1 Department of Dermatology, China-Japan Friendship Hospital, National Center for Integrated Traditional Chinese and Western Medicine, 100029 Beijing, China
2 Department of Dermatology, Shunyi Hospital, Beijing Hospital of Traditional Chinese Medicine, 101300 Beijing, China
3 China-Japan Friendship Clinical Medical College, Beijing University of Chinese Medicine, 100029 Beijing, China
Abstract
Psoriasis is an enduring inflammatory skin disorder defined by recurring attacks, distinguished primarily by red patches and scaly skin. Quercetin, a kind of natural flavonoid compound, is widely found in various vegetables, fruits, and Chinese herbs. Quercetin is a multifaceted compound with a wide range of potential health benefits. In addition to antioxidant, cardiovascular protection, and anti-tumor effects, quercetin has shown potential in regulating immune and inflammation effects. In the initial stages, in vivo studies have demonstrated that quercetin positively affects psoriasis and is connected with the phosphatidylinositol 3-kinase (PI3K)/Protein Kinase B (AKT)/glucose transporter 1 (GLUT1) signaling. Nevertheless, the precise mechanism by which quercetin influences the PI3K/AKT/GLUT1 signaling cascade in the context of psoriasis remains uncertain.
The aim of this study was to investigate the potential therapeutic influence of quercetin on psoriasis and the relationship with the PI3K/AKT/GLUT1 signaling pathway.
A mouse model for psoriasis induced by imiquimod was employed to assess alterations in the morphology of skin lesions and their histopathological characteristics. Cell Counting kit-8 (CCK-8) assay was used to assess the impact of proliferation of HaCaT human keratinocyte cells. HaCaT cells were examined using flow cytometry for the influence of quercetin on apoptosis. Additionally, Western blot analysis was used to evaluate the protein expression levels in the PI3K/AKT/GLUT1 signaling pathway.
concerning pathological alterations, the mice in the model group exhibited characteristic alterations associated with psoriasis. The extent of excessive keratinization in the epidermis and hypertrophy of the spinous layer observed in each quercetin dosage group was less pronounced compared to the model group. The CCK-8 assay laid out that quercetin can suppress the proliferation of HaCaT cells. Furthermore, it was found that quercetin facilitates the apoptosis of these cells. Analysis of immunoblotting demonstrated that the intervention of quercetin in HaCaT cells led to modifications in the proteins related to the PI3K/AKT/GLUT1 signaling pathway.
Through in vivo and in vitro experiments, this study shows that quercetin may play a therapeutic role in psoriasis and inhibit the PI3K/AKT/GLUT1 signaling pathway.
Keywords
- psoriasis
- quercetin
- HaCaT
- glycometabolism
- PI3K/AKT/GLUT1 signaling pathway
Psoriasis is a common chronic autoimmune dermatological disorder characterized by the presence of thick, silvery scales and patches that are itchy and dry [1]. This condition involves an accelerated growth of keratinocytes and a markedly reduced cellular turnover rate [2]. This skin disorder impacts more than 60 million individuals worldwide, and about 2%–3% of the global population are affected by this disease [3, 4]. Moreover, psoriasis is associated with numerous other health issues, including anxiety, psoriatic arthritis, and various cardiometabolic disorders [1, 5]. Research has indicated that among individuals diagnosed with psoriasis, the occurrence of metabolic syndrome is around 32% [6]. Moreover, the elderly suffering from metabolic syndrome are at an increased likelihood of developing psoriasis when compared to those who do not have this condition [7]. In addition, the abnormal metabolism-induced inflammation may also cause other psoriasis comorbidities, such as depression [8]. This disrupted metabolic process contributes to the emergence of risk factors linked to severe incidents such as heart attacks and blood clots, which may significantly impact patients’ longevity [9, 10, 11]. One of the primary objectives for managing psoriasis in the future might involve reversing the current inflammatory damage while enhancing the manifestations of inflammatory complications [12].
The metabolism of immune cells is significantly influenced by glycolysis. Research has shown that the glycolytic pathway metabolism is enhanced in patients with psoriasis [13]. Upon T cell antigen receptor (TCR) signaling activation, naive T cells transition to a state of enhanced glucose metabolism, which facilitates their development into effector T cells [9].
Glucose transporter 1 (GLUT1) belongs to the glucose transporter family and has the function of exchanging substrates on the plasma membrane, affecting different physiological and pathological activities [14]. It takes an irreplaceable part in glucose metabolism, as well as affects various physiological and pathological processes by participating in glycolysis. The glycolytic pathway mediated by GLUT1 is crucial in the pathophysiological processes of varied diseases [15, 16, 17]. GLUT1 is a selective essential substance for the proliferation of keratinocytes related to injury and inflammation, and its inhibition provides a new therapeutic strategy for psoriasis [18]. The GLUT1 protein is not only involved in the pathogenesis of psoriasis; but also, in its progression and severity. It may influence the onset of psoriasis by facilitating excessive proliferation of the epidermal layer, Inflammation, and the formation of new blood vessels [19]. Research has shown that certain traditional Chinese medicine decoction may alleviate imiquimod-induced psoriasis by inhibiting bone marrow-derived inhibitory cell glycolysis induced by the p21/hypoxia-inducible factor-1
Quercetin is a polyphenolic flavonoid compound with chemical names of 3,3’, 4’, 5,7-pentahydroxyflavone. There are promising effects of quercetin and its derivatives on diabetes, bacteria, inflammation, and antioxidants [21]. Previous study has shown that the main active ingredient in the QingRe Liangxue Formula for treating psoriasis might be quercetin [22]. Quercetin can exert mechanisms related to glucose metabolism through multiple pathways through Glut1. The combination of quercetin with superparamagnetic iron oxide nanoparticles is capable of modulating the expression of miR-29, which in turn enhances the levels of glucose transporters and insulin-like growth factor-1 (IGF-1) while also diminishing the complications associated with diabetes [23].
