1 Department of Urology, Tulane University School of Medicine, New Orleans, LA 70112, USA
2 London Women’s Clinic, W1G 6AP London, UK
Abstract
The prevalence of male infertility attributed to oxidative stress (OS) is a growing concern globally. Traditional methods to treat male infertility have some limitations, including low efficacy and invasiveness. Additionally, assisted reproductive procedures, such as in vitro fertilization and intracytoplasmic sperm injection, are expensive and carry higher risks. These challenges underscore the need for innovative solutions. A multidisciplinary approach is imperative, drawing insights from fields such as reproductive biology, nanotechnology, and clinical research to effectively combat male infertility caused by OS. Recent advancements in nanobiotechnology provide a promising opportunity to tackle male infertility caused by OS. These advancements enable the design and development of nanoantioxidants (nanoAOXs) and drug delivery systems tailored to the male reproductive environment. This review highlights the recent progress in the rational design of nanomaterials, with a specific focus on nanoAOXs for managing male infertility associated with OS.
Keywords
- antioxidants
- nanoantioxidants
- oxidative stress
- infertility
- male
Clinicians define infertility as occurring when a couple cannot conceive after one year of regular, unprotected intercourse [1], which could be attributed to the male, female, or both partners [2, 3]. The World Health Organization (WHO) in its latest report states that around 1 in 6 individuals of reproductive age globally experience infertility [4]. On average, the global prevalence of couple infertility is approximately 9%, with variations observed between developed (3.5% to 16.7%) and less developed countries (6.9% to 9.3%) [5]. In the United States, up to 12% of men encounter fertility issues [6]. Notably, male factors contribute significantly to infertility in 20–30% of cases and up to 50% of couple infertility [7].
Male infertility is a complex condition influenced by a range of factors, including genetic, environmental, lifestyle, and medical factors [8, 9, 10]. Various pathologic causes of male infertility include testicular, pretesticular, and extratesticular hormonal factors [11, 12, 13]. Idiopathic male infertility (characterized by no known identifiable causes) accounts for 30–40% of cases [14].
Oxidative stress (OS) due to elevated production of reactive oxygen species (ROS) is a common risk factor for idiopathic male infertility [15, 16]. Sperm rich in polyunsaturated fatty acids (PUFAs) are particularly susceptible to ROS-induced damage, resulting in reduced motility, count, and increased DNA fragmentation [17]. Antioxidants that neutralize these oxidants play a critical role in maintaining the redox homeostasis of a cell [18]. Antioxidants can be taken as single supplements or in combination, including vitamin A, vitamin C (Vit C), vitamin E (Vit E), carnitine, N-acetyl cysteine, coenzyme Q10 (CoQ10), and lycopene, along with important cofactors such as zinc (Zn), selenium (Se), and folic acid [18]. The application of nanotechnology in the pharmacotherapy of male infertility has shown some benefits, such as improved oral bioavailability, increased stability through the extension of drug half-life, targeted drug delivery, and reduced adverse effects [19, 20, 21]. This review summarizes the current research on OS-related male infertility and the therapeutic potential of nanoantioxidants (nanoAOXs) in improving testicular function and sperm parameters.
Approximately 30–80% of males who struggle with infertility issues are reported to have increased ROS levels in their seminal fluid [16]. Endogenous sources of ROS include leukocytes and abnormal/immature spermatozoa. Exogenous factors are more diverse and encompass environmental pollutants, radiation, infections, inflammation, male genital tract conditions such as varicocele and cryptorchidism, and lifestyle-related factors including tobacco and nicotine use (smoking and vaping), high-fat diets, and alcohol consumption [16, 22]. It is well known that spermatozoa produce ROS at two distinct sites—the sperm plasma membrane and the mitochondria—through the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system and the NADH-dependent oxidoreductase (diaphorase), respectively [23]. ROS, such as hydroxyl radicals, superoxide anion, and hydrogen peroxide, are mainly produced by mitochondria during the oxidative phosphorylation process [24]. Due to unique aspects of sperm structure, such as cell membranes enriched with PUFA [22] and a lack of protective antioxidants in sperm cytoplasm, they are very sensitive to OS. Additionally, the large surface area compared to the limited cytoplasm makes sperm more susceptible to damage. Spermatozoa membranes rich in PUFA could be damaged by ROS through lipid peroxidation (LPO) reactions, leading to the production of mutagenic molecules such as malondialdehyde (MDA) [25]. When in excess, ROS induce sperm DNA fragmentation (SDF) [26] and impair sperm maturation, thereby increasing the risk of recurrent pregnancy loss and preterm delivery [22]. The excessive accumulation of ROS affects sperm mitochondrial function, adenosine triphosphate (ATP) production, and sperm motility [22]. These intracellular changes affect proteins and molecular signaling pathways in sperm, resulting in programmed cell death and apoptosis [27, 28]. Infertile men show significantly elevated levels of apoptotic pathway indicators in their sperm.
Several factors can influence ROS production levels, including leukocyte activation from infection or inflammation, radiation exposure, and toxin exposure [16]. Excessive levels of ROS and reduced natural antioxidants lead to an imbalance, resulting in OS. The ATP outflow from cells and decreased viability can cause sperm morphological defects and impaired motility, which ultimately reduce sperm quality and fertility potential [29, 30]. Studies have shown that sperm lack cytoplasmic enzyme repair systems, leaving DNA damage unrepaired and chromatin poorly packaged, which increases apoptotic pathway activation [30, 31]. OS could increase the percentage of unrepaired single- or double-stranded DNA through direct or indirect pathways. The direct impact of OS on DNA bases is the formation of adducted 8-hydroxy-2-deoxyguanosine (8-OHdG), which eventually induces apoptotic pathways. The indirect effect of OS on DNA damage results in the production of MDA, a mutagenic byproduct of LPO [32]. It has also been demonstrated that reduced antioxidant levels can trigger excessive ROS production in semen [33]. For instance, Vit C or Zn deficiency results in increased SDF and male infertility [34, 35, 36].
