1 Department of Zoology, College of Science, King Saud University, 11451 Riyadh, Saudi Arabia
2 Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, 11451 Riyadh, Saudi Arabia
Abstract
We investigated chitosan’s protective effects against tertiary butylhydroquinone (TBHQ)-induced toxicity in adult male rats, focusing on cognitive functions and oxidative stress in the brain, liver, and kidneys.
Rats were divided into four groups (n = 8/group): (1) Control, (2) Chitosan only, (3) TBHQ only, and (4) Chitosan + TBHQ.
TBHQ exposure led to significant cognitive impairments and increased oxidative stress, marked by elevated malondialdehyde (MDA) and decreased superoxide dismutase (SOD) and glutathione (GSH) levels. Behavioral tests, including the Morris Water Maze (MWM) as well as Passive Avoidance Learning (PAL) tasks, confirmed memory and learning deficits in the TBHQ group. Histopathological analysis showed damage in the brain, liver, and kidney tissues of TBHQ-exposed rats. Chitosan treatment significantly mitigated these effects, reducing oxidative stress markers and preserving tissue integrity. These findings suggest that chitosan’s antioxidant properties may provide a therapeutic benefit against TBHQ-induced neurotoxicity and organ damage.
These findings suggest that chitosan exerts potent neuroprotective effects, potentially through its antioxidant and anti-inflammatory properties, and could serve as a therapeutic agent against TBHQ-induced toxicity.
Keywords
- TBHQ
- chitosan
- cerebral cortex
- antioxidant
- toxicity
- behavioral test
The food packaging industry is rapidly expanding, and food preservatives such as tertiary butylhydroquinone (TBHQ), a potent phenolic antioxidant is widely employed to inhibit oxidative spoilage and prevent rancidity [1]. TBHQ has a wide range of applications in the food industry [2]. It can effectively delay the oxidation of oils, improve food stability, and significantly prolong the shelf life of oil- and fat-rich foods. Additionally, TBHQ has many applications other than food preservation [1]. It is utilized in cosmetics, coatings, biofuels, pharmaceutical products, and industries as a stabilizer to hinder autopolymerization.
The recommended daily intake of TBHQ, with acceptable benefits, is 0.7 mg/kg [3]. TBHQ has been yielded contradictory findings, indicating that TBHQ exhibits both prooxidative and antioxidative effects, and it may also be cytotoxic and genotoxic. TBHQ causes breaks on the single-strand DNA in human cells, including the major endothelial cell model, human umbilical vein endothelial cell (HUVEC), according to in vivo and in vitro genotoxicity findings, where A549 and HUVECs lung cancer cells were used to test the potential cytotoxicity of TBHQ, and the results revealed significant necrosis and apoptosis in the treated cells [3]. The maximum permitted amount of TBHQ in food is 200 mg/kg according to the Chinese National Standards and US Food and Drug Administration (USFDA) [2]. Reports are limited on the danger levels of TBHQ. A previous study categorized TBHQ as a class 4 compound: mice that received an oral dose of 300–2000 mg/kg died, and they used 2000 mg/kg in this study [4]. If used in excess, TBHQ may have harmful consequences for human health, such as cytotoxicity, mutagenicity, and carcinogenicity. Numerous countries have set a number of stringent criteria to limit the use of TBHQ and maintain food safety [2]. TBHQ can trigger the production of 8-hydroxydeoxyguanosine via increasing reactive oxygen species (ROS) such as superoxide anions in thymus DNA. As a result of ROS production, the genotoxicity assessment of 400 mg/kg in mouse organs indicated DNA damage in stomach cells after 24 hours and increased DNA migration in the liver and kidneys [5].
Preservative use carries risks, but Samal et al. [6] argued that it is crucial to recognize its value to the packaged food industry. It takes time and effort to identify safe and natural preservatives such as chitosan. Chitosan, which is produced by partial or complete deacetylation of chitin, is the second most prevalent polysaccharide after cellulose [7]. Cellulose derivatives, which are considered safe for human health, are used to create biopolymers such as chitosan by N-deacetylating chitin [8]. Chitin is present in a variety of places in nature, such as exoskeletons of crustaceans, insects, mollusks, and fungi [9]. Chitosan is bioactive, non-toxic, and degradable. It is used in food manufacturing because of its antibacterial, antioxidant, and film-forming properties [10].
Chitosan is widely employed in various biomedical and biological applications, including as a scaffold for tissue engineering, drug carriers, and water purification systems. Chitosan has gained considerable attention in recent decades because of its distinctive biological properties [11]. Chitosan and its derivatives have also been used in the pharmaceutical, cosmetic, wastewater treatment, and wound-healing industries [12, 13]. In a previous study by Yu et al. [14], recent developments in chitosan drug delivery systems for treating brain diseases have been highlighted. Oligopolysaccharide-like chitosan acts as an antioxidant against cell toxicity [15]. Chitosan oligosaccharides (COS) reportedly have significant anti-inflammatory and antioxidant activities [16] such as a strong antioxidant activity against lipid oxidation in food [17]. In a mouse model of colorectal cancer, oral administration of COS (500 mg/kg/day) reduced the tumor size by approximately 60% [16]. The brain, liver, and kidneys as the organs of focus stems from their susceptibility to oxidative stress and their prominent roles in the metabolism of xenobiotics. The brain is highly sensitive to oxidative damage due to its high lipid content and oxygen utilization. Additionally, the liver and kidneys serve as key detoxification organs, metabolizing xenobiotics such as drugs and foreign compounds. The interrelationship between these organs is critical, any damage in one organ can exacerbate damage in others through systemic inflammatory and oxidative responses.
This study assessed the potential protective effects of chitosan against TBHQ-induced toxicity in the cerebral cortex by assessing the levels of brain enzymes, results of behavioral tests, oxidative stress, and antioxidants in rats treated with chitosan and TBHQ.
