1 Department of Pharmaceutical Sciences, School of Health Science and Technology, UPES, Campus Bidholi Dehradun, 248007 Uttarakhand, India
2 Department of Pharmacology, Delhi Pharmaceutical Sciences and Research University (DPSRU), 110017 New Delhi, India
3 Department of Pharmacognosy, College of Pharmacy, King Khalid University, 62529 Abha, Saudi Arabia
4 Department of Pharmacology & Toxicology, College of Pharmacy, Prince Sattam Bin Abdulaziz University, 11942 Al-Kharj, Saudi Arabia
Abstract
Identifying novel biomarkers is a reliable approach to predict and diagnose human diseases as well as manage individual responses to therapeutic drugs. Heat shock proteins (HSPs) are molecular chaperones that play a major role in maintaining protein stability and folding. Many studies suggested their association with multiple types of diseases. HSPs from different categories play different roles; therefore, it is important to identify HSPs and their function to understand their biological functions clearly. This comprehensive review was performed to evaluate the role of HSPs as predictive biomarkers in cardiovascular diseases. The original publications related to HSPs from 2010 to 2024 were identified by using the keywords “heat-shock proteins”, “HSP in cardiovascular disorders” and “HSP in atherosclerosis”. The regulatory pathways involved in HSPs’ functioning are the important points of discussion in this review. HSPs play a critical role in key cellular processes, including apoptosis regulation, protein folding, immune responses, genomic stability, and DNA repair. Aberrant expression of HSPs causes dysregulation of these pathways resulting in the development and progression of diseases. A comprehensive understanding of HSPs in cardiovascular diseases and their associated regulatory pathways can have significant implications for disease intervention, diagnosis, and prognosis. In this review paper, we have highlighted the importance of HSPs as versatile biomarkers and their importance as targets for the therapeutic management of cardiovascular diseases.
Keywords
- heat shock protein
- cardiovascular diseases
- atherosclerosis
- predictive biomarker
Heat shock proteins (HSPs) were discovered by serendipity in the laboratory of one of the Italian scientists Ferruccio Ritossa. This discovery took place when he examined the chromosomes of Drosophila melanogaster exposed to high temperature showing a distinct puffing phenomenon. This observation hypothesized that the puffing phenomenon occurs because of the gene activation causing heightened protein expression caused by heat stress. Therefore, the “heat shock protein” term was coined [1]. Heat shock protein can also be referred to as cellular stress response, which is a fundamentally physiological process observed in all living organisms. It includes modulation of gene expression characterized by an expansion of HSP expression. Many research studies revealed that elevations in the level of HSPs could strengthen cell survival and protect cellular proteins from damage and aggregation [2]. Apart from heat stress-induced HSPs several other factors like environmental, chemical, and non-stress physiological agents and disease conditions can elevate the expression of HSP levels [3]. HSPs can be classified depending on the molecular masses like HSP70 which denotes that a subset of HSPs shows a molecular weight of 70 kDa. Several HSP variants are found with similar attributes or significant differences that led to an increased demand for a more organized and unified classification arrangement system for these HSPs. This comprehensive review was performed to evaluate the role of HSPs as predictive biomarkers in cardiovascular diseases. The inclusion criteria for the selection of the manuscript include a wide range of analysis of HSPs and their molecular mechanism in cardiovascular diseases, evaluation of therapeutic approaches targeting HSPs, and insights into the application of HSPs in clinical practice.
This novel approach helps in simplifying and streamlining the nomenclatures for human HSPs. Initially, the nomenclature was HSP110, HSP90, and HSP70, which is still in use however the nomenclature was redesigned as HSPA, HSPB, HSPC, and HSPH in turn. Additionally, each member was assigned one numerical identifier to enhance clarity and consistency for naming these diverse interrelated proteins like HSPA1, HSPA2, HSPA4, etc. Table 1 (Ref. [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20]) represents the classification and description of HSPs. HSPs act as molecular chaperones, help in regulating new proteins by modulating folding/unfolding steps to attain correct configuration, and also help in the transport of proteins across the intracellular membranes [21]. As shown in Table 1, HSPs are classified into different families as per molecular weight like 40, 60, 70, 90, and 110 kDa. Fig. 1 represents the role of heat shock transcription factor-1 (HSF-1) regulating HSP genes. HSF-1 plays an important role in protection of cell from necrosis caused by the deposition of misfolded proteins by driving the transcription of HSPgenes. This transcriptional stimulation depends upon the HSF-1 trimerization and its binding with identifiable sequences within the HSP genes [22].
