1 Department of Oral and Maxillofacial Surgery, University Hospital Regensburg, 93053 Regensburg, Germany
Abstract
Dental follicle cells (DFCs) are dental stem cells that can only be obtained from tooth germs or after extraction of unerupted wisdom teeth. For many years, DFCs have been studied in basic research and preclinical studies in regenerative dentistry, as they are involved in both the development of the periodontium and tooth eruption. Since the first isolation, the number of studies with DFCs has increased. This article summarizes the most important articles of the last five years to provide an overview of current research topics. The focus was on basic research and preclinical research. Basic research includes articles on tooth development and tooth eruption, as well as research into molecular mechanisms during osteogenic differentiation. In addition, articles on preclinical research with DFCs focused on regenerative therapies and immunotherapies are also discussed. These new studies show that DFCs have improved our understanding of periodontal development and regeneration. DFC research is important for the regenerative dentistry of the future; however, preclinical studies indicate that significant progress is still needed before DFCs can be integrated into routine clinical practice.
Keywords
- tissue engineering
- osteogenic differentiation
- tooth development
- immune therapy
- periodontal regeneration
Human dental follicle cells (DFCs) were first isolated and characterized as dental stem cells approximately 20 years ago [1]. Human DFC are special somatic stem cells because they cannot be obtained from the body at any time, but are lost during the development of the tooth when the follicle disappears. They can therefore play an important role in biological studies of tooth development. These multipotent dental stem cells can either be used in basic research for molecular studies under in vitro conditions or in different applications of regenerative dentistry such as tissue engineering. As the number of studies on DFCs has increased over the years, it is important to categorize and summarize recent developments in DFC research. This article has taken on this task and is divided into different chapters: tooth developmental, stem cell culture conditions, senescence, molecular processes of osteogenic differentiation, tissue engineering and immunomodulation/extracellular vesicles.
The dental follicle is an important tooth germinal tissue as it is involved in both tooth root development and tooth eruption. New insights into tooth root development have been gained through single-cell transcriptome analyzes using murine dental follicle cells and their progeny such as cementoblasts and periodontal ligament cells [2]. Ono and co-workers identified parathyroid hormone-related protein (PTHrP) as a specific marker for undifferentiated cells in murine dental follicles [2, 3]. Another possible marker for undifferentiated human dental follicle cells is the fibroblast activation protein-
Another study showed that murine dental follicles differ from incisor or molar tooth germs. In a study published in 2021, He et al. [7] isolated cells from murine tooth germs, which came from either the incisor or molar germ. These cells were cells from the cervical loop, Hertwig’s epithelial sheath and follicular cells, which were isolated from either the incisor (I_DFC) or the molar (M_DFC) from postnatal rats on the seventh day. The study investigated to what extent conditioned medium from dental epithelial cells (cervical loop, Hertwig epithelium), which are likely to control both tooth root formation and tooth eruption, influences the properties of I_DFCs or M_DFCs. Interestingly, there were clear differences. The osteogenic differentiation and mineralization abilities of I_DFCs were stronger than those of M_DFCs. Both cervical loop- and Hertwig’s epithelial sheath cell-derived CM enhanced these abilities of I_DFCs, whereas they showed the opposite effect of M_DFCs. Overall, immunohistochemical studies on tooth germ tissues from mice suggested that I_DFCs expressed markers for bone formation rather than bone resorption relative to M_DFCs. This may be related to tooth eruption, which could control DFCs in tooth germs of molars [7]. In this context, Wang et al. [8] were able to show that bleomycin, which has a known cytostatic effect, can prevent tooth eruption when administered locally to the dental follicle. According to the authors’ conclusion that this outcome does not have a cytotoxic effect, signaling pathways inhibited by bleomycin may provide initial clues as to which factors may play a role in tooth eruption. The molecular mechanism of bleomycin in DFCs is also related to osteogenic differentiation (see below). As suggested by the studies with PTHrP positive DFCs (see above), there are subpopulations of undifferentiated DFCs that differ significantly. It will be important in the future to further characterize these populations and their roles in tooth eruption and periodontal formation.