This study utilized thirty-two adult male Wistar rats that weighed 200
Rats were adapted for 1 week and then classified into 4 groups (n = 8/group) as follows: (1) Control: administered the vehicle, 5% carboxymethylcellulose (CMC), daily for 3 weeks. (2) Chitosan: orally administered chitosan (200 mg/kg) daily for 3 weeks [12, 16, 18]. The chitosan dose was selected based on a study by Ozcelik et al. [19], who reported a protective effect of this dose against acetaminophen-induced hepatotoxicity. (3) TBHQ: orally administered an accumulative dose of TBHQ (1500 mg) over 3 weeks (500 mg/kg/week). (4) Chitosan and TBHQ: administered TBHQ and chitosan (200 mg/kg/day) daily for 3 weeks. The measured effect of these treatments on body weight before and after euthanasia is shown in Figs. 1,2.
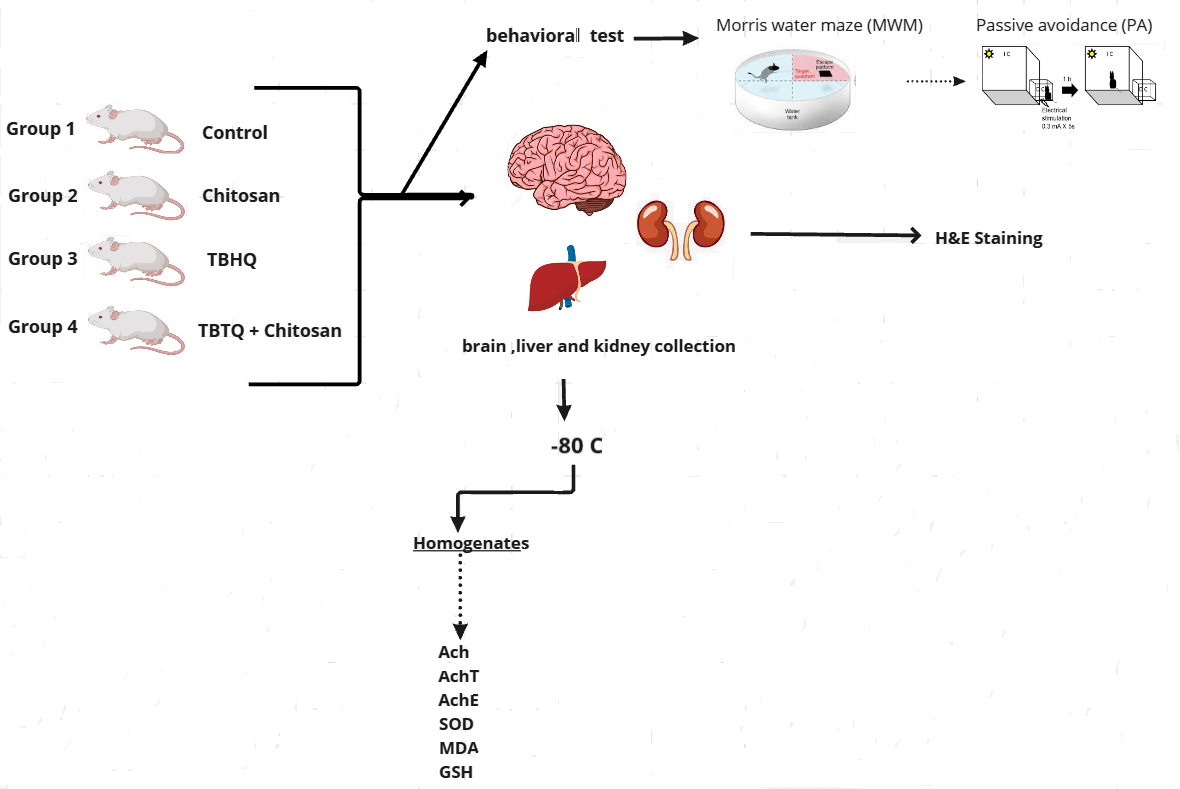 Fig. 1.
Fig. 1. Schematic diagram for the experimental setting. Ach, acetylcholine; AchE, acetylcholinesterase; AchT, acetyltransferase; GSH, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; TBHQ, tertiary butylhydroquinone. Created with Miro board.
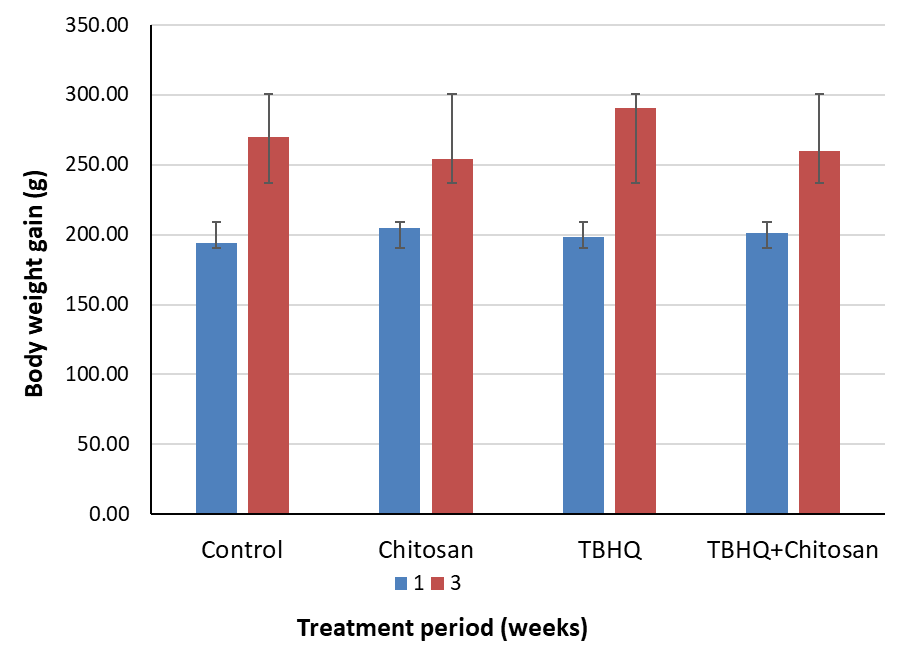 Fig. 2.