Currently, it remains unclear if quercetin contributes to the enhancement of psoriasis by influencing GLUT1 and altering glucose metabolism. In light of this, research was conducted utilizing imiquimod-induced psoriasis models in animals along with HaCaT cell systems to explore the underlying mechanisms of quercetin’s effect on psoriasis. This aims to enhance the understanding of the traditional Chinese medicine approaches to psoriasis, probe additional natural compounds, and provide a foundation for future applications.
Imiquimod cream (IMQ, 5%, w/w) cream was bought from Mingxin Pharmaceutical Company (Chengdu, China, H20030128). Quercetin (Q111273), LY294002 (L9908), and Dimethyl sulfoxide (DMSO, D5879) were purchased from Sigma (St. Louis, MO, USA). Tripterygium glycosides were a product of Zhejiang DND Pharmaceutical company (Zhejiang, China, Z33020422). GLUT1 antibody was obtained from Abcam (Cambridge, MA, USA, ab115730). Cell Counting Kit-8 (CCK-8) was acquired from Dojindo Laboratories (Kumamoto, Japan, CK04-01). Annexin V-FITC/PI was purchased from BD Pharmingen™ (New Jersey, NY, USA, 556547). Recombinant human tumor necrosis factor-
Based on previous studies by other researchers [24, 25], dissolve 60 mg, 120 mg, and 240 mg of quercetin standard in 20 mL of 0.5% Sodium carboxymethyl cellulose (CMC-Na, Sinopharm Group Co. Ltd., Beijing, China, 9004-32-4), solution, respectively, to prepare quercetin solutions with concentrations of 30 mg/kg, 60 mg/kg, and 120 mg/kg. According to the long-term foundation and experience of our research group, dissolve 10 mg of Tripterygium glycosides in 15 mL of physiological saline to prepare a Tripterygium glycosides oral solution with a concentration of approximately 7.56 mg/kg.
A total of 47 male BALB/c mice (6–8 weeks, 18–20 g) were purchased from Beijing Vitong Lihua Laboratory Animal Technology Co., Ltd. (Beijing, China, SCXK, 2016–0011). Human immortalized keratinocytes (HaCaT, 1101HUM-PUMC000373) were purchased from the Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China), supplemented with 10% FBS (Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences) and 1% penicillin-streptomycin. All cell lines were validated by short tandem repeats (STR) profiling and tested negative for mycoplasma. Cells were all cultured in a humidified incubator at 37 °C and 5% CO2.
The BALB/C mice were assigned randomly into six groups, including blank control group, model (IMQ) group, positive control group (GTW), low-dose quercetin (QCL), medium-dose quercetin (QCM), as well as the groups treated with a high concentration of quercetin (QCH), including seven mice in the control group and eight mice in the treated groups. Using imiquimod treatment to establish a psoriasis mouse model [26]. Apart from the control mice, all subjects in the experiment were treated with a 5% solution of imiquimod. The treatment was administered once daily to a 2
Groups receiving low, medium, and high doses of quercetin were administered concentrations of 30 mg/kg, 60 mg/kg, and 120 mg/kg respectively. The GTW group received a gavage of a suspension containing Tripterygium glycosides at an approximate concentration of 7.56 mg/kg. The control group and the model group were administered a solution with a concentration of 0.9% saline solution daily.
The experiment was approved by the Experimental Animal Management Committee of the Clinical Research Institute of China-Japan Friendship Hospital, and the ethics review number is 190109.
In mice, the assessment of lesions resembling psoriasis and the severity of the condition involves evaluating parameters such as redness, skin thickness, and the presence of scales. Each parameter is evaluated on a scale from 0 to 4 points, representing no, mild, moderate, and severe, with the respective scores being 1, 2, 3, and 4 [27]. The overall score reflects the condition of the inflammatory lesions. The overall score is determined by summing the values from three different parameters, with a maximum possible score of 12. Monitor the alterations in the mouse dermal lesions and inflammation by creating a trend chart for the PASI score. It was evaluated back-to-back by two professional researchers skilled in PASI and reviewed by a third professional researcher.
After the experiment, the mice were euthanized by means of cervical dislocation under the guidelines of the Institutional Animal Care and Use Committee to ensure that the suffering to the animals was minimized. The skin lesions were gathered and then preserved in a mixture of 10% formaldehyde and paraffin wax after 7 days. Subsequently, carry out the standard H&E staining procedure on the specimens. The specimens underwent a series of traditional processes, including gradient alcohol dehydration, clearing, wax infiltration, embedding, sectioning, spreading, drying, dewaxing, staining with hematoxylin, differentiation with hydrochloric acid, eosin staining, further dehydration through an alcohol gradient, and finally sealing. Following the staining with hematoxylin and eosin, photographs were captured using a light microscope set at 200
On the eighth day, samples were obtained from the dorsal skin of the mice, which were quickly frozen in liquid nitrogen and frozen at
| Gene | Primer | Sequence (5′ to 3′) |
|---|---|---|
| Mus Akt | Forward | CGCTTCTATGGTGCGGAGATT |
| Mus Akt | Reverse | CAGCCCGAAGTCCGTTATCTT |
| Mus Glut1 | Forward | CCGATGTGACCCGAGACCTG |
| Mus Glut1 | Reverse | GCACCACAGCGATGAGGATG |
| Mus | Forward | CGTTGACATCCGTAAAGACCTC |
| Mus | Reverse | ACAGAGTACTTGCGCTCAGGAG |
In a 96-well plate, HaCaT cells, which are in the logarithmic growth phase, were plated at a density of 5
Each set consists of six duplicates. Following the addition of the medication, curing at 37 °C Incubate with 5% CO2 for 24 hours, 48 hours 72 hours later. The intervention involving the drugs was conducted at each designated time interval, the medications were removed individually, and the wells were treated with a blank medium infused with 10% CCK-8 and maintained at 37 °C for four hours. An enzyme-linked immunosorbent assay (ELISA) reader was utilized to measure the optical density (OD) at a wavelength of 450 nm for each well. This experiment was conducted in triplicate.
