1 Nanjing University of Chinese Medicine, 210029 Nanjing, Jiangsu, China
2 Department of Gynecology, Kunshan Hospital of Traditional Chinese Medicine, Kunshan Affiliated Hospital of Nanjing University of Chinese Medicine, 215300 Kunshan, Jiangsu, China
3 Department of Gynecology of Traditional Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, 210029 Nanjing, Jiangsu, China
†These authors contributed equally.
Abstract
Polycystic ovary syndrome (PCOS) is a prevalent gynecological endocrine and metabolic disorder in women, with an incidence rate of 10–13%. The etiology of PCOS is multifaceted, involving genetic predisposition, environmental influences, lifestyle factors, and endocrine metabolic dysregulation. Iron, a critical mineral, not only plays a role in regulating female physiological functions and the progression of PCOS but also requires careful management to avoid deficiency. However, excess iron can trigger ferroptosis, a form of nonapoptotic cell death characterized by the accumulation of lipid peroxides. While numerous studies have explored ferroptosis in patients with PCOS and animal models, the precise mechanisms and therapeutic implications remain inadequately understood. This review seeks to elucidate the pathophysiology of PCOS and the contributory factors of ferroptosis. Additionally, we examine the diverse manifestations of ferroptosis in PCOS and evaluate its role. Furthermore, we introduce ferroptosis-related traditional Chinese medicines that may enhance the understanding of PCOS pathogenesis and aid in the development of targeted therapies for ferroptosis in PCOS.
Keywords
- polycystic ovary syndrome
- ferroptosis
- traditional Chinese medicine
- therapies
Polycystic ovary syndrome (PCOS), a complex disorder, is the primary cause of infertility and metabolic disturbances in women of reproductive age. Globally, the prevalence and incidence rates of PCOS in 2019 were 1677.8 and 59.8 per 100,000, respectively, reflecting increases of 30.4% and 29.5% since 1990 [1]. Diagnosis of PCOS is established when a woman presents with two or more of the following criteria: clinical and/or biochemical hyperandrogenism, anovulation