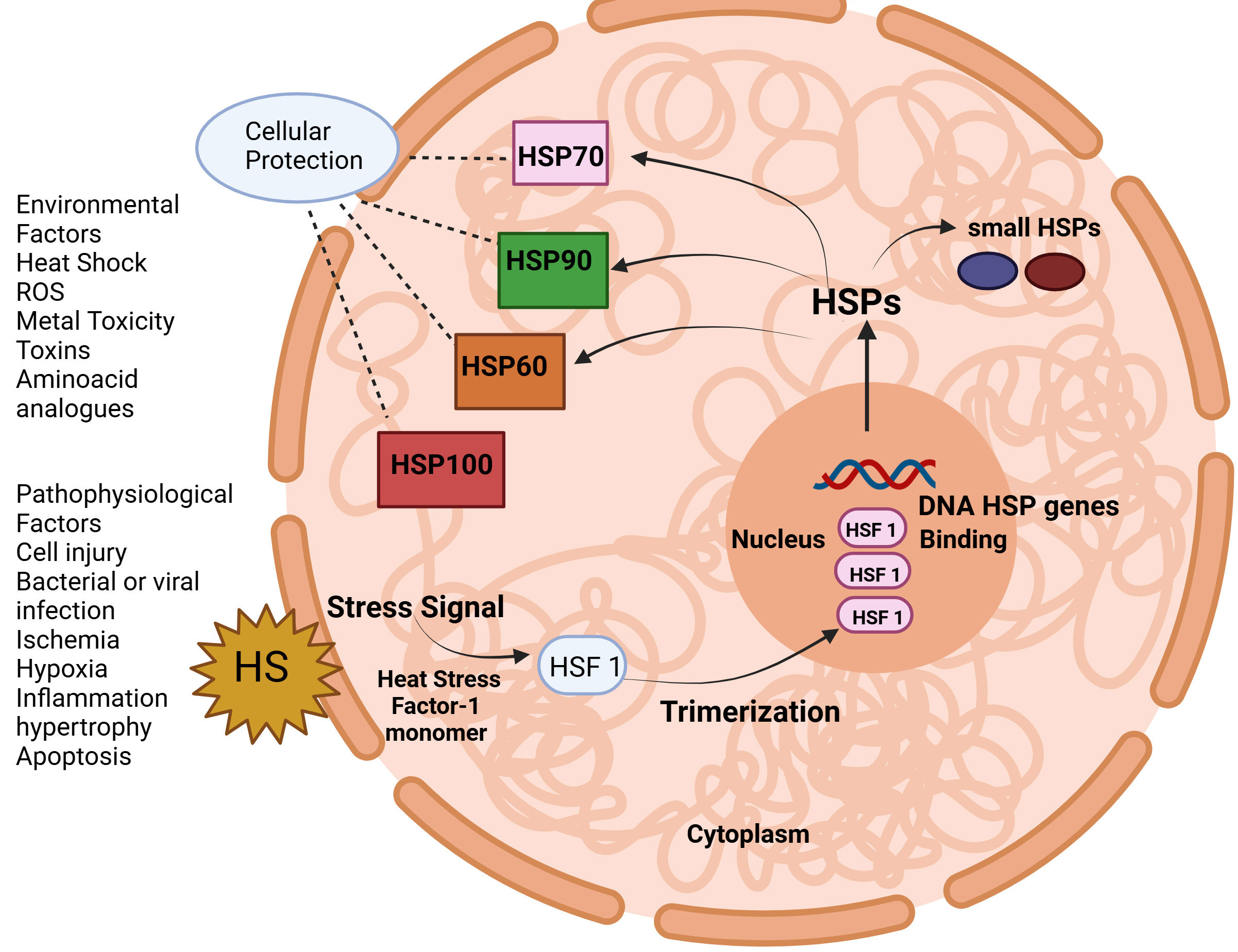 Fig. 1.
Fig. 1. The heat shock protein response is regulated by heat shock transcription factor 1 (HSF 1). ROS, reactive oxygen species. (Created with Biorender.com).
| Heat shock proteins | Description | Location | Overexpression of HSPs in Diseases | References |
| Large heat shock proteins (HSPs) Family | High molecular weight proteins with chaperone activity; Protein disaggregation and refolding. | HSP110-Cytosol | Myocardial infarction | [4] |
| HSP110 | GRP170-Endoplasmic reticulum (ER) | ER stress, Ischemic injury, and heart failure | [5] | |
| glucose-regulated protein (GRP)170 | ||||
| HSP90 Family | 90 kDa in size; stabilizing and assisting in folding of a wide range of client. proteins involved in cellular growth and signaling. | HSP90AA1-Cytosol | Atherosclerosis, Heart failure | [6] |
| HSP90AA1 | HSP90AB1-Cytosol | Atherosclerosis, Hypertension | [7] | |
| HSP90AB1 | GRP94-Cytosol, ER | Ischemia-reperfusion, myocardial infarction | [8, 9] | |
| GRP94 | ||||
| HSP70 Family | 70 kDa in size, protein folding, transportation, and degradation. | HSP70-Cytosol | Atherosclerosis | [10] |
| HSPA1A, HSPA8, and HSPA5 | HSPA1B-Cell surface | Ischemic injury, heart failure | [11] | |
| HSPA8-Cytosol | Ischemic stroke | [12] | ||
| HSPA5-ER | Ischemia and heart failure | [13] | ||
| HSP60/Chaperonin Family GroEL and GroES are HSP60 proteins in bacteria | Also known as chaperonins, they are involved in the folding of other proteins inside cellular compartments, particularly mitochondria, and chloroplasts. | GroEL and GroES are involved in the protein folding of Cytosol, mitochondria, chloroplast | Heart failure and atherosclerosis | [14] |
| HSP40/DnaJ Family | Co-chaperones; assist in protein folding and refolding. Their conserved J-domain is essential for their function. | Cytosol | Ischemia | [15] |
| Small HSP (sHSP) Family | 20–30 kDa in size; role in preventing protein aggregation. They are often found in various cellular compartments and protect proteins from stress-induced damage. | HSP10-Mitochondria | Myocardial infarction | [16] |
| HSP10 | HSP27-Cytosol/Nucleus | Myocardial infarction | [17] | |
| HSP27 | ||||
| HSP110 Family | HSP110 is similar to HSP70 and functions as molecular chaperones; protein folding and unfolding processes. | HSP110-Cytosol | Myocardial Infarction, Cancer | [4, 18] |
| HSP27 Family | HSP27, also known as HSPB1; plays a role in protecting cells from stress-induced damage and preventing protein aggregation. | HSP27-Cytosol/Nucleus | Cellular Dysfunctions | [19] |
| HSP20/Alpha-Crystallin Family | Small HSPs like alpha-crystallin in the eye lens. They have chaperone activity and help maintain the transparency of the lens. | HSP20-Mitochondria | Cardiovascular disorders, eye lens disorders | [20] |
HSPs are activated in response to a wide variety of stressors like cold, heat, oxidative stress, inflammation, infections, toxins, and several other factors. High levels of HSPs can act as an indicator of cellular stress. In addition, dysregulation of HSPs can lead to several systemic diseases like cancer, autoimmune, cardiovascular, and neurodegenerative diseases. Therefore, understanding the role of HSPs can help in predicting and diagnosing multiple pathological conditions. In this paper, we have investigated the regulatory pathways and mechanisms involved in the regulation of HSPs and their role as predictive and prognostic biomarkers in systemic diseases.