Herein is the CCK-8 calculation formula.
formula = [(Ac – As)/(Ac – Ab)]
• As is the absorbance value of the experimental group, that is, the absorbance value of the cell group with the substance to be measured added.
• Ab is the absorbance value of the blank group, that is, the absorbance value of the culture medium without cells.
• Ac is the absorbance value of the control group, that is, the absorbance value of the cell group without adding the substance to be measured.
Take HaCaT cells with good growth status and divide them into 9 groups, namely blank control group (Control), simulated psoriasis disease group (Model A), disease+PI3K/AKT pathway inhibition group (Model B), disease+high-dose quercetin group (Model A+QCH), disease+medium dose quercetin group (Model A+QCM), disease+low-dose quercetin group (Model A+QCL), disease+inhibition+high-dose quercetin group (Model B+QCH), disease+inhibition+medium dose quercetin group (Model B+QCM), and disease+inhibition+low-dose quercetin group (Model B+QCL).
When the cells grew to 70% of the bottom of the well, the blank control group culture medium was replaced with DMEM complete culture medium. The Model A group, Model B group, and two intervention groups were all added with prepared TNF-
Inoculate HaCaT cells (1
Western blots were used to determine whether quercetin affected AKT, pAKT, and GLUT1 proteins of HaCaT cells in each group (cell grouping and intervention measures are described earlier). Add 100 µL of RIPA lysis buffer and 1 µL of protease inhibitor to the cells in a 6-well plate. Place on ice and scrape along all directions with cells. Collect the liquid in the well, centrifuge at 4 °C, 12,000 rpm, for 5 minutes. Collect the supernatant. BCA assay kit was used to determine the concentration of total protein in lysis buffer. Equal amounts of protein were subjected to 10% SDS-PAGE and transferred onto a PVDF membrane (Millipore, Billerica, MA, USA), which was then sealed in 5% skim milk. PVDF membrane was incubated overnight with primary antibodies against AKT (catalog number 