1 Pavlov Institute of Physiology of the Russian Academy of Sciences, 199034 Saint Petersburg, Russian
Abstract
Interferons (IFNs) are ototoxic drugs leading to vestibular and auditory disorders. This study investigated the effect of pro-inflammatory cytokine IFN-α2b on the afferent glutamatergic synaptic transmission of the vestibular end organ, focusing on ionotropic glutamate receptors (iGluRs).
In order to characterize the role of IFN-α2b in the glutamatergic synaptic transmission in vestibular epithelium, we investigated its influence on responses evoked by D,L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA) and kainic acid (kainate). This was carried out using external perfusion of the vestibular apparatus and multiunit recording of afferent firing activity of semicircular canal ampullary nerve fibers. The change in the ratio of the maximum frequency of pulse activity to the preceding background was chosen as a criterion for evaluating the evoked responses of glutamate receptor (GluR) agonists.
Acute perfusion of the vestibular apparatus with IFN-α2b and AMPA did not alter the AMPA-evoked response. However, a significant increase in the response was observed 15 min after cessation of drug application and washing with normal solution (paired-samples t-test p = 0.018; n = 20). IFN-α2b significantly increased the kainate-evoked response during cytokine application (Wilcoxon signed-rank test p = 0.016; n = 11), and further potentiates the response 15 min after rinsing with normal solution, compared to the test value (Wilcoxon signed-rank test p = 0.05; n = 11). IFN had no effect on NMDA-induced responses. AMPA receptors (AMPARs) potentiated by IFN-α2b increase NMDA-evoked responses (Repeated measures analysis of variance [ANOVA RM], p = 0.028; n = 10).
IFN-α2b stimulates AMPARs and kainate receptors (KARs) through various mechanisms but has no direct effect on NMDA receptors (NMDARs). Interferon-activated AMPARs can stimulate NMDARs activity, thereby altering synaptic plasticity of the glutamatergic afferent synapse in vestibular epithelium.
Keywords
- interferon
- vestibular epithelium
- AMPA receptors
- kainate receptors
- NMDA receptors
- neuroimmunomodulation
Cytokines are a well-studied and heterogeneous group of soluble mediators of innate and acquired immunity [1]. Interferons (IFNs) are cytokines with antiviral properties, released from host cells in response to aberrant RNA or DNA of viruses, and acting through host toll-like receptors (TLRs) [2, 3, 4].
The natural protective function of IFN is widely used in medical practice in the treatment of cancer, multiple sclerosis and severe viral infections [5]. However, the use of high doses of IFN in long-term therapy may be accompanied by severe side effects leading to interruption of treatment [6]. It is generally accepted that a wide range of severe adverse reactions during IFN therapy is associated with the cytokine’s influence on various mediator systems. According to medical statistics, interferon is an ototoxic substance that leads to acute loss of hearing and balance [7].
To date, pharmacological tools used to correct vestibular dysfunctions are extremely limited due to insufficient study of the molecular mechanisms involved in the processing of sensory information in the structures of the inner ear.
The vestibular epithelium is a highly specialised tissue that ensures the conversion of the subtle mechanical stimuli perceived by the hair cell into the bioelectrical activity of nerve fibres. The glutamatergic, cholinergic, dopaminergic, and opioid systems provide this process. Afferent glutamatergic synaptic transmission is extremely sensitive to the influence of the external and internal environment, including the influence of active molecules expressed during inflammation [8, 9, 10, 11].
The active role of the immune system in the functioning of inner ear structures is the subject of intense research at the molecular and cellular level [12]. The data on the influence of pro-inflammatory cytokines on mediator processes in the vestibular epithelium are extremely limited and the only data have focused on tumour necrosis factor (TNF
The influence of pro-inflammatory cytokines IFNs on synaptic processes of the inner ear has not been investigated so far. Neither receptors for different types of IFNs and their localisation in the vestibular epithelium have been identified, nor the functional role of supporting cells, which are thought to function as glia that play a key role in the expression of cytokines during inflammation, has been investigated.
We have been researching the effect of IFN-
We were able to show for the first time that afferent glutamatergic synaptic transmission in the vestibular epithelium could be a target of IFN type I. IFN-
The function of glutamate in the amino acid synapse is carried out by close functional interaction of ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs) localised in the same synapse and providing high speed and plasticity of afferent synapse functioning. This paper continues the previous research by focusing on the mechanisms of IFN influence on iGluRs.
The electrophysiological method of recording the pulse activity of afferent fibres in contact with the frog vestibular organs is a common technique that allows us to study the mechanisms of synaptic transmission in the vestibular epithelium in vitro. The study was conducted on 60 isolated vestibular capsules of adult frogs Rana temporaria with a body length of 5–7 cm at room temperature. Each frog was anesthetized by immersion in 0.1% MS 222 (3-aminobenxoic acid ethyl ester) until all signs of breathing activity ceased. They were then decapitated and the bone with the membranous labyrinth it enclosed was excised. The scheme for obtaining the vestibular capsule was agreed with the ethical committee of the Pavlov Institute of Physiology (No. 09/18 dated 09/18/2023). The opened cartilaginous capsule with all preserved structures of the inner ear was separated and then placed in a recording chamber, perfused constantly with a physiological solution approximating the composition of perilymph (in mM): NaCl 117, KCl 2.5, NaHCO3 1.2, NaH2PO3
The reagents used in the experiments were: D,L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (Sigma, St. Louis, MO, USA), N-methyl-D-aspartate (NMDA) (Sigma), kainic acid (kainate) (Sigma) and IFN alpha-2b (IFN-
In order to investigate the effect of IFN-
To investigate whether the cytokine would trigger iGluRs at brief temporal exposures, simultaneous perfusion of the synaptic region with a solution of cytokine and iGluRs agonist was used. In this case, the perfusion time was 35–40 s and it’s minimum time was dictated only by the dynamics of the development of the agonist evoked response.
