1 Hephaestus Laboratory, School of Chemistry, Faculty of Sciences, Democritus University of Thrace, Kavala University Campus, St. Lucas, 65404 Kavala, Greece
2 Department of Biological Sciences, University of Limerick, V94PH61 Limerick, Ireland
3 Bernal Institute, University of Limerick, V94PH61 Limerick, Ireland
4 Health Research Institute (HRI), University of Limerick, V94PH61 Limerick, Ireland
Abstract
Neurodegenerative disorders (NDs), including dementia, Alzheimer’s disease (AD), and Parkinson’s disease (PD), are age-related diseases closely associated with chronic inflammation, oxidative stress, gene mutations, autoimmune-derived inflammation, and other external risk factors. They are characterized by progressive neuronal loss, cognitive decline, and/or motor dysfunction, with chronic inflammation being a key player in intensifying NDs’ occurrence. One of the most important molecular inflammatory mediators linking inflammation to NDs is the platelet-activating factor (PAF) and its pivotal signaling for regulating neuroinflammation, apoptosis, and neuronal damage. Dysregulation of PAF activity and metabolism/levels, along with overexpression of its receptor (PAF-R) have been associated with exacerbated inflammatory responses, further aggravating neurodegeneration. This article highlights the role of PAF in neurodegeneration, with a particular focus on novel insights into the potential medicinal use of PAF inhibitors for the prevention and treatment of neurodegenerative diseases. We evaluate the recently proposed concept of targeting the PAF signaling pathway through either natural and/or synthetic inhibitors or a combination of both. It explores the potential of these inhibitors to offer significant preventative and therapeutic benefits against NDs, likely through anti-inflammatory anti-aging effects and by slowing down the disease progression and preserving cognitive and motor dysfunction. Current status and future perspectives of such therapeutic approaches are also discussed.