or ovulatory dysfunction, and the presence of multiple ovarian follicles, known as polycystic ovary morphology [2]. PCOS can affect women across various age groups, with adolescents and those of reproductive age primarily exhibiting menstrual irregularities, acne, hirsutism, and infertility, while older women are more susceptible to diabetes, hyperlipidemia, and cardiovascular diseases [3]. Given its unclear etiology and wide-ranging clinical manifestations, there is no definitive cure for PCOS; symptomatic management remains the cornerstone of treatment, focusing on lifestyle modifications and pharmacotherapy [4, 5, 6]. The disorder’s unknown origin and lack of a cure significantly impact patients’ quality of life, fertility, and long-term health outcomes.
Iron, an essential trace element, is crucial for maintaining overall health, yet it must be carefully managed to prevent deficiency. Ferroptosis, a form of regulated cell death, was first identified in cancer cells induced by erastin, as described by Dixon et al. [7]. The mechanistic understanding of ferroptosis has advanced rapidly (Fig. 1), particularly following the discovery of the cystine-import-glutathione-glutathione peroxidase 4 (GSH-GPX4) pathway, which highlighted phospholipid hydroperoxides (PLOOHs) as key executors of ferroptosis. Recent research has also identified GPX4-independent ferroptosis surveillance pathways. The mechanisms underlying PLOOH synthesis, particularly the role of polyunsaturated fatty acids (PUFAs) as PLOOH precursors, have been extensively studied [8]. Increasing attention is being directed towards the role of ferroptosis in PCOS [9, 10, 11, 12, 13]. To provide clarity on this emerging area, it is necessary to summarize and critically assess the existing research, identifying key findings, ongoing challenges, and areas for future investigation. This review aims to elucidate the ferroptosis-related mechanisms involved in PCOS pathogenesis and treatment, while also examining traditional Chinese medicines targeting ferroptosis as potential therapies for PCOS.
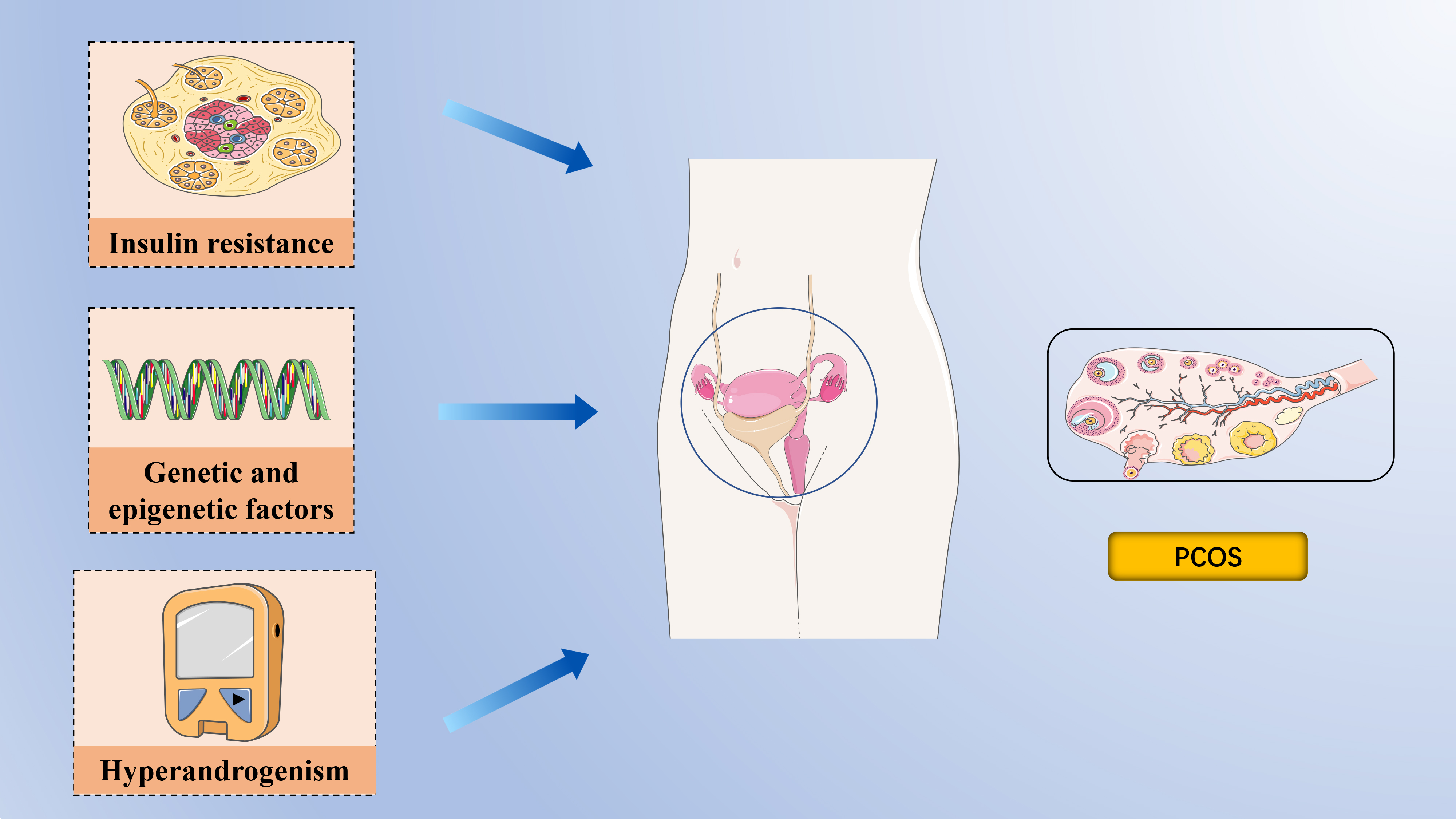
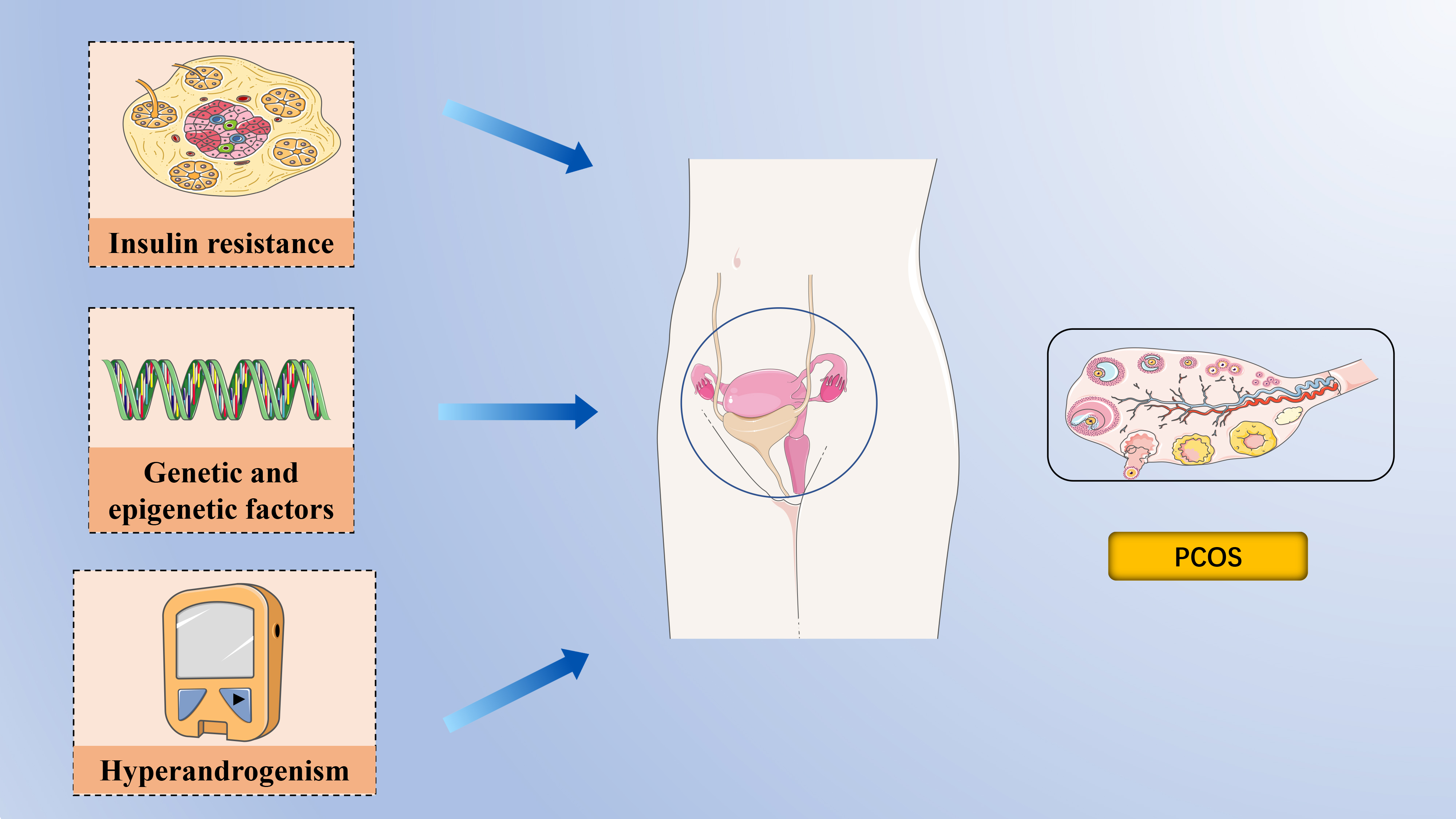 Fig. 1.