The effect of iGluR agonists was evaluated as a percentage of the ratio of the maximum frequency of impulse activity of an iGluR agonist evoked response to previous resting activity level. The effect of IFN-
Statistical analysis of the results was performed with normalised data. Initially, the data were analysed for deviations from normal distribution using the Shapiro-Wilk criterion, as well as for the presence of outliers. The effect of IFN-
Analysis of experimental data showed that IFN-
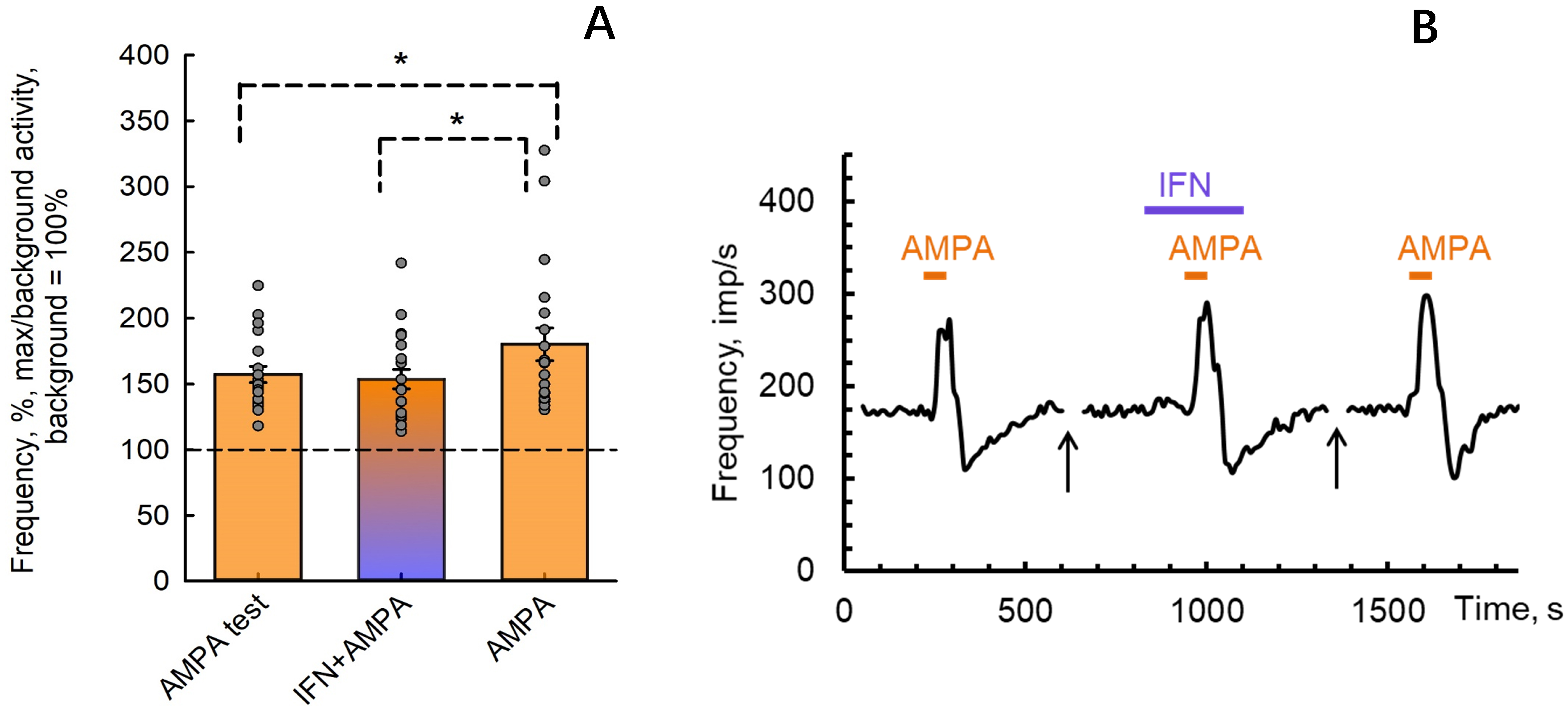 Fig. 1.
Fig. 1. The change of D,L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) evoked responses (2 µM) in posterior semicircular ampullary nerve before (test), against preliminary 2 min interferon-
Preliminary 2-minute perfusion of synaptic area with IFN-
However, 15 min after cessation of the combined action of IFN-
Brief 30–40 s perfusion of the synaptic area with solutions of AMPA and IFN-
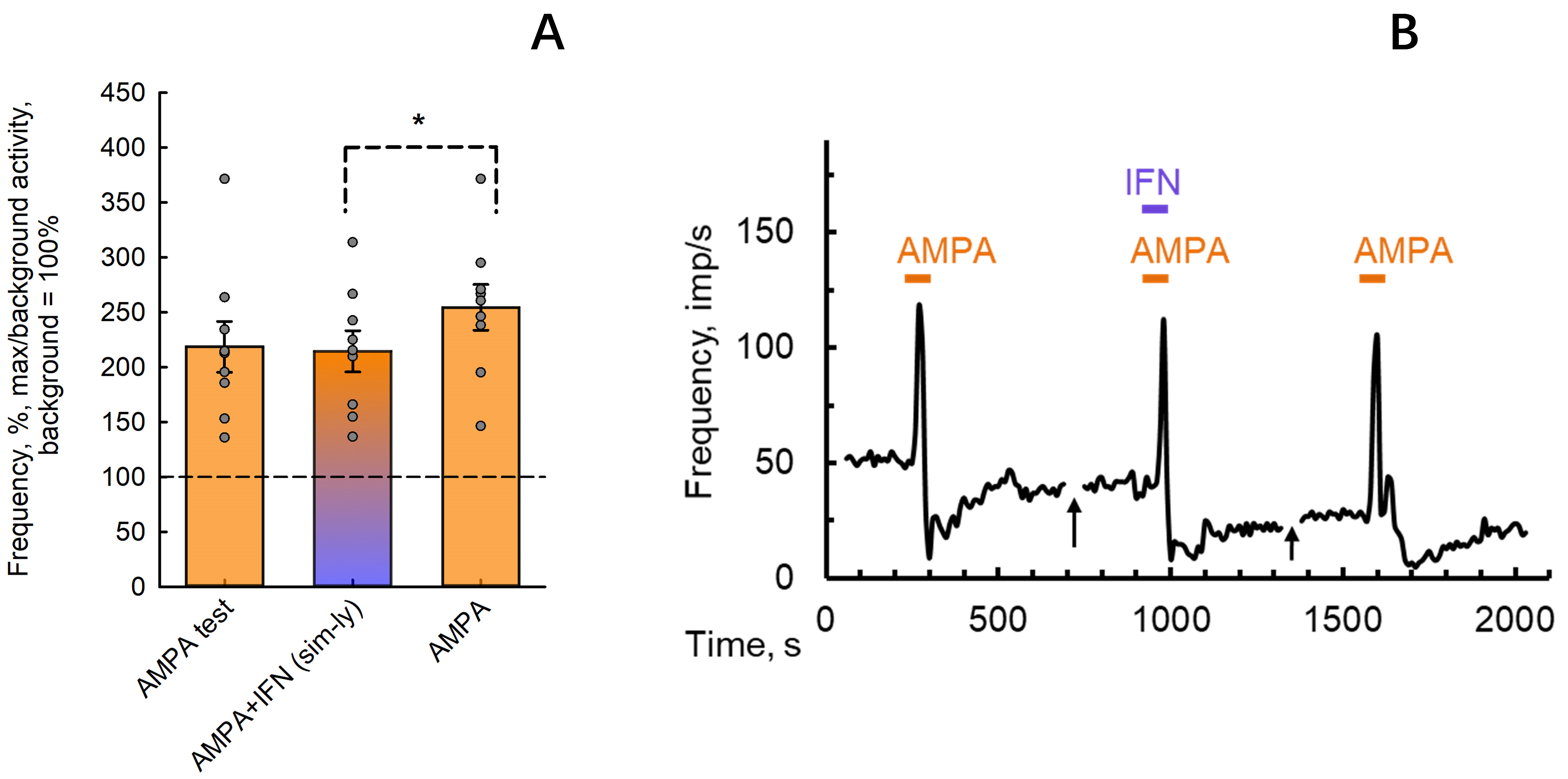 Fig. 2.