The relationship between the mesodermal dental germ tissues, the dental follicle and the apical papilla remains unclear in tooth development and has also been an area of research. While stem cells from the apical dental papilla are the actual precursors of odontoblasts and other cell types of the dental pulp/dentin complex, the odontoblastic differentiation or trans differentiation potential of DFC is unclear. However, a new study could help clarify this. This study showed that cells from harmatomatous, hyperplastic calcifying dental follicles had an odontoblastic phenotype [9]. These mineralizing cells expressed typical odontoblast markers such as dentin sialoprotein (DSP) or nestin. While this study suggests an odontoblastic potential of DFCs, another study also demonstrated strong interactions between cells of the dental follicle and the apical papilla. It was shown here that apical papillary cells inhibit the differentiation of DFCs via the Hedgehog signaling pathway [10]. This appears to be related to the secretion of osteoglycin (OGN) by apical papillary cells. The results of these two studies suggest a close relationship and essential interactions between the cells of the dental follicle and the dental papilla.
In recent years, studies have also been carried out into the importance of epigenetic factors in tooth development. One study examined the expression levels of long-non-coding RNAs in dental follicles that came from impacted wisdom teeth [11]. Here, the RNA maternally expressed 3 (MEG3) and non-coding RNA activated by DNA damage (NORAD) were induced in the dental follicles. However, these data do not allow conclusions to be drawn about the molecular mechanisms that led to the cystic lesion of the dental follicle. Another study looked at the chromatin organization of Hox genes [12]. The study examined the methylation of histone proteins and was able to show clear differences that could suggest a causal connection between the expression of HOX genes and the methylation patterns of the promoters. The authors showed this particularly for the promoters of undifferentiated dental follicle cells and alveolar osteoblasts [12]. However, the tight regulation of HOX gene clusters appears to be related to tooth formation and, to a lesser extent, to periodontal development and tooth eruption. One factor for the tight regulation of HOXA2 gene is for example is the long non-coding RNA HOTAIRM1 (see also below for molecular mechanism in osteogenic differentiation) [13]. However, a study on epigenetic factors during osteogenic differentiation of DFCs have become more common in recent years [14].
Research investigating molecular mechanisms should be as important in the study of tooth development as studies of cell populations outlined above. The work on molecular mechanisms is probably even more important, as it must be remembered that cells, especially after isolation, are very heterogeneous and can have very different properties. Examples of this are given in the next chapter.
An important topic of research with dental follicle cells is isolation and cell culture. A major concern with the initial isolations of DFCs was the extent to which contamination with epithelial cells might have occurred [15]. These could, for example, be remnants of Hertwig epithelial cells. This concern is entirely justified, as epithelial cells can often be seen in histological specimens of dental follicles [16]. However, there has always been interest in epithelial cells from the dental follicle, with a major focus on the question of the extent to which these epithelial cells can be converted into mesenchymal cells (EMT) not only in order to obtain another source of periodontal progenitor cells. Interestingly, in recent years a working group was able to isolate epithelial cells from human dental follicles [17, 18]. They cultured them in serum-free medium. Interestingly, EMT took place in the presence of serum and the cells became ectomesenchymal DFCs. It remains unclear with which oral epithelial cell type these isolated cells can best be compared and what the proportion of native epithelial cells is in DFC cultures after EMT, since DFC culture media are mainly not serum-free.
In this context, it should be noted that the cell culture conditions have a major influence on the properties of the DFCs, such as the surface properties and the cell culture medium. The plasticity of the cells should also not be forgotten, which, in combination with factors that cannot be further determined, determines the selection of stem cells that define the quality of the DFCs.