Fig. 1. The pathophysiology of polycystic ovary syndrome. Epigenetic and transcriptomic signatures have been identified in both daughters and sons of women with polycystic ovary syndrome (PCOS), supporting the role of epigenetic inheritance alongside genetic factors in the syndrome. Insulin Resistance is a central feature of PCOS, and the consequent hyperinsulinemia impairs glucose utilization, reduces follicular glucose metabolism, diminishes energy supply to follicles, and ultimately hampers follicular growth and development. Hyperandrogenemia is a hallmark of PCOS and a critical focus during clinical evaluation. Created with PowerPoint (Microsoft Corporation, Redmond, WA, USA).
Despite being the most prevalent endocrinopathy in women of reproductive age, the factors contributing to the development of PCOS remain poorly understood (Fig. 1).
Genetic studies have significantly advanced our understanding of PCOS, shedding light on various diagnostic criteria, gender-specific manifestations, reproductive potential, and mental health implications. Initial genome-wide association studies (GWAS) on Han Chinese and European women identified 16 loci associated with PCOS [14]. Further, Day et al. [15] provided the first genetic evidence linking PCOS with male phenotypes, identifying 14 loci, including novel ones such as microtubule-associated protein, RP/EB family, member 1 (MAPER1), zinc-finger and BTB domain-containing protein 16 (ZBTB16), and plasminogen receptor (PLGRKT). The extensive involvement of PCOS in multiple genome-wide association studies has facilitated the use of Mendelian randomization (MR) analyses to pinpoint potential causal factors of the syndrome[16]. MR studies assess causality by leveraging genetic variants associated with specific exposures, thus minimizing the confounding factors often present in epidemiological studies [17, 18]. According to MR findings, PCOS is associated with high body mass index (BMI), insulin resistance, elevated fasting insulin levels, and reduced levels of sex hormone-binding globulin (SHBG) [15, 16, 19, 20, 21]. Moreover, genetic variants linked to delayed menopause in women appear to be associated with PCOS [22].
Over the past decade, growing evidence has highlighted alterations in epigenetic programming in women with PCOS. DNA methylation has been documented across various cell types, including peripheral mononuclear blood cells, ovarian tissue, granulosa cells, skeletal muscle, umbilical cord blood, and endometrium [23]. An epigenetic signature linked to PCOS has been detected in the umbilical cord blood of neonates born to mothers with PCOS, predisposing these offspring to reproductive, metabolic, and neuropsychiatric conditions [24, 25, 26, 27]. Notably, PCOS-like traits have been observed in the offspring of mice without direct exposure to dihydrotestosterone or anti-Mullerian Hormone (AMH) during pregnancy, suggesting the possibility of epigenetic inheritance [14, 28]. The transgenerational transmission of reproductive and metabolic phenotypes in mice is associated with transcriptional changes and mitochondrial function in oocytes, DNA methylation in ovaries, and alterations in small non-coding RNA in sperm, indicating that PCOS may be transmitted through epigenetic modifications in germ cells [14]. Interestingly, epigenetic and transcriptomic signatures have been identified in both daughters and sons of women with PCOS, further supporting the role of epigenetic inheritance alongside genetic factors in the syndrome [22, 24].
Hyperandrogenemia is a hallmark of PCOS and a critical focus during clinical evaluation. In PCOS, androgen secretion is often prematurely elevated, contributing to early-onset insulin resistance [29]. Disruptions in lipid metabolism within visceral adipocytes are a well-known cause of insulin resistance; however, androgen overexposure can independently and directly induce insulin resistance [30, 31, 32, 33, 34]. Under the influence of follicle-stimulating hormone (FSH), the ovarian granulosa cells convert androgens into estrogens, yet in most patients with PCOS, elevated androgen levels persist, impacting multiple organ systems [35]. Low levels of sex hormone-binding globulin (SHBG) further exacerbate the condition by increasing the levels of free testosterone and androstenedione, which are readily available to bind to androgen receptors in various tissues [36].
Insulin resistance (IR) is characterized by a diminished ability of insulin to facilitate glucose uptake and utilization, leading the body to compensate by secreting excessive insulin, resulting in hyperinsulinemia to maintain stable blood glucose levels [37, 38]. IR is a central feature of PCOS, and the consequent hyperinsulinemia impairs glucose utilization, reduces follicular glucose metabolism, diminishes energy supply to follicles, and ultimately hampers follicular growth and development [39, 40]. Studies have investigated the cellular and molecular mechanisms underlying insulin action and glucose uptake in insulin-target tissues, such as adipose and skeletal muscle, in both lean and obese PCOS individuals [41, 42]. Although insulin receptor affinities appear similar between PCOS and non-PCOS women, patients with PCOS exhibit reduced insulin binding in adipose tissues, leading to decreased glucose uptake and insulin sensitivity [29]. Additionally,
There is a strong correlation between obesity, metabolic syndrome, and elevated oxidative stress levels, with “obesity and insulin resistance” being the metabolic syndrome component most closely linked to increased oxidative stress [43]. The cardiovascular system suffers when hyperglycemia elevates inflammatory markers and oxidative stress [44, 45]. Chronic inflammation and oxidative stress, in turn, contribute to
Intracellular iron metabolism maintains a delicate equilibrium involving iron uptake, distribution, storage, utilization, and efflux [8]. Typically, most cells absorb iron through the interaction between transferrin (TF) and its receptor, transferrin receptor 1 (TFR1) [47]. The Fe3+-bound TF forms a complex with TFR1, which is then endocytosed into cellular endosomes. The downregulation of TFR1 enhances cellular tolerance to ferroptosis, whereas its overexpression increases intracellular iron levels, thereby reducing this tolerance [48]. The reduction of Fe3+ to Fe2+ by six-transmembrane epithelial antigen of prostate 3 (STEAP3) contributes to the formation of a labile iron pool (LIP), with Fe2+ subsequently transported by zinc transporter 8/14 (ZIP8/14) or divalent metal transporter 1 (DMT1) [49]. The iron within LIP can be utilized by mitochondria, stored in ferritin, or exported via ferroportin.