Poor bioavailability and low stability of conventional antioxidant formulations have driven the development of advanced drug delivery systems, such as nanoparticles (NPs), nanoemulsions, and liposomes [37]. These innovative systems could easily cross the blood-testis barrier, providing higher concentrations in targeted tissues [38] and ultimately improving drug efficacy with minimal toxicity [39]. Due to their size, shape, surface hydrophilicity or hydrophobicity, elasticity, and surface charge, NPs affect cellular uptake and pharmacokinetics of loaded drugs (e.g., antioxidants or hormones) and increase their efficacy even at lower doses [21, 40, 41]. Upon administration, NPs are absorbed through several pathways, including various forms of endocytosis (clathrin-mediated, caveolae-mediated, and caveolae-independent), cellular uptake processes (phagocytosis, pinocytosis, and micropinocytosis), and direct transport methods (passive diffusion, electroporation, and microinjection) [42, 43, 44]. The intracellular trafficking of NPs is pivotal in determining their ultimate target within cellular components [40] and therapeutic effects [44]. NPs, internalized through endocytosis, become enclosed within endosomes (membrane-lined vesicles) [44]. This trafficking is primarily governed by three types of endosomes (early, late, and recycling) and the Golgi apparatus [43]. The early endosome, which contains endocytosed material, partially fuses with the recycling endosome, while the remnant part is differentiated into the late endosome. This dynamic process is facilitated by cellular microtubules, which play a crucial role in enabling NPs to pass the cell-membrane-nuclei path [42, 43].
One of the antioxidant NP formulations recently investigated is cerium oxide nanoparticles (CeO2NPs). Their increased surface area-to-volume and reversible transition between the two cationic forms of cerium (Ce3+ and Ce4+) effectively neutralize ROS, including hydroxyl and nitric oxide radicals [45, 46, 47]. Notably, this study investigates citrate-coated CeO2NPs, which lead to significant accumulation of antioxidants in mitochondria without inducing an immune response [45]. Furthermore, some metallic NPs containing manganese (Mn), copper (Cu), or iron (Fe) can function like a superoxide dismutase (SOD) enzyme that neutralizes ROS through catalytic mechanisms [48, 49]. In addition, the evaluation of carboxymethyl inulin-coated iron oxide nanoparticles (Fe2O3NPs) has demonstrated high stability and targeted delivery [50]. Conjugated Vit C-NPs, including silica-coated gold (Au) NPs and polymeric micelles, have shown intracellular antioxidative activity at lower concentrations [51].
A substantial body of research has explored the effects of NPs on embryo culture, shedding light on their potential benefit for assisted reproductive technology (ART) [21]. Komninou et al. [52] assessed the effect of melatonin-loaded lipid-core nanocapsules in an in vitro bovine-cultured embryo. The results indicated lower levels of the proapoptotic BAX gene and apoptosis-related CASP3 gene but higher levels of the OS-related catalase (CAT) gene and superoxide dismutase 2 (SOD2) gene. These effects led to reduced OS and apoptotic cell death, ultimately improving embryo quality and indicating enhanced intracellular bioavailability of melatonin [52].
NPs, with their established capabilities in drug delivery, enhanced bioavailability, and reduced toxicity, represent a novel approach that has attracted considerable attention for their potential use in diagnosing and treating male infertility [53]. Based on their activity and chemical structure, antioxidants fall into two distinct categories, enzymatic and nonenzymatic antioxidants [54], which are present in both cellular and extracellular environments [55]. Enzymatic antioxidants, aided by trace elements like Zn, Fe, Mg, and Cu, facilitate the conversion of ROS into hydrogen peroxide, which is subsequently metabolized into water [55]. Several nonenzymatic antioxidants, like Vit C, Vit E, melatonin, curcumin, plant polyphenols, and reduced glutathione, work by neutralizing free radicals and stopping their chain reactions [56]. Although antioxidants play a crucial role in protecting cells from OS damage and show promise in treating male infertility, they have certain limitations. Low solubility and stability represent major limitations, resulting in variable bioavailability [57]. The utilization of nanoAOX enhances durability, increased cellular uptake, and targeted delivery [54].
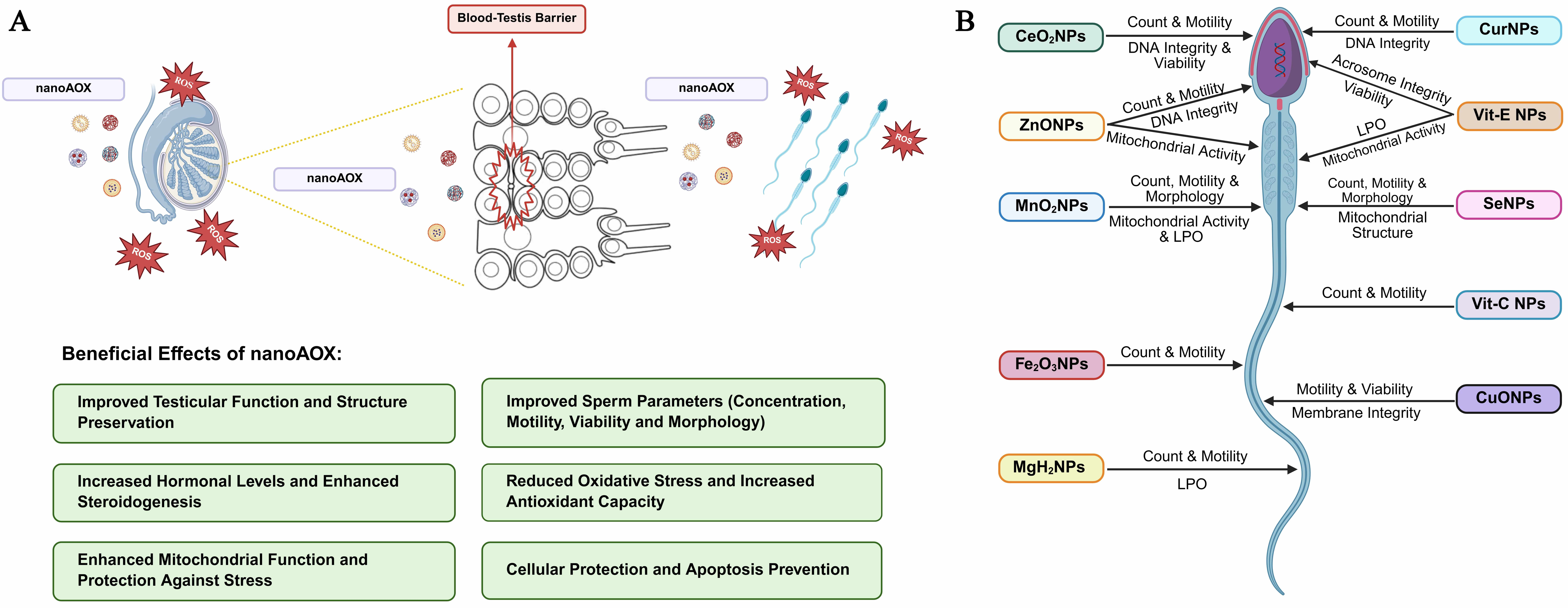
Nanoencapsulated antioxidants are protected from gastrointestinal degradation, thereby improving bioavailability and therapeutic efficacy while reducing cytotoxic damage compared to nonencapsulated formulations. For example, increased active intracellular uptake of polymeric NPs in the small intestine results in enhanced targeted drug delivery [58]. A nanoencapsulated formulation of Vit C, for instance, can prolong the drug’s half-life [59]. Due to their antioxidative properties, NPs are beneficial to sperm function and may improve male infertility (Fig. 1) [19]. Various methods have been used to produce NP-containing antioxidants, including supercritical fluid technology, solvent displacement templating, emulsion/solvent evaporation, and nanoprecipitation [60]. The positive effects of nanoAOXs on male reproductive systems, as documented in animal studies, are presented in Table 1 (Ref. [61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83]).