Another topic of stem cell culture is cryopreservation. The quality issue of cryopreserved cells compared to freshly isolated DFCs has long been unclear, but two recent publications addressed this issue [19, 20]. Interestingly, both studies came to different conclusions. While Raik et al. [19] showed that dental stem cells, including DFCs, are very robust and do not require complicated preservation protocols, AlHindi and Philip [20] showed a large variation in osteogenic differentiation potential in fresh and cryopreserved samples. Although cryopreservation for research purposes generally does not cause major problems, future research projects in the field of cell therapies and/or stem cell banking should examine this topic in more detail.
In recent years there have also been some studies that have compared the differentiation potential of DFCs with other mesenchymal stem cells, particularly from dental tissue. Qu et al. [21] compared the osteogenic differentiation of DFCs with dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), and alveolar bone derived mesenchymal stem cells (ABMSCs). While DFCs showed higher proliferation and apoptosis rate, ABMSCs and PDLSCs showed the highest osteogenic ability, followed by DPSCs. DFCs had the lowest potential [21]. In contrast to this study, Perczel-Kovách et al. [22] and coworkers showed that DFCs, PDLSCs, and DPSCs had similar functional osteogenic differentiation capacities, although their expression profiles of key osteogenic markers showed significant differences. This study also showed that the expression of stem cell markers such as STRO-1 should not necessarily be used to assess the quality of stem cells, but rather functional studies should be carried out [22]. Petrescu et al. [23] were able to achieve similar results in a comparison with DPSCs. They showed that the expression of osteogenic markers was higher in DFCs than in DPSCs after differentiation [23]. On the other hand, another study showed that DPSCs have an excellent potential in biomineralizing cells, which can be further enhanced by 17
Finally, a new comparative proteome study has also been published in recent years. The proteome of DFCs was compared with that of closely related stem cells of the apical papilla (SCAP) [26]. The authors found that 12 proteins were significantly upregulated and 4 were significantly downregulated, and concluded that the high expression of cluster of differentiation (CD)13 and Myristoylated alanine-rich C-kinase substrate (MARCKS) could explain that DFCs have a higher proliferation ability than SCAP [26]. However, such generalizations should always be treated with caution, as there are differences within different isolations of stem cell cell-lines. For example, there may be differences between stem cells that come from the same donor or even from the same cell culture [27, 28]. Moreover, we were also able to show that, for example, a higher endogenous expression of PTHrP is associated with greater osteogenic differentiation potential in DFCs [6].
Cellular senescence is a problem in stem cell therapy and needs to be controlled and understood. Induction of cellular senescence in DFCs reduces typical features of somatic stem cells such as osteogenic differentiation or cell proliferation [29], but the actual cause of senescence is still unclear and a major focus of DFC research [30]. One study showed that senescence is induced more quickly in pre-senescent cells with shortened telomeres than in comparably old cells with longer telomeres [31]. Interestingly, P53, which is a well-known cell cycle regulator protein and a marker of cellular senescence, is not associated with the induction of cellular senescence in DFCs [32]. In contrast to P53, the cell cycle protein E2F1 is involved in the induction of cellular senescence and it is also discussed to what extent DNA repair plays a role in the induction of senescence [32].
Although a recently published study showed that repair sites for DNA double-strand breaks appear after mild irradiation but do not cause radiation-induced senescence despite cell cycle arrest [33], another study showed that an impaired expression of DNA repair protein DNA-protein kinase (DNA-PK) inhibits cellular senescence [34]. Although DNA-PK contributes to the induction of cellular senescence, inhibition of DNA-PK did not lead to the restoration of senescent DFCs [34]. Rather, inhibition of DNA-PK appears to inhibit osteogenic differentiation and the import of glucose and glycolysis, which is also associated with a suppressed osteogenic differentiation potential [34]. Cellular senescence is associated with increased demand of glycolysis or the “glycolytic metabotype”, which can be induced by activation of 5′adenosine monophosphate-activated protein kinase (AMPK), and decreased autophagy [35]. These last two studies suggest that stimulation (by AMPK inducer metformin) or inhibition of glycolysis (by DNA-PK inhibitor NU7441) are associated with the induction or inhibition of cellular senescence, respectively [34, 35]. Overall, induction of signals that inhibit apoptosis, such as activation of protein kinase B (AKT) [34], and support cell cycle progression, such as the extracellular signal-regulated kinases (ERK) signaling pathway [36], appear to be involved in the induction of senescence.