9272, Cell Signaling Technology, Danvers, MA, USA, 1:4000), pAKT (catalog number 4060, Cell Signaling Technology, USA, 1:1000), and GLUT1 (catalog number ab115730, Abcam, USA, 1:1000) at 4 °C, followed by incubation with horseradish peroxidase-labeled goat anti-rabbit Immunoglobulin G (IgG) (H+L) (catalog number BF03008, Beijing TDY Biotech Co., Ltd., Beijing, China, 1:8000) and goat anti-mouse IgG (H+L) (catalog number BF03001, Beijing TDY Biotech Co., Ltd., 1:8000) at 25 °C for 40 minutes. Perform imprinting detection using enhanced chemiluminescence assay (Millipore, USA). The internal reference is
Using SPSS.22 software (https://www.ibm.com/products/spss-statistics) to analyze the results. All tests were conducted using a two-sided test, and p
In terms of changes in skin lesions, the model group mice showed typical “psoriasis-like” changes such as erythema, hypertrophy, as well as scales, while the quercetin group had milder erythema, hypertrophy, and scales compared to the model group at the same time point. In terms of PASI score, the quercetin group had significantly lower scores for erythema, hypertrophy, and scales (Fig. 1). Compared to the blank control group, the model group mice showed typical “psoriasis-like” changes, and the degree of epidermal hyperkeratosis and spinous hypertrophy in each dose group of quercetin was milder than that in the model group. In terms of epidermal thickness, the model group was significantly higher than the blank control group (130.93
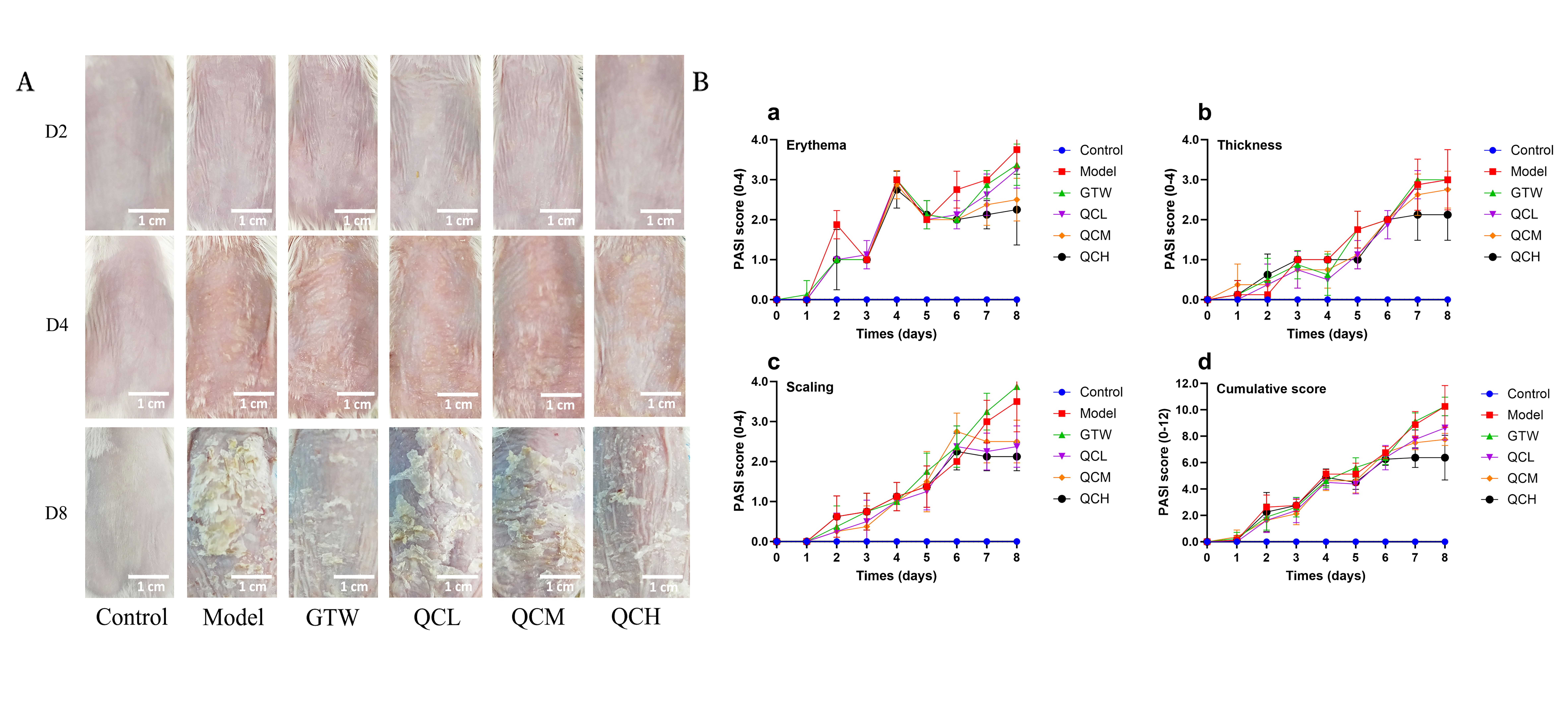 Fig. 1.
Fig. 1. Quercetin reduced the IMQ-induced psoriasis skin lesions. (A) Map of stage changes of target lesion on the back of mice (Scale bar = 1 cm) (B) PASI scoring trend (n = 6). B-a: Erythema PASI scores; B-b: Thickness PASI scores; B-c: Scaling PASI scores; B-d: Total PASI scores. IMQ, Imiquimod cream; PASI, Psoriasis Area and Severity Index; GTW, positive control group; QCL, low-dose quercetin; QCM, medium-dose quercetin; QCH, high concentration of quercetin.
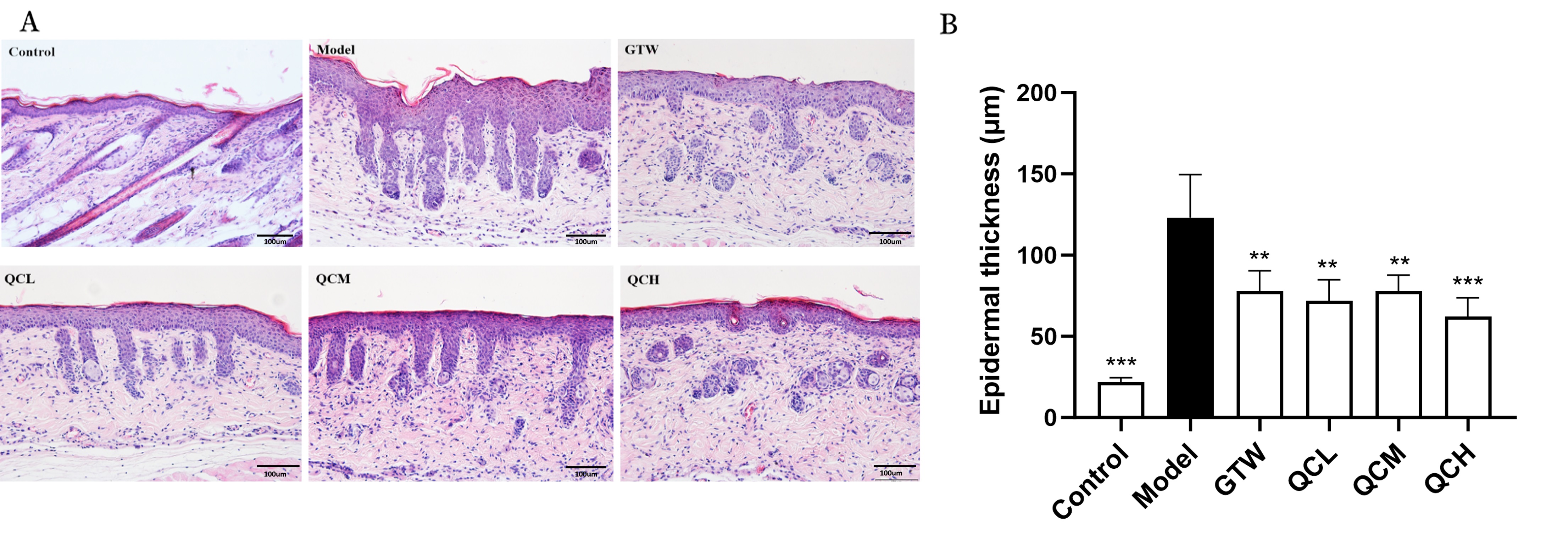 Fig. 2.