In cases of abnormal iron transport, upregulation of TFR1 coupled with downregulation of FTH1 leads to Fe2+ overload [50]. The excessive Fe2+ reacts with hydrogen peroxide, producing hydroxyl radicals through the Fenton reaction, which directly catalyzes lipid peroxide formation and ultimately promotes ferroptosis [51]. Despite being a key feature of ferroptosis, the regulatory mechanisms governing iron overload remain incompletely understood. Disruptions in intracellular iron balance, such as increased iron uptake, diminished iron storage, enhanced ferritinophagy, and restricted iron efflux, can lead to iron overload. This iron excess triggers the Fenton reaction, resulting in the generation of reactive oxygen species (ROS) and the activation of iron-dependent enzymes that accelerate lipid peroxidation, causing oxidative damage to cell membranes and inducing ferroptosis [52].
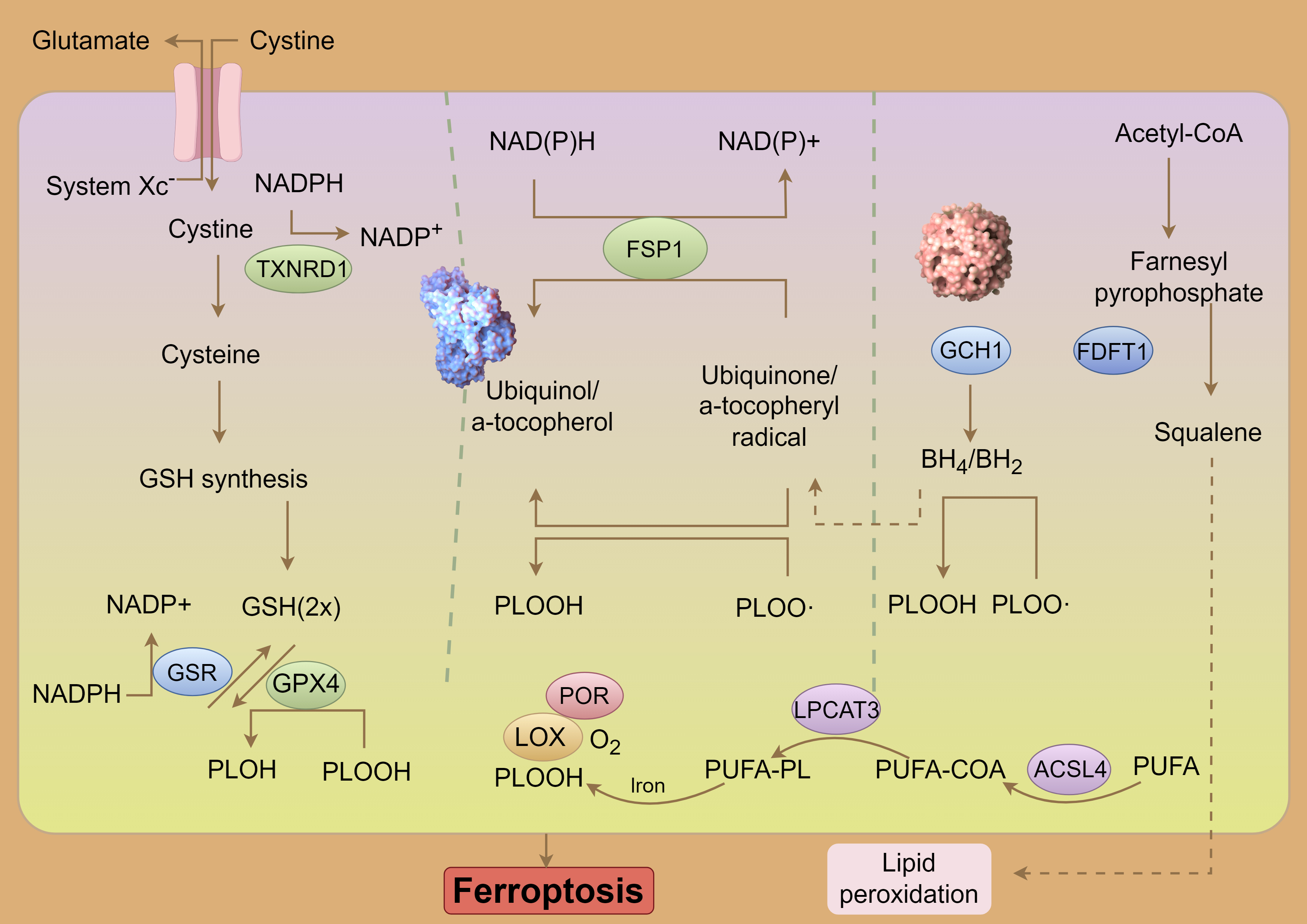
Glutathione (GSH) serves as the primary intracellular antioxidant in mammals, synthesized from glutamate, cysteine, and glycine through the enzymatic actions of glutamine cysteine ligase (GCL) and glutathione synthetase (GSS) [53]. As a critical antioxidant, GSH is predominantly produced by System Xc–, which facilitates the exchange of intracellular glutamate for extracellular cystine [54]. Among the precursors for GSH synthesis, cysteine is considered rate-limiting due to its relatively low intracellular concentration. When GSH levels are rapidly depleted, System Xc– (composed of subunits solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2)) compensates by exporting glutamate and importing extracellular cystine into cells [7]. A deficiency of SLC7A11 in mice results in cell death, which can be mitigated by ferroptosis inhibitors; however, paradoxically, overexpression of SLC7A11 also induces ferroptosis, suggesting that SLC7A11 acts as an executor of ferroptosis [55]. In several cancer types, SLC7A11 expression is markedly upregulated, thereby inhibiting ferroptosis and promoting cancer cell proliferation, invasion, and resistance to chemotherapy [56]. Specifically, overexpression of SLC7A11 in HeyA8-R cells has been shown to induce apoptosis, inhibit colony formation, and enhance sensitivity to paclitaxel. Conversely, low SLC7A11 expression in patients with ovarian cancer is associated with poorer overall survival, progression-free survival, and post-progression survival [57]. GPX4, a well-characterized ferroptosis suppressor, plays a pivotal role in mitigating the accumulation of iron-dependent lipid peroxides, thereby regulating various physiological processes [58]. GPX4 functions by reducing lipid hydroperoxides to less harmful lipid hydroxides in a GSH-dependent manner, effectively preventing ferroptotic cell death triggered by lipid peroxidation. Mice with a Gpx4 knockout exhibit embryonic lethality, while those with conditional Gpx4 knockout display immune system dysfunction, liver abnormalities, kidney issues, and neurological disorders [59]. In summary, intracellular depletion of GSH or inactivation of GPX4 can lead to ferroptosis, underscoring the importance of maintaining metabolic homeostasis within the Xc–/GPX4 system to prevent ferroptosis (Fig. 2).