A recently published study showed how senescence can be prevented [37]. Interestingly 50 mM curcumin (50 mM) inhibited senescence in DFCs, which showed no effect on cell size, but restored the ability to proliferate. Curcumin is supposed to work by downregulating senescence marker P16 and restoring expression of proliferation marker E2F1, among other things. In addition, curcumin also restored the osteogenic differentiation potential in DFCs by inducing osteogenic markers such as Runt-related transcription factor (RUNX2) and osteopontin (OPN) [37]. However, the activated signaling pathways and the “metabolic status” in the treated cells remain unclear. It is also not clear to what extent the results obtained in the studies with DFCs on senescence are reproducible. Interestingly, Dasi et al. [37] suggest that P53 is involved in cellular senescence of DFCs, but they did not contradict previous observation that cellular senescence in DFCs is independent of P53 expression [32]. However, the expression of P53 was induced after curcumin treatment, but experiments after inhibition of P53 showed that this factor did not induce cellular senescence of DFCs [37].
Elucidating the process of osteogenic differentiation is one of the main research topics of DFCs and a comprehensive review article has been published recently [14]. A current model for the molecular mechanism is also presented in Fig. 1. However, this article summarizes the last trends of this research.
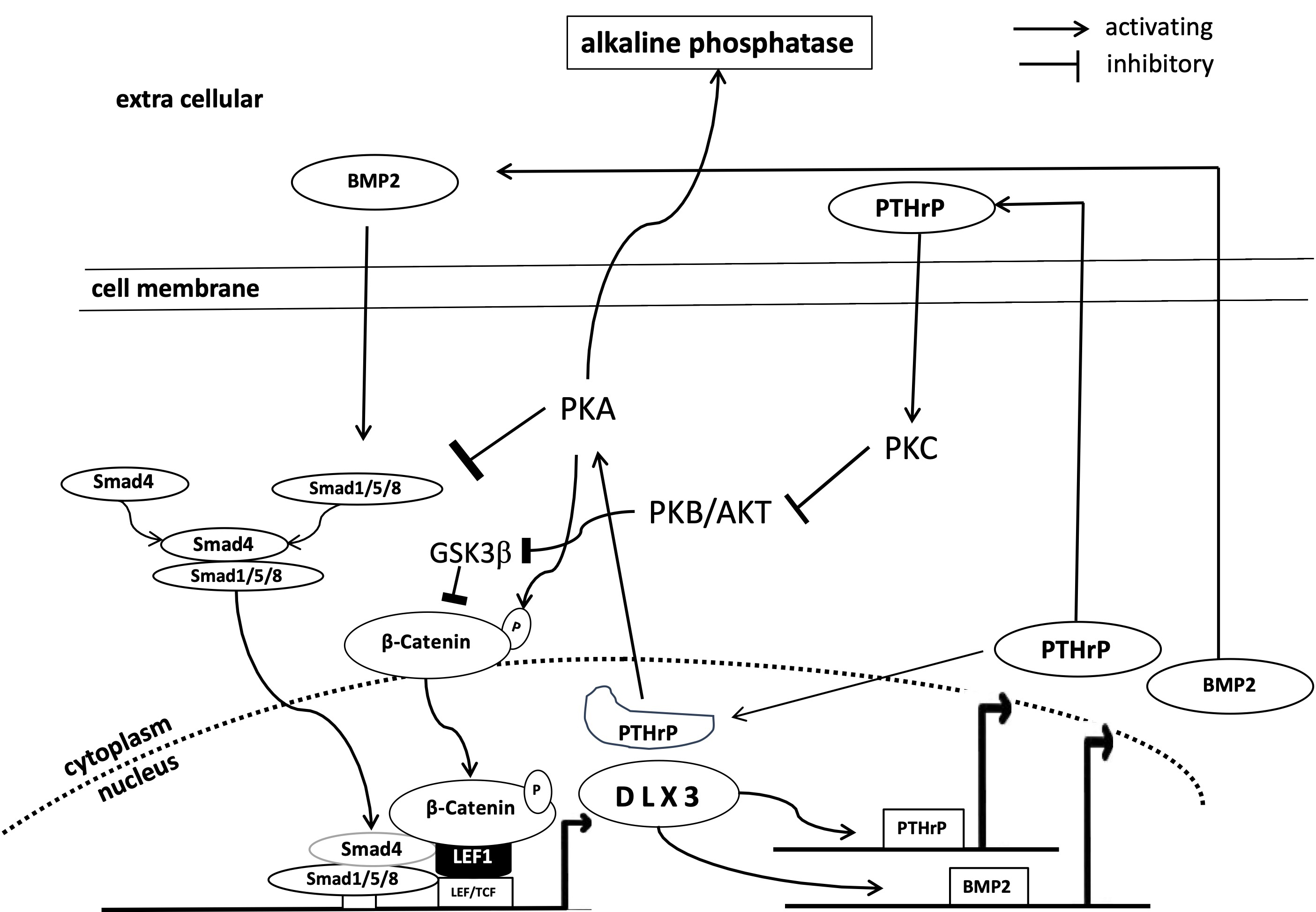 Fig. 1.
Fig. 1. Simplified representation of the DLX3 associated mechanisms of osteogenic differentiation of DFCs. AKT, protein kinase B; BMP2, bone morphogenetic protein 2; DLX, distal-less homeobox transcription factor; GSK, glycogen synthase kinase; LEF, lymphoid enhancer-binding factor; PKA, protein kinase A; PKB, protein kinase B; PKC, protein kinase C; PTHrP, parathyroid hormone-related protein; Smad, small mothers against decapentaplegic; LEF/TCF, lymphoid enhancer factor family/T cell factor; DFCs, dental follicle cells.