Keywords
- PAF
- neurodegenerative diseases
- aging
- Alzheimer’s disease
- Parkinson’s disease
- polar lipids
- phenolics
- anti-inflammatory
- natural amphiphilic bioactives
- neuroprotection
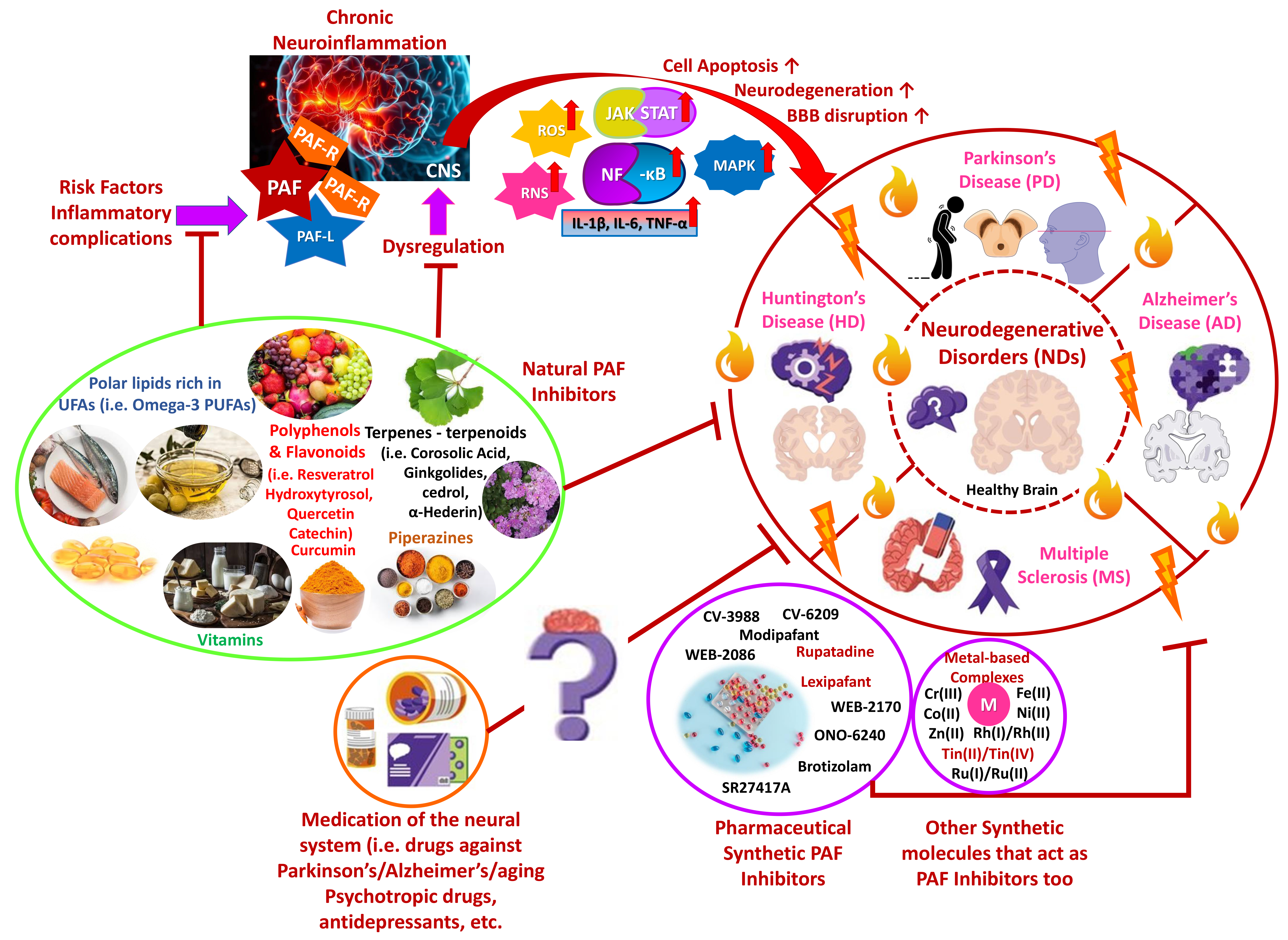
Neurodegenerative disorders (NDs), including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD), as well as multiple sclerosis (MS), are age-related diseases closely associated with chronic inflammation, oxidative stress, gene mutations, and other external factors. The disorders are characterized by progressive neuronal loss, cognitive decline, and/or motor dysfunction [1, 2, 3]. Interestingly, chronic inflammation is an important factor in intensifying NDs’ occurrence. Among the key inflammatory mediators, platelet activating factor (PAF) is considered for a long time pivotal in regulating neuroinflammation, apoptosis, and neuronal damage [1, 2, 4]. PAF dysregulation has been associated with exacerbated inflammatory responses, further aggravating neurodegeneration [4, 5]. Recent research suggests that targeting the PAF signaling pathway through natural and synthetic inhibitors may offer significant therapeutic benefits in managing NDs, potentially slowing disease progression and preserving cognitive and motor dysfunction [6, 7]. Furthermore, understanding how PAF interacts with other inflammatory pathways and mediators in the brain may provide novel insights into PAF inhibitors’ medicinal potential toward NDs’ pathogenesis [4, 5, 7]. Therefore, PAF’s role in neurodegeneration and potential strategies for its modulation based on emerging evidence are discussed, including our previous work on PAF inhibitors in inflammatory diseases (Fig. 1).