Fig. 2. Dynamics of changes of D,L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) evoked responses (2 µM) in the afferent fibers of the posterior semicircular canal nerve before, during simultaneous (sim-ly) short-term perfusion of the vestibular apparatus with interferon-
Importantly, as with the 2-minute cytokine pre-application, the AMPA-evoked response to simultaneous presentation of AMPA and IFN-
Comparison of the results of the above two series suggests the potentiating delayed effect of IFN-
Brief simultaneous perfusion of the synaptic area with solutions of kainate (2 µM) and IFN (10 ng/mL) increased significantly the ratio of the maximum frequency of pulse activity relative to the preceding background (216.5
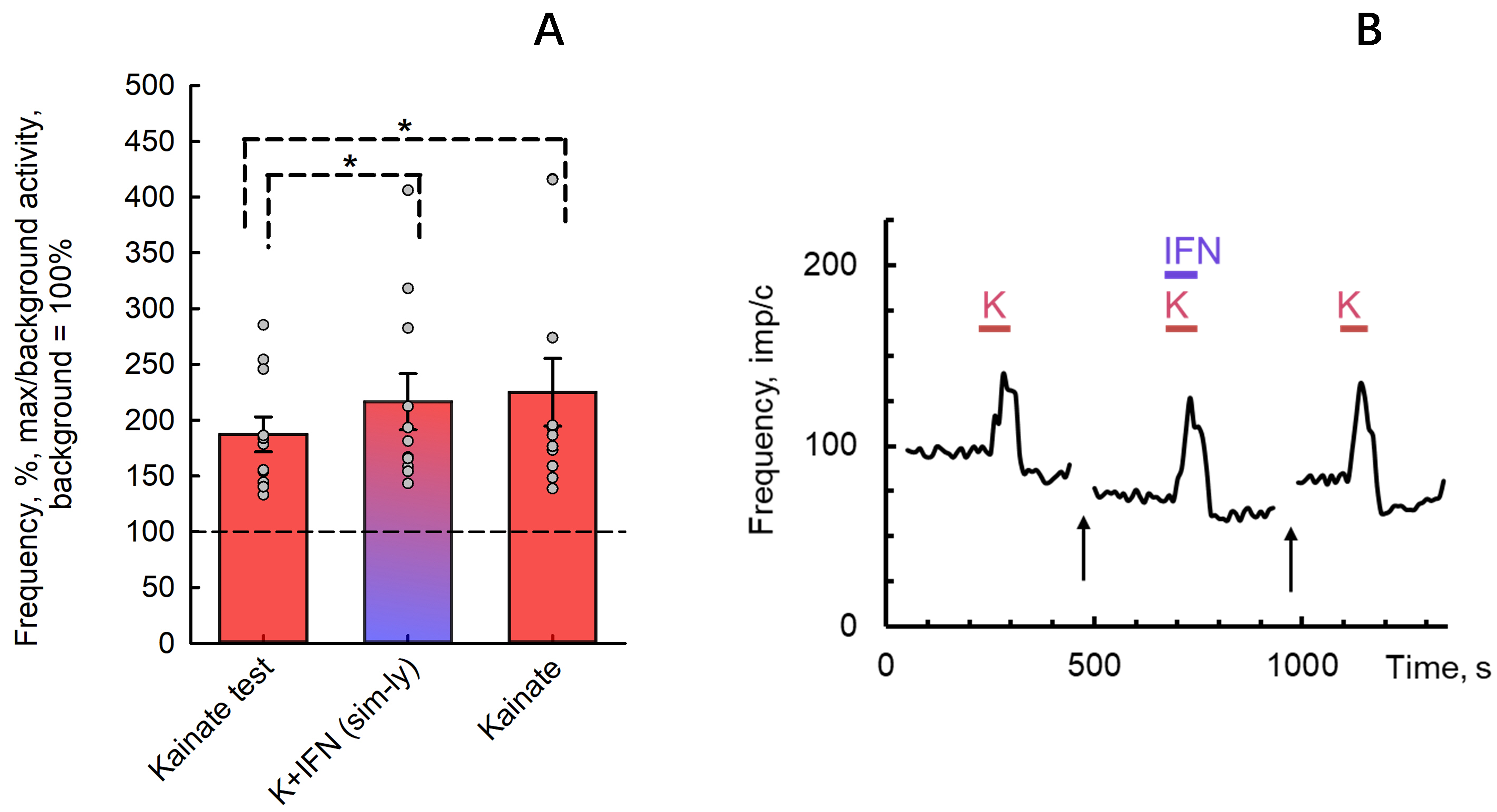 Fig. 3.