This comprehensive review was conducted to evaluate the important mechanism of HSPs as potential therapeutic targets and predictive biomarkers in cardiovascular disease. Appropriate Literature published between 2010 and 2024 was identified from databases like PubMed, Web of Science, and Scopus by using the keywords “heat shock proteins”, “HSPs in cardiovascular disease”, “HSPs as biomarkers” and “HSPs in atherosclerosis”. Research studies focusing on HSPs’ physiological pathways involving protein folding, HSP expression, immune response, inflammation, and oxidative stress were included, while non-English or unrelated articles were excluded. Results on experimental studies, HSP expression, and their association with cardiovascular disease were extracted and explored. Special consideration was given to the molecular pathways and mechanisms involving HSPs and their implications for interventions, safeguarding a thorough evaluation of their biological role and clinical impact. In this comprehensive review all the figures were created by using BioRender Software (M5V 2J1, Spadina Ave, Toronto, Ontario, Canada).
Small HSPs are widely distributed in several tissues and are found to be heat-inducible (HSP22) and are cell-type dependent. They play a major role in cell survival under stress conditions. HSP10 and HSP alpha crystalline represent tissue-restricted patterns and are supposed to play an important role in cell differentiation, development, and tissue-specific functions [23]. Apart from molecular chaperon functions in the folding and unfolding of proteins preventing aggregation, these HSPs are involved in different functions like maintenance of cytoskeleton structure, cell cycle, cellular differentiation, protein degradation, stress tolerance, apoptosis, and cellular signal transduction. Fig. 2 represents the types of small HSPs and their tissue distribution. Class I HSPs include HSP27,
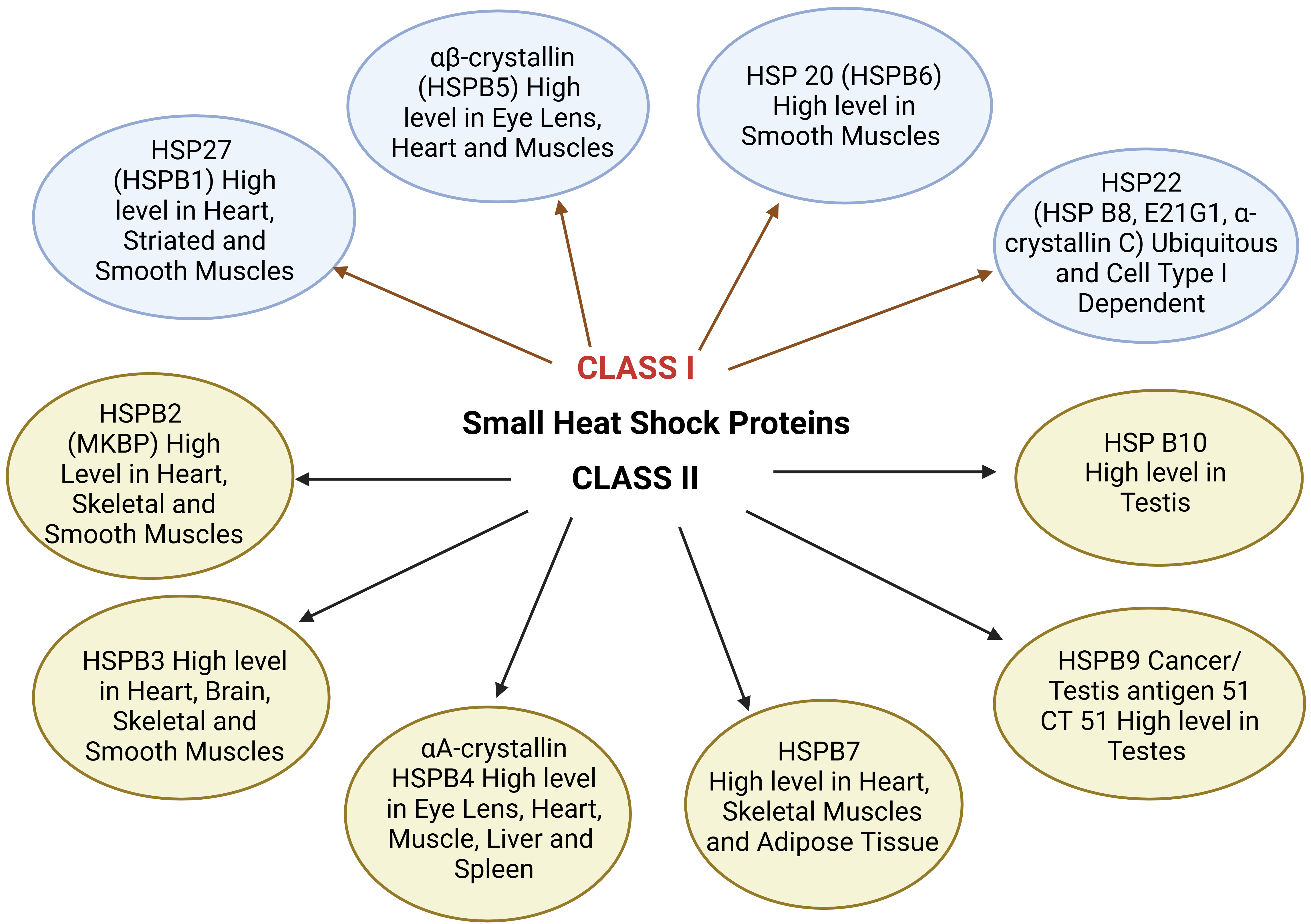 Fig. 2.