Fig. 2. Quercetin reduced the IMQ-induced psoriasis skin lesions on pathology. (A) Hematoxylin-eosin staining of the skin in control, model, GTW, QCL, QCM, and QCH group (HE, 200
The real-time fluorescence quantitative PCR results exhibited that the relative expression levels of Akt mRNA and Glut1 mRNA in the skin lesions of the model group mice were significantly higher than those in the blank control group (p
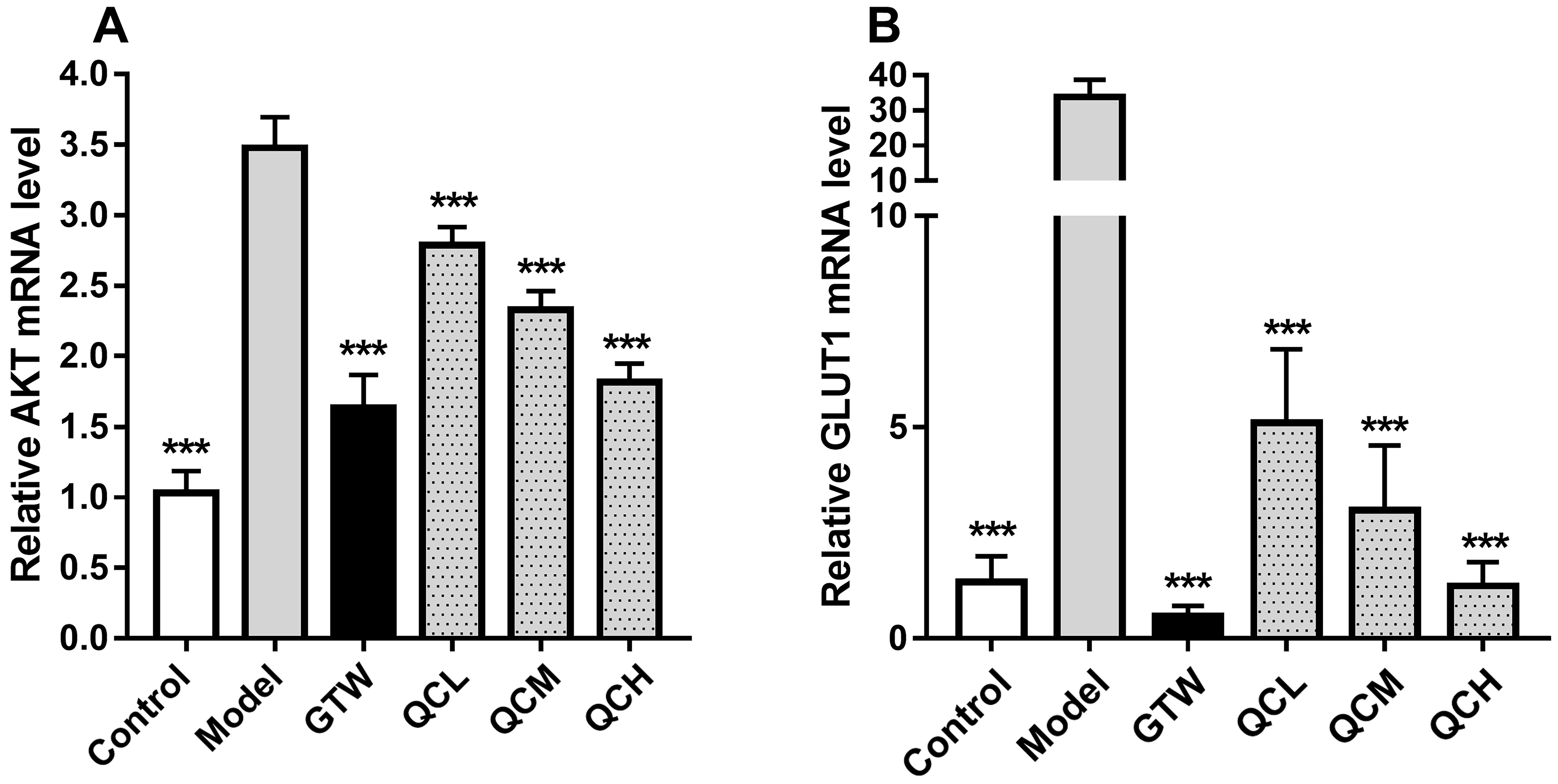 Fig. 3.
Fig. 3. Quercetin decreased the expression of Glut1 mRNA and Akt mRNA in target lesions of mice. (A) Expression of Akt mRNA in the skin lesions of mice in each group. (B) Expression of Glut1 mRNA in the skin lesions of mice in each group. ***p
We performed the CCK-8 assay to examine the half maximal inhibitory concentration (IC50) value of the quercetin against the HaCaT cell. The results of CCK-8 assay exhibited that quercetin could inhibit the HaCaT cell proliferation. The IC50 values of quercetin on HaCaT cell inhibition at different administration times were 24 hours, IC50 = 160.3 mM (Ig 2.205); 48 h, IC50 = 73.28 mM (Ig 1.865); 72 h, IC50 = 42.20 mM (Ig 1.625) (Fig. 4).