One trend is the discovery of more or less specific inducers and inhibitors of the osteogenic differentiation. For example, Wei et al. [38] showed that the expression of periostin supports osteogenic differentiation and the maintenance of cell viability in an inflammatory microenvironment. Periostin appears to be involved in the suppression of cellular stress, which, as the authors have shown, has a negative effect on differentiation. Cellular stress is caused by oxidative stress, among other things, and reactive oxidative species (ROS) inhibit osteogenic differentiation of DFCs [39]. However, a study with N-acetylcysteine, which has an antioxidant effect, have shown that in the right dosage it can also improve cellular properties, particularly osteogenesis and senescence [40]. The authors showed that this positive effect on the osteogenic differentiation is mediated via AKT, which has already been shown to be involved in the differentiation of DFCs [41]. Moreover, a natural product can also influence the differentiation of DFCs. A study showed that puerarin, an isoflavone glycoside, promoted osteogenic differentiation of rat DFCs via the activation of the nitric oxide pathway [42]. On the other hand, there are also molecules or proteins that have an inhibitory effect on osteogenic differentiation. For example, antidiuretic hormone inhibits osteogenic differentiation of DFCs via V1a receptors and a phospholipase C-associated signaling pathway, resulting in increased cytoplasmic calcium concentration and inhibition of mineralization [43]. Interestingly, the known inhibitory effect of bleomycin on tooth eruption (see above) could also be attributed to the inhibition of osteogenesis in DFCs via activation of the Transforming growth factor (TGF)-
Another study indicates that AMPK and the downstream activated process of autophagy inhibit osteogenic differentiation of human dental follicle cells [45]. These processes are particularly activated when the cell is lacking energy and indicate that hunger signals disrupt differentiation. In line with this hypothesis, another study was able to show an increased energy supply through glycolysis and oxidative phosphorylation during differentiation [46]. It can therefore be assumed that an increased energy level and/or processes associated with it play an essential role in osteogenic differentiation and that manipulation of regulatory proteins of this process, such as AMPK, inhibits osteogenic differentiation [45].