Fig. 2. Change in rats’ weights. TBHQ, tertiary butylhydroquinone.
High-molecular-weight chitosan (CAT No. 419419), TBHQ (CAT No. 112941), and CMC (as a universal solvent) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs were always dissolved in 5% CMC at desired concentrations in the experimental procedure; 5% CMC was used as the vehicle.
Spatial and long-term memory functions were assessed using the Morris Water Maze (MWM) and passive avoidance learning (PAL) tests.
The MWM test involves 5 days of training. The maze consists of a hidden platform within a large circular pool filled with water divided into four quadrants (West, South, East, and North), with the hidden platform positioned in the Southwest quadrant (to equalize the swimming distance). Three trials are conducted each day (90 s each), and the rat is released from one quadrant to find the hidden platform [20].
The PAL test was conducted as described by Alfaris et al. [20]. The device (50
Immediately after the PAL test, rats were returned to their cages for 48 h. Subsequently, they were anesthetized. Anesthesia was induced using a ketamine/xylazine hydrochloride mixture (1.9 mg/kg), and euthanasia was performed through neck dislocation. The skull was opened, and the brain was quickly removed and placed on ice. The cerebral cortex and hippocampus were identified and dissected into two identical halves.
Four brains were immediately placed in 10% buffered formalin and sent to the pathology laboratory for routine histological staining with hematoxylin and eosin [21]. The hippocampi of all other brains were harvested by an expert pathologist, snap-frozen in liquid nitrogen, and used for biochemical and molecular studies.
The frozen parts of the brain and hippocampal tissues were homogenized twice via sonication in phosphate buffered saline (pH 7.4) for 300 s (twice). All tissues were centrifuged at 12,000 g for 10 minutes. The supernatants were isolated and stored at –80 °C until used for biochemical analysis [22].
Acetylcholine (Ach) levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (CAT No. STA-603; Cell Biolabs Inc., San Diego, CA, USA) [23, 24]. Choline acetyltransferase (AChT) levels were measured using an ELISA kit (CAT No. SEB929Ra; Cloud-Clone Corp., Houston, TX, USA) [25]. Acetylcholinesterase (AChE) levels were measured using an assay kit (CAT. No. ab138871; Abcam, Cambridge, UK) [26]. The tests were performed according to the manufacturer’s instructions for 8 rats per group and run in duplicate.
2.6.2.1 Superoxide Dismutase
Superoxide dismutase (SOD) catalyzes the decomposition of superoxide free radicals and provides the first line of defense against oxygen poisoning [27]. SOD levels were measured using an ELISA kit (CAT No. ab238535; Abcam) [28, 29]. The test was performed according to the manufacturer’s instructions for 8 rats per group and run in duplicate.
2.6.2.2 Glutathione
Glutathione (GSH) has many important cellular and extracellular functions, including ROS detoxification [30]. GSH levels were measured using an ELISA kit (CAT No. MBS265966; MyBioSource, CA, USA). The test was performed according to the manufacturer’s instructions for rats per group and run in duplicate.
Lipid peroxidation was evaluated based on malondialdehyde (MDA) levels in the brain measured using an ELISA kit (CAT No. 10009055; Cayman, MI, USA) [31]. The test was performed according to the manufacturer’s instructions 8 rats per group and run in duplicate.
A histological assessment was performed on each of the four groups. The samples were fixed for 24 hours in 10% buffered formalin, then dehydrated in a succession of ethanol solutions (VWR Chemicals BDH, Radnor, PA, USA), cleaned in xylene (Fisher Chemicals, Loughborough, UK), and embedded in paraffin wax (Sigma-Aldrich, Darmstadt, Germany). Sections of the tissue block, each 4 µm thick, were cut out and stained with hematoxylin and eosin. Following DPX , which is a mixture of distyrene, a plasticizer, and xylene (VWR Chemicals BDH) mounting, the stained tissues were allowed to air dry. The tissues were then inspected using a light microscope (Olympus BX51, Tokyo, Japan) [32, 33]. The pathology scores in the cerebral cortex and medulla oblongata were assessed through evaluating the pyknosis of the cells, while these scores were assessed in the cerebellum via detecting the degeneration of Purkinje cells. For liver and kidney samples, the pathology scores were assessed through counting the hemorrhagic and inflammatory cells with five different fields of 4
Data were assessed by GraphPad Prism software version 8 (GraphPad Software, LLC, San Diego, CA, USA). Normality and analysis were conducted using the Kolmogorov–Smirnov and 2-way analysis of variance tests, respectively. One-way ANOVA followed by Tukey’s post hoc analysis were used for analysis. The significance lever was set as p
During treatment periods at week 1 and week 3, all rats were observed and no death were found, and they appeared physically healthy. Although the body weight of the rats changed in all groups, the percentage of weight change differed between the groups after 3 weeks. The percentage of weight change was significantly lower in the chitosan group than in the control group (p
TBHQ-treated rats took significantly longer time to find the platform over days 3, 4, and 5, than control and chitosan-treated rats. TBHQ and chitosan group significantly decreased the swim time needed to find the platform on days 3, 4, and 5 compared with TBHQ group. These values of TBHQ and chitosan group were not significantly different from those of the control and chitosan groups (Fig. 3A).