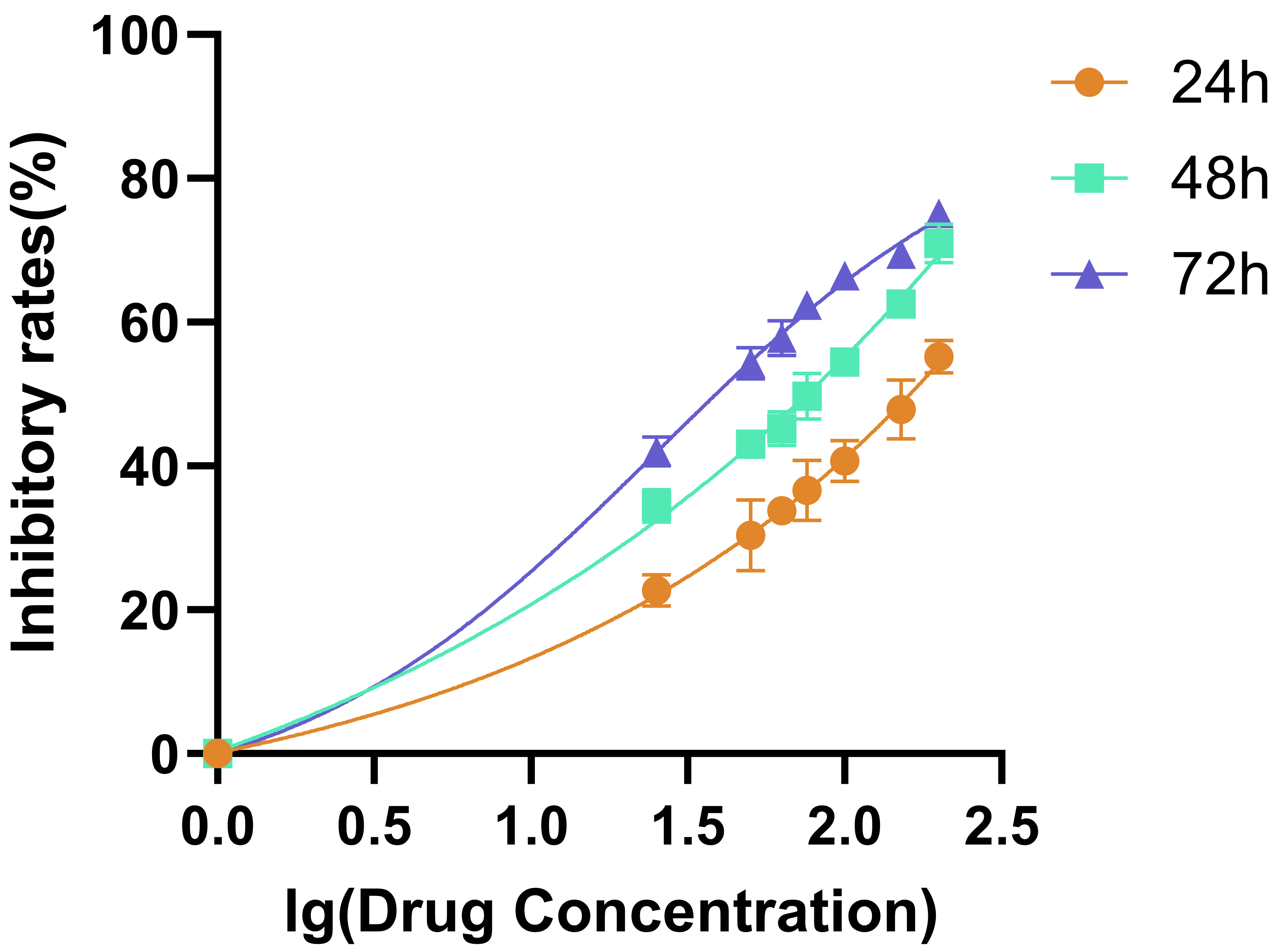 Fig. 4.
Fig. 4. The inhibitory effect of quercetin on HaCaT cells.
The results of cell apoptosis showed that the apoptosis rate of Model A group was significantly lower than that of the blank control group (p
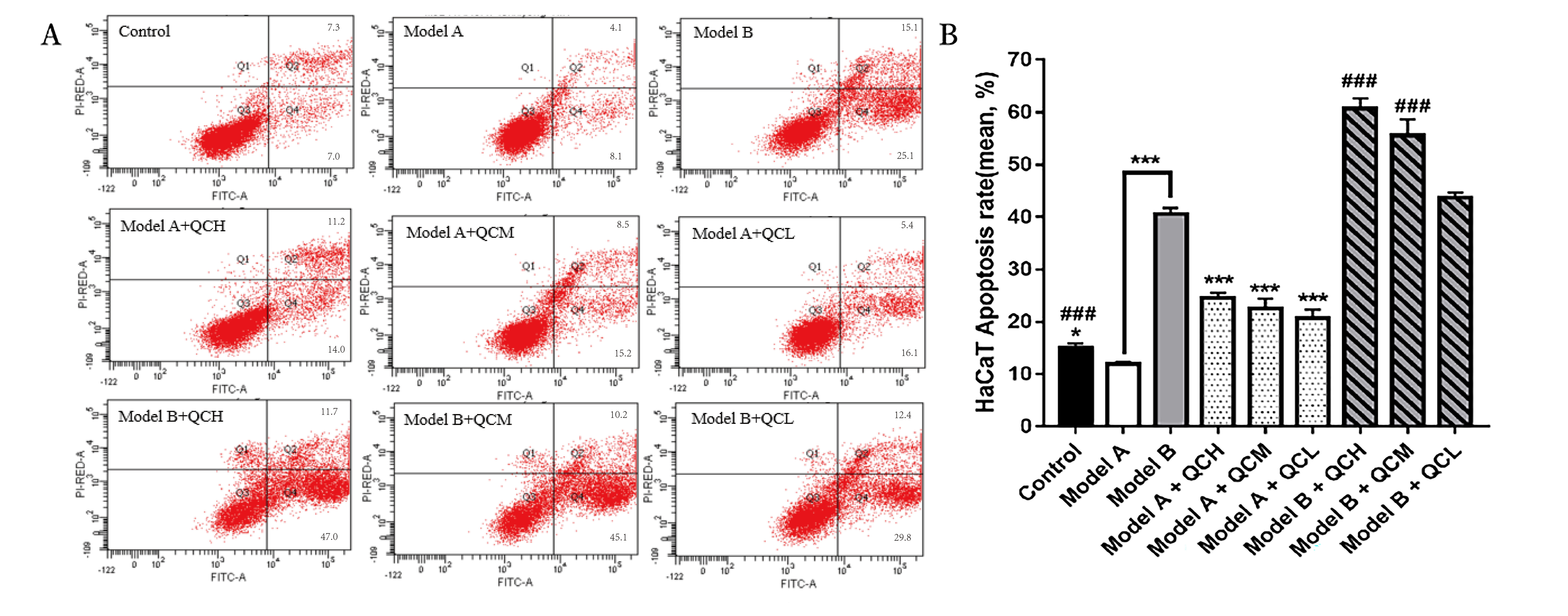 Fig. 5.