PTHrP, which has been studied for many years, is important for osteogenic differentiation (Fig. 1) because it connects important parts of the osteogenic differentiation machinery (bone morphogenetic protein (BMP)-pathway, WNT-pathway and AKT and protein kinase C (PKC)). A negative feedback loop has been demonstrated between PTHrP and the BMP signaling pathway through which PTHrP and the osteogenic differentiation are induced [47, 48]. It has also been shown that the nuclear localized form of PTHrP, which is highly associated with the osteogenic differentiation potential of DFCs [6], is also involved in the activation of protein kinase A, which induces the expression of DLX3 and downstream the osteogenic differentiation [49]. On the other hand, another study seems to show that the expression of the receptor of PTHrP (PTH1R) also positively influences osteogenic differentiation [50]. Important in this context is a study that showed that PTHrP is involved in the expression of protein kinase C, which regulates the activation of
Other factors have also been found that are probably not directly related to PTHrP. Examples are the transcription factors nuclear factor 1 C-type (NFIC) or ALF transcription elongation factor 4 (AFF4), which promote the proliferation and osteogenic/cementogenic differentiation of DFCs [54, 55]. Moreover, endosomal sorting complexes vacuolar protein sorting 4 homolog B (VPS4B) [56], gap junction communication membrane protein Connexin 43 (CX43) [57], ion channel protein transient receptor potential melastatin 7 (TRPM7) [58] and the mechanosensitive ion channel Piezo1 [59] were discovered to be involved in the osteogenic differentiation of DFCs. However, the context of the underlying mechanisms is largely unknown, so PTHrP and the PTHrP-related signaling pathway have been and will likely continue to be the most fruitful targets for exploring the molecular mechanisms of osteogenic differentiation of DFCs.
In the investigation of the molecular mechanisms of differentiation, another focus is on nucleic acids such as non-coding RNAs associated or proteins associated with epigenetics. The long non-coding RNA HOXA transcript antisense RNA, Myeloid-Specific 1 (HOTAIRM1) has been mentioned earlier in this article to regulate the expression of homeobox gene HOX2A and to be involved in the osteogenic differentiation of DFCs [13]. A recent study showed that HOTAIRM1 upregulates the oxygen-sensing histone demethylases lysine demethylase 6B (KDM6A/B) and inhibits the methyltransferase enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) in a hypoxia inducible factor 1 subunit alpha (HIF-1
All studies in this section show that the mechanism of osteogenic differentiation is far from being completely understood. However, it can be assumed that with the help of individual key proteins or signaling pathways, an understanding can be achieved in the near future that will enable targeted control of osteogenic differentiation. Key proteins could be PTHrP and PKC. It is difficult to assess whether these key proteins or its pathways have potential effects on clinical application such as supporting bone regeneration. There are substances, such as the PKC inhibitor Göttingen (GÖ)6976, which, based on in vitro study [52], could be well suited for bone regeneration. However, osteogenesis is a complex process that is slowly becoming better understood, but not yet in all its complexity. In order to understand this precisely, thorough basic research is still required.
Tissue Engineering is another major DFC research topic. In tissue engineering, stem cells are combined with biomaterials to obtain functional organs for transplantation under ex vivo conditions. One problem is obtaining enough cells for therapy. Suitable carriers were therefore sought that would support the mass cultivation of multipotent stem cells. A previous study showed that one product could be nanofiber microspheres (NFM), which support the proliferation, metabolic activity and differentiation of DFCs [63]. Another possibility is an agarose-based spheroid culture. This cell culture method increased the stem cell capacity and also promoted the differentiation potential of DFCs [64]. The future will also show to what extent the formation of so-called organoids can support stem cell cultivation for regenerative therapy [65].