Fig. 2. Small HSPs and their tissue distribution. MKBP, myotonic dystrophy protein kinase binding protein; CT, cancer/testis.
HSP90 proteins are ubiquitous molecular chaperones that promote the folding and unfolding of proteins thus helping in preventing aggregation [29]. In mammalian cells, two isoforms HSP90
Multiple co-chaperones associated with HSPs play a significant role in assisting the chaperone functions. These co-chaperones include HSC70 interacting protein (HIP), Bcl-2 linked athanogene (BAG-1), HSPs organizing proteins (HOP), HSP40 family, and p23. HIP was first recognized as an HSP90 co-chaperone due to its ability to react with the ATPase site of HSC70 and enhance HSC70-related interactions by alleviating its ADP-bound form. HIP is a 50 kDa protein found in cytosol. Afterward, it was also reported to interact with BAG-1 binding to HSP70 protein competitive antagonism and therefore considered as HSP70 co-chaperone [42, 43]. BAG-1 co-chaperone is identified as a protein linked to HSP70 and shown to act as a suppressor of HSP70. It helps in protein refolding by forming ternary complexes with HSP70 substrates which are stable. The mechanism behind this function is because of the presence of BAG-1 that alters HSP70 conformation. Subsequently, the binding domain in the substrates becomes less susceptible to digestion of proteins by protease enzyme still in the presence of HSP40 and nucleotides [44]. Thus, when BAG-1 is occupied in the process of releasing peptides from HSP70, the HIP protein is simultaneously competing with BAG-1 in maintaining HSP70 in its peptide-binding stage. HOP alternatively called stress-inducible protein 1, acts as a co-chaperone that engages with HSP90 and HSP70 families in the cytosol by distinct repeating tetratricopeptide domains. It is assumed to play an important role in facilitating substrate protein folding by regulating the functions of chaperones of HSP90 and HSP70. Therefore, it serves as an important physical linkage between chaperones and body systems like progesterone and glucocorticoid hormone receptors [45]. ATP hydrolysis by ATPase is the rate-limiting step predominantly responsible for HSP70 chaperone functions. Therefore, the prime target for regulation of this protein function is ATP hydrolysis caused by the HSP40 family that acts to elevate the ATP turnover rate and strengthen the chaperone activity. The natural turnover rate of stable ATPase activity of HSP70 is very low to fuel the HSP70 chaperone functions even when the substrate is there. Hence, the synchronized potential of HSP70 and HSP40 is required to ensure the effective functioning of HSP70 [46]. Protein Kinase R (PKR) facilitates the antiviral response of the host body and belongs to the eukaryotic initiation factor 2a (eIF-2a) kinase family. It is found to be associated with functions like apoptosis and tumor suppression. HSP90 chaperones regulate PKR. This molecular chaperone complex delivers this function when a p23 acidic protein, a cochaperone binds with this protein in an ATP-dependent manner. They bind with each other through the kinase domain and N-terminal ds RNA binding domain. Separation of either p23 or HSP90 causes apoptosis [47]. HSP60 featured a cavity known as “Anfinsen-cage” chaperones that provide a sheltered, protected closed environment that aids in proper substrate folding. The important steps of protein folding occur in the presence of a protected environment of chaperones in functions. Protein unfolding occurs through a multidirectional extension of chaperones by loosening the compact protein and collapsing of inner structure, and during extension, the water molecules enter inside the hydrophobic region, which is an important step in chaperone functions [48]. In addition, the client protein is completely covered and isolated from its external surroundings by forming a “cap” by assembling HSP10 heptamer. This “cap” remains in its original place until it is released, permitting the target protein to be freed once ATP hydrolysis occurs and the new target binds to different sites of the chaperone machine. Researchers investigating the HSP60 family have highlighted the important role of HSP10 in both initiation and continuation of the chaperone activity [48]. This review suggested the synergistic action of HSP families and their co-chaperones in a coordinated manner with the exception role of BAG-1 on HSP70. Therefore, the synergistic and antagonistic interactions between HSP proteins and their co-chaperones play a major role in modulating the activities of HSPs and contribute to the development of novel therapeutic approaches for several diseases [49].