Fig. 3. The change of kainate evoked responses (2 µM) in afferent fibers in the posterior semicircular canal nerve before, during simultaneously (sim-ly) short-term perfusion of the vestibular apparatus with kainic acid (K) and interferon-
To answer the question about the effect of IFN
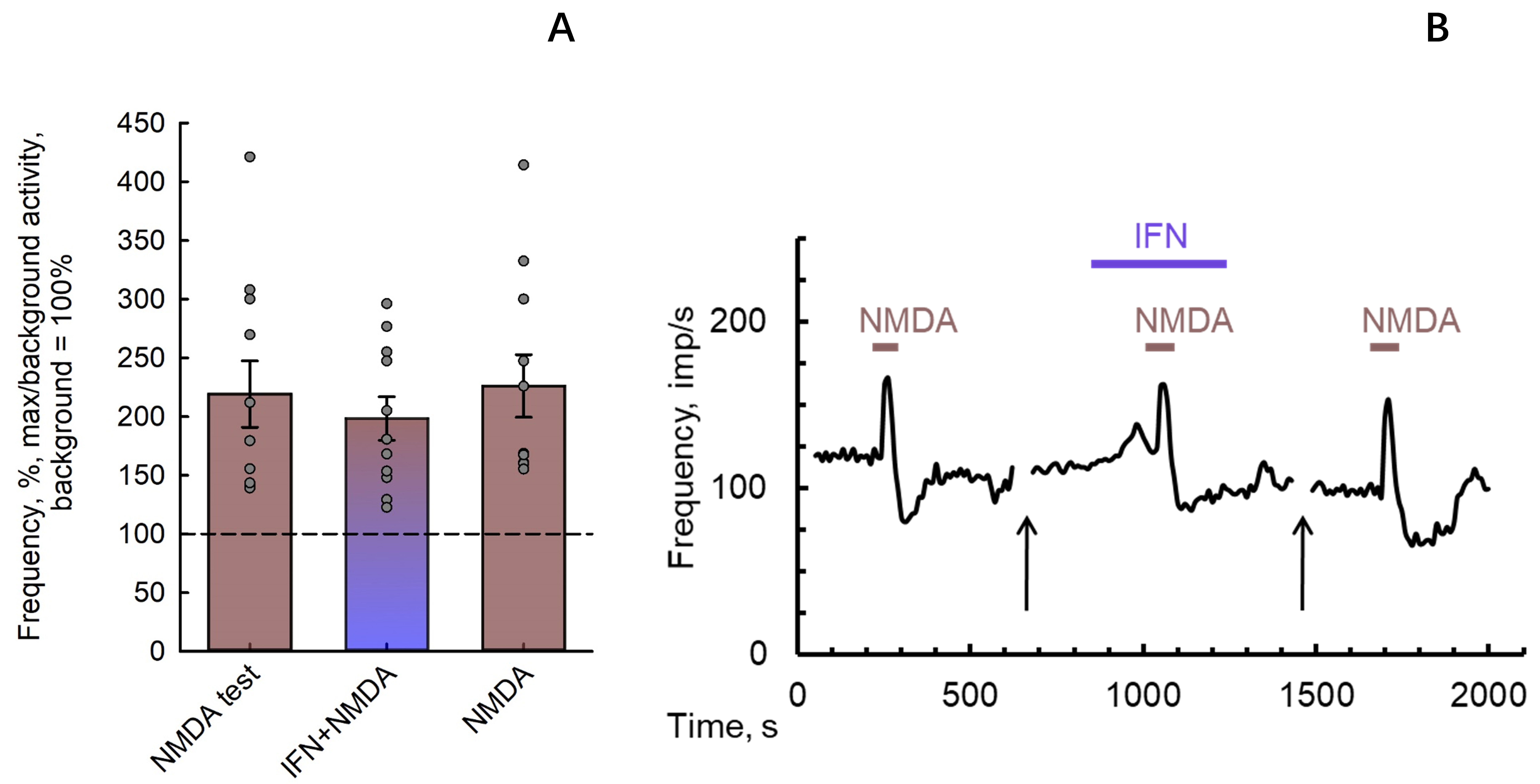 Fig. 4.
Fig. 4. Absence of the effect of interferon-
The ratio of the maximum frequency of pulse activity to the preceding background activity level differed significantly between NMDA and IFN-
Analysis of the obtained results allows us to conclude that interferon IFN-
As follows from the results of this work, IFN exerted a delayed potentiating effect on AMPA- and kainate-evoked responses (Figs. 1,2,3). To answer the question of whether IFN-
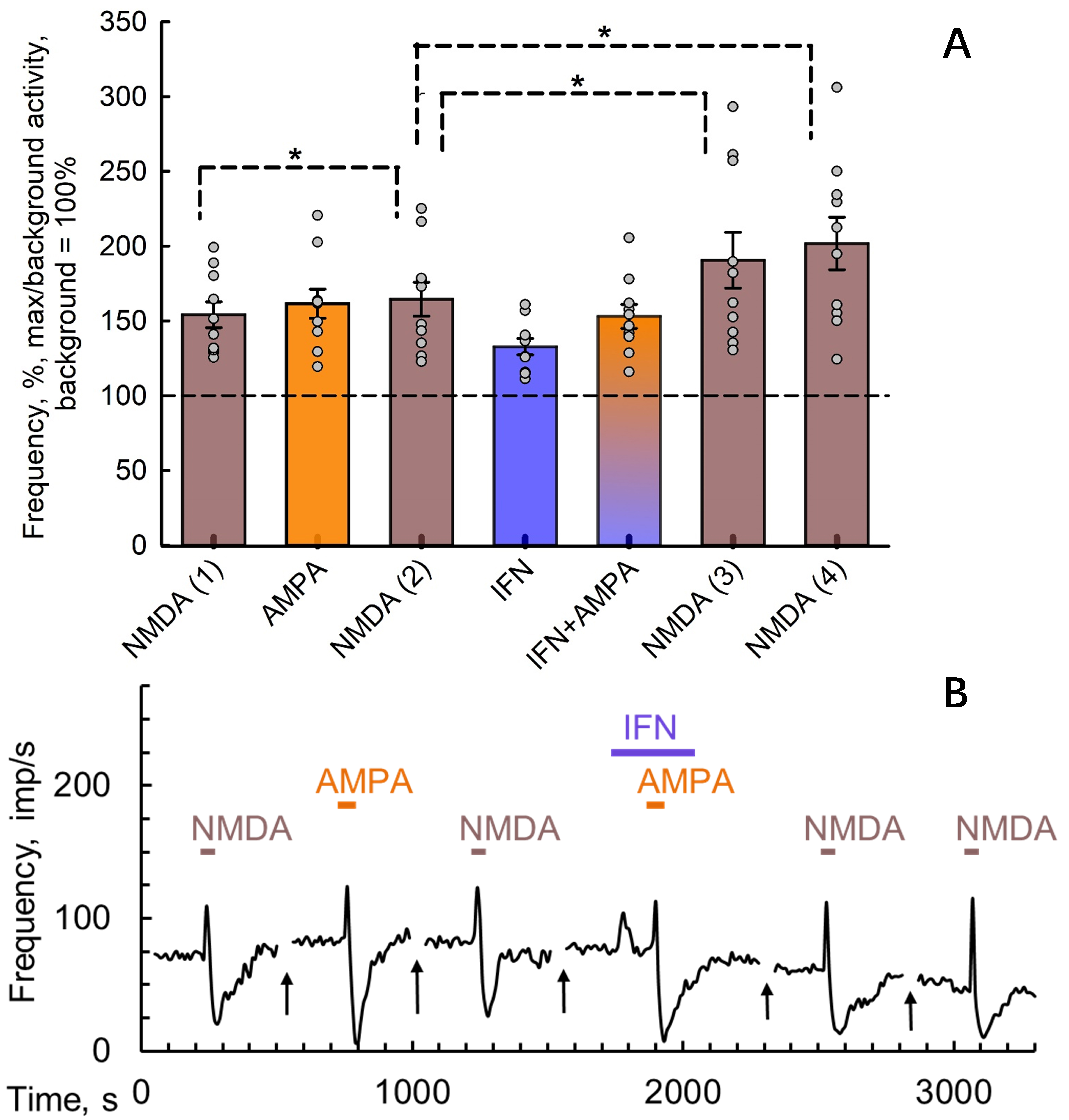 Fig. 5.
