications due to their simplicity and scalability. However, they often yield lower-purity products with co-isolated contaminants like non-EVs proteins [96]. Despite this, precipitation remains a favored technique for its efficiency and ease of use.
Microfluidics-based techniques (MF) represent a cutting-edge approach for EVs isolation, enabling precise separation from small sample volumes [97]. These systems integrate size-based, immunoaffinity, and dynamic separation mechanisms to achieve high purity and yield. Microfluidics devices like the ExoTIC chip demonstrate significant potential [98], offering superior performance compared to traditional methods like Polyethylene glycol (PEG) precipitation or ultracentrifugation. MF ability to process complex analytical tasks at the microscale makes them suitable for applications requiring high specificity and efficiency. However, microfluidic methods often demand advanced equipment and expertise, which may limit their widespread adoption.
In conclusion, each EVs isolation method offers unique benefits and limitations, and the choice of methodology depends on the specific application, sample type, and desired purity or yield. While traditional methods like ultracentrifugation remain dominant, newer approaches like microfluidics and immunoaffinity capture are gaining traction due to their precision and advanced capabilities. Combining multiple techniques often provides the best outcomes, balancing yield, purity, and efficiency [99]. The ongoing evolution of isolation technologies continues to enhance our ability to study EVs and their role in biological and clinical research.
Proper characterization of EVs involves evaluating their size, morphology, surface charge, density, biomarker expression, yield, and purity. This analysis employs a combination of methods that target distinct structural and biochemical attributes. The following techniques encompass the most used approaches (Table 4, Ref. [2, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114]).
| Method | Advantages | Limitations | Applications |
| Transmission Electron Microscopy (TEM) [100, 101] | High resolution ( | Dehydration process can distort morphology, resulting in a “cup-shaped” appearance. | Confirming EVs presence, assessing sample quality, and analysing size/morphology at the single-vesicle level. |
| Cryogenic Electron Microscopy (Cryo-EM) [102, 103] | Preserves EVs in their natural aqueous state, avoiding artefacts from dehydration. Maintains ultrastructure and membrane integrity. | Requires specialized equipment and complex sample preparation. | Imaging biomolecules in their native environment, ideal for EVs analysis in biological fluids. |
| Scanning Electron Microscopy (SEM) [104, 105] | Generates three-dimensional images, highlighting surface features. Useful for surface morphology studies. | Provides less detail on internal structures, does not show vesicles in their hydrated state. | Surface morphology studies of EVs, visualizing spherical vesicles rather than cup-shaped ones. |
| Atomic Force Microscopy (AFM) [106, 107] | High-resolution topography imaging ( | Requires precise experimental conditions for reproducibility (e.g., probe type, temperature, and scan speed). | Nanoscale imaging of EVs’ topography, measuring shape, molecular composition, and biomechanics in their natural state. |
| Resistive Pulse Sensing (RPS) [108, 109] | High resolution for size and concentration, measures vesicles between 50–1000 nm in diameter. Reliable quantitative tool. | Limited to particles passing through a microscopic aperture, may miss some vesicles. | Quantification of EVs size and concentration, reliable with TEM counts for size determination. |
| Nanoparticle Tracking Analysis (NTA) [110] | High throughput, real-time analysis, measures particles (30–1000 nm), including fluorescently labelled EVs. | Less detailed compared to TEM, may struggle with larger or highly concentrated samples. | Size and concentration determination, real-time analysis of EVs, and studies involving fluorescently labelled vesicles. |
| Flow Cytometry (FCM) [111, 112] | High throughput, allows multiparametric analysis (size, shape, biomarkers) using beads or dyes. | Difficulty in detecting small particles due to background noise and light scattering overlaps. | Semi-quantitative analysis of EVs subpopulations, identifying cellular origin through antigen detection. |
| Imaging Flow Cytometry (IFCM) [113] | Combines flow cytometry with microscopy, offering higher resolution for submicron-sized particles. | Standardization and reproducibility challenges persist, still limited by background noise. | Submicron particle analysis, EVs identification, and characterization through both flow cytometry and imaging techniques. |
| Western Blotting (WB) [2, 114] | Detects EVs proteins using specific antibodies, including surface (e.g., CD9, CD63) and internal proteins (e.g., Tsg101). | No universally exclusive biomarkers for EVs subtypes, requiring complementary methods to ensure specificity. | Identification of EVs proteins, confirming sample purity and specificity, and characterization of EV subtypes. |
EVs, extracellular vesicles; TEM, transmission electron microscopy; TSG101, Tumor Susceptibility 101.