Tytell et al. [48] demonstrated the release and uptake of HSPs and observed that these proteins can be transferred from glial cells to axons. Another research team investigated the release of HSPs from cultured cells of rat embryo model [50]. Some studies reported the release of HSPs like HSP70, HSP90, and GP96 from necrotic cells that induces the activation of APC-like dendritic cells and macrophages through pathway nuclear factor Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-
| Heat shock proteins | Description | Location | Role of HSPs in diseases |
| HSP10 | Functions as molecular chaperone-assisted protein folding. | Mitochondria | Overexpression of HSP10 shows chronic atrial fibrillation. |
| HSP27 | Small HSPs with 27 kDa molecular weight, plays an important role in cellular stress response and function as molecular chaperones. | Cytosol and Nucleus of the cells | Overexpression of HSP27 shows acute Atrial fibrillation. |
| HSP32 | Protects against cellular oxidative stress. | Cytosol and Nucleus | Found in the hemeoxygenase-1 enzyme and is involved in heme degradation. Performs antiatherogenic functions. |
| HSP47 | Plays an important role in collagen synthesis and clotting. | Extracellular matrix and Endoplasmic reticulum | HSP47 expression is reduced during prolonged immobilization, which protects against thrombi inflammation and thereby prevents clot formation. |
| HSP60 | Plays an essential role in protein folding and mitochondrial assembly. | Mitochondria and cytosol | Overexpression in atrial fibrillation, atherosclerosis, coronary artery disease, acute heart failure. |
| HSP65 | Functions as mitochondrial chaperonin. | Mitochondria | Overexpression of HSP65 in coronary artery disease. |
| HSP70 | The protective role against cellular oxidative stress plays an important role in protein folding. | Cell surface, cytosol, nucleus, and mitochondria | HSP70 levels have an inverse relationship with atrial fibrillation. |
| Overexpression of HSP70 in atherosclerosis and acute heart failure. | |||
| HSP70 is an endogenous antigen in hypertension induced by innate and adaptive immune response that induces essential hypertension. | |||
| HSP90 | Molecular chaperones regulate the stability and maturation of proteins. | Cytoplasm, endoplasmic reticulum, mitochondria, and nucleus | HSP90 inhibition reduces necroptosis of cells. |
The HSPs family responds to numerous pathological conditions like hypoxia, infection, radiation, variation in temperature, and stress. In cardiovascular disorders, HSPs regulate protein function, folding, and localization by acting as molecular chaperones. They prevent damage to the cardiomyocytes and thereby help in maintaining proteostasis. Several circulatory disorders are caused by derailment of cellular proteins, causing loss of proteostatic control in cells that results in the expression of HSPs. In addition to this, upregulation of genes encoding HSPs is done by activated heat shock factor 1, which is the central regulator of heat shock response. Few clinical cases show the association of atrial fibrillation with the collapse of HSPs. In Atrial fibrillation, derailment of cellular protein and electropathological remodeling are caused by the impairment in heat shock response. Elevated expression level of HSP27 was observed in patients with paroxysmal atrial fibrillation that may protect cardiomyocytes against myolysis. An increased level of HSP27 proteins is found to be associated with acute atrial fibrillation and to a lesser extent with structural impairment [53]. In line with this, studies show that HSP70 levels have an inverse relationship with atrial fibrillation postoperatively in patients who undergo coronary artery bypass surgery [54, 55]. Another study represents the overexpression of mitochondrial HSPs (HSP60 and HSP10) in atrial myocytes from patients suffering from atrial fibrillation [56]. HSP27, HSP70, HSP60, and HSP10 have shown their direct or indirect association with the progression and development of atrial fibrillation therefore, this study suggested the possibility of using HSPs as potential predictive biomarkers [57].
HSP60 and HSP70 families have been extensively investigated by researchers to identify their role in vascular diseases like atherosclerosis, and they determined HSP60, which shows distinctive localization in atherosclerotic lesions as compared to non-atherosclerotic arterial walls. Overexpression of HSP70 is observed in several cell types like macrophages, monocytes, dendritic, and smooth muscle cells in an advanced stage of atherosclerosis [58, 59]. A population-based study revealed that an elevated level of HSP60 is associated with low-density lipoprotein cholesterol levels [60]. HSP32 was found in the heme oxygenase-1 enzyme that plays an important role in heme degradation. Heme degradation is activated by stressors and therefore performs antiatherogenic actions like reduction in adhesion of monocytes, scavenging reactive oxygen species, and chemotaxis [61]. Investigating the role of HSPs in atherosclerosis and their association with infectious diseases is one of the important fields of current research, as endothelial cells of damaged arteries show overexpression of HSP60. Due to the homology between human HSP60 and microbial HSP60, the activation of the immune response against microbes may be responsible for damage to the arterial endothelial cells and atherosclerosis [62]. Ischemic heart disease (IHD) and its connection with HSPs have been thoroughly investigated by some researchers. Their study includes the extent and presence of myocardial infarction, coronary artery disease (CAD), ischemia, and cardiac protection [56]. Considerably, among patients diagnosed with CAD, research study revealed a significant association between the presence and severity of the disease and the presence of antibodies directed against HSP60 and HSP65 [56]. Increased levels of antibodies were consistently reported in tandem with an elevated number of diseased vessels as defined by coronary atherosclerosis scores [56]. The extent of CAD disease is associated with high levels of HSP65 and HSP60, but it was also reported that some specific HSPs have a protective influence on the heart. Researchers reported that elevated levels of human HSP70 are associated with a reduced risk of CAD, suggesting a multifaceted and intricate role of HSPs in the context of atherosclerosis linked with coronary arteries [63, 64].