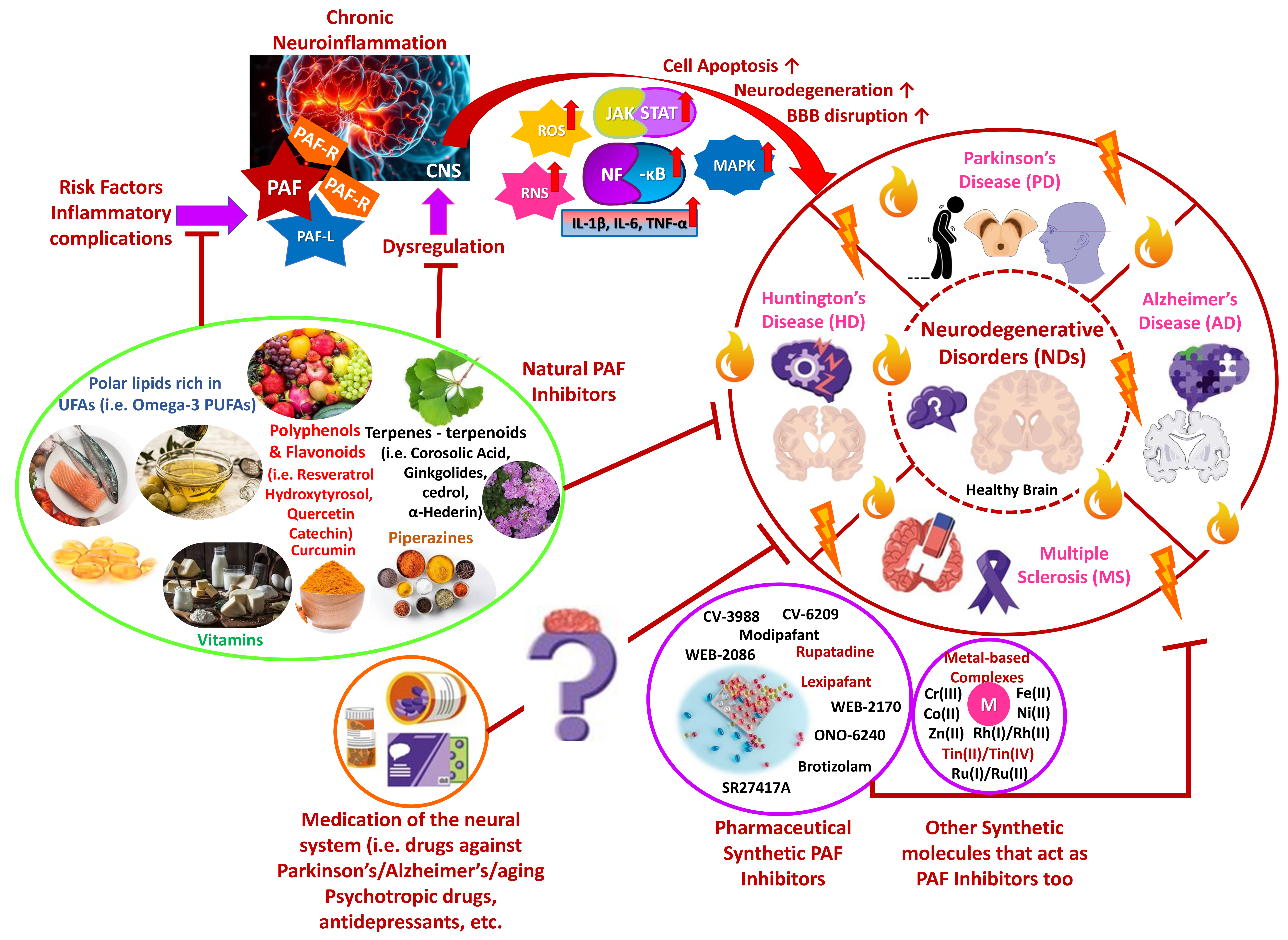 Fig. 1.
Fig. 1.
PAF-associated inflammatory induction of NDs and
Health-Promoting Benefits of PAF-inhibitors against NDs. Chronic inflammation
manifestations highly influence NDs, and PAF, as an inflammatory mediator, may
exacerbate their occurrence. Natural and synthetic PAF inhibitors may possess
therapeutic benefits against NDs progression, while several psychotropics are
believed to be potent PAF inhibitors in NDs (Parts of this figure were obtained
from https://www.freepik.com and https://smart.servier.com). PAF, platelet activating factor; NDs,
neurodegenerative disorders; PAF-R, platelet activating factor receptor; PAF-L,
platelet activating factor-like molecule; CNS, central nervous system; ROS,
reactive oxygen species; RNS, reactive nitrogen species; JAK, Janus kinase; STAT,
signal transducer and activator of transcription; NF-
PAF is a potent phospholipid mediator implicated in multiple pathophysiological processes as well as chronic inflammatory manifestations, contributing to chronic disorders like cancer or neurodegeneration. In the central nervous system (CNS), PAF and PAF-like molecules (PAF-Ls) are synthesized by several types of cells, including inflammatory and neural cells which act as signaling agents, regulating neuronal communication and immune responses. However, PAF’s dysregulation contributes to excessive neuroinflammation, oxidative stress, and neuronal apoptosis [3, 4, 6, 8, 9]. Reportedly, elevated PAF levels have been linked to cognitive impairment in AD and PD, reactive oxygen and nitrogen species (reactive oxygen species (ROS) and reactive nitrogen species (RNS), respectively) formation, pro-inflammatory cytokine release, cell apoptosis induction, and other pathophysiological responses, like those affecting the neural system [4, 8, 9, 10, 11].