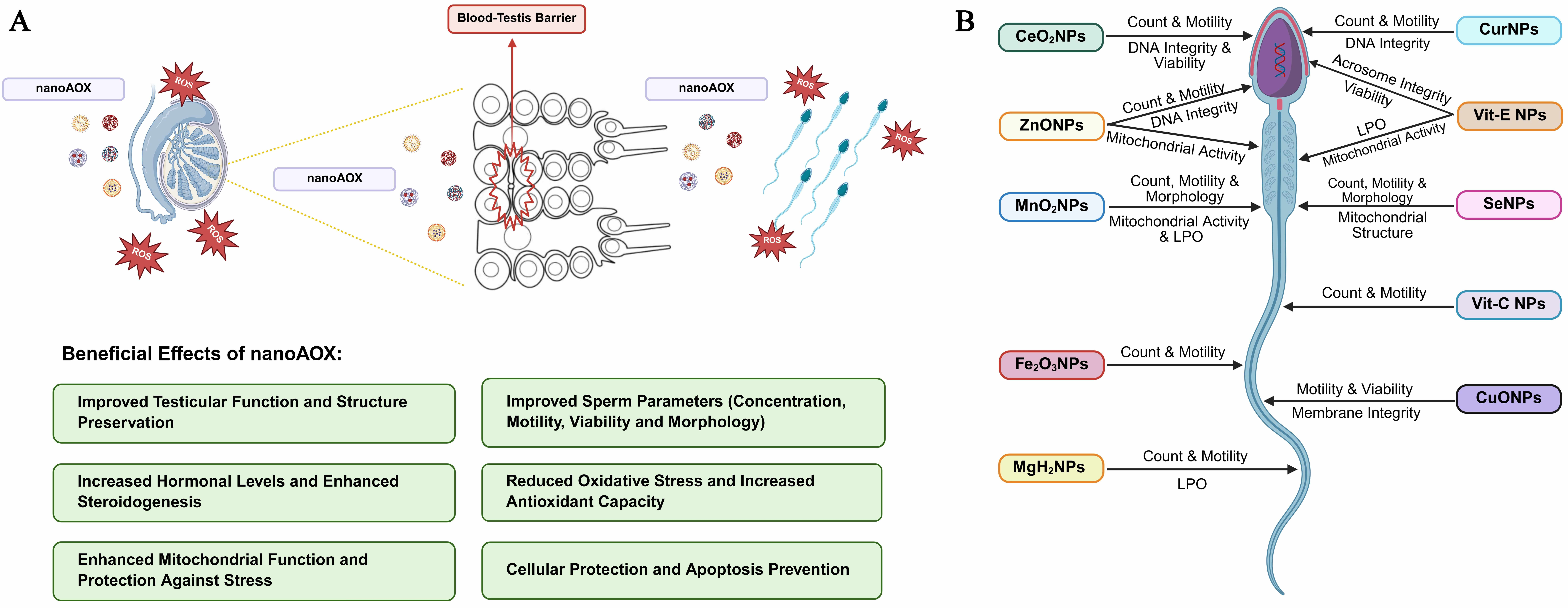 Fig. 1.
Fig. 1.
Protective role of nanoAOXs in enhancing male reproductive function. (A) Beneficial effects of nanoAOXs in improving testicular function and sperm parameters. Created in BioRender. Panner Selvam, M. (2025) https://BioRender.com/mg1p7ut. (B) Individual nanoAOX and their beneficial effects on sperm function. Created in BioRender. Panner Selvam, M. (2025) https://BioRender.com/0e6u31c. Abbreviations: nanoAOXs, nanoantioxidants; LPO, lipid peroxidation; ROS, reactive oxygen species; CeO2NPs, cerium dioxide nanoparticles; CurNPs, curcumin nanoparticles; ZnONPs, zinc oxide nanoparticles; MnO2NPs, manganese dioxide nanoparticles; SeNPs, selenium nanoparticles; Fe2O3NPs, iron oxide nanoparticles; CuONPs, copper oxide nanoparticles; MgH2NPs, magnesium hydride nanoparticles.