Transmission Electron Microscopy (TEM) is a key tool for examining EVs morphology and structure due to its high resolution (~1 nm). It is widely used for confirming EVs presence, assessing sample quality and analyzing size and morphology at the single-vesicle level [100]. However, the dehydration process during TEM sample preparation can distort EVs morphology, often resulting in a characteristic “cup-shaped” appearance. Despite this limitation, TEM is essential for detecting impurities and ensuring integrity [101].
Cryogenic Electron Microscopy (Cryo-EM), a variant of TEM, preserves EVs in their natural aqueous state by cryogenically freezing them [102]. This technique avoids artefacts from dehydration and fixation, maintaining ultrastructure and membrane integrity. Cryo-EM is ideal for imaging biomolecules in their native environment and is particularly effective for EVs analysis in biological fluids [103]. In fact, for electron microscopy methods, sample preparation steps like fixation, adsorption, and negative staining are critical and can alter EVs ultrastructure. Cryo-EM minimizes such artifacts, making it preferable for observing native features.
Scanning Electron Microscopy (SEM) generates three-dimensional images of EVs by scanning the sample with a focused electron beam [104]. It highlights surface features, showing EVs as spherical vesicles rather than the cup shape seen in TEM [105]. SEM is valuable for surface morphology studies but provides less detail about internal structures.
Atomic Force Microscopy (AFM) analyses EVs topography at the nanoscale, offering high-resolution imaging (~1 nm) [106]. It measures shape, abundance, biomechanics, and molecular composition. AFM can study EVs in their natural state with minimal sample preparation [107]. However, it requires precise experimental conditions (e.g., probe type, temperature, and scan speed), which can limit reproducibility.
Resistive Pulse Sensing (RPS) quantifies EVs size and concentration by measuring changes in electrical resistance as particles pass through a microscopic aperture [108]. It detects vesicles ranging from 50 to 1000 nm in diameter and provides higher size resolution than other methods like dynamic light scattering or nanoparticle tracking analysis (NTA) [109]. RPS produces results consistent with TEM counts, making it a reliable quantitative tool.
NTA is a widely applied technique in EVs research [110]. It tracks the Brownian motion of vesicles (30–1000 nm in size) using laser light scattering microscopy combined with a camera system. NTA software determines particle size and concentration based on the Stokes-Einstein equation. Compared to TEM and flow cytometry, NTA offers advantages such as higher throughput and real-time analysis also of fluorescently labelled EVs.
Flow Cytometry (FCM) analyses EVs subpopulations semi-quantitatively by detecting surface biomarkers using beads or dyes [111]. It enables simultaneous multiparametric analysis of physicochemical characteristics (size, shape, and biomarkers) at high throughput. However, traditional FCM has limitations in detecting small particles like EVs due to background noise and light scattering overlaps. To address these issues, high-sensitivity flow cytometers with improved forward scatter detection and fluorescence amplification have been developed [112]. Also, Imaging Flow Cytometry combines flow cytometry and microscopy, allowing the analysis of submicron-sized particles with higher resolution [113]. FCM excels in studying EVs and identifying their cellular origin through antigen detection, but standardization and reproducibility challenges persist.
Western Blotting (WB) identifies EVs proteins by separating them via SDS-PAGE and detecting them with specific antibodies [114]. Common markers include surface proteins (e.g., CD9, CD63, CD81) and internalized proteins (e.g., Tsg101, Heat shock protein 70) [2]. However, no universally exclusive biomarkers differentiate EVs subtypes. Thus, WB is often used in combination with other techniques to ensure sample purity and specificity.