PAF, specifically, interacts with its receptor (PAF-R), stimulating downstream
pro-inflammatory pathways like the rapid activation of the nuclear factor
kappa-light-chain-enhancer of activated B cells (NF-
Considering PAF’s pathogenic role in neuroinflammation, PAF inhibitors represent a promising therapeutic strategy for ND treatment. Several PAF antagonists of natural and synthetic origin have displayed neuroprotective effects by mitigating and reducing oxidative stress, inflammation, and neuronal apoptosis [4, 10], and especially several amphiphilic molecules from various natural sources [4, 5, 7, 15, 16, 17]. PAF inhibitors not only suppress PAF-driven inflammation but also modulate the activity of immune cells, therefore aiding in restoring immune balance in ND conditions. For instance, natural amphiphilic bioactives (NABs) and synthetic inhibitors, have already shown the potential to regulate microglial and astrocytic activation, thus reducing neuroinflammation [4, 7, 18, 19, 20]. Biomarkers such as PAF levels in cerebrospinal fluid and the PAF-R expression levels in brain tissues, may serve as indicators for identifying patients most likely to benefit from PAF-targeted therapies based on PAF inhibitors. PAF-associated biomarkers could also aid in monitoring therapeutic efficacy and personalizing mitigation strategies [6, 8, 9, 12, 14].
Among these, several dietary marine derived, dairy-derived and plant-derived
compounds have been identified as PAF antagonists, especially NABs that possess
higher bioavailability by being able to surpass complex barriers more
efficiently, like the BBB [4, 7, 10]. The most prominent example is natural
bioactives from the Ginkgo biloba tree, which is rich in ginkgolides that
antagonistically inhibit PAF binding on its receptor and thus, effectively reduce
PAF-signaling and activity which results in less PAF-associated neuroinflammation
and oxidative stress [4, 21, 22, 23]. Similarly, NAB-like phenolics and polar
lipids (phospholipids and glycolipids) rich in unsaturated fatty acids (i.e.,
omega-3 polyunsaturated fatty acids (PUFAs)) mainly of marine origin, but also
from other sources (i.e., olive oil, dairy fermented products, apple pomace,
etc.) have exhibited great promise in attenuating PAF-associated inflammatory
signaling and thereby, PAF-induced neurotoxicity [4, 7, 10, 16, 17, 24, 25, 26]. Such
polar lipids for example, can inhibit PAF antagonistically due to structural
homology. Additionally, also due to their amphiphilic nature, they transfer more
effectively their PUFA content, such as their docosahexaenoic acid (DHA), into
the brain. PUFA can regulate membrane fluidity and reduce neuroinflammatory
cytokines’ production (i.e., astrocytes), limiting NDs progression [7, 17, 26].
Other NABs, like bioactive polyphenols and flavonoids (i.e., curcumin, catechin,
quercetin, resveratrol, etc.), also demonstrate favorable PAF-inhibitory and
neuroinflammation-suppressive outcomes [4, 27, 28, 29, 30, 31]. Furthermore, other natural
bioactives like piperazines, terpenes, and terpenoids, such as piperine,
withaferin A, cedrol,
Pharmacological, synthetic PAF inhibitors, such as rupatadine and lexipafant, have also displayed notable anti-inflammatory and neuroprotective effects. Such compounds can block PAF-R activation, preventing downstream inflammatory manifestations, suppressing neuronal damage, and thus, optimizing BBB integrity [4, 5, 17, 34, 35]. Moreover, modipafant, brotizolam, WEB-2086, WEB-2170, CV-6209, CV-3988, ONO-6240, and SR27417A have similarly exhibited anti-PAF, anti-inflammatory and neuroprotective benefits [4, 9, 17, 36].