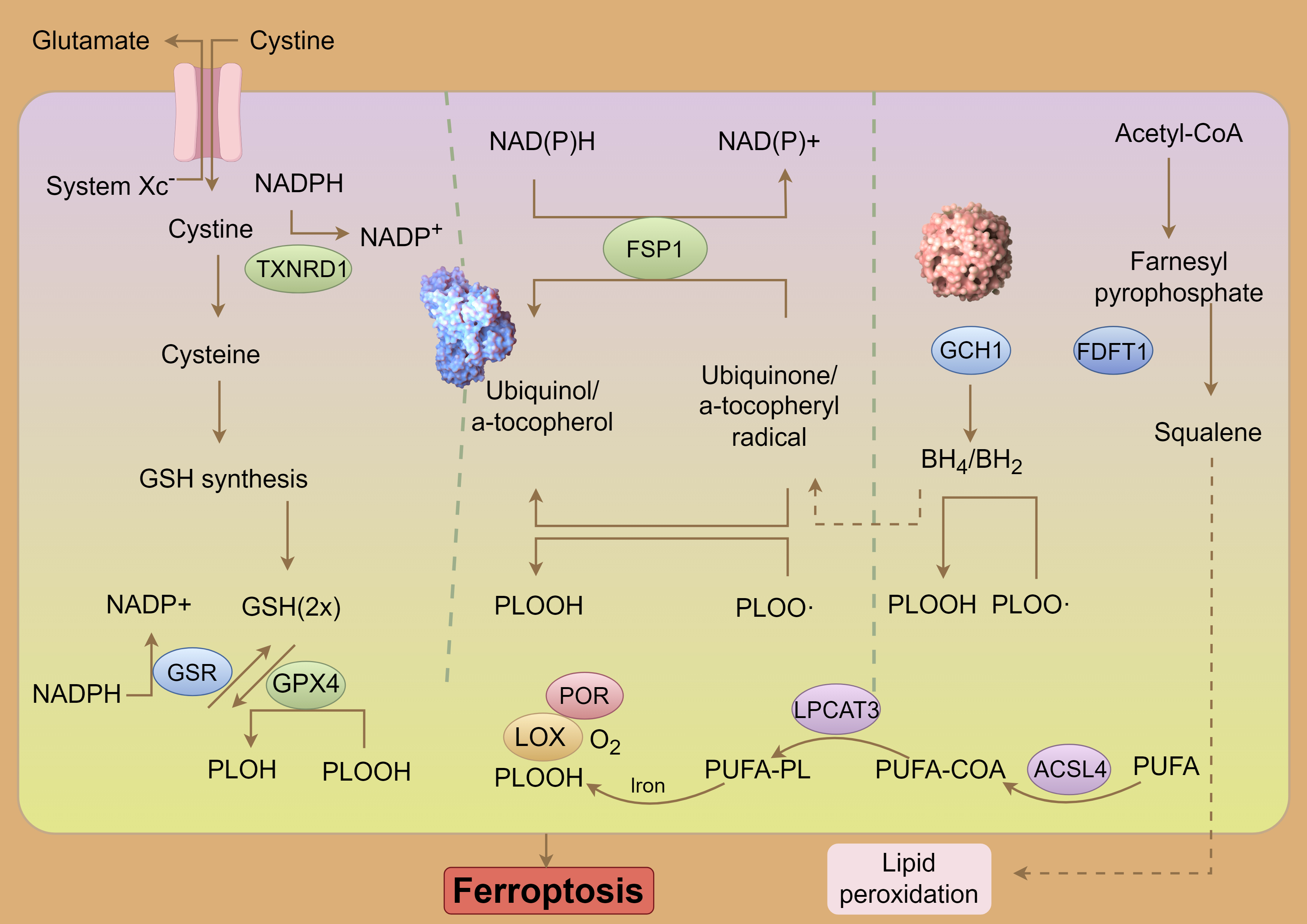 Fig. 2.