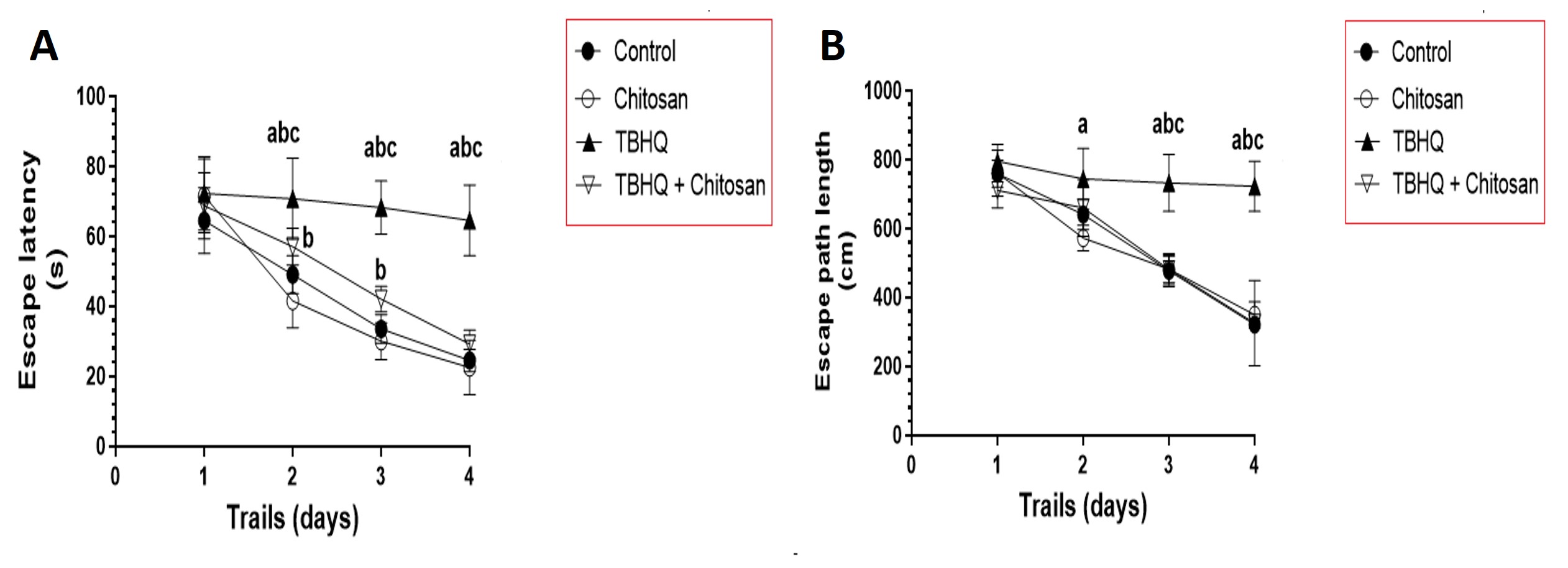 Fig. 3.
Fig. 3. Distance Crossed to Find the Rats’ Hidden Platform in the Morris Water Maze (MWM) Test. (A) Time to find the hidden platform (escape latency) during the Morris Water Maze test over 4 consecutive days. Data are presented as mean
Rats treated with TBHQ took significantly longer to find the platform over days 3, 4, and 5, than control and chitosan-treated rats. TBHQ and chitosan group had less swim distance travelled to find the rescue platform on days 3, 4, and 5 compared with TBHQ group. These values of TBHQ and chitosan group were not significantly different from those of the control and chitosan groups (Fig. 3B).
TBHQ reduced the number of crosses over the hidden platform during the probe trial on day 5 of the MWM test compared to other groups. TBHQ-treated rats spent fewer probe trials finding the hidden platform than control rats. TBHQ and chitosan treatment significantly increased the crossing time during the probe trial compared with TBHQ treatment and showed normal behavior as compared to the other groups (Fig. 4A).
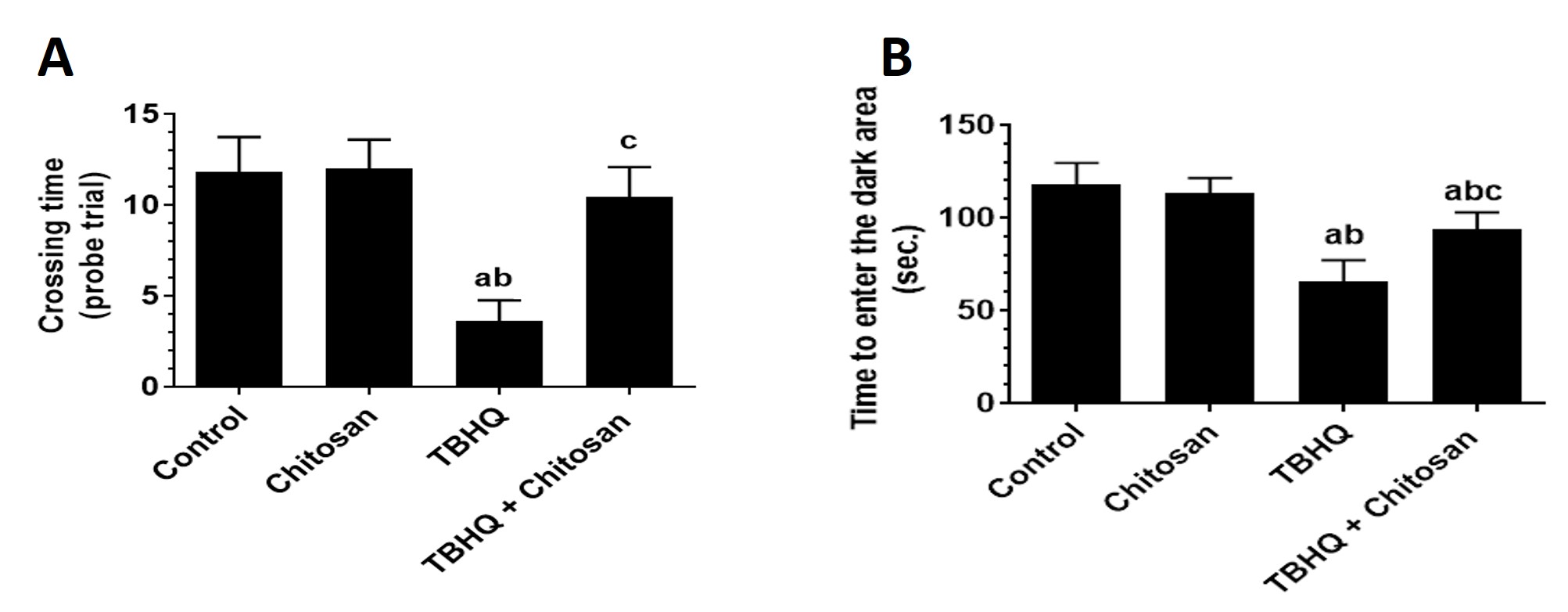 Fig. 4.