Recently, another class of synthetic PAF inhibitors has gained attention: several metal-based complexes with anti-inflammatory and antithrombotic properties were identified, including synthetic ones based on Cr(III), Co(II), Zn(II), Mn(II), Fe(II), Ni(II), Rh(I)/Rh(II), Ru(I)/Ru(II), and Tin(II)/Tin(IV), as well as metal-based porphyrins, which have also been recently highlighted to own significant anti-inflammatory potential, suggesting a potent link between their activity and NDs prevention [4, 9, 17, 35, 37, 38, 39].
The role of PAF signaling in neurodegeneration highlights its significance as a therapeutic target in managing NDs. Evidence suggests that modulating PAF activity via natural and synthetic inhibitors or metal-based complexes, can reduce neuroinflammation, oxidative stress, and neuronal apoptosis, offering many neuroprotective and immune response benefits. Interestingly, PAF inhibition has shown promise in preserving cognitive and motor functions by attenuating inflammatory cascades [4, 7, 10]. However, further research is needed to understand the long-term effects of such inhibitors and their interactions with other therapeutic agents. Bridging the gap between preclinical findings and clinical applications, will pave the way for novel, more personalized mitigation strategies [4, 7, 10, 17].
Despite the promising potential of PAF inhibitors, whether of natural or synthetic origin, several challenges must be addressed. First and foremost, the complexity of PAF signaling, PAF’s crosstalk with multiple inflammation-linked cascades, bioavailability, and the need to enhance BBB penetration of not NAB PAF inhibitors must be addressed. Advances in nanotechnology and drug delivery systems may enhance the bio-availability of PAF inhibitors at the CNS, which may improve their therapeutic efficacy [4, 7, 10]. At the same time, PAF inhibition’s impact on neuronal plasticity and cognitive function, and individual variations in NDs progression, should remain a key focus of ongoing research [4, 7, 10]. Currently, there is a lack of targeted human clinical trials and valid animal-based studies investigating the efficacy of PAF inhibitors in NDs like dementia, AD and PD [5, 12, 14, 23, 25, 40, 41]. Future research should focus on translating preclinical findings into clinical applications, including targeted trials exploring the vast therapeutic potential of PAF inhibition in such neuroinflammation-associated conditions [4, 5]. Furthermore, PAF-driven neuroinflammation also facilitates peripheral immune cells’ recruitment into the CNS, by enabling BBB disruption; thereby, inhibiting PAF signaling may offer several additional neuroprotective benefits [4, 5, 13].
An intriguing question, though, remains: what exactly is the interaction of PAF and its signaling pathways with medication affecting the neural system (i.e., drugs against aging, dementia, AD and PD, psychotropic drugs, antidepressants, etc.) that may influence the PAF-related activity in NDs? Given the established link between inflammation and neurological and psychiatric conditions, it is believed that certain associated medications, like the aforementioned ones, may exert either beneficial anti-PAF and anti-inflammatory effects or detrimental ones [42]. The ones with beneficial anti-PAF effects may also positively impact ND progression and overall mental health due to this interaction, which needs further attention [42]. Future studies should focus on optimizing PAF-targeted therapeutic interventions and exploring their synergy with existing treatment solutions, paving the way for innovative future management of NDs tailored to an individual’s specific needs [4, 7, 10, 17].
PAF plays a pivotal role in neuroinflammation and neurodegeneration and, thus, is a compelling agent for therapeutic interventions. Modulating PAF signaling through natural and synthetic inhibitors, presents a promising strategy to mitigate neuronal damage and preserve cognitive function, by suppressing many inflammation-related mediators and cascades. However, further optimization of current PAF inhibitors, exploration of propitious PAF-targeted therapy, and investigation of the potential of psychotropic medication against NDs is still warranted.
AD, Alzheimer’s disease; BBB, blood-brain barrier; CNS, central nervous system; DHA, docosahexaenoic acid; HD, Huntington’s disease; IL-1
Conceptualization, AT; software, AT, TA and AMG; validation, AT; investigation, AT and TA; writing—original draft preparation, TA and AT; writing—review and editing, AT and AMG; visualization, AT; supervision, AT; project administration, AT. All authors contributed to editorial changes in the manuscript. All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We thank the School of Chemistry, the Faculty of Sciences of the Democritus University of Thrace, and the Department of Biological Sciences of the University of Limerick for their continuous support.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.