Fig. 2. System Xc–-GSH-GPX4 pathway and ferroptosis. NADPH, Nicotinamide Adenine Dinucleotide Phosphate (Reduced); NADP, Nicotinamide Adenine Dinucleotide Phosphate; TXNRD1, Thioredoxin Reductase 1; GSH, Glutathione (Reduced form); PLOH, Phospholipid Alcohol; PLOOH, Phospholipid Hydroperoxide; PLOO-, Phospholipid Peroxyl Radical; FSP1, Ferroptosis Suppressor Protein 1; POR, Cytochrome P450 Oxidoreductase; LOX, Lipoxygenase; PUFA-PL, Polyunsaturated Fatty Acid-Containing Phospholipid; LPCAT3, Lysophosphatidylcholine Acyltransferase 3; COA, Coenzyme A; ACSL4, Acyl-CoA Synthetase Long-Chain Family Member 4; PUFA, Polyunsaturated Fatty Acid; GCH1, GTP Cyclohydrolase 1; FDFT1, Farnesyl-Diphosphate Farnesyltransferase 1; BH4/BH2, Tetrahydrobiopterin/Dihydrobiopterin; GSR, glutathione-disulfide reductase. By Figdraw (https://www.figdraw.com/#/).
Lipid peroxidation is a key initiator of ferroptosis, where PUFAs undergo oxidation by reactive oxygen species (ROS) through enzymatic or nonenzymatic reactions, resulting in the formation of lipid peroxides [60]. Due to the presence of unstable carbon-carbon double bonds, PUFAs are particularly susceptible to lipid peroxidation, thus playing a significant role in the induction of ferroptosis [61]. Free PUFAs are first esterified by acyl-CoA synthetase long-chain family member 4 (ACSL4) and then incorporated into membrane phospholipids by lysophosphatidylcholine acyl-transferase 3 (LPCAT3). Once integrated into the membrane, they are oxidized by lipoxygenases into toxic lipid peroxides. The activities of ACSL4 and LPCAT3, which increase the PUFA content in phospholipids, are therefore critical in promoting ferroptosis under various pathophysiological conditions.
The ferroptosis signaling network is complex, involving numerous regulatory factors, including transcription factors such as tumor protein 53 (p53), nuclear factor E2-related factor 2 (Nrf2), and AMP-activated protein kinase (AMPK), which modulate the expression of genes involved in ferroptosis. The role of p53 in ferroptosis regulation is particularly nuanced, as it can exert both pro- and anti-ferroptotic effects through distinct molecular mechanisms. p53 has been shown to repress the transcription and non-translational expression of SLC7A11, leading to reduced GSH synthesis and decreased Cys2 uptake, thereby promoting ferroptosis [62, 63]. Additionally, p53 may transactivate the polyamine catabolizing enzyme spermidine/spermine N1-acetyltransferase 1 (SAT1), which in turn induces ferroptosis by increasing the expression of ALOX15 [64]. Nrf2, a redox-sensitive transcription factor, plays a vital role in maintaining cellular redox balance by interacting with antioxidant response elements (ARE) and Kelch-like ECH-associated protein 1 (Keap1) [65]. Through regulating downstream genes, Nrf2 regulates ferroptosis by promoting GSH synthesis, reducing ROS production, and upregulating NADPH synthesis [66]. However, constitutive activation of Nrf2 can enhance tumorigenesis, increase ROS detoxification, modulate the redox state, and confer resistance to many anticancer drugs [67]. In various cancers, Keap1 loss has been implicated in chemoradiation resistance [68]. Specifically, elevated Nrf2 expression may confer resistance to cisplatin—a commonly used chemotherapy drug in head and neck cancer—by modulating ROS production and promoting cancer stem cell formation [69]. AMPK, which monitors cellular energy status, also plays a critical role in regulating ferroptosis. Activation of AMPK through energy stress has been shown to inhibit ferroptosis by influencing redox homeostasis and iron metabolism [70, 71].
Ferroptosis has been implicated in the pathogenesis of PCOS, as evidenced by elevated serum ferritin levels and increased ROS levels in the leukocytes and granulosa cells of the ovary in patients with PCOS [72, 73, 74]. A study involving 257 premenopausal women revealed that serum ferritin levels were significantly higher in patients with PCOS compared to those without the condition, independent of obesity, indicating the presence of iron overload [75]. The ovarian dysfunction in PCOS, leading to delayed menstruation, hypomenorrhea, or amenorrhea, reduces one of the primary pathways for iron loss in women, which is regular menstruation [75]. Moreover, serum ferritin levels have been linked to the severity of menstrual dysfunction, suggesting that iron storage may contribute to the iron retention effects observed in cases of menorrhagia among some patients with PCOS [76]. Additionally, iron overload in PCOS may be exacerbated by compensatory hyperinsulinemia resulting from insulin resistance, which promotes tissue iron uptake while inhibiting iron release from macrophages [77, 78]. This excess iron can negatively impact glucose metabolism, worsening the metabolic abnormalities associated with PCOS, such as increased insulin resistance and heightened luteinizing hormone sensitivity. These metabolic disruptions can further impair ovulatory functions and affect the embryonic implantation process in PCOS [79]. Recent analyses of datasets (GSE155489 and GSE168404), along with real-time quantitative polymerase chain reaction (qPCR) validations involving 33 patients with PCOS and 7 controls, have identified JUN and HMGA1 as critical transcription factors in the context of PCOS [80].