Fig. 5. Cross talk of AMPA and NMDA receptors in the absence and presence of interferon-
For this purpose, in the first part of the experiment, comparison of the NMDA-evoked responses before (NMDA (1) in Fig. 5A) and after AMPA exposure (NMDA (2) in Fig. 5A) allowed us to assess the effect of AMPARs activation on NMDARs. In the second part of the experiment, the vestibular apparatus was perfused for 2 min with IFN-
Since the analysis of data distribution in some series of experiments revealed significant differences from normal, the Friedman test, Wilcoxon paired comparisons and in addition ANOVA RM analysis of variance for dependent variables were applied. The ratio of maximum frequency of impulse activity relative to the preceding background during NMDA application (50 mM) was found to increase 15 min after AMPA application (2 µM) (Wilcoxon signed-rank test p = 0.093; Paired-Samples t-test p = 0.043; n = 10), which implies minimal delayed potentiation of NMDARs by AMPARs 15 min after cessation of AMPA application and washing of the synaptic area in normal solution. Subsequent perfusion of the synaptic area with IFN and IFN+AMPA solutions had a significant effect on NMDA responses (ANOVA RM F(2.18) = 5.019, p = 0.019,
15 min after cessation of co-perfusion of the synaptic area with IFN and IFN+AMPA solutions the ratio of maximum response frequency to the preceding background of NMDA responses was 190.5
Moreover, there was no significant difference between NMDA-induced responses 15 and 30 min after cessation of IFN + AMPA application (Wilcoxon signed-rank test p = 0.309; n = 10), suggesting a prolonged delayed stimulatory effect of interferon-activated AMPARs on NMDARs.
Thus, our data suggest that IFN-
There is now no doubt that the nervous and immune systems are in continuous interaction. Under normal conditions, the release of inflammatory mediators usually represents an adaptive and regulated brain response to immune signals. When the immune challenge becomes prolonged and/or uncontrolled, the subsequent inflammatory response leads to maladaptive synaptic plasticity and brain disorders [2]. A growing body of evidence indicates that some of the immune system proteins, in addition to their immunological function, play a critical role in the formation, function and modulation of synaptic contacts [16, 17, 18]. This group includes proteins of innate immunity (Dscam, pentraxins, C1q and C3 compliments), the major histocompatibility complex class I (MHCI) family of proteins, and also pro-inflammatory cytokines (TNF
IFNs are known to have anti-inflammatory and immunomodulatory effects against viral and malignant cells [29]. In vivo IFNs are produced in the CNS by neurons, astrocytes and microglia, as well as immune cells: macrophages, monocytes, T-lymphocytes—at a low physiological level [30, 31].
IFNs, belonging to the group of mediators of innate and acquired immunity [1], are divided into three types according to the types of membrane receptors to which the cytokine binds [32].
Signalling from the interferon type I receptor (IFNRI) and activation of the Janus kinase 1/signal transducers and activators of transcription (JAK/STAT) pathway is a hallmark of viral control and host defence [4]. In addition to their protective function, IFNs, in a dose-dependent manner, exert multidirectional effects on synaptic processes.
Modulatory and toxic effects of different types of IFNs have been described in the CNS. Clinical and experimental data indicate that low doses of IFNs don’t cause severe side effects. Neurotoxic effects of IFNs in the glutamatergic system at the neuronal level are associated with activation JAK/STAT (Janus kinase 1/signal transducers and activators of transcription) cascade, and with direct and indirect impact on the various subtypes of iGluRs [33, 34]. IFNs alter the dendritic tree pattern, the number and nature of synapses, specifically decrease the levels of presynaptic protein vesicular glutamate transporter and postsynaptic density protein-95 [35].
The analysis of the mechanisms of IFNs influence on synaptic transmission is based mainly on the data obtained for CNS structures. The modulatory effects of IFN differ significantly in different CNS structures. IFN not only has both excitatory and inhibitory effects in various structures of the CNS, but also causes multidirectional effects on neurons of the same structure [33, 35, 36]. IFN-
Data of clinical and experimental physiology provide numerous evidences of ototoxic effect of IFN on inner ear functions [40, 41, 42, 43, 44]. One of the causes of vestibular disorders may be impaired synaptic transmission in vestibular organs, which is reflected in changes in the nature and ratio of spontaneous and evoked activity in synaptic structures. This results in the balance problems, from minor sensations of instability to severe vertigo attacks. Our studies demonstrate for the first time that IFN-
In our experiments, interferon increased the level of background activity of afferent fibres in contact with the epithelium of the posterior semicircular canal [13]. Despite the fact that the background activity of afferent fibres of the vestibular epithelium is due to activation of AMPARs [46], it seems unlikely that the observed increase in the level of background activity may be directly due to changes in AMPAR activity, since neither simultaneous nor sequential application of cytokine with AMPA changed the ratio of maximal activity to the preceding background.
The increase in the resting activity level of afferent fibres may be explained by direct interaction of cytokine with IFNRI, so far not identified in the vestibular epithelium, or with opiate receptors, to which cytokine binds specifically [47, 48]. In the CNS IFNs inhibit neuronal activity of the spinal cord, reduce pain responses by acting on µ-opiate receptors [37, 49].