| Study reference | NanoAOX and dose | Study group | Aim/objective | Outcomes |
| Sadraei et al. [61] | CurNPs (4 mg) | Wistar rats (n = 70) | To determine the impact of CurNPs on spermatogenic processes and sperm functionality in varicocele rat models. | – Improved sperm concentration and motility. |
| – Reduced ROS levels, LPO, and SDF. | ||||
| – Improved testicular function. | ||||
| Ahmed-Farid et al. [62] | Cur nanoemulsion (5 mg/kg) | Wistar rats (n = 40) | To investigate the impact of Cur nanoemulsion on reproductive performance and spermatogenesis in juvenile rats on a PDD. | – Improved reproductive performance. |
| – Decreased OS and nitrosative stress. | ||||
| – Increased testes weight, total sperm count, progressive motility. | ||||
| Sarawi et al. [63] | Liposomal nano Cur (80 mg/kg) | Wistar rats (n = 40) | To examine the therapeutic potential of Cur and liposomal nano Cur in preventing CuSO4-induced testicular OS, inflammatory responses, and apoptotic processes. | – Protected against Cu-induced testicular damage, OS, inflammatory responses, and apoptotic processes. |
| – Enhanced cell proliferation, sex hormone circulation, and steroid hormone production. | ||||
| Mohamed and Abdelrahman [64] | ZnONPs (10 mg/kg) | Wistar rats (n = 40) | Examining ZnONPs’ ability to prevent nicotine-induced damage to testis and epididymis. | – Decreased OS and preserved seminiferous tubules structure. |
| – Boosted expression of steroidogenic enzymes. | ||||
| Lokman et al. [65] | ZnONPs (4 mg/kg) | Wistar rats (n = 28) | To evaluate the effects of ZnONPs treatment on aluminum-induced reproductive toxicity. | – Decreased inflammation and histopathological alterations of testis. |
| – Reduced apoptotic markers and increased Ki-67 expression in spermatogenic cells. | ||||
| Zhang et al. [66] | ZnONPs (50 & 100 mg/kg) | Jinzhong ram lambs (n = 16) | To explore the effects of ZnONPs on the semen parameters, seminal plasma antioxidant enzyme activities, and epididymal Cu-Zn SOD expression. | – Improved semen parameters, enhanced anti-oxidase activity in seminal plasma, and increased Cu-Zn SOD expression. |
| Yazdanshenas et al. [67] | ZnONPs (10 & 10-3 mM) | Holstein bulls (n = 4) | To assess the effect of different concentrations of ZnONPs on spermatozoa quality and pregnancy outcome after freeze-thawing process. | – Decreased MDA levels and improved mitochondrial activity. |
| – No improvement in fertility rates. | ||||
| Jahanbin et al. [68] | ZnONPs (10 mM) | Holstein bulls (n = 4) | To investigate the effect of varying concentrations of ZnONPs on sperm quality, membrane integrity, function, and mitochondrial activity after freezethaw cycles. | – Improved plasma membrane and mitochondrial functions. |
| – No significant difference in motility parameters (total and progressive), membrane integrity, and abnormal morphology. | ||||
| Shi et al. [69] | SeNPs (0.3 mg/kg) | Boer goat bucks (n = 42) | To study the effect of SeNPs on testicular structure, sperm quality, and GPX enzyme activity. | – Enhanced GPX activity and selenium content in testicular tissue and semen. |
| – Preserved membrane integrity and mitochondria midpiece structure. | ||||
| Shi et al. [70] | SeNPs (0.3 mg/kg) | Taihang black goats (n = 40) | To study the effect of SeNPs on growth performance, Se concentration, and antioxidant status. | – Increased final body weight, serum antioxidant enzymes activity (GPX, SOD, CAT). |
| Hozyen et al. [71] | SeNPs (0.5 mg/kg) | Sprague Dewley rats (n = 32) | To evaluate SeNPs ability to prevent deltamethrin-induced damage to sperm quality, oxidative balance, sexual behavior, and the histological structure of the testes and epididymis. | – Improved sperm parameters, serum testosterone level, and enhanced antioxidant biomarkers, blood TAC, and GPX activity. |
| Asadpour et al. [72] | SeNPs (0.5 µg/kg) | Mice (n = 28) | To investigate the therapeutic ability of SeNPs on aflatoxin B1-induced reproductive toxicity. | – Inhibited aflatoxin B1-induced damage to the testis. |
| – Improved sperm parameters, in vitro fertilization, and embryo production. | ||||
| Khalaf et al. [73] | SeNPs (2 mg/kg) | Rats (n = 90) | To evaluate the role of SeNPs in alleviating Bisphenol A-induced testicular toxicity. | – Improved antioxidant activity, genetic changes, and restoration of testicular morphology. |
| Zhang et al. [74] | SeNPs (2 mg/kg) | Sprague Dawley rats (n = 40) | To determine whether SeNPs can prevent NiSO4-induced testicular damage. | – Decreased NiSO4-induced cell death in testicular tissue. |
| – Reduced proapoptotic markers (Bak, cytochrome c, caspase-9, caspase-3) and elevated anti-apoptotic Bcl-2 expression. | ||||
| Alrashidi and Gomaa [75] | SeNPs (0.4 mg/kg) | Wistar rats (n = 35) | To investigate SeNPs protective potential against MSG-induced reproductive damage. | – Elevated antioxidant enzymes, decreased MDA levels, and inhibited testicular injury. |
| Pardhiya et al. [76] | MnO2NPs (12.5 mg/kg) | Wistar rats (n = 24) | To counteract the harmful effect of microwave exposure by using BSA-conjugated MnO2NPs. | – Increased testosterone levels and antioxidant status and decreased LPO in testis. |
| – Improved sperm parameters, membrane integrity, testicular morphology, and mitochondrial activity. | ||||
| Paskeh et al. [77] | CoQ10 (0.02 mg/kg) and Fe2O3NPs (0.03 mg/kg) | Wistar rats (n = 48) | To investigate the combined protective potential of CoQ10 and Fe2O3NPs against hyperthermia-induced sperm damage. | – Fe2O3NPs along with CoQ10 improved sperm parameters and prevented scrotal hyperthermia-induced spermatogenic cell death. |
| Afshar et al. [78] | Fe2O3NPs coated with Cur (5.4 µg/240 µL) | Mice (n = 18) | To determine whether Fe2O3NPs containing curcumin can protect spermatogenesis from prolonged scrotal hyperthermia. | – Increased testis volume, length of seminiferous tubules, sperm count, and stereological parameters. |
| – Increased testosterone levels. | ||||
| – Decreased TUNEL-positive cells and increased c-kit, STRA8, and PCNA expression. | ||||
| Moridi et al. [79] | CeO2NPs (30 mg/kg) | Wistar rats (n = 36) | To evaluate the effect of CeO2NPs on OS and sperm parameters after malathion exposure. | – Protective effect on sperm count, motility, and viability. |
| – Increased TAC and total thiol group. | ||||
| – Restored testicular changes induced by malathion. | ||||
| Raeeszadeh et al. [80] | Vit C-NPs (200 mg/kg) | Wistar rats (n = 42) | To evaluate the effect of Vit C-NPs on lead-induced testicular histological changes, sperm parameter alterations, OS, and hormonal imbalances. | – Improved sperm parameters and enhanced antioxidant enzyme activity (GPX, SOD, CAT). |
| – Increased testosterone, LH, and FSH levels. | ||||
| Jurado-Campos et al. [81] | Vit E nanoemulsion (12 mM) | Rams of Manchega breed (n = 7) | To evaluate the feasibility of Vit E nanoemulsion to improve sperm quality during transportation. | – Protected kinematic and physiological characteristics of sperm during transportation. |
| – Improved mitochondrial activity, sperm viability, and acrosome integrity. | ||||
| – Reduced ROS and LPO levels. | ||||
| Jurado-Campos et al. [82] | Vit E nanoemulsion (9 mM) + Vit E hydrogels (1 mM) | Red Deer (n = 6) | To investigate the cryoprotective potential of Vit E nanoemulsion on sperm. | – Prevented exogenous OS, reduced ROS and LPO levels. |
| – Enhanced sperm viability and kinematic parameters while maintaining mitochondrial activity. | ||||
| Sánchez-Rubio et al. [83] | Vit E nanoemulsion (12 mM) | Red Deer (n = 11) | To evaluate the protective effects of Vit E nanoemulsions against OS. | – Reduced ROS production and LPO with enhanced sperm motility parameters. |
| – Preserved mitochondrial activity and acrosomal integrity. |
Abbreviations: nanoAOXs, nanoantioxidants; CurNPs, curcumin nanoparticles; ZnONPs, zinc oxide nanoparticles; SeNPs, selenium nanoparticles; MnO2NPs, manganese dioxide nanoparticles; CoQ10, coenzyme Q10; Vit C-NPs, vitamin C nanoparticles; BSA, bovine serum albumin; CAT, catalase; CuSO4, copper sulfate; FSH, follicle-stimulating hormone; GPX, glutathione peroxidase; LPO, lipid peroxidation; LH, luteinizing hormone; MDA, malondialdehyde; MSG, monosodium glutamate; OS, oxidative stress; PDD, protein-deficient diet; ROS, reactive oxygen species; SeNPs, selenium nanoparticles; SDF, sperm DNA fragmentation; SOD, superoxide dismutase; TUNEL, terminal uridine nick end labeling; TAC, total antioxidant capacity.