Overall, each method has unique advantages and constraints. While microscopy techniques (TEM, SEM, Cryo-EM, and AFM) provide high-resolution visualization of EVs morphology, they often require extensive sample preparation, which can introduce artefacts. On the other hand, RPS, NTA, and FCM allow high-throughput quantitative analysis but may lack the structural detail provided by microscopy. On the other side, RPS and NTA excel in particle size distribution and concentration analysis, but they may require complementary methods like TEM for visual confirmation of sample integrity. FCM offers detailed phenotypic profiling but struggles with small particle detection without specialized equipment. For these reasons, no single method provides a comprehensive characterization of EVs. A combination of approaches is often necessary to analyse various aspects, including morphology, size, concentration, purity, and biomarker composition, as stated in the last ‘Minimal Information for Studies of Extracellular Vesicles’ (MISEV2023) released by the International Society for Extracellular Vesicles [1]. As a simplified workflow: TEM or Cryo-EM for structural analysis, NTA or RPS for size and concentration, FCM and WB for markers profiling. In conclusion, EVs characterization is a multifaceted process that requires integrating diverse analytical techniques. Each contributes distinct insights into EVs properties. By leveraging these tools, researchers can comprehensively study EVs physical and biochemical attributes, paving the way for their potential applications in diagnostics and therapeutics.
By definition, biomarkers are quantifiable indications that offer vital details about a range of physiological and pathological bodily processes [115]. Because EVs are found in biological fluids like blood, urine, and synovial fluid, they are perfect biomarker carriers, allowing for minimally invasive “liquid biopsy” techniques for diagnosis and treatment monitoring [116]. By monitoring changes in disease-associated biomarkers, which ought to correspond with the advancement of the disease or its amelioration during treatment, they may also be used in clinical settings to monitor patient responses to medication. EVs have shown great promise in recent years for the detection and characterisation of biomarkers associated with a variety of illnesses, such as infectious, cardiovascular, musculoskeletal, neurodegenerative, and cancerous conditions.
Biomarkers of metastasis and progression associated with cancer. Because of their role in the progression of tumours [117] and their metastasis [118], EVs have attracted a lot of attention in cancer research. EVs comprising a variety of bioactive chemicals are released by tumor cells and are demonstrated to aid in angiogenesis, immunological suppression, premetastatic niche creation, and tumor microenvironment change [119]. EVs help deliver oncogenic cargo to target cells, including DNA fragments, oncogenic proteins, and miRNAs, causing alterations that encourage tumor growth and metastasis [120, 121, 122]. A favorable environment for the deposition of metastatic cells can be created by mediating this communication between tumor cells or between tumor cells and cells in other organs [123]. EVs markers for cancer diagnosis and surveillance frequently include cancer biomarkers including CD63, CD9, and CD81. Potential EV biomarkers of many cancer types include proteins like epithelial cell adhesion molecule (EpCAM) [124], human epidermal growth factor receptor 2 (HER2) [125], and epidermal growth factor receptor (EGFR) [126].
EVs content for the detection of cancer. EVs are appealing candidates for use in early cancer diagnosis because of the makeup of particular biomolecules within them, such as distinct miRNA and protein signatures [127]. A less intrusive option to traditional tissue biopsy is provided by the analysis of liquid biopsy samples made up of isolated EVs from body fluids [128]. Researchers hope to create non-invasive diagnostics that can identify cancer in its early stages by recognizing EV indicators particular to the disease. For example, research has demonstrated that EVs miRNA profiles can be used to differentiate between healthy people and people with different kinds of cancer [129, 130]. malignancy diagnostics could be revolutionized by the capacity to identify changes in EV cargo that are linked to malignancy.
Biomarkers for neurodegenerative diseases. Early detection of neurodegenerative illnesses places a heavy strain on international healthcare systems [131]. EVs may be useful instruments for comprehending the processes that underlie neurodegeneration [132]. These vesicles can transport disease-specific cargo that represents the diseased condition of the brain and can pass through the blood-brain barrier [133]. It has been demonstrated that EVs from patients with neurological disorders such as Alzheimer’s [134] and Parkinson’s [135] diseases contain misfolded proteins called amyloid-beta and alpha-synuclein, respectively. Studying EVs gives researchers a way to look at how disease progresses and provides insights into how abnormal proteins travel throughout the neural system [136].