Iron overload has the potential to contribute to the development of PCOS by disrupting redox homeostasis, leading to an increase in ROS production [81]. In patients with PCOS, oocytes exhibit mitochondrial dysfunction, redox potential imbalance, and heightened oxidative stress, as revealed by a targeted metabolomic assay using follicular fluid [82]. GSH and FSH are crucial in regulating follicular apoptosis, with ROS playing a significant role in this process. Inhibition of GSH synthesis has been shown to increase sinus follicular atresia in rats and exacerbate follicular dysplasia in women with PCOS [83]. Moreover, the uptake of iron by granulosa cells via the transferrin receptor leads to substantial ROS production, mitochondrial autophagy activation, lipid peroxidation, and ferroptosis, ultimately inhibiting follicle development [83]. In PCOS, miR-93-5p has been observed to increase apoptosis and ferroptosis by downregulating nuclear factor-kB, elevating ROS and malondialdehyde (MDA) levels, and stabilizing GPX4 expression [84]. Additionally, in mouse PCOS models, fatty acid desaturase 2 (FADS2) has been identified as a ferroptosis-related gene through bioinformatics analysis and experimental validation. Overexpression of FADS2 inhibited ferroptosis-related markers and elevated GSH, GPX4, and TFR1 levels in Human Ovarian Granulosa Tumor Cell Line (KGN) cells. Peroxisome proliferator activated receptor alpha (PPAR-
Mitochondrial dysfunction contributes to or exacerbates hyperandrogenism, insulin resistance, and obesity, leading to disrupted follicular development and negatively impacting the menstrual cycle and reproductive health in women with PCOS [90]. Ferroptosis may also occur in the uterus of pregnant women with PCOS, reducing endometrial receptivity. Transmission electron microscopy has revealed that during pregnancy in women with PCOS, mitochondrial volume is reduced, mitochondrial membrane density increases, mitochondrial cristae are absent, and the outer mitochondrial membrane is ruptured [91]. Mitophagy, triggered by increased ROS during ferroptosis, may further alter mitochondrial function. Increased transferrin receptor protein 1 (TFRC) expression leads to elevated iron content, promoting mitochondrial aggregation and mitophagy through NADPH oxidase 1 (NOX1) translocation to PTEN-induced putative kinase 1 (PINK1). This process results in cytochrome C release into the cytoplasm, ACSL4 activation, lipid peroxidation, and inhibition of follicle development, suggesting that targeting the TFRC/NOX1/PINK1/ACSL4 signaling pathway could be a therapeutic approach for PCOS by modulating folliculogenesis [92].
Traditional Chinese medicine (TCM) offers significant benefits and demonstrates substantial therapeutic effects in treating PCOS, with fewer side effects compared to conventional treatments. TCM has been shown to reduce insulin levels, regulate lipid metabolism, and increase both ovulation and pregnancy rates in patients with PCOS [93]. TCM treatments encompass a broad range of approaches, including syndrome differentiation, classic prescriptions, acupuncture, moxibustion, and the use of single Chinese herbs. The flexibility of TCM formulations allows for adjustments based on individual patient needs, providing a personalized treatment approach.
Baicalein, a flavonoid derived from the roots of Scutellaria baicalensis Georgi [94], has garnered attention for its diverse biological activities, including scavenging oxygen radicals, antipyretic and analgesic effects, anti-inflammatory properties, inhibition of neovascularization, anti-tumor activity, and antibacterial and antiviral effects [95, 96, 97, 98, 99, 100, 101]. In a study using a PCOS rat model treated with baicalein, researchers assessed oxidative stress and inflammation levels in serum and ovaries, conducted tissue analyses, and performed RNA sequencing [102]. The findings indicated that baicalein treatment led to reduced oxidative stress, decreased lipid peroxidation, lowered chronic inflammation, and modulated mitochondrial function and ferroptosis in PCOS rat ovaries. Mechanistically, baicalein treatment reversed the downregulation of glutathione peroxidase and ferritin heavy chain 1, suggesting that baicalein could improve PCOS prognosis by reducing oxidative stress and ferroptosis [102].
Berberine, an isoquinoline alkaloid derived from the Chinese herb Rhizoma Coptidis, is well-known for its anti-inflammatory, antibacterial, and antiviral properties [103, 104, 105]. Modern research has highlighted berberine’s effectiveness in preventing and treating organ damage, cardiovascular diseases, and metabolic diseases, with no significant adverse effects reported [106, 107, 108]. In the study [109] involving patients with PCOS and PCOS cell models (human ovarian granulosa cells treated with dihydrotestosterone), berberine was found to modulate Circ_0097636 expression, which was downregulated in PCOS conditions. Berberine treatment partially alleviated the inhibitory effects of dihydrotestosterone on cell proliferation, while promoting apoptosis, inflammation, ferroptosis, and reducing oxidative stress [109]. Furthermore, berberine upregulated Circ_0097636 and SIRT3 expression while downregulating miR-186-5p expression, ultimately ameliorating cell injury and ferroptosis through the regulation of the Circ_0097636/miR-186-5p/SIRT3 pathway [109].
In addition to baicalein and berberine, a review by Malik et al. [110] examined 13 clinical studies on plants and phytochemicals effective against PCOS, some of which have been reported to influence ferroptosis in other diseases (Table 1, Ref. [110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127]). These phytochemicals and herbal drug formulations not only offer alternatives for PCOS management but may also provide synergistic effects when combined with conventional treatments. However, extensive clinical trials are necessary to determine the optimal dosages and treatment durations for these compounds, both alone and in conjunction with other medications, to effectively manage PCOS.