For bone tissue engineering stem cells are not only combined with biomaterials but growth factors are also required. However, biomaterials are the main subject for bone tissue engineering research. Although there are different topics the main goal is the compatibility of biomaterials and stem cells. In a current study, E et al. [66] combined a nanohydroxyapatite/collagen/poly(l-lactide) scaffold with DFCs and recombinant human BMP2. With this combination, DFCs showed better osteogenic differentiation ability than alveolar marrow-derived mesenchymal stem cells, demonstrating the potential of DFCs for alveolar bone regeneration. In a more sophisticated study, the micromechanical compatibility between cell and substrate was investigated to achieve energetically favorable mechanotransduction that guides the stem cell [67]. The results of this study suggest that maximal mechanical interaction can only occur when cell stiffness and substrate stiffness (local scaffold stiffness) are comparable, which is important for stem cell proliferation or differentiation. Authors of this study used atomic force microscopy in combination with a cell to investigate the mechanical properties [67]. Another study used a real-time bioimaging system to investigate the success of tissue formation under in vivo conditions [68]. Interestingly, a previous study of this group focused on 3-dimensional (3D) culture of DFCs on biomaterials for bone regeneration. They were able to show that 3D scaffolds loaded with DFCs cultured under dynamic conditions showed higher tissue growth and stronger osteogenic differentiation than dental pulp cells [69]. Two other studies showed that stem cell properties of DFCs benefit from low-intensity pulsed ultrasound [70] or de-differentiation of already differentiated cells [71]. Moreover, xenogeneic dentin matrix [72] and a lyophilized, platelet-rich fibrin that forms a composite with gelatin and bioadhesive bone cement [73] were evaluated as scaffolds for biomineralization and for the regeneration of alveolar bone defects. These studies demonstrate again that advances that increase our understanding of the cultivation and differentiation of DFCs will bring us closer to the goal of successful bone tissue engineering.
Further studies have focused on the regeneration or tissue engineering of the periodontium. One problem in the differentiation of DFCs is the oxidative stress that is generated due to the formation of an extracellular matrix [40, 74]. A study by Zhang et al. [74] attempted to solve the problem and their results suggest that N-acetylcysteine can significantly protect the viability and stem cell property of DFCs under oxidative stress and achieve better and longer lasting effects in bioroot grafts. Interestingly, in a subsequent study on periodontal ligament regeneration, the group of Lan et al. [75] showed that administration of rosiglitazone (RSG), an agonist of the adipogenic transcription factor peroxisome proliferator activated receptor gamma (PPAR-
In addition to dental therapies significantly more attempts have been made to use DFCs for tissue engineering. DFCs are also being investigated for the regeneration of nerve tissue [78], cartilage [79, 80], tendons [81] and wound healing [82]. However, these studies are still at an early stage and it is unclear to what extent DFCs are actually suitable for such treatments. The studies mentioned are only preliminary results and need to be confirmed by further similar studies. It is important to note that basic research will be particularly important to better understand the key molecular processes required to generate tissues from non-dental tissues using DFCs.
The use of dental stem cells such as DFCs for immune modulation is an important goal of current pre-clinical and/or clinical research. This stem cell-based treatment is part of immunotherapy and generally weakens immune cells of patients suffering from autoimmune diseases or periodontitis, for example [83, 84]. An obvious application of DFCs is periodontitis and it has recently been shown in pre-clinical experiments that a successful use is not only due to the formation of new tissue, but also to the modulation of immune cells involved in the degradation of periodontal tissues [85]. Extracellular vesicles, which were derived from lipopolysaccharide (LPS) stimulated DFCs, facilitate periodontal regeneration through macrophage reprogramming via the formation of M2 macrophages, which, for example, suppress bone resorption by osteoclasts [85]. Moreover, Wei et al. [86] demonstrated a possible mechanism. They showed that recombinant periostin, which is matricellular protein and highly expressed in transplanted DFCs, facilitates this macrophage reprogramming through the integrin-