Fig. 4. Number of Times Rats Crossed the Hidden Platform in MWM test and Time Required to Enter the Dark Room in the passive avoidance learning (PAL) Test. (A) Number of crosses over the hidden platform that was removed during the probe trial on day 5 of the Morris Water Maze test. Data are presented as mean
Avoiding entry into the dark room after electrical stimulation indicated intact memory during the PAL test. TBHQ-treated groups showed a significant decline in the time required to enter the dark room compared with control and chitosan-treated rats (Fig. 4B).
The TBHQ group showed a significant decrease in Ach levels in the cerebral cortex and hippocampus compared with the control and chitosan groups (Fig. 5A).
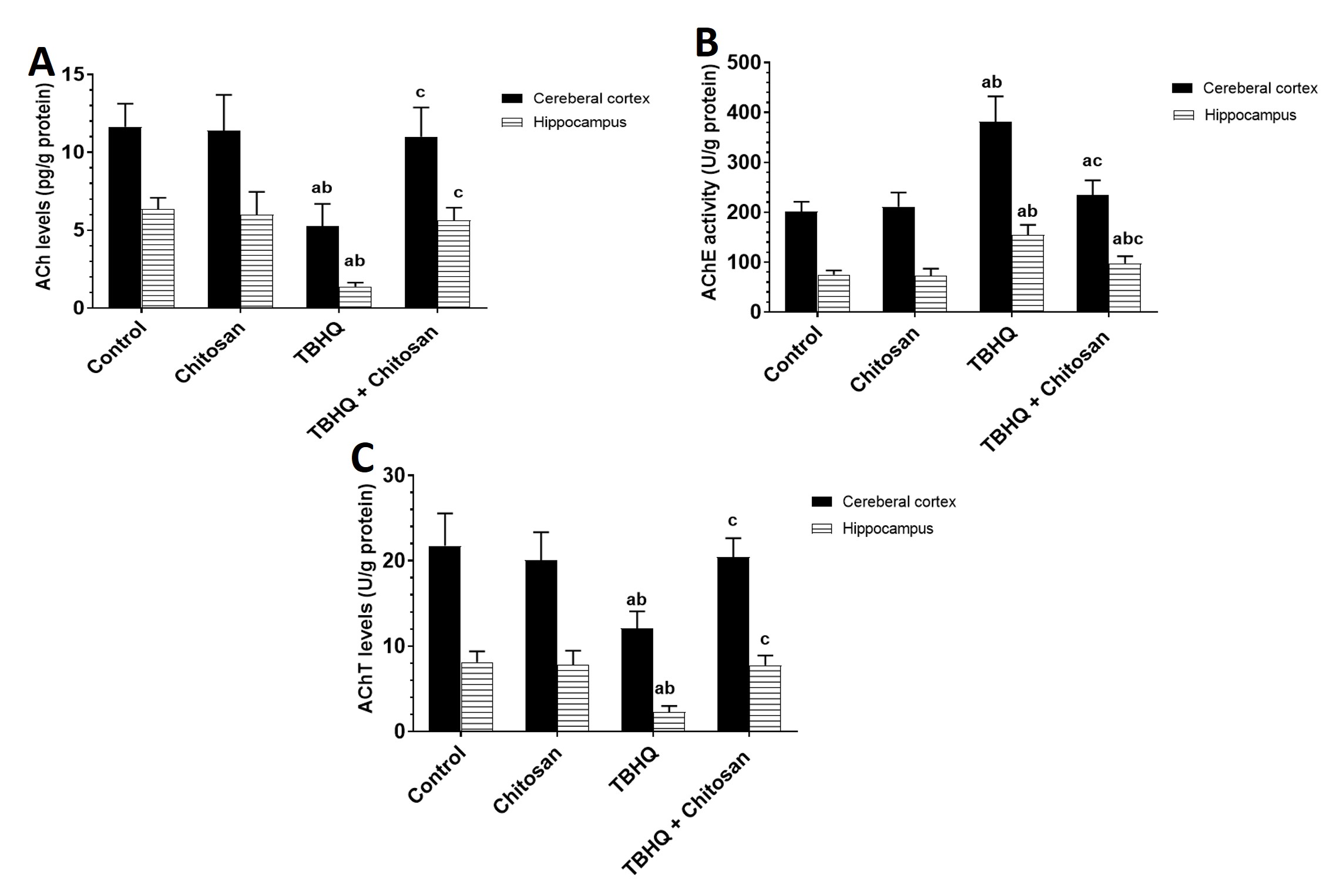 Fig. 5.