In vestibular organs, opiate receptors, which are represented by µ- and
The increase in the level of background activity of afferent fibres upon IFN influence may also occur indirectly through an increase in the glutamate content in the synaptic cleft, which is carried out through various mechanisms. For example, IFN may change the properties of the basal membrane of the hair cell, which entails a change in the amount of spontaneously released mediator. It has been shown that in the CNS IFN changes membrane properties at the subthreshold level, increasing the resistance and time constant of the membrane [39].
Another mechanism affecting the level of background activity of afferent fibres in the vestibular epithelium under the IFN influence may be the change in the level of the astrocytic glutamate-aspartate transporter (GLAST), which clears the synaptic cleft of excessive neurotransmitter and thus ensures accurate and rapid transmission of information from the hair cell [51]. According to cited auther, IFN decreases the level of GLAST. The opposite effect, an increase in GLAST levels, was observed when interferon receptor (IFNAR) was inhibited [52].
Glutamate release from the basal membrane of the hair cell can also be modulated indirectly, through a possible depression of the efferent system. In amphibians, efferent fibres are known to contact directly with type II hair cells [53], supporting the complex and fine tuning of afferent glutamatergic transmission by balancing excitatory and inhibitory influences with the participation of cholinergic, dopaminergic and opioid systems [54]. It is known that acetylcholine (ACh) can have different effects on the level of background activity in the sacculus, utriculus and semicircular canals depending on the type of ACh receptors it affects [54, 55, 56]. In the semicircular canals, ACh increases background activity level by acting on muscarinic ACh receptors [57].
In the efferent synapses in the cochlea and in vestibular organs, ACh is co-localised with dopamine (DA), which is tonically released from efferent fibres and has a neuroprotective effect on glutamatergic transmission through activation of D1 and D2 dopamine receptors [58, 59, 60, 61]. DA has been shown to reduce the level of background afferent fibre activity and responses elicited by the application of glutamate and agonists of iGluRs and mGluRs. It is conceivable that suppression of DA release by IFN or inhibition of postsynaptic DA receptor activity may lead to increased level of background activity in afferent fibres.
As indicated previously, all subtypes of iGluRs have been identified in the vestibular apparatus. Under our experimental conditions, IFN had different effects on NMDARs, AMPARs, and KARs.
In accordance with current evidence KARs are tetramers associated of different combinations of the GluK1-GluK5 subunits. In CNS KARs are involved in the synaptic plasticity process, including long-term potentiation (LTP) and long-term depression (LTD), at presynaptic and postsynaptic levels using ionotropic and metabotropic mechanisms [62, 63].
GluK1-3 has been shown to form a functional homomeric channel. High-affinity GluK4-GluK5 subunits, characterised by a slow deactivation rate, do not function independently but must co-assemble with GluK1-GluK3 subunits for channel function. In contrast, receptors containing the GluK3 subunit, unlike other AMPARs/KARs, desensitise more rapidly at low agonist concentrations, rendering them insensitive to spillover from neighbouring synapses.
The functional role of different KARs in synaptic plasticity (including binding specificity, ion channel permeability and channel block) depends on the composition of the subunits and on the auxiliary subunits (neuropilin- and tolloid-like 1) Neto1 and (neuropilin- and tolloid-like 2) Neto2, which influence their synaptic trafficking [64, 65, 66, 67]. Modulation of MAP (mitogen-activated protein kinase) activity has been shown to be associated with a change in ion channel conformation from a ligand-bound fully open state to a fully closed state [68, 69, 70].
It is accepted that synaptic efficiency depends on the number of receptors on the synaptic membrane, which is regulated by the processes of endocytosis, recycling, exocytosis and lateral diffusion of receptors, including the phosphorylation process [71]. The combination of the above mechanisms provides a fine regulation of synaptic plasticity in various CNS structures at the pre- and postsynaptic level.
In contrast to CNS vestibular KARs are poorly studied. The first direct information about postsynaptic KARs in the vestibular epithelium was obtained in electrophysiological experiments on afferent synapses of the posterior semicircular canal of the axolotl (Ambystoma tigrinum). Block of synaptic transmission induced by a high-Mg2+ and low-Ca2+ solution did not alter the responses of the glutamate agonists AMPA and kainate, suggesting a postsynaptic influence of kainate and AMPA [72].
Immunoblotting of proteins from inner ear cell homogenate revealed 5 bands associated with KAR subunits with different molecular masses around 48,000 Mr. Immunocytochemical staining with polyclonal and monoclonal antibodies was detected on dendrites of afferent fibres, but not on hair cells or efferent fibres [73]. Immunoreactivity to kainate GluR5&6 and KA1&2 subunits was also detected on membranes of afferent fibres [74]. In vestibular ganglia culture, currents induced by kainate application were inhibited by the AMPA-kainate receptor antagonist CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) and trimetazidine, suggesting postsynaptic localisation of KARs [75]. KARs associated with G protein activation were not detected in vestibular epithelium.
Importantly, in our experiments IFN-
Since, as mentioned above, KARs have not been identified on hair cells, it can be argued that in our experiments IFN modulated kainate evoked responses at the postsynaptic level.
Although the structure of AMPARs and KARs is different, pharmacological tools that reliably distinguish between AMPARs and KARs have not been developed sufficiently [69]. Taking into account the fact that kainate is partially an agonist of AMPARs, it can be assumed that the delayed potentiation of KARs may be due not only to activation of KARs themselves, but also to activation, at least in part, of AMPARs. At the same time, we cannot exclude the possibility that IFN triggers common hypothetical mechanisms that lead to increased trafficking of both AMPARs and KARs to the synaptic membrane surface.
The background activity of afferent fibres was found to be due to glutamate release from hair cells and activation of postsynaptic AMPARs [46]. It was logically expected that one of the possible mechanisms for the increase in the background activity of afferent fibres upon cytokine exposure would be a change in the activity of AMPARs. However, this hypothesis was not confirmed by experimental data, because the ratio of the maximum frequency of pulse activity during AMPA application to the preceding level of background activity did not change during both short-time and long-time acute IFN applications.
At the same time, a delayed potentiating effect of the cytokine on AMPA-evoked responses has been observed 15 min after cessation of all exposures and washing vestibular epithelium in normal solution with both short-term and long-term IFN exposure. This indicates that the delayed potentiating effect of cytokine on AMPA-evoked responses is independent of the duration of IFN exposure. This suggests that IFN acts as a trigger of the delayed potentiating response of AMPARs.