Peripheral vascular disease (PVD) typically refers to persistent ischemia affecting the lower part of the body because of atherosclerosis. Individuals with PVD show a mortality rate 3 times higher than that of control in terms of age and gender. This high mortality is primarily credited to the presence of comorbidity caused by atherosclerotic conditions in other vascular areas [65]. Research investigations suggested the involvement of HSPs in the progression of atherosclerotic PVD and most importantly, the impact of physical exercise. A study showed 20 PVD patients with a higher level of circulating HSP70 as compared to control subjects [65]. In addition, there is also an elevation found in the level of anti-HSP70 antibodies, which is not significant. The same study shows a significant rise in the level of HSP60 antibodies in PVD patients, exhibiting a positive correlation with the severity of the disease [66]. Another study also reported the same significant rise in the level of anti-HSP70 antibodies in PVD subjects with ischemia, suggesting a probable correlation between antibodies and the severity of the disease [67]. Diabetes mellitus is found to be one of the important risk factors for PVD. One study reported 67 type I and type II diabetic subjects have elevated levels of IgA class anti-HSP70 antibodies in contrast to individuals with no diabetes. This study evidenced that IgA antibodies may be involved in vascular diseases with diabetes mellitus and HSP70 may act as an autoantigen in disease progression and pathogenesis [68]. The treatment approach for PVD patients is transluminal angioplasty. However, balloon angioplasty develops stretching and physical strain that can trigger several adverse outcomes like lipid accumulation, platelet adhesion, smooth muscle proliferation, and plaque formation. Interestingly, it has been observed that mechanical stress caused by balloon angioplasty leads to the production of HSP70 in the smooth muscle cells of the blood vessels. These discoveries suggest that HSP70 expression plays a significant role in blood vessel’s response to injury, an early stage in the development of atherosclerosis [69].
DVT is a cardiovascular disease that involves the formation of blood clots in deep veins, mostly in the legs, caused by blood stasis, injury to the blood vessels, and changes in blood composition. Probable risk factors include prolonged immobilization, surgery, certain medical conditions, and genetic predisposition. A major concern with DVT is that a clot may detach and travel to the lungs, causing a pulmonary embolism, which can be life-threatening. Immobilization-associated DVT is initiated by blood stasis in veins, resulting in endothelial cell hypoxia. Site-directed recruitment of platelets to the activated endothelium results in the activation of innate immune cells, including neutrophils and monocytes, and the plasmatic coagulation system. This sequel of events results in deleterious thrombosis and inflammation, the underlying major mechanism of DVT. Platelet HSP47 plays a crucial role in thrombosis and thereby in blood clot formation by recruitment of thrombin to platelets and activates them to form platelet aggregates. HSP47 also forms blood clots by activating polymorphonuclear leukocytes (PMNs) generating neutrophil extracellular traps (NETs) that comprise proteins and DNA as shown in Fig. 3. NETS provide a temporary structure for procoagulant molecules, red blood cells, and platelets. HSP47 expression is reduced during prolonged immobilization, which protects against thrombo-inflammation and thereby prevents clot formation [70].
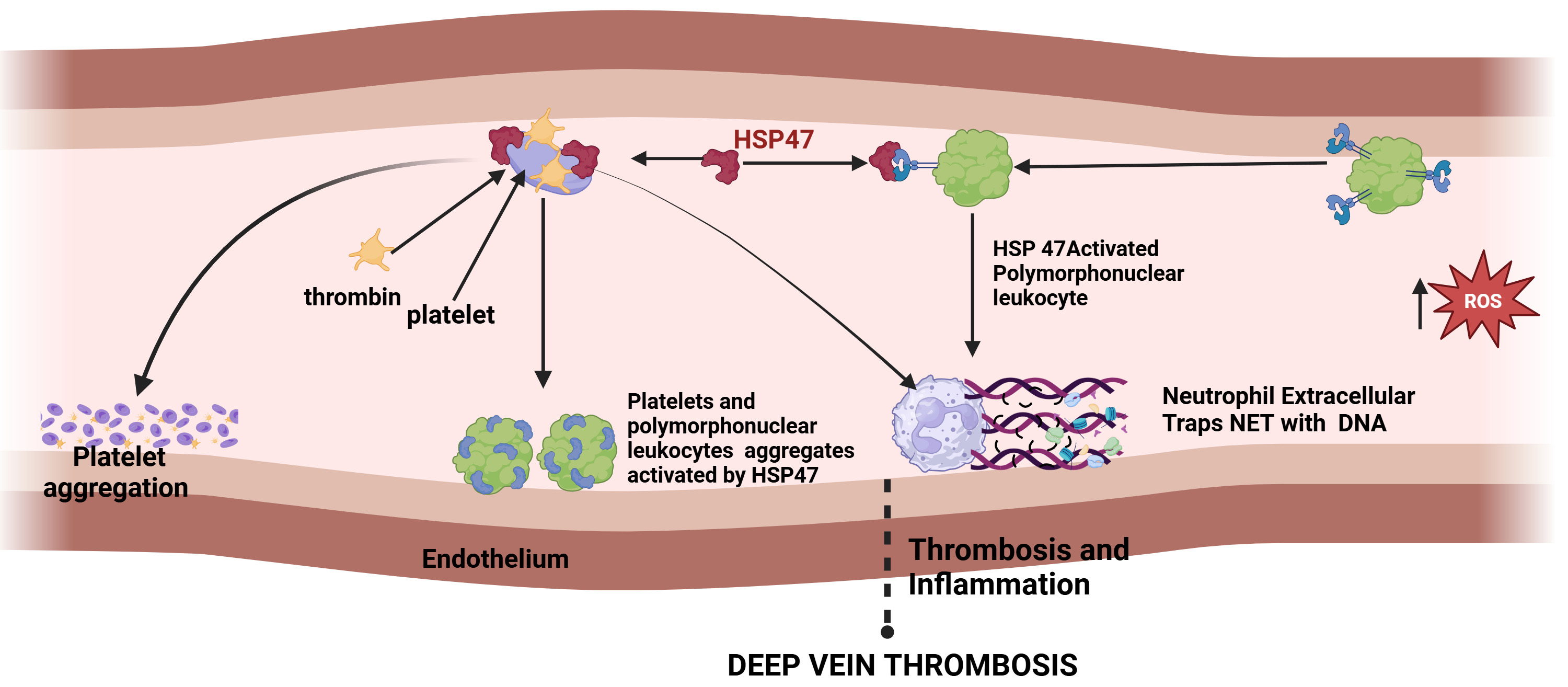 Fig. 3.