Curcumin nanoparticles (CurNPs) have shown beneficial effects on male fertility by enhancing various sperm parameters: count, concentration, motility, and morphology [84]. Furthermore, curcumin can potentially suppress OS and reduce tissue atrophy [85]. CurNPs demonstrated several beneficial effects, including reduced levels of ROS and LPO, decreased sperm DNA fragmentation, and improved testicular function in rats with varicocele-induced infertility [61]. Daily supplementation with CurNPs could improve reproductive performance and spermatogenesis in juvenile rats on a protein-deficient diet (PDD). Conversely, malnutrition and protein deficiency trigger excessive production of ROS and reactive nitrogen species. Methionine-deficient diets typically cause MDA levels to progressively increase while glutathione levels notably decrease, accompanied by significant reductions in CAT and SOD enzyme activities. The study also showed that a PDD resulted in a significant decrease in testes weight and sperm parameters such as total count and progressive motility. Biochemically, testicular tissue exhibited reduced adenylate energy charge, increased 8-OHdG levels, along with OS and nitrosative stress. Additionally, the study revealed a negative correlation between sperm concentration and progressive motility and several factors such as elevated 8-OHdG levels, increased OS, disrupted cell energy, and elevated amino acid (essential and nonessential) levels in seminal plasma. The findings indicated that CurNPs prevented the detrimental effects associated with PDD [62].
Exposure to Cu compounds has been linked to harmful effects on the pituitary-gonadal axis and steroidogenesis, including testicular OS, apoptosis, and diminished sperm quality [86, 87]. Both curcumin and liposomal CurNPs reduce testicular oxidative injury, inflammation, and apoptosis caused by copper sulfate (CuSO4) exposure in rats [63]. They also improved cell proliferation, normalized circulating sex hormone levels, and enhanced steroidogenesis. These protective effects were linked to activation of the nuclear factor erythroid 2-related factor/heme oxygenase-1 (Nrf2/HO-1) signaling pathway and an overall rise in antioxidant levels. Activation of Nrf2/HO-1 signaling has been shown to protect the testis from OS, inflammation, and apoptosis caused by heavy metals [88]. Notably, CurNPs provide stronger protection than curcumin alone, likely due to their improved properties.
Animal studies have shown that zinc oxide nanoparticles (ZnONPs) significantly reduce apoptosis, enhance spermatogonia, and reduce oxidative damage to the sperm cell membrane due to their potent antioxidant properties [66, 89]. Omu et al. [90] found that Zn deficiency was associated with elevated OS, as marked by increased LPO, caspase-3 activity, and tumor necrosis factor-alpha levels.
Mohamed and Abdelrahman [64] analyzed the harmful reproductive effects of cigarette smoke exposure and evaluated ZnONPs as a potential protective agent against nicotine-induced male reproductive dysfunction. Nicotine-exposed rats treated with ZnONPs showed reduced OS and increased expression of steroidogenic enzymes. Coadministration of nicotine and ZnONPs to rats led to the development of a thin connective tissue capsule with blood vessels and maintained the normal structural organization of the seminiferous tubules [64].
Research showed that aluminum chloride (AlCl3) given orally to rats resulted in testicular oxidative damage, marked by elevated MDA and nitric oxide levels and reduced glutathione content and CAT activity. When these rats received daily intraperitoneal ZnONP injections prior to AlCl3 exposure, the reproductive toxicity from AlCl3 was significantly reduced. Furthermore, ZnONP treatment improved testicular inflammatory, apoptotic, and reproductive markers; corrected histopathological changes; and enhanced Ki-67 immunoreactivity in spermatogenic cells [65].
Dietary supplementation with ZnONPs enhanced epididymal semen quality, seminal antioxidant activity, and Cu-Zn SOD expression in young rams [66]. The addition of ZnONP to bull semen extender likewise decreased MDA levels while enhancing mitochondrial activity [67]. Additionally, ZnONPs dose-dependently improved sperm plasma membrane function while preserving motility characteristics [68].
Selenium nanoparticles (SeNPs) have a positive impact on semen quality through their antioxidant properties and ability to protect against OS. SeNPs are also reported to scavenge ROS and inhibit LPO [91, 92] to protect spermatozoa from oxidative damage [93]. SeNPs demonstrated superior efficacy compared to other Se forms (sodium selenite and selenized yeast) in enhancing antioxidant enzyme activity, including glutathione peroxidase (GPX), SOD, and CAT, while simultaneously improving sperm quality [69, 70, 94]. Hozyen et al. [71] conducted a study in which they orally administered SeNPs (0.5 mg/kg bwt) to male rats with deltamethrin-induced infertility. The results indicated improvements in sperm parameters as well as increased levels of antioxidants, GPX activity, and testosterone levels [71]. Likewise, multiple rat studies have demonstrated that SeNPs improve sperm parameters by reducing OS damage [72, 73, 74].