Potential for tracking the course of the disease and early neurodegeneration diagnosis. There is potential for early diagnosis and disease monitoring with the discovery of certain EVs markers in neurodegenerative disorders [137]. In order to track the course of diseases and evaluate the effectiveness of therapeutic measures, researchers are investigating the use of EV biomarkers as diagnostic tools. There may be a window of opportunity for intervention if changes in the cargo content of EVs occur before clinical symptoms [138]. The use of customized medicine techniques to treat neurodegenerative illnesses may be made possible by tracking how EVs change in response to treatment, which may yield important information about the effectiveness of the treatment [139].
Biomarkers for cardiovascular disease. By transporting bioactive compounds implicated in angiogenesis, inflammation, and oxidative stress, EVs contribute to cardiovascular homeostasis [140]. Because these vesicles can deliver cardioprotective substances to injured tissues, they have been linked to heart regeneration and repair processes [141]. Furthermore, EVs made from endothelial cells have molecular cargo that indicates a person’s vascular health [142]. MicroRNAs and proteins found in EV cargos are being studied by researchers as possible markers of vascular disorders and heart function [143].
Cardiovascular disorders, including atherosclerosis. Atherosclerosis and other cardiovascular etiology and development mechanisms have been better understood thanks to the content analysis of EVs [144]. Lipids, miRNAs, and inflammatory chemicals found in EVs produced from atherosclerotic plaques aid in the development and rupture of the plaque [145]. Researchers have discovered possible indicators for evaluating plaque susceptibility and forecasting cardiovascular events by examining EVs [146]. Additionally, using their innate transport capacities to deliver therapeutic cargo to particular cell types, EVs are being investigated as carriers for targeted medication delivery in cardiovascular therapy [147].
Joint disorders biomarkers. EVs have a crucial role in joint structure homeostasis [148]. In rheumatoid arthritis (RA), synovial fluid EVs are highly heterogeneous, often platelet-derived, and frequently express autoantigens like vimentin and fibrinogen [149]. These EVs exhibit pro-inflammatory activity, inducing leukotriene production in neutrophils. The presence and abundance of specific synovial fluid EVs may also be linked to disease severity or progression. For instance, CD66b EVs are more prevalent in established RA compared to early RA. Additionally, a weak correlation was also reported between anti-citrullinated peptide antibody titers, CD4 EVs, and annexin V/CD45 EVs [150]. In osteoarthritis (OA), synovial fluid EVs are enriched with cytokines and biomarkers, including lncRNAs and circular RNAs, which vary with disease stage. For example, EVs associated hsa-circ-0104595 in synovial fluid has the potential to be transported via bodily fluids and could serve as diagnostic biomarker [151]. Gender-specific differences are noted, with distinct protein and miRNA profiles in male and female OA patients [152, 153]. EVs content mirrors senescent chondrocytes, suggesting diagnostic and therapeutic monitoring potential [154]. Thus, EVs from synovial fluid offer a valuable tool to study RA and OA progression and evaluate treatment outcomes.
Infectious disease biomarkers, the case of Coronavirus disease 2019 (COVID-19). Recent studies highlight the diagnostic and prognostic potential of EVs content analysis for COVID-19 [155, 156]. Proteomic profiling, EVs phenotyping, and RNA analysis have been employed to distinguish COVID-19 patients from healthy individuals and classify disease severity [157]. Notably, fibrinogen alpha, beta, and gamma chains in EVs showed high diagnostic accuracy, with AUC values of 0.94, 0.90, and 0.93, respectively, achieving high sensitivity (83%–92%) and specificity (86%–97%). Other proteins, including fibronectin, complement subcomponent C1r, and serum amyloid P, also demonstrated robust diagnostic potential with AUC values above 0.91 [155]. Likewise, EVs RNA analysis identified 114 differentially expressed coding RNAs and 10 non-coding RNAs between COVID-19 patients and healthy individuals [157]. These RNAs are associated with immunity, inflammation, cell cycle regulation, and apoptosis, offering insights into disease pathogenesis and potential biomarkers. For severity classification, critically ill patients exhibited elevated levels of C-reactive protein (CRP), alpha-1-acid glycoproteins, chemokine ligand 7, and zinc-alpha-2-glycoprotein, and reduced complement component 4 binding protein alpha, distinguishing them from less severe cases [155].