Fig. 3. The sequence of events in thrombosis and inflammation and role of HSP47 in deep vein thrombosis. (Created with Biorender.com).
In kidneys, the immune cell infiltration comprises macrophages and T cells in case of salt-sensitive hypertension. Experimental models suggested that these cells have an impact on specific cell populations which causes depletion of cells and hypertension. Using this approach researchers revealed a prohypertensive impact on immune cells, CD 4, CD 8T, B cells, T17 cells, and macrophage infiltration. On the other hand, it also demonstrated the anti-hypertensive role of regulatory T cells (Tregs) by regulating vascular injury and immune homeostasis. It has been hypothesized that the inflammation might be set in motion by localized cellular damage caused by renal vasoconstriction. This injury causes ischemia, triggering the release of damage-associated molecular patterns stimulating the innate immune response. This process leads to the introduction of specific endogenous proteins that initiate adaptive immune responses. In these circumstances, HSP70 and proteins modified by isoketals play an important role as endogenous antigens of importance in the pathogenesis of hypertension [71]. Fig. 4 represents the importance of HSP70 as an endogenous antigen in hypertension induced by an innate and adaptive immune response that induces essential hypertension. When tolerance to this protein is induced, it causes the production of regulatory T-cell response driven by interleukin (IL)-10, which efficiently safeguards against salt-induced hypertension and inflammation [71].
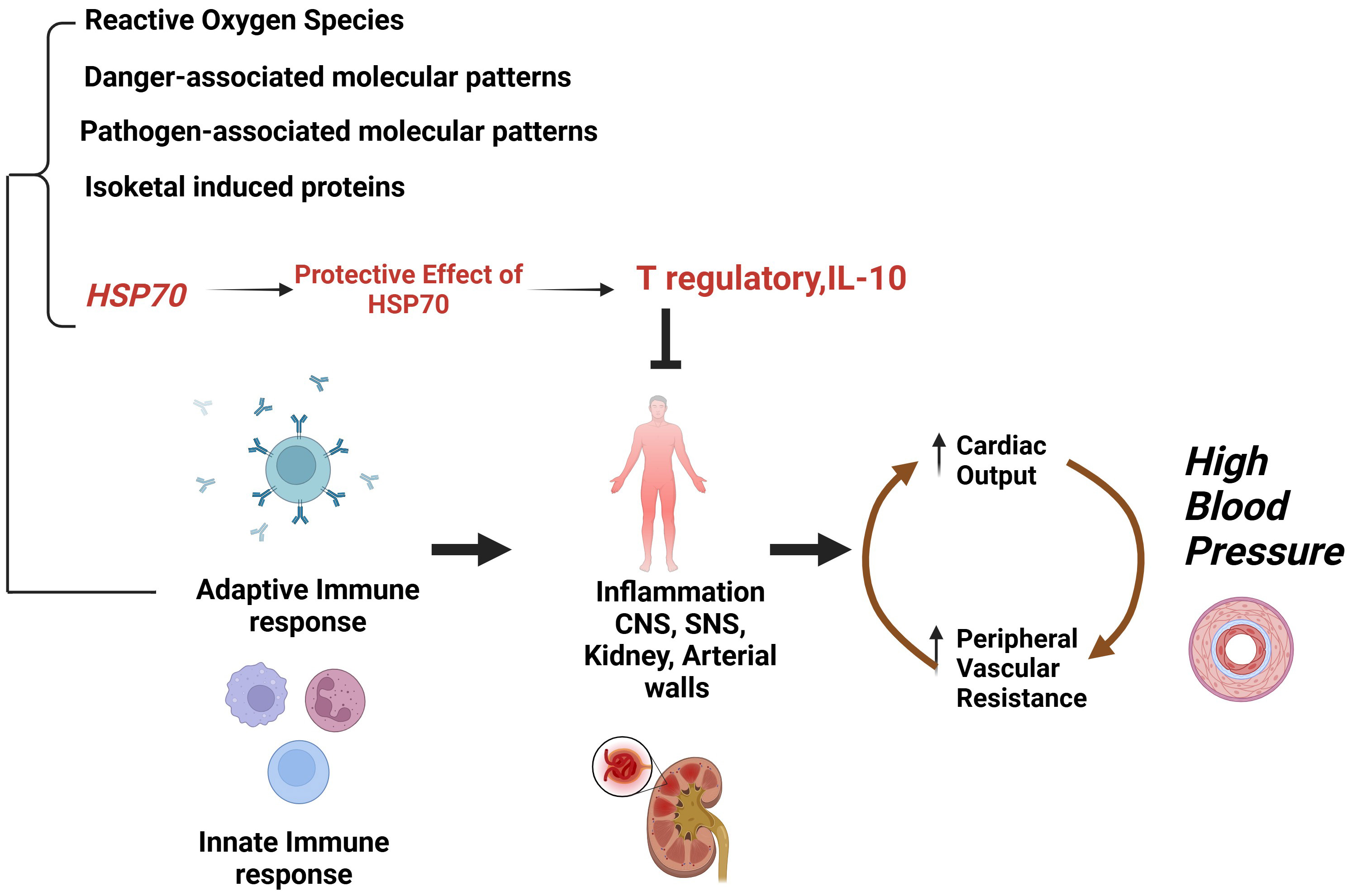 Fig. 4.
Fig. 4. Importance of HSP70 as endogenous antigen in hypertension induced by an innate and adaptive immune response that induces essential hypertension. IL-10, interleukin 10; CNS, central nervous system; SNS, sympathetic nervous system. (Created with Biorender.com).