| Subject | Clinical results | Ferroptosis-related mechanisms |
| Curcumin | Decreased fasting plasma glucose dehydroepiandrosterone levels [111] | Act as a ferroptosis inhibitor by suppressing the ROS formation and increasing the HO-1 and Nrf2 expression [112]; |
| Promote ferroptosis via the PI3K/Akt/mTOR pathway in colorectal cancer cell [113] | ||
| Resveratrol | Increased menstruation and decreased hair loss [114] | Inhibit ferroptosis and decelerate heart failure progression by Sirt1/p53 pathway activation [115]; |
| Alleviate diabetic periodontitis-induced alveolar osteocyte ferroptosis by SLC7A11/GPX4 pathway [116]; | ||
| Attenuated high intensity exercise training-induced inflammation and ferroptosis via Nrf2/FTH1/GPX4 pathway [117] | ||
| Quercetin | Decreased testosterone [110] | Alleviate acute kidney injury by inhibiting ferroptosis [118]; |
| Induce ferroptosis in gastric cancer cells by the p-Camk2/p-DRP1 and NRF2/GPX4 Axes [119]; | ||
| Alleviate high-fat diet-induced hepatic lipotoxicity by targeting mitochondrial ROS-mediated ferroptosis [120]; | ||
| Alleviate ferroptosis accompanied by reducing M1 macrophage polarization in neutrophilic airway inflammation [121]; | ||
| Prevent the ferroptosis of oligodendrocyte progenitor cells by inhibiting the Id2/transferrin pathway [122] | ||
| Hesperidin | Decreased waist circumference [123] | Mitigate oxidative stress-induced ferroptosis in nucleus pulposus cells by Nrf2/NF-κB axis [124]; |
| Promote diabetic wound healing by inhibiting ferroptosis by activating SIRT3 [125] | ||
| Catechin | Weight reduction [126] | Play anti-inflammatory effects through mediating ferroptosis [127] |
ROS, Reactive Oxygen Species; HO-1, Heme Oxygenase 1; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; PI3K/Akt/mTOR, Phosphoinositide 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin; SLC7A11/GPX4, Solute Carrier Family 7 Member 11/Glutathione Peroxidase 4; FTH1, Ferritin Heavy Chain 1; p-Camk2/p-DRP1, Phosphorylated Calcium/Calmodulin-Dependent Protein Kinase 2/Phosphorylated Dynamin-Related Protein 1; NF-
PCOS, characterized by insulin resistance and hyperandrogenism, is a significant risk factor for the early onset of type 2 diabetes and cardiovascular disease. This condition has profound reproductive implications, including irregular menstrual cycles, anovulatory infertility, an increased risk of pregnancy complications, and endometrial cancer. Additionally, PCOS is linked to psychological disorders such as anxiety, depression, eating disorders, psychosexual dysfunction, and negative self-image, all of which contribute to a diminished health-related quality of life. One of the critical aspects of PCOS is its connection to fetal reproductive dysfunction, which is closely associated with dysregulated iron homeostasis and a subsequent cascade of ferroptosis. Although various genetic and metabolic mechanisms regulate ferroptosis, current research on ferroptosis regulation in PCOS remains limited, inconsistent, and insufficient. In PCOS rat models, alterations in ferroptosis-related genes, increased levels of glutathione and malondialdehyde, activation of ERK/p38/JNK phosphorylation, decreased glutathione and malondialdehyde levels, and increased iron deposition in the gravid uterus have been observed. Additionally, mitochondria exhibiting electron-dense cristae, a key feature of ferroptosis-related mitochondrial morphology, were noted [90]. Another study linked fetal loss in rats to excessive ROS production in the placenta, mitochondrial dysfunction, and disrupted superoxide dismutase 1 (SOD1) and Keap1/Nrf2 antioxidant responses [128]. Despite these findings, identifying specific ferroptosis-related targets and signaling pathways in PCOS remains challenging. However, it is clear that inhibiting ferroptosis can effectively treat functional disorders associated with this form of cell death. Ferrostatin-1 (Fer-1), a first-generation aromatic amine, has been shown to inhibit ferroptosis and prevent lipid peroxidation accumulation [129]. In PCOS cell models, treatment with Fer-1 successfully inhibited apoptosis, oxidative stress, and ferroptosis [86]. This protective effect may be mediated through the activation of the Tet enzyme and DNA methylation, suggesting that Fer-1 could be a potential therapeutic drug for PCOS. This review aims to elucidate the pathophysiology of PCOS and the contributory factors of ferroptosis (Table 2). Further research into how currently marketed drugs regulate ferroptosis is warranted. Early integration of these drugs into clinical treatment protocols could lead to the development of new therapeutic approaches for managing PCOS.
| The pathophysiology of polycystic ovary syndrome | Epigenetic and transcriptomic signatures have been identified in both daughters and sons of women with PCOS, supporting the role of epigenetic inheritance alongside genetic factors in the syndrome; |
| Insulin Resistance is a central feature of PCOS, and the consequent hyperinsulinemia impairs glucose utilization, reduces follicular glucose metabolism, diminishes energy supply to follicles, and ultimately hampers follicular growth and development; | |
| Hyperandrogenemia is a hallmark of PCOS and a critical focus during clinical evaluation. | |
| Mechanism of ferroptosis | The iron excess triggers the Fenton reaction, resulting in the generation of reactive oxygen species (ROS) and the activation of iron-dependent enzymes that accelerate lipid peroxidation, causing oxidative damage to cell membranes and inducing ferroptosis; |
| Intracellular depletion of GSH or inactivation of GPX4 can lead to ferroptosis, underscoring the importance of maintaining metabolic homeostasis within the Xc–/GPX4 system to prevent ferroptosis; | |
| Lipid peroxidation is considered as an initiation signal of ferroptosis, in which polyunsaturated fatty acids (PUFAs) are oxidized by ROS through enzymatic or nonenzymatic reactions, resulting in lipid peroxides; | |
| The ferroptosis signaling network involves multiple regulatory factors, and transcription factors can modulate downstream genes involved in ferroptosis, such as such as p53, Nrf2 and AMPK; | |
| Lipid peroxidation is a key initiator of ferroptosis, where PUFAs undergo oxidation by ROS through enzymatic or nonenzymatic reactions, resulting in the formation of lipid peroxides. |
PCOS, Polycystic Ovary Syndrome; GSH, Glutathione (Reduced form); GPX4, Glutathione Peroxidase 4; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; AMPK, AMP-Activated Protein Kinase; PUFAs, Polyunsaturated Fatty Acids.
JA, QZ, ZC, QL and XG wrote the first draft and reviewed the design. CX and XFJ performed literature searches and drafted Figures and Tables. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This review was funded by The Traditional Chinese Medicine Science and Technology Program in Jiangsu (MS2021057), The Suzhou Integrated Chinese and Western Medicine Research Fund (SYSD2021207) and Xia Guicheng Gynecology Expert Team of Jiangsu Provincial Hospital of Traditional Chinese Medicine (01201804).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.