EVs have a long circulating half-life, are well tolerated by the body, and can penetrate cellular membranes [158], making them suitable for targeting specific cell types [159] as therapeutic agents. These characteristics also make EVs promising candidates for drug delivery systems [160]. Additionally, MSCs-derived EVs have shown therapeutic potential in reducing tissue injury [161]. Despite the broad clinical applications of EVs, standardized methods for their isolation and analysis are required to meet regulatory standards set by agencies like the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), enabling their use as drug delivery systems and therapeutic agents. Here, the most promising and in advanced stage applications of EVs as therapeutic tools.
Recently, clinical trials have begun to explore the therapeutic benefits of EVs, primarily utilizing particles derived from human cells or plant specimens. According to data from ClinicalTrials.gov (https://clinicaltrials.gov/), EVs are being investigated for diverse applications, including biomarkers, therapeutic agents, drug delivery systems, and cancer vaccines. To ensure the safe and effective use of EVs in clinical settings, production must adhere to Good Manufacturing Practice (GMP) standards [162]. The GMP-grade production involves careful selection of cell types, culture environments, and cultivation systems, as well as the use of appropriate culture media [163]. Post-production purification typically follows a multi-step process to ensure product quality. Another critical aspect of GMP compliance is the establishment of robust characterization and identification methods, which evaluate both the physical properties and bioactivity of the EVs [164]. The regulatory landscape for advanced therapies, including EVs-based products, has also evolved. The EMA provides scientific guidance for classifying advanced therapy medicinal products (https://www.ema.europa.eu/en/human-regulatory-overview/advanced-therapy-medicinal-products-overview/guidelines-relevant-advanced-therapy-medicinal-products). This guidance acknowledges significant advances in cellular and molecular biotechnology, which have driven the development of novel therapies such as gene therapy, somatic cell therapy, and tissue engineering. These advancements create new opportunities to address diseases and dysfunctions, paving the way for innovative biomedicine. Moreover, EMA outlines comprehensive guidelines for researchers working on advanced therapies, offering recommendations on regulatory compliance, product classification, and quality standards [165]. These frameworks are essential for translating scientific progress into effective clinical applications, ensuring that emerging therapies meet safety and efficacy standards.
EVs-based vaccines have shown potential against infectious diseases [166] and tumour-derived EVs prompt strong anti-tumour immune responses [167]. Molecules on EVs, such as Major histocompatibility complex (MHC) and costimulatory proteins, enhance immune responses, and advances in profiling EVs cargo have identified active agents for cancer therapy [167]. As an example, dendritic cell (DC)-derived EVs were shown to be safe and effective in clinical trials, showing higher biostability, bioavailability, and lower costs compared to traditional therapies. Phase I trials (NCT01159288) using DC-EVs for metastatic melanoma demonstrated safety but limited T cell responses, while Phase II trials for small cell lung cancer showed enhanced Natural killer (NK) cells activity but failed to achieve significant progression-free survival [168]. Combining DC-EVs with NK therapies or optimizing DC lines could improve outcomes and reduce costs since EVs small size and shape allow extended circulation and efficient cell communication, underscoring their therapeutic potential.