HSP60 is naturally found in most types of cells, primarily detected inside the mitochondria, some of which are also found in the cytosol. Their level is increased in response to several stressors like oxidative stress, anoxia, infection, and inflammation. HSP60 protein plays an important protective role in preventing injury caused by these stressors by maintaining three-dimensional protein structure and thus preserving cellular equilibrium [72]. Some studies reported that HSP60 relocated into the myocardial cell surface before getting released into the bloodstream in subjects with AHF [73, 74]. Interestingly, elevations in the level of HSP60 on the plasma surface and the presence of serum HSP60 in the hearts of human and animal model have shown their associations with increased apoptosis of myocardial cells measured by activation of caspases and fragmentation of DNA as well as modulation of immune response. Therefore, HSP60’s role can be attributed to an antigen showing these effects [73]. A study investigated the link between HSP60 and distinct endpoints of readmission/death of subjects with heart failure. Their study reported that a total of 132 patients with acute heart failure were admitted and there is a significant association between HSP60 level and end point of readmission/death in subjects with AHF [74]. The exact mechanism behind the association of HSP60 with acute heart failure remains uncertain. Future research is required to determine their precise function in risk assessment and its capacity to be treated as a target for therapeutic interventions. In one study researchers confirmed the alterations in the HSPs (HSP27 and HSP70) at an early stage of cardiac remodeling in animal models. Also, they determined the plasma concentration of these HSPs, HSP27, HSP70, and HSP90, in 222 patients at different stages by using the enzyme-linked immunosorbent assay method. Their results show a positive correlation of heart failure with HSP70. Moreover, they have not observed any significant alterations in HSP27 and HSP90 which suggests that HSP70 is the potential predictive biomarker for the early detection of cardiac failure [75]. Another model of myocardial ischemia demonstrated the role of HSP70 in the onset of the apoptotic process [76]. Increased levels of HSP70 in the endothelial and myocardial cells confer a protective mechanism on the cardiovascular system enhancing the heart’s resilience to myocardial injury. These effects are associated with the reduction of oxidative stress and apoptosis prevention ultimately causing repair of endothelial damage [77, 78]. HSP70 present in myocardial cells activates the enzyme superoxide dismutase present in the mitochondria that inhibits the movement of apoptosis-inducing factor and phosphorylated eukaryotic elongation factor 2 into the nucleus of the cell causing enhanced functioning of mitochondria and inhibition of apoptosis [79]. Further, high levels of aldehyde dehydrogenase 2 in mitochondria cause the accumulation of 4-hydroxynoneal during ischemic injury to cardiac muscles. This accumulation induces the proapoptotic response by inhibiting HSP70 levels and activating the c-Jun N-terminal kinase (JNK)/p53 pathway [80]. This process may have a significant function in the causation of heart failure, and this can be counteracted by HSP70 expression. Moreover, it has been demonstrated that intracellular HSP70 has been found to possess a protective effect on the heart whereas extracellular HSP70 seems to promote apoptosis [81]. HSP90 modulates the necroptosis regulating proteins like receptor-interacting protein (RIP) 1, 3 which programmed for cell death, a study conducted in an animal model. It is expressed in different types of cells like cardiomyocytes and is involved in the activation and maintenance of their functions. Recent study investigates that HSP90 inhibition reduces the necroptosis of cells by inhibiting RIP pathways [82]. Endothelial nitric oxide synthase (eNOS) is a protein that is regulated by HSP90 by identifying its correct function and folding. This enzyme is important for generating nitric oxide that regulates the dilation of blood vessels, reduces inflammation, and prevents atherosclerosis [6]. Molecular chaperones like HSP70, HSP90, the carboxyl terminus of HSC70-interacting protein (CHIP), and BAG-3 are important for preventing protein’s native functions and folding under conditions of oxidative stressor improper foldings or mutations caused by post-translational modifications like phosphorylation. Misfolded proteins cannot be refolded by molecular chaperones as they are focused on degradation mechanisms to prevent noxious accumulation of these misfolded proteins. After this, the misfolded proteins are labelled with ubiquitin in the Ubiquitin-Proteasome Pathway for degradation by 26S proteasome, which involves a joint effort between molecular chaperones, ubiquitin stimulating enzymes E1, conjugating enzymes E2 and ligase enzymes E3 facilitating substrate specificity. Subsequently, the misfolded proteins are then transported to lysosomes by the HSP70 complex for lysosomal degradation. Followed by lysosomal degradation, the aggregates are cleared by autophagosomes. In case any misfolded protein aggregates escape by autophagosomes then they accumulate and contribute to toxicity ultimately causing cardiac impairment [83].
HSP inhibitors have evolved as a potential therapeutic target for the treatment of cardiovascular complications. A natural compound, Geldanamycin is an HSP90 inhibitor that plays a significant role in reducing necroptosis of cardiomyocytes thus improving cardiac functions. It modulates the expression and function of HSP90 and is found to be effective in the treatment of cardiac diseases. It inhibits the signalling pathway in cardiac fibroblasts and cardiomyocytes by acting on profibrotic transforming growth factor-beta (TGF-
However, the exact mechanism of HSPs in atherosclerosis and atherogenesis remains unclear, some studies suggested interlink between the expression of HSPs, immune reactions, and inflammation plays a major role in atherosclerosis progression [92, 93]. The intensity of HSP expression determines the severity of atherosclerosis. In immune reactivity, the lesion shows the localized deposition of
JU, MN, KYT, SR and MNA designed the study. JU, MN, KYT, SR and MNA wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through a Large Research Project under grant number RGP2/580/45.
This research was supported by Deanship of Research and Graduate Studies at King Khalid University.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.