The current literature on the effect of type I IFN
Similar data on the effect of IFN
On the other hand, IFNs may exert toxic effects on CNS neurons via AMPARs. On mouse cortical neurons IFN-
Comparison of our results with the few current data [34, 76] demonstrates the principal possibility of interaction of different types of IFNs with AMPARs. The modulatory effect of type I and type II IFNs on AMPARs can be manifested in wide time intervals (from minutes to days) and trigger neuroimmunomodulatory and neurotoxic processes. The study of the mechanisms of influence of different types of IFNs on AMPARs is of special interest and requires close investigation, since hyperactivation of AMPARs may cause excitotoxicity, destruction of glutamatergic synapse.
According to the literature, AMPARs are tetramers consisting of dimers composed of GluA1/GluA2 and GluA2/GluA3 subunits [77, 78, 79, 80]. The function of AMPARs depend on subunit composition and on different auxiliary proteins (associate with the AMPAR subunits during their assembly). Each of AMPAR subunit has a large N-terminal extracellular domain (NTD), highly conserved extracellular ligand-binding domain (LBD), Transmembrane domains (TMD) 1–4 and C-terminal intracellular domain (CTD). It is the TMD2 that forms the pore-lining region and responsible for the channel properties of the receptor. If TMD2 of GluA2 is subject to nucleotide editing (conversion of glutamine (Q) to an arginine (R) at position 607)—the charge of the cannel pore changes from neutral to negative resulting in to formation of Ca2+-Impermeable AMPAR (CI-AMPAR). Ca2+-Permeable AMPARs (CP-AMPARs)—those lacking an edited GluA2 subunit—have a higher single-channel conductance than CI-AMPAR containing calcium-impermeable receptors. It is accepted that change of subunit composition or increase of GluA1:GluA2 ratio in synaptic zone may serve as a molecular base of short term plasticity.
We propose that interferon causes an increase in the number of CP-AMPARs in the synaptic zone either by the nucleotide editing of GluA2 at position 607 (R/Q), follow by a change in the channel charge, or by increasing the traffic of CP-AMPARs using auxiliary proteins.
Quantity of AMPARs in the synapse is in dynamic equilibrium between the synaptic and extrasynaptic membranes and the intracellular space through the mechanisms of AMPA exocytosis, lateral diffusion and clathrin-dependent endocytosis [81, 82].
The increase or decrease in the number of AMPARs on the synaptic surface is a key mechanism of synaptic plasticity revealed during LTP and LTD, and under functional stress. For example, it has been shown that accelerated AMPAR incorporation is observed during LTP [83]. In contrast, a decrease in AMPARs is associated with LTD or with the removal of desensitised AMPARs from the synaptic zone [82, 84, 85].
The process of AMPAR recycling has been identified in the central and peripheral nervous system: in the synapses of ascending fibres of Purkinje cells [86], in the cortex [87], in the hippocampus [82], and in structures of the inner ear [88]. Specifically, suppression of AMPAR endocytosis in cochlear neuronal cultures upon exposure to a glutamate agonist and acoustic loading results in excitotoxic responses to acoustic stimuli that were normally not excitotoxic. These included vacuolization in the nerve terminals and spiral ganglion, as well as irreversible threshold shifts. These data suggest that endocytosis of AMPAR plays an important protective role during excitotoxicity [88].
The process of dynamic equilibrium between synaptic, extrasynaptic and intracellular space can be modulated by endogenous ligands: insulin [89], stress hormone [90], and the pro-inflammatory cytokine TNF
The effect of TNF
In hippocampal and cortical cultures, glial TNF
It was shown that in hippocampal neurons TNF
There are no direct data indicating a change in AMPARs trafficking to the synaptic area upon exposure to IFN. We hypothesise that changes in the number of synaptic membrane receptors under the influence of proinflammatory cytokines is a common mechanism for the central and peripheral nervous systems, and that in our experiments IFN-
The study of the mechanisms of IFN influence on the synaptic recycling of AMPARs is a necessary step in the development of methods to prevent excitotoxicity and nerve cell death in inner ear structures.
Activation of AMPARs is accompanied by the entry of Ca2+ into the cell and subsequent membrane depolarization. In our experimental conditions, IFN-
The results of our work indicate that different subtypes of iGluRs are affected by IFN to varying degrees. Analysis of literature data and our results indicate that IFN has a complex effect on glutamatergic synaptic transmission in the structures of the inner ear. It is known that AMPARs and NMDARs are co-localized on the hair cell and on the postsynaptic membrane.
Summarizing the data obtained, we suggest that IFN leads to a delayed activation of AMPARs, which causes long-term membrane depolarisation and activation of NMDARs (presumably by removing Mg2+ from the cationic channel of NMDAR and subsequently induced an influx of Ca2+ in cell) (Fig. 6).
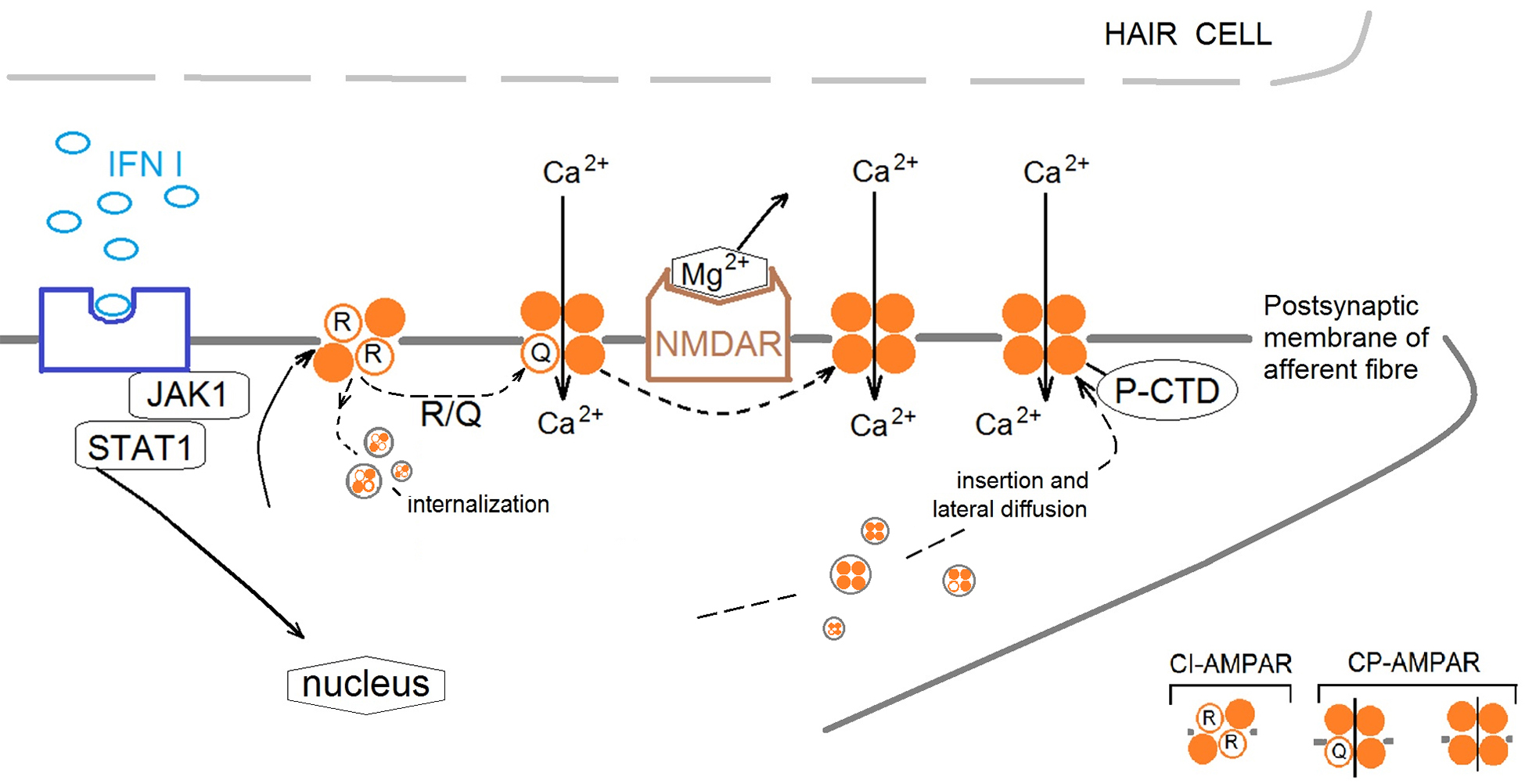 Fig. 6.