Research has shown that SeNP administration effectively counteracts the adverse effects of monosodium glutamate (MSG). Regardless of dosage, MSG administration resulted in significantly reduced testosterone levels and notably increased OS markers. Nevertheless, SeNPs provided effective protection against MSG-induced damage, demonstrating strong antioxidant activity that significantly boosted antioxidant enzyme levels and reduced MDA levels. These findings indicate that SeNPs protect against testicular injury while enhancing antioxidant status [75]. Additionally, Se enhances the testicular architecture and sprerm production by effectively scavenging ROS produced by MSG [75]. Through improved antioxidant capacity and reduced MDA levels, SeNPs effectively restored testosterone levels.
Manganese dioxide nanoparticles (MnO2NPs) possess antioxidant-mimicking properties and can inhibit apoptosis [95, 96]. Their antioxidant capacity is evident in their ability to boost endogenous antioxidant-enzyme levels and reduce the OS marker MDA. Microwave exposure triggers OS, which in turn affects reproductive parameters [97]. MnO2NPs at 12.5 mg/kg protect the reproductive system against microwave-induced modifications [76]. As an antioxidant mimic, MnO2NPs neutralize microwave-induced OS, protecting cells and tissues from further damage.
Fe2O3NPs have strong potential owing to their superior drug-carrying capacity and enhanced targeting ability, which are derived from their magnetic and biological properties [98]. Fe2O3NPs, along with CoQ10, exhibited antioxidant properties and significantly improved semen parameters. This combination represents a promising therapeutic approach for addressing hyperthermia-induced scrotal damage and associated infertility [77]. Moreover, curcumin-coated Fe2O3NPs boosted testosterone levels, increased germ cell proliferation, and decreased apoptotic sperm cells in mice subjected to testicular hyperthermia [78].
CeO2NPs with their autocatalytic, antioxidant, and regenerative properties, stand out as exceptional therapeutic agents that target pathologies associated with chronic OS and inflammation [99, 100]. CeO2NPs, known for their antioxidant, autoregenerative, and low-toxicity properties, have shown promising therapeutic results for male health and fertility [45, 101, 102]. Recent findings reveal that CeO2NPs function as antioxidant agents by scavenging hydroxyl and nitric oxide radicals and mimicking SOD and CAT activity [103, 104] and CAT mimetic activity [105]. Karakoti et al. [106] demonstrated that CeO2NPs can efficiently neutralize superoxide, a harmful metabolic byproduct of the cell. The antioxidative capabilities of CeO2NPs hold significant potential for positively impacting the progression of male infertility [45].
Oral administration of CeO2NPs (1 mg/kg, single dose) resulted in accelerated spermatogenesis in reproductively active male rats. Further, CeO2NPs, even at a higher dose (100 mg/kg), did not significantly affect either sperm parameters or the structural-functional integrity of the reproductive system [107]. A 10-day regimen of CeO2NPs led to increased sperm count and improved quantitative sperm parameters. It also significantly lowered serum LPO levels while boosting CAT and SOD activity [45]. Moridi et al. [79] divided 36 male Wistar rats into six groups and treated them with different concentrations of CeO2NPs, malathion, or CeO2NPs + malathion. Administration of CeO2NPs remarkably enhanced testicular sperm viability compared to the malathion-treated group. Additionally, sperm parameters were notably improved in the rats coadministered with CeO2NPs and malathion, which demonstrates the protective effect of CeO2NPs against malathion-induced toxicity in the rat testis. Further, the CeO2NPs- administered group exhibited higher total antioxidant capacity and thiol levels than the malathion-exposed group. CeO2NPs mimic the properties of the SOD enzyme, effectively reducing testicular MDA levels at both 15 mg/kg and 30 mg/kg dosages, with the latter showing statistically notable improvement [79].
Vitamin deficiencies, particularly of Vit C, B1, and B6, have been linked to increased risk of cadmium and lead (Pb) toxicity [108]. Vitamin supplementation is important to protect against free radical damage, which effectively prevents LPO in organs. For instance, the protective effects of Vit C on the liver, kidneys, brain, and testes against oxidative damage induced by Pb exposure have been shown. Exposure to arsenic and Pb triggers OS, apoptosis, and cytotoxicity, which can ultimately cause male infertility [80]. Vit C-NPs and other antioxidant complexes act as therapeutic agents against spermatogenic toxicity by targeting apoptosis-inducing gene expression. Chakraborty and Jana [51] demonstrated that NPs (polyaspartimide and Au) conjugated with Vit C provide protection against OS. Male rats supplemented with Vit C-NPs showed improved sperm parameters, increased testosterone, LH and FSH levels, and enhanced antioxidant enzyme (GPX, SOD, CAT) activity. In another study, Jurado-Campos et al. [81] reported that treatment with Vit E nanoemulsion significantly reduced ROS production and LPO levels under OS conditions in ram sperm. Similar effects have been observed in studies involving bull [109, 110], boar [111], and human [112] sperm.
Nanoemulsion of Vit E demonstrated a protective effect by lowering ROS and LPO levels and reducing DNA fragmentation. Additionally, it enhanced sperm viability and preserved mitochondrial activity in red deer spermatozoa [82]. Sánchez-Rubio et al. [83] observed the protective effects of Vit E nanoemulsions against OS using red deer epididymal sperm. These nanoemulsions enhanced motility parameters such as progressivity and sperm velocity while preserving mitochondrial activity. They effectively reduced ROS production, prevented LPO following OS, and safeguarded the integrity of the acrosome, thereby preventing cell death [83].
ROS generation is one of the most common consequences of diabetes mellitus [113]. Diabetes mellitus directly impacts male fertility by inducing OS, with a subfertility prevalence rate of 51% among diabetic patients [114]. It is well established that diabetes mellitus leads to adverse changes in sperm count, quality, and function [115]. Furthermore, diabetes mellitus negatively affects male fertility at multiple levels, including ejaculation, endocrine regulation of spermatogenesis, erection, semen volume, as well as sperm vitality and motility [116].
ZnONPs have been reported to increase sperm parameters and function by mitigating OS in diabetic rats [117]. Further, ZnONPs offer a safer therapeutic option for diabetic male rats, providing OS protection through antioxidant and anti-inflammatory mechanisms while maintaining low toxicity profiles [118].