Fig. 5. Effects of Treatments on Cholinergic System in the Hippocampus and Cerebral Cortex. (A) Levels of Ach in the cerebral cortex and hippocampus. Data are presented as mean
Under normal conditions, AChE was as low as that in the control group. However, AChE levels in the TBHQ group were significantly higher in the cerebral cortex and hippocampus than in the control and chitosan groups. The cerebral cortex in the TBHQ and chitosan group was significantly different from that in the control and TBHQ groups. The hippocampi in the TBHQ and chitosan group were significantly different from those in the control, chitosan, and TBHQ groups (Fig. 5B).
AChT levels in the TBHQ group were significantly lower than those in the control and chitosan groups. The TBHQ and chitosan group was significantly different from the TBHQ group. The chitosan group was slightly different from the control group (Fig. 5C).
The chitosan group showed a highly significant difference compared with the control group. The enzymatic activity of GSH was significantly lower in the TBHQ group than in the control and chitosan groups. The TBHQ and chitosan group showed significantly different GSH levels in the brain parts (cerebral cortex and hippocampus), liver, and kidney compared with the control, chitosan, and TBHQ groups (Fig. 6A,C,E).
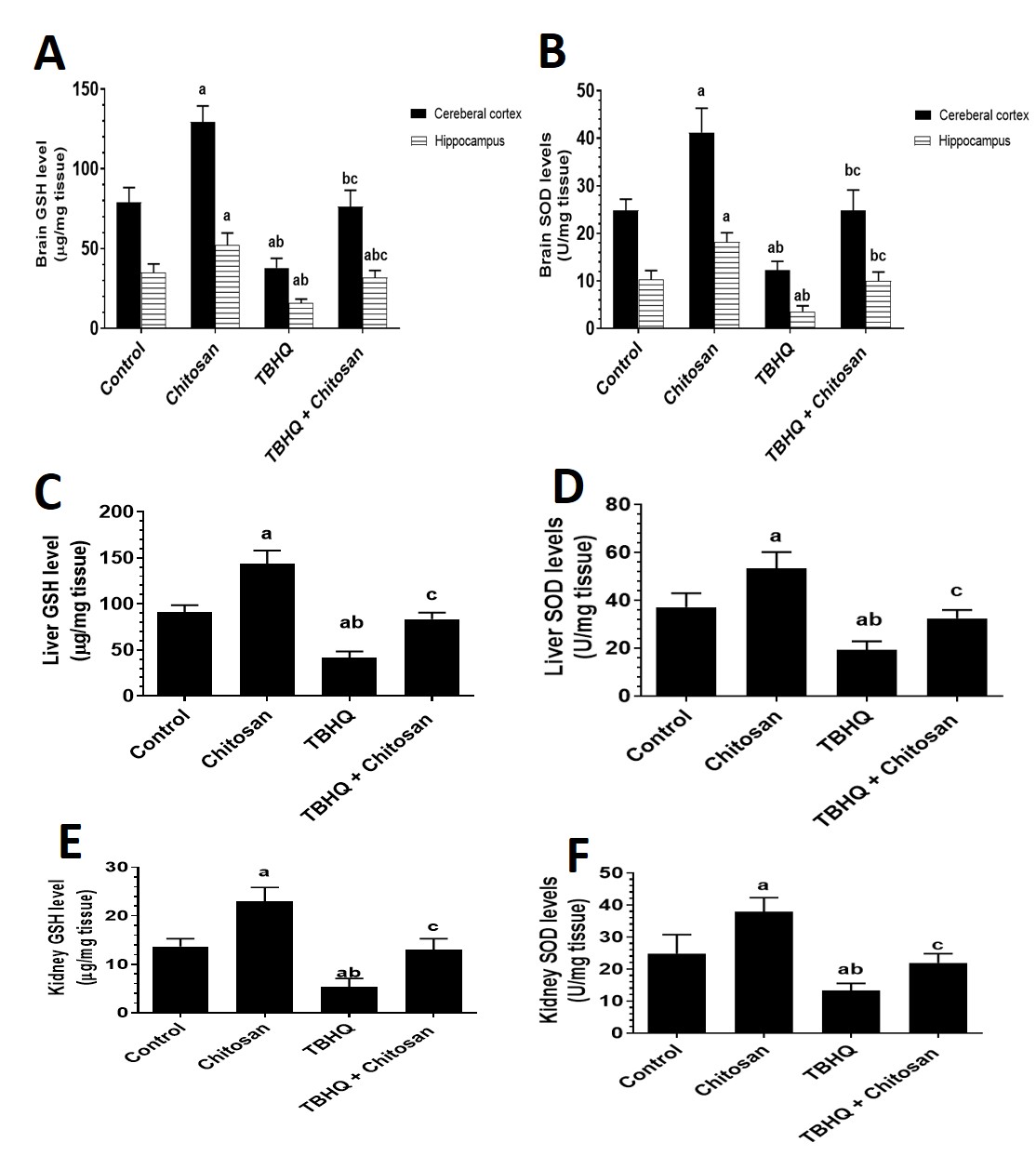 Fig. 6.
Fig. 6. Effects of Treatments on Oxidative Stress in the Hippocampus and Cerebral Cortex. (A) Levels of GSH in the cerebral cortex and hippocampus. (B) Levels of SOD in the cerebral cortex and hippocampus. (C) Levels of GSH in the livers of all groups of rats. (D) Levels of SOD in the livers of all groups of rats. (E) Levels of GSH in the kidneys of all groups of rats. (F) Levels of SOD in the kidneys of all groups of rats. Data are presented as mean
The values of SOD levels was significant higher in the brain parts (cerebral cortex and hippocampus), liver and kidney in the chitosan group than in the control group. The enzymatic activity of SOD was significantly lower in the TBHQ group than in the control and chitosan groups. The TBHQ and chitosan groups differed significantly from both the chitosan-only and TBHQ-only groups (Fig. 6B,D,F).
The activity of MDA was significantly different in the TBHQ group compared with the control and chitosan groups. The MDA level was significantly lower in the TBHQ and chitosan group than in the TBHQ group and relatively lower in the chitosan group than in the control group (Fig. 7A–C).