Fig. 5. Results of apoptosis rate of HaCaT cells in each group. (A) Flow cytometry of HaCaT cell apoptosis in each group. (B) Results of apoptosis rate of HaCaT cells in each group. *p
The Western Blot results showed that the pAKT/AKT in the Model A group was significantly higher than that in the Control group (p
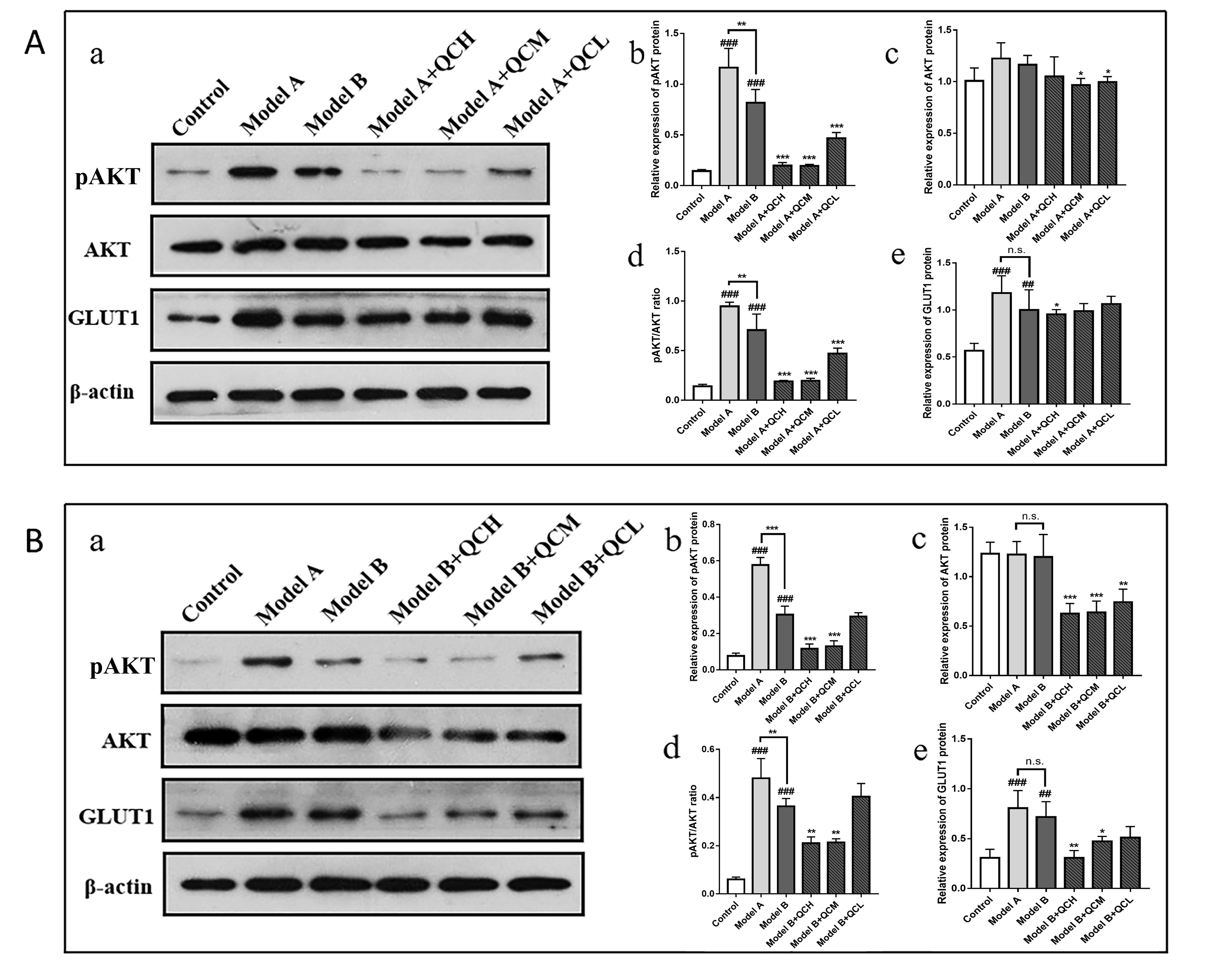 Fig. 6.