Fig. 6. Proposal mechanism of IFN type I influence on cross-talk of AMPA receptors (AMPARs) and NMDA receptors (NMDARs) in the frog vestibular epithelium. IFN initiates the restructure of the AMPAR subunit composition in the postsynaptic membrane of afferent fiber by activation Janus activated kinases 1/signal transducer and activator of transcription 1 (JAK1/STAT1) cascade. Changes in synaptic strength are accompanied by changes in the composition of AMPAR subunits in the synapse due to the movement of receptors lacking GluA2 subunits to and from the postsynaptic membrane. IFN increases the ratio of Ca2+-Permeable AMPA Receptors (CP-AMPAR) to Ca2+-Impermeable AMPA Receptors (CI-AMPAR), presumably through two mechanisms: (1) By increasing the amount of CP-AMPAR in the extrasynaptic zone and increasing traffic of CP-AMPAR into the synaptic membrane by phosphorylation of (C)-terminal domain (P-CTD). (2) By phosphorylation of transmembrane domain 2 at 607 sequence (R-Q) resulting in changing the charge of the channel pore and increase of Ca2+ entrance and/or removing CI-AMPAR from the synaptic zone by internalization. The activation of CP-AMPARs in synaptic zone leads to relieve NMDARs pore blockaded by Mg2+ to permit Ca2+ entrance into the postsynaptic membrane and initiate signaling cascades.
At the presynaptic level, this is accompanied by activation of the glutamatergic ribbon synapse and abundant release of glutamate from the hair cells. At the postsynaptic level, it is accompanied by activation of postsynaptic glutamate receptors. In the vestibular epithelium, it is AMPARs that trigger the excitation process, determine the background activity of the afferent synapse, which maintains current excitability of the postsynaptic membrane and together with other GluRs provide instant transmission of information from the hair cell.
It is known that interferon disrupts the function of the glutamate transporter, leading to excess glutamate in the synaptic cleft, resulted in hyperactivation of GluRs and disruption of the balance of positive and negative process. In accordance of current evidence appropriate NMDAR activation is essential for neuronal survival and physiological functions, excessive activation contributes to pathological changes including cell death. It was suggested that the degree of excitotoxicity depends on the magnitude and duration of synaptic and extra-synaptic NMDAR co-activation [93]. While low-dose NMDA preferentially activated synaptic NMDAR, higher doses progressively activated increasing amount of extra-synaptic NMDAR along with synaptic NMDAR and triggered cell death program.
The delayed IFN-induced increase in AMPAR increase NMDA responses and changes cross talk of NMDAR and non-NMDA receptors.
The presented data show for the first time that type I IFN has different effects on NMDAR, AMPAR and KAR. IFN has no direct effect on NMDARs, but stimulates AMPARs and KARs by involving different mechanisms. IFN-activated AMPARs can stimulate NMDAR activity, thereby altering synaptic plasticity of the glutamatergic afferent synapse and modifying afferent flow from the vestibular periphery to the CNS.
Thus, the effect of IFN on the functions of iGluRs is a common and important mechanism of functional interaction between the immune and nervous systems in inner ear structures, and IFN type I can be considered as an neuroimmunomodulator in vestibular end organs.
Ach, acetylcholine; AMPA, D,L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AMPAR, AMPA receptor; ANOVA RM, repeated measures analysis of variance; CNS, central nervous system; CI-AMPAR, Ca2+-Impermeable AMPA Receptors; CP-AMPAR, Ca2+-Permeable AMPA Receptors; CTD, (C)-terminal domain; DA, dopamine; EPSC, excitatory postsynaptic currents; GLAST, astrocytic glutamate-aspartate transporter; GluR, glutamate receptor; GluA1-GluA2, subunits of AMPAR; GluK1-GluK5, subunits of KAR; IFN, interferon; IFNAR, interferon receptor; IFNRI, the interferon type I receptor; IFNR
All data reported in this paper will be shared by the lead contact upon request.
IVR suggested idea and designed the research study. IVR and TVT performed the research and data analysis. EAV provided statistical data analysis. TVT and EAV designed the figures. IVR wrote the manuscript with input from all authors. AGM was involved in discussing the results and analyzing the data for the paper. All authors discussed the results and commented on the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All procedures were agreed with the ethical committee of the Pavlov Institute of Physiology (No. 09/18 dated 09/18/2023). and were performed in accordance with the ethical principles set out in the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes.
The authors would like to thank O.V. Shamova, Corresponding Member of the Russian Academy of Sciences, Head of the Pathology Department of the Institute of Experimental Medicine, Saint-Petersburg, Russia, for support of the project and E.A. Protasov, Leading Engineer of this department, for interferon purification.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