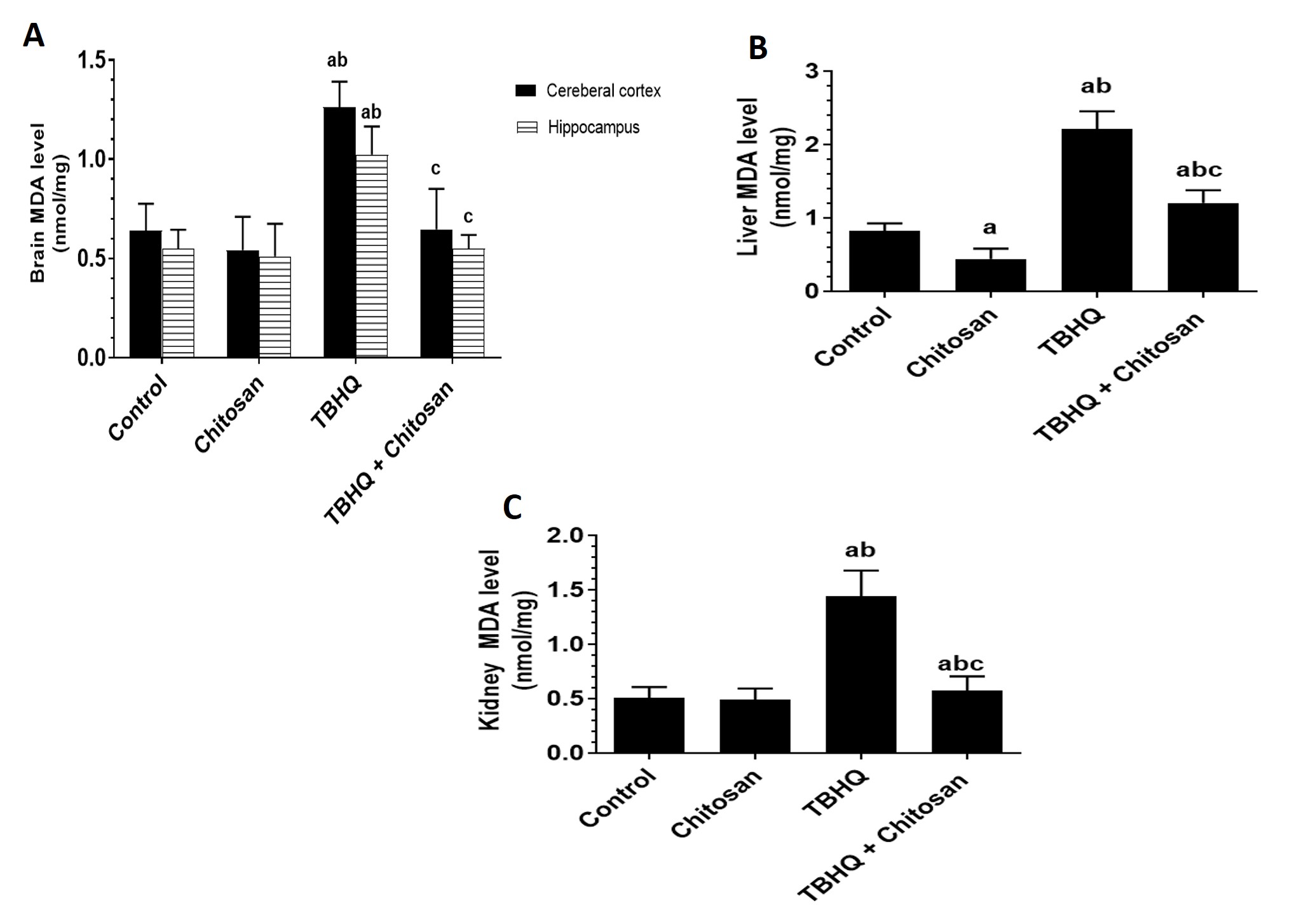 Fig. 7.
Fig. 7. Effects of Treatments on MDA in the Brain, Liver and Kidney. (A) Levels of MDA in the cerebral cortex and hippocampus. (B) Levels of MDA in the livers of all groups of rats. (C) Levels of MDA in the kidneys of all groups of rats. Data are presented as mean
The cerebral cortex in the control and chitosan groups (Fig. 8A,B) exhibited normal architecture. In contrast, the TBHQ group (Fig. 8C) exhibited pyramidal cell distribution, pyknosis (thin arrow), and neurocyte chromatolysis (NCH) thick arrow. The Chitosan + TBHQ group showed in significant pyramidal cell protection from nuclear shrinkage (PKC) and NCH resulting from TBHQ (Fig. 8D).