EVs are also used to treat various diseases [169], with almost 100 studies registered under ClinicalTrials.gov at end of 2024 (
Despite promising results, concerns about safety and side effects persist, as highlighted by the FDA’s warnings against unapproved EVs products causing adverse effects [170]. To enhance EVs production and therapeutic potential, strategies such as preconditioning stem cells, genetic modifications, and combining EVs with biomaterials have been employed [171]. For instance, norepinephrine and N-methyldopamine can boost EVs secretion from MSCs without affecting their modulatory functions [172]. Other culture conditions methods include hypoxic, acidic, or lipopolysaccharide-rich conditions [173]. Additionally, plant- and microbial-derived EVs have been explored, although their safety requires further evaluation [174, 175]. Other challenges in clinical applications include the low yield of EVs (typically
EVs can serve as carriers for small RNA molecules, such as miRNAs, anti-miRNAs, circular RNAs (circRNAs), and short interfering RNAs (siRNAs), which play distinctive roles in gene regulation and tumour suppression. Delivering miRNAs through EVs represents since a long time a promising strategy for gene regulation and cancer treatment [176]. miRNAs, noncoding RNAs capable of influencing gene expression, are often linked to various diseases [177]. Many miRNAs are implicated in tumour suppression, making their reintroduction a novel therapeutic avenue [178]. However, miRNAs are rapidly degraded in vivo, which limits their therapeutic potential. EVs can encapsulate and deliver miRNAs, improving their stability and efficacy [179]. circRNAs are a class of non-coding RNAs (ncRNAs) that regulate gene expression both at the transcriptional and post-transcriptional levels by interacting with miRNAs and inhibiting their activity [180]. Recent studies have revealed that several circRNAs, similar to miRNAs, may also exhibit anticancer properties [181]. siRNAs, also known as silencing RNAs, are double-stranded non-coding RNA molecules typically ranging from 20 to 24 base pairs in length [182]. siRNAs have been recognized as a promising therapeutic option for viral infections and cancer. While siRNAs can target and silence specific cancer-causing oncogenes, their delivery to targeted tissues or cells may be hindered by the immunogenic properties of siRNA or rapid degradation in the bloodstream [183]. As a result, EVs are being explored as a means to deliver siRNAs to specific sites effectively [184]. While EVs delivery of siRNA is a promising approach, questions remain about its specificity and efficiency. Alongside small RNAs, EVs also allow the encapsulation and transport of messenger mRNAs, which face similar challenges in vivo, such as instability and immunogenicity. Encapsulation in EVs enhances the delivery and efficacy of mRNA-based therapies [185]. However, if EVs are considered a promising strategy for delivering small RNAs, their yield for mRNA encapsulation is low. Nevertheless, the challenge of incorporating and releasing large amounts of mRNA has been recently addressed by enhancing encapsulation through biological modification of cell sources and cellular nanoporation techniques [186].
EVs are also being explored for protein drug delivery, leveraging their membrane protein composition and structural stability. These vesicles naturally incorporate MHC-I proteins, which can stimulate immune responses against tumours. Drug loading techniques for engineered EVs are categorized into endogenous and exogenous methods. Endogenous loading involves transfecting donor cells with genetic modifications to produce desired proteins, nucleic acids, or drugs, which are then encapsulated in EVs [187]. A prominent example is using the Clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 system for gene editing. EVs carrying CRISPR/Cas9 can facilitate non-invasive gene editing in cancer cells inducing apoptosis [188]. Exogenous loading involves isolating EVs and adding drugs or bioactive cargo externally. This method offers simpler protocols and is more scalable [189]. Techniques include co-incubation, electroporation, sonication, freeze–thaw cycles, and extrusion. In co-incubation, EVs are mixed with drugs, retaining membrane integrity, ideal for small hydrophobic molecules. Electroporation uses electric fields to create temporary pores in EVs membranes, allowing hydrophilic molecules like peptides to enter. The same procedure is the most used for miRNA encapsulation. While effective, electroporation can lead to aggregation and instability. Sonication involves applying sound waves to induce membrane pores, permitting the entry of small molecules. Although effective, it requires careful control to avoid compromising EVs integrity. The freeze–thaw cycle method involves alternating temperatures to promote drug incorporation but can reduce EVs stability. Lastly, extrusion uses a lipid extruder to load drugs, effective for encapsulation but requiring precise control to avoid EVs damage.
Chemical drug delivery via EVs offers another exciting application, particularly for drugs with poor water solubility or rapid degradation. To improve the effectiveness of anti-cancer drug therapy, various chemical drugs have been incorporated into EVs. These formulations are continuously being developed to enhance the bio distribution and pharmacokinetics of the drugs, while minimizing their side effects. The most studied disease is cancer. As examples, a paclitaxel-loaded macrophage-derived EVs modified with AA–PEG, enhanced lung cancer targeting and survival [190]. THP-1-derived EVs were loaded with doxorubicin and miRNA for breast cancer, demonstrating reduced tumour growth and improved survival [191]. Also, siS100A4/cationic bovine serum albumin combination in EVs to treat lung cancer reduced metastatic nodules was reported [192]. The therapeutic potential of EVs extends to delivering bioactive molecules like curcumin, a compound known for its anticancer properties. EVs-mediated delivery of curcumin induced apoptosis in pancreatic cancer cells, suggesting that EVs could enhance the pharmacological effects of natural compounds [193]. Similar promising results were obtained for treating joint-related diseases like RA. Research highlights various approaches, such as using dexamethasone-loaded EVs functionalized with folic acid to target damaged areas in RA, improving joint morphology and bone density in mice [194].