It should be noted that this immunotherapeutic aspect in research with DFCs has increased in recent years. This topic also includes work with extracellular vesicles that could be obtained from cell cultures with DFCs. In continuation of previous studies on immunomodulation and periodontal regeneration, Huang et al. [85] recently demonstrated that extracellular vesicles isolated from LPS-preconditioned DFC cultures (see above [85]) inhibit apoptosis in periodontal cells and alveolar bone loss in an animal model of periodontitis [90]. Interestingly, similar results with vesicles from DFCs were also shown by another group, who could particularly demonstrate increased cell proliferation on periodontal ligament (PDL) cells [91]. These new results further demonstrate the versatility of DFC-derived extracellular vesicles for periodontal regeneration. However, while the signaling pathways involved for example in anti-apoptosis and macrophage reprogramming have been at least partially elucidated, the responsible molecules of the extracellular vesicles that trigger these biological processes remain unclear. However, in further experiments, Yi et al. [92] were able to show that the matrix vesicles activate PKC in supporting osteogenesis, which again shows that this kinase plays an important role in the regulation of osteogenic differentiation (see above). Another study showed that bacteria are also direct targets of these small vesicles because they prevent growth and biofilm formation by Porphyromonas gingivalis. They also prevent the adhesion and invasion of gingival epithelial cells, thereby reducing the expression of pro-inflammatory cytokines and bacterial invasion of gingival epithelial cells. This treatment also resulted in less bone loss in animal experiments [93]. Interestingly, isolation of extracellular vesicles does not seem to be absolutely necessary, as a medium conditioned by DFCs can also increase the regenerative capacity of the inflamed dental pulp of rats via a paracrine pathway [94]. The use of DFCs vesicles also does not seem to be absolutely necessary, as vesicles from non-dental stem cells such as adipogenic stem cells also lead to regeneration of periodontal tissue [95]. It will be interesting to see to what extent extracellular vesicles from different stem cells differ and whether there are also similarities that can explain these therapeutic successes.
The research focus with DFCs in recent years has been on basic research and preclinical research. The studies with and on DFCs in recent years have shown that PTHrP is not only an interesting marker for stem cells, but also plays a special role during osteogenic differentiation. In the future, it will be important to further characterize this PTHrP positive cell population and its role in tooth eruption and periodontal formation.
Senescence is also an important research area with DFCs. Even if the results on the development of cellular senescence of DFCs seem to contradict each other, cell cycle and crucial metabolic processes are important keywords for future research projects. Finally, preclinical research is becoming more and more exciting, with not only tissue regeneration but also immunotherapy playing an important role. The use of components of DFCs such as extracellular vesicles is also a groundbreaking development, especially when considering clinical feasibility. These biological products are much easier to characterize and may even be replaced by cell-free products based on new research results in the future. However, extensive research is still needed. The next few years will determine the direction in which DFC-based research and therapies will develop.
The results of the studies summarized here indicate that DFC cell lines are heterogeneous in their properties. However, it is unlikely that these differences are solely due to the isolation method, as previous experiments have shown differences between cell lines despite identical isolation methods. This also applies to different cell lines derived from the same donor [27]. However, it can also be assumed that the isolation method influences the properties of the cell. New methods could help to better understand the properties of DFCs. The development of new technologies such as single-cell RNA sequencing or CRISPR/Cas9 could help to solve open questions in DFC research. The new sequencing techniques could help to answer questions about different subpopulations and also help to develop new concepts for identifying certain (differentiated) cells [96]. Using the clustered regularly interspaced short palindromic repeats/CRISPR-associated 9 (CRISPR/Cas9) method, DFCs could be manipulated in a targeted manner, for example to optimize osteogenic differentiation. These could be important steps towards clinical application. However, studies are also necessary that show a proof of concept of the feasibility of the therapies. So care must be taken to ensure that there are suitable animal models that are very similar to human application in order to obtain approval for clinical trials based on their reliability and safety. For clinical trials, there must also be clear parameters that allow an assessment of success. However, it can be assumed that the first attempts could be disappointing and that there is still a lot to learn here. A new direction in therapy will then be necessary. Basic research in dental cell biology could help here.
CM designed the study, performed the search and analyzed the data, wrote the manuscript. The author read and approved the final manuscript. The author has participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The author declares no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