Sperm banking is a well-established method for preserving fertility [119]. Sperm cryopreservation has also become a promising solution for addressing many male infertility issues. Over the past five decades, various methods for sperm freezing have been developed, making it an integral component of ART [120]. Semen cryopreservation has a negative impact on sperm parameters such as motility, morphology, viability, and DNA integrity [121] and can increase ROS generation in spermatozoa, substantially reducing their fertilization potential [122]. Spermatozoa as such is vulnerable to free radical attacks. Their plasma membranes are rich in unsaturated fatty acids, while their minimal cytoplasm provides limited free-radical scavenging capacity [123]. Animal studies reporting the positive effects of nanoAOX in semen cryopreservation are summarized in Table 2 (Ref. [121, 124, 125, 126, 127, 128]).
| Study reference | nanoAOX and dose | Study group | Findings/results |
| Elshamy et al. [121] | CuONPs (60 ppm/mL) | Zaraibi bucks (n = 5) | – Increased sperm motility, viability index, membrane integrity, antioxidant marker expression, DNA integrity, and decreased MDA level. |
| Isaac et al. [128] | ZnONPs (100 µg/mL) | Human (n = 40) | – Decreased MDA levels, prevented LPO at the membrane level. |
| Safa et al. [124] | SeNPs (1%) & Vit E (5 µg/mL) | White Leghornroosters (n = 12) | – Reduced LPO and enhanced antioxidant levels in semen plasma. |
| – Better maintained sperm quality and morphology with Vit E-NPs or SeNPs compared to Se and Vit E alone. | |||
| Khalil et al. [125] | SeNPs (0.5 & 1 µg/mL) | Friesian bulls (n = 5) | – Improved post-thaw sperm quality and IVF outcomes. |
| – Reduced apoptosis, LPO and MDA concentration. | |||
| – Increased TAC in seminal plasma. | |||
| Khalique et al. [126] | CeO2NPs (25 & 50 µg/mL) | Beetal bucks (n = 5) | – Reduced ROS and LPO, and improved antioxidant enzyme activity. |
| – Improved motility (total and progressive), velocity, plasma. membrane and acrosomal integrity, viability, and DNA integrity. | |||
| – Improvement in pregnancy rate. | |||
| Bisla et al. [127] | Fe2O3NPs (100 ppm) | Murrah buffalo (n = 4) | – Improved semen quality parameters, especially mean post-thaw motility, and DNA integrity. |
| – Decreased MDA levels and increased TAC and SOD levels. |
Abbreviations: nanoAOXs, nanoantioxidants; CuONPs, copper oxide nanoparticles; SeNPs, selenium nanoparticles; CeO2NPs, cerium oxide nanoparticles; Fe2O3NPs, iron oxide nanoparticles; Vit E-NPs, vitamin E nanoparticles; TAC, total antioxidant capacity; LPO, lipid peroxidation; MDA, malondialdehyde; IVF, in vitro fertilization; ROS, reactive oxygen species; SOD, superoxide dismutase.
SeNPs have shown promising results in mitigating OS during cryopreservation. Safa et al. [124] demonstrated that semen enrichment with SeNPs and Vit E increased antioxidant levels in seminal plasma of roosters and potentially humans, by reducing LPO after freeze-thaw cycles. NP forms (Vit E-NPs and SeNPs) were more effective than conventional Se and Vit E in maintaining sperm quality and morphology [124]. Furthermore, both SeNPs and ZnONPs protected cryopreserved spermatozoa by reducing apoptosis and improving sperm quality [125, 129].
CeO2NPs have been studied for their ability to retain oxygen and serve as ROS scavengers, protecting the viability of ram sperm cells during the cryopreservation process [130]. CeO2NPs possess antioxidant properties that can alleviate OS during the cryopreservation process and enhance the activity of antioxidant enzymes. Particularly, adding 25 and 50 µg/mL CeO2NPs improved motility velocity, viability, plasma membrane, acrosome, and DNA integrity of sperm [126].
Elshamy et al. [121] evaluated the effectiveness of cupric oxide nanoparticles (CuONPs) when used as cryo-extender additives in preserving sperm quality after thawing. The results indicated that seminal plasma removal prior to cryopreservation had detrimental effects on sperm parameters. CuONPs supplementation provided broad-spectrum improvements in sperm function (motility, viability), cellular integrity (membrane, DNA), and antioxidant status (biochemical markers, reduced MDA). Seminal plasma enriched with high CuONPs concentrations apparently reduces the negative impact of cryopreservation on sperm quality [121].
Fe2O3NPs coated with anti-ubiquitin antibodies were used to deplete dead/damaged spermatozoa in freshly ejaculated semen. Nano-purified semen samples that were treated with Fe2O3NPs (2.0 µg/mL) conjugated to anti-ubiquitin antibodies improved sperm DNA integrity by removing dead or damaged spermatozoa in buffalo ejaculates, serving as a promising approach to reduce OS and enhance post-thaw semen quality [127].
Studies have demonstrated that ZnONPs significantly reduce MDA levels, indicating that these NPs effectively scavenge free radicals generated during the freeze-thaw process and protect spermatozoa from oxidative damage [128, 131]. Nano-vitamins showed protection against LPO in cryopreserved semen, with possible implications in the field of ART [21]. Jurado-Campos et al. [132] utilized a Vit E nanoemulsion to preserve ram sperm, supporting the use of nano-controlled-release antioxidants to prevent OS in an ART setting.
Utilizing cytotoxic chemotherapies for the treatment of malignancies can cause long-term gonadotoxicity and Leydig cell impairment. These negative effects, which depend on the dose and type of chemotherapy agent, can impact male reproductive health, specifically testicular function. Animal studies reporting the positive effects of nanoAOX on radiation and chemotherapeutic agents are summarized in Table 3 (Ref. [133, 134, 135, 136, 137, 138]).