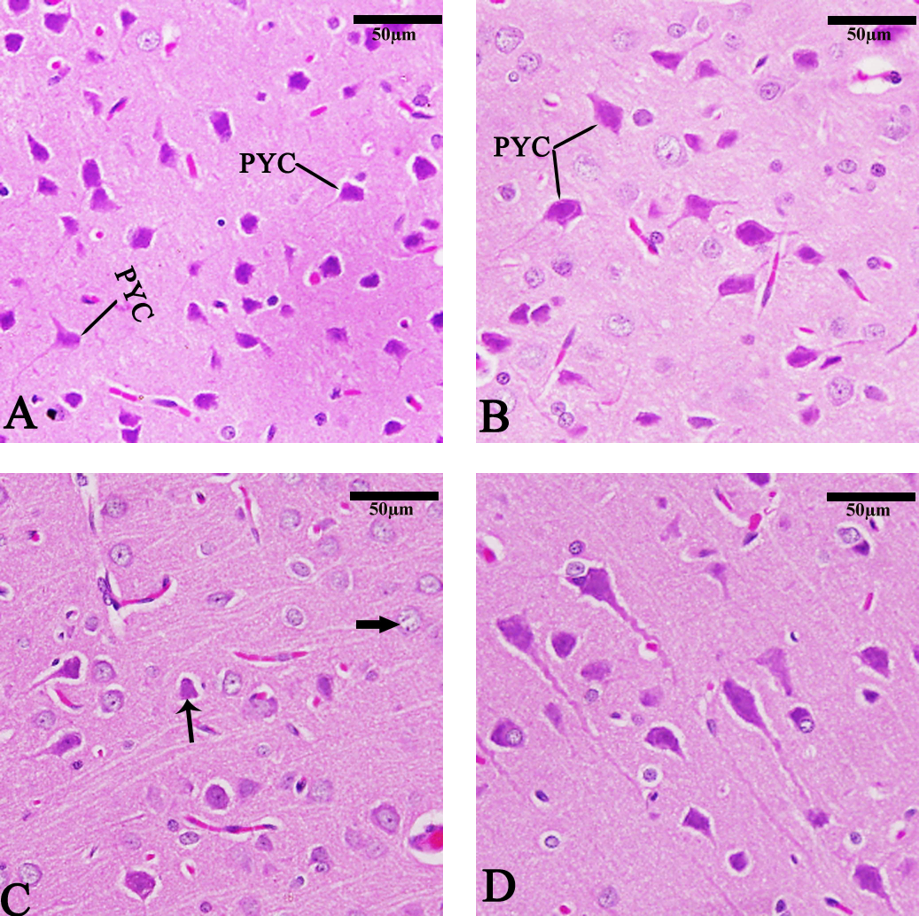 Fig. 8.
Fig. 8. Photomicrograph of sagittal sections of the cerebral cortex showing pyramidal cell distribution (PYC), pyknosis (PKC) (thin arrow), and neurocyte chromatolysis (NCH) (thick arrow). (A) Control group, (B) Chitosan group, (C) TBHQ group, (D) Chitosan and TBHQ group (hematoxylin and eosin, 400
The cerebellum in the chitosan group (Fig. 9A,B) showed normal sections. In the TBHQ group (Fig. 9C), observed degenerated or abnormal Purkinje cells (PCL). The TBHQ and chitosan group (Fig. 9D) exhibited protection of the PCL, internal granular layer, and molecular layer compared to the TBHQ group.
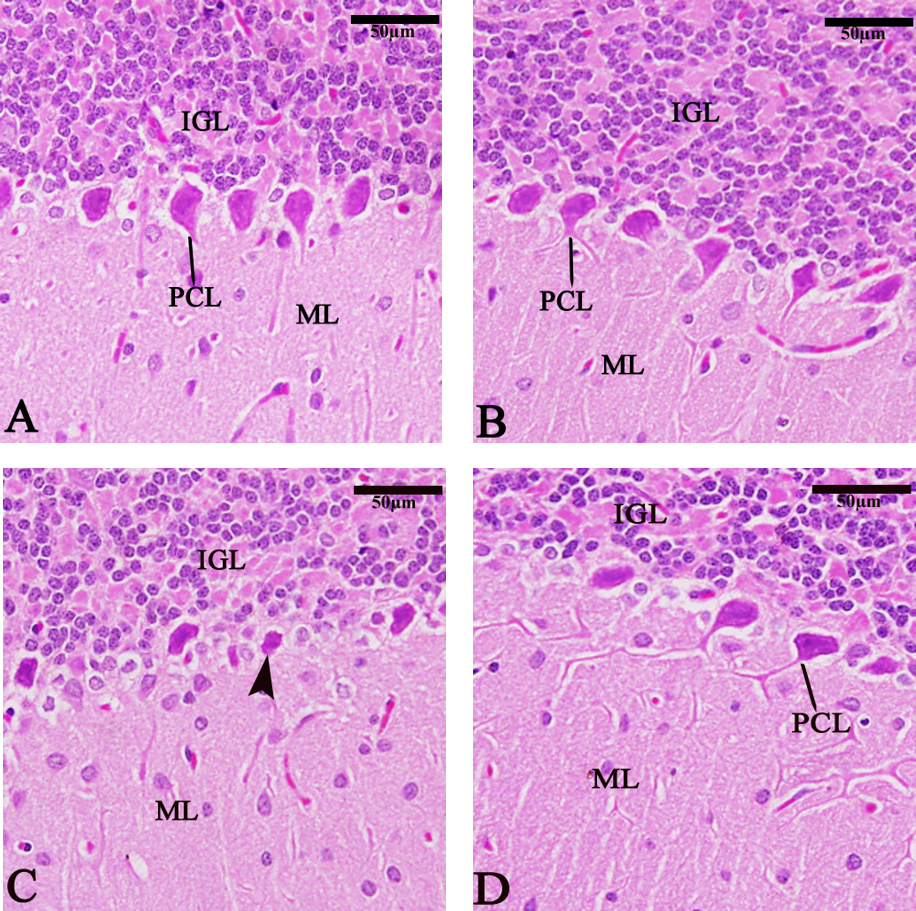 Fig. 9.
Fig. 9. Photomicrograph of sagittal sections of the cerebellum showing the Purkinje cell layer (PCL), degenerated Purkinje cell (arrowhead), internal granular layer (IGL), and molecular layer (ML). (A) Control group, (B) Chitosan group, (C) TBHQ group, (D) Chitosan and TBHQ group (hematoxylin and eosin, 400
Sagittal sections of the medulla oblongata showed normal medullary neurons (MN) (Fig. 10A). The chitosan group (Fig. 10B) showed a relative increase in MN size compared with the control group. The TBHQ group showed a decrease in MN size and PKC nuclear shrinkage (Fig. 10C). The TBHQ+ Chitosan group (Fig. 10D) showed the protective antioxidant role against TBHQ toxins.