Fig. 6. Relative expression levels of AKT, pAKT and GLUT1 in HaCaT cells in each group. (A) a. the Western blot results in HaCaT cell in Model A group. b–e. the relative expression levels of pAKT,AKT,pAKT/AKT, and GLUT1 proteins in HaCaT cells of Model A groups. ##p
Psoriasis, an autoimmune condition, is shaped by the interplay of environmental and hereditary elements. The development of psoriasis is significantly influenced by the roles of interleukin-23 (IL-23) and T helper cell 17 (Th17) cells, which are intricately linked to both genetic predispositions and immune responses [1]. Currently, there are no conclusive therapies available for treating psoriasis. Current therapeutic approaches are capable of achieving prolonged management or extended periods without redness. While numerous individuals with psoriasis have attained effective management through biological therapies, a portion of patients still exhibit either an initial lack of response or a diminished response over time to these agents [28]. The practice of traditional Chinese medicine offers a long-standing approach to managing psoriasis, with various herbal formulations and preparations demonstrating significant promise in alleviating the condition [29]. It is, therefore, quite intriguing to investigate and create additional therapeutic agents for psoriasis derived from historical remedies, utilizing contemporary technological advancements.
Quercetin, a natural polyphenolic flavonoid derived from fruits and vegetables, is renowned for its significant antioxidant and anti-inflammatory effects, making it a promising candidate for addressing conditions such as dermatitis, psoriasis, and skin aging [30]. Research indicates that quercetin can reduce inflammation associated with psoriasis lesions. Nonetheless, the precise way in which it operates remains uncertain. Studies indicate that the therapeutic effects of Xiao-chai-hu Decoction on psoriasis might be attributed to the regulation of keratinocyte growth, along with the suppression of inflammation and the reduction of vascular proliferation. Findings from network pharmacology indicate that quercetin ranks among the four primary active constituents of Xiao-chai-hu Decoction beneficial for the treatment of psoriasis [31]. Additionally, quercetin has gained recognition for its role in the formulation of novel materials aimed at reducing inflammation and combating oxidative stress in skin care applications [30, 32].
The intricate interconnection between immune response and metabolic processes in long-standing skin inflammation has been garnering increasing interest. Psoriasis manifests through an overproduction and irregular maturation of keratinocytes, accompanied by significant infiltration of inflammatory cells [33], indicating a higher energy demand to sustain its biological processes. The alteration in glucose metabolism, particularly through aerobic glycolysis, is intricately linked to the processes of cellular differentiation and functionality. Studies indicate that epithelial tissue primarily relies on glycolysis for its metabolic processes [34, 35, 36]. Upon stimulation, immune cells engage in the glycolytic metabolic pathway [37]. Crucial elements in the transport of glucose include the localization and expression levels of GLUT1, with prior research indicating that GLUT1 protein levels are heightened in the epidermis affected by psoriasis [20, 38]. In psoriasis, the increased expression of Glut1 leads to enhanced glycolytic activity. The response of keratinocytes to IL-17 stimulation is linked to the involvement of HIF-1
The investigation revealed an atypical glycolytic process prevalent in psoriasis, primarily influenced by Glut1, which might be modulated by the PI3K/AKT signaling cascade. The PI3K/AKT signaling cascade is frequently engaged in various biological processes and associated with malignancies, metabolic disorders, and nephrolithiasis, it plays a significant role in their pathological development [42, 43, 44]. The PI3K/AKT pathway participates in various processes, including the aging of cells within the context of psoriasis [45, 46]. The findings of this research suggest that the signaling pathway of PI3K/AKT/GLUT1 may serve as an innovative link between inflammatory responses and metabolic processes in psoriasis, further supporting the therapeutic potential of traditional Chinese medicine, exemplified by quercetin, in modulating this pathway. Targeting the PI3K/AKT/GLUT1 pathway represents a promising approach for psoriasis treatment, while quercetin, a flavonoid compound, offers fresh perspectives for discovering additional therapeutic agents for this condition.
There are certain constraints within this research. Initially, the study focused solely on the detection of Glut1, without evaluating additional molecules or proteins that participate in glycolytic processes, including HIF-1. Secondly, an investigation into glycolysis related to T cells was not conducted. Future studies will aim to explore the metabolic pathways linked to T cells in psoriasis, alongside understanding how traditional Chinese medicine influences these mechanisms. Investigating these biochemical pathways could uncover new potential targets for therapeutic interventions and medications.
To conclude, the findings of this research indicate that quercetin may be potential therapeutic medicine for psoriasis and may be associated with its modulation of the PI3K/AKT/GLUT1 signaling pathway. The potential of quercetin for future investigations and practical medical uses appears to be quite promising. Our research represents a significant advancement in the field of the treatment of psoriasis with Traditional Chinese Medicine and offers a novel perspective on finding a new bioactive ingredient for the treatment of psoriasis. Future investigations will delve deeper into how its therapeutic effects are mediated.
The datasets used during the current study are available from the corresponding author on reasonable request.
YPB—Conceptualization, Supervision, and Funding Acquisition. JM—Investigation, Writing - original draft. FFW—Data curation, Writing - review and editing. LW and YW—Validation. NL—Methodology. DDW and WBJ—Visualizaiton. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The research was approved by the Animal Ethics Committee of the Clinical Research Institute of China-Japan Friendship Hospital (approval NO. 190109)and all experiments were performed following the Animal Ethics Code and Operational Guidelines. All efforts were made to minimize animal suffering and discomfort in compliance with the 3R principles.
Not applicable.
This work was supported by the National Natural Science Foundation of China (Grant No. 81803135, 81904210).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