Collectively, these studies underscore the versatility of EVs in delivering therapeutic agents, including RNAs, proteins, and chemical drugs. Their unique properties—biocompatibility, stability, and specificity—make them promising candidates for advancing treatment strategies. Researchers continue to explore the intricacies of EVs biogenesis, cargo selection, and delivery mechanisms to optimize their application in clinical settings.
EVs have emerged as crucial players in intercellular communication and a wide array of biological processes (Table 5). Recent advancements in EVs research have significantly enhanced our understanding of their roles in disease mechanisms, diagnostics, targeted therapies, and drug delivery systems. Despite the progress, challenges remain, such as the standardization of EV isolation methods and the need for more comprehensive studies on their biogenesis and functional diversity. As a corollary, as advancements in EVs-based diagnostics and therapies continue, ethical concerns become increasingly important. These innovations raise questions about consent, privacy, and unintended consequences. Developing robust ethical frameworks is essential to address issues such as obtaining informed patient consent for the collection, storage, and sharing of EVs-derived data. Moreover, the potential for off-target effects and unexpected outcomes in EVs-based treatments underscores the need for rigorous preclinical and clinical testing to ensure patient safety and well-being. Addressing these ethical and safety challenges is critical to fostering responsible development and application of EVs technologies in healthcare. Overall, EVs represent a fascinating and rapidly evolving field with the potential to revolutionize biomedical science and healthcare in the years to come.
| Role | Applications | Advantages | Challenges |
| EVs as Biomarkers | - Disease diagnostics (e.g., cancer, neurodegenerative disorders). | - Non-invasive or minimally invasive sampling (e.g., liquid biopsies). | - Need for standardized isolation and analysis techniques. |
| - Prognostic indicators for treatment outcomes. | - High specificity and sensitivity due to their molecular cargo (e.g., proteins, RNA, lipids). | - Variability in EV composition across individuals and conditions. | |
| - Real-time monitoring of disease progression. | |||
| EVs as Therapeutic Tools | - Immune modulation (e.g., suppressing or enhancing immune responses). | - Natural origin and biocompatibility reduce risks of immunogenicity. | - Understanding and controlling EV biogenesis and release. |
| - Promoting tissue regeneration (e.g., cartilage, bone, and neural repair). | - Ability to target specific cells or tissues. | - Ensuring safety and efficacy in clinical applications. | |
| Anti-inflammatory treatments. | |||
| EVs as Drug Delivery Systems | - Targeted delivery of small molecules, nucleic acids, and proteins. | - Inherent targeting capabilities reduce off-target effects. | - Scaling up production for clinical use. |
| - Protection of therapeutic cargo from degradation. | - Addressing heterogeneity in EV populations. | ||
| - Overcoming biological barriers (e.g., blood-brain barrier). | |||
| Enhancing drug stability and bioavailability. |
EVs, extracellular vesicles.
Raw data are available at: https://osf.io/8k9vd/?view_only=62c8f639bbcc4c94b16a8b1b2cfd765a.
ER: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Software, Resources, Supervision, Validation, Visualization; ER: Writing – original draft; Writing – review & editing. ER: Read and approved the final manuscript. ER: Has participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Author wishes to acknowledge all members of Laboratorio di Biotecnologie Applicate all’Ortopedia for useful discussion.
Funded by the European Union - Next Generation EU - NRRP M6C2 - Investment 2.1 Enhancement and strengthening of biomedical research in the NHS - Project Code PNRR-MCNT2-2023-12377836 (CUP Master C43C24000460001).
The author declares no conflict of interest.
During the preparation of this work, the author used ChatGPT to check spelling and grammar throughout the text. After using this tool, the author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