| Study reference | nanoAOX and dose | Study group | Radiation or chemotherapeutic agents | Findings/results |
| Badkoobeh et al. [133] | ZnONPs (5 mg/kg) | Wistar rats (n = 24) | Doxorubicin | – Improved TAC, plasma testosterone, LH, sperm count, and DNA integrity. |
| Anan et al. [134] | ZnONPs (5 mg/kg) | Sprague Dawley rats (n = 60) | Cyclophosphamide | – Inhibited caspase enzyme expression. |
| – Enhanced mitochondrial functional with decreased proapoptotic factors and cytochrome c levels. | ||||
| Mohammadi et al. [135] | ZnONPs (5 mg/kg) | Mice (n = 15) | Cyclophosphamide | – Prevented seminiferous tubule epithelial disorganization and spermatogenic cell loss. |
| Rezvanfar et al. [136] | SeNPs (2 mg/kg) | Wistar rats (n = 32) | Cisplatin | – Improved serum testosterone, sperm quality, DNA integrity and spermatogenesis. |
| – Reduced free radical levels. | ||||
| Mobaraki et al. [137] | AuNPs (12.5 µg/mL) | Wistar rats (n = 30) | Cyclophosphamide | – Decreased apoptosis during spermatogenesis and altered expression caspase-3, caspase-9, Bax, Bcl2. |
| – Reversed hormonal and morphological alterations of testis. | ||||
| Ma et al. [138] | MgH2NPs (10 µg/mL) | Mice (n = 44) | X-ray irradiation using KUBTEC XCELL 225 system (225 kV, 13.2 mA, 1 Gy/min dose rate) | – Increased sperm density, motility and maintained spermatogenesis. |
| – Reduced irradiation-induced ROS and lipid damage. | ||||
| – Significantly reduced testicular apoptosis and reversed irradiation-induced G2/M phase cell cycle arrest. |
Abbreviations: nanoAOX, nanoantioxidant; ZnONPs, zinc oxide nanoparticles; SeNPS, selenium nanoparticles; AuNPs, gold nanoparticles; MgH2NPs, magnesium hydride nanoparticles; TAC, Total antioxidant capacity; ROS, reactive oxygen species; MDA, malondialdehyde; LH, luteinizing hormone.
A study of adult Wistar rats confirmed that doxorubicin triggers LPO, as evidenced by increased levels of MDA. Doxorubicin readily autoxidizes in the presence of oxygen, producing superoxide and other ROS, thereby stimulating LPO [119]. The findings indicated that doxorubicin-induced reproductive toxicity is associated with heightened OS.
Treatment with ZnONPs ameliorated doxorubicin-induced male gonadotoxicity by attenuating OS through their antioxidant activity [133]. ZnONPs also exhibit anti-apoptotic effects in cyclophosphamide-induced testicular damage in male rats via suppression of caspase expression or improvement of mitochondrial function. This leads to reduced levels of cytochrome c and apoptosis-inducing factors, which act as intrinsic cellular death signals [134]. ZnONPs also exhibit potent antioxidant activity, effectively preventing both testicular tissue changes and the decrease in spermatogenic cell numbers resulting from cyclophosphamide treatment [135].
Cisplatin, an anticancer drug with male reproductive toxicity, causes significant reproductive damage. Oral SeNPs administration was able to restore spermatogenesis, improve sperm quality (motility and DNA integrity), and mitigate oxidative damage [136]. AuNPs synthesized utilizing Nasturtium officinale (AuNPs-NO) showed significant cytoprotective and antioxidant properties against cyclophosphamide-induced testicular damage in rats, effectively preventing spermatogenic apoptosis [137]. Magnesium hydride (MgH2), a hydrogen-storage nanomaterial, provides protective effects by suppressing inflammation, apoptosis, and pyroptosis and by alleviating cell cycle arrest caused by irradiation. Under in vitro conditions, MgH2 also reduces MDA levels via dual mechanisms: inhibition of ROS generation and elimination of hydroxyl radical (.OH) through H2 production [138].
Disease characteristics and treatment responses differ among patients due to factors such as genetics, phenotype, blood hemostasis, physiological stress, lifestyle habits, and environmental conditions [139, 140]. These variations lead to the development of a novel delivery system, assuring higher efficacy and treatment safety [140]. NPs, with their unique features, such as a higher surface-to-volume ratio due to their small particle size, compatibility with formulating drugs that have poor bioavailability, and the ability for controlled and targeted delivery, present a promising approach to treatment [139]. Personalized treatment, with the help of omics sciences, including genomics, transcriptomics, proteomics, and metabolomics, reveals information about the genetic profile of infertility development. Genetic factors influencing nutrition metabolism can impact the antioxidant status and fertility chance. Determining exact genetic factors related to nutrition could enable personalized antioxidant supplementation for managing male infertility [141]. One approach for customized treatment involves utilizing nanocarrier functions activated by individuals’ intracellular enzymes. However, NP-mediated personalized treatment also faces challenges, such as potential nanocarrier toxicity, increased clearance time of loaded medicine, uncertainty about biodistribution, stability and storage complexities, regulatory approval difficulties, and economic concerns [142].
While the current findings provide promising insights based on preclinical studies, the translation of these results to human applications remains uncertain. To date, no large-scale clinical trials have been conducted to confirm the safety, efficacy, and pharmacokinetics of this approach in humans. Bridging this gap requires careful consideration of species differences, appropriate dosing strategies, and long-term safety profiles. Additionally, regulatory hurdles, including compliance with guidelines set by agencies such as the United States Food and Drug Administration or the European Medicines Agency, must be addressed before clinical implementation. Future studies should prioritize human-based investigations to validate the therapeutic potential and ensure successful clinical translation.
The management of male infertility associated with OS presents a significant challenge, requiring innovative approaches to improve treatment outcomes. Antioxidants have emerged as promising agents for mitigating OS and improving semen quality and sperm function. In this regard, the advent of nanotechnology has opened new opportunities for enhancing the effectiveness of antioxidant therapy in male infertility. NanoAOXs, characterized by enhanced durability, cellular uptake, and targeted delivery, can more effectively neutralize ROS and reduce OS damage in sperm cells. Additionally, nanoAOXs offer improved bioavailability and stability compared to conventional antioxidants, enhancing their therapeutic potential in managing male infertility. While traditional antioxidant therapy remains a valuable option in male infertility management, nanoAOXs offer a more potent and targeted approach. Their ability to overcome the limitations of conventional antioxidants and optimize treatment outcomes highlights their potential as a foundation of future infertility treatments. Further exploration and advancements in nanoAOX therapy offer hope for enhancing reproductive outcomes with reduced failures and addressing the complex issues of male infertility linked to OS.
MKPS conceived the idea. ZB, YE, and MKPS contributed to the writing of the manuscript. ZB, YE, RF and SB assembled the figures and tables. ZB, YE, RF, SB, SCS, and MKPS contributed to the study design, data interpretation, and critical review of the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The authors would like to thank Scott Bailey, PhD (Editor, Department of Urology, Tulane University School of Medicine, USA) for editing this manuscript.
This research received no external funding.
Author Renata Finelli was employed by the London Women’s Clinic, London, UK. The remaining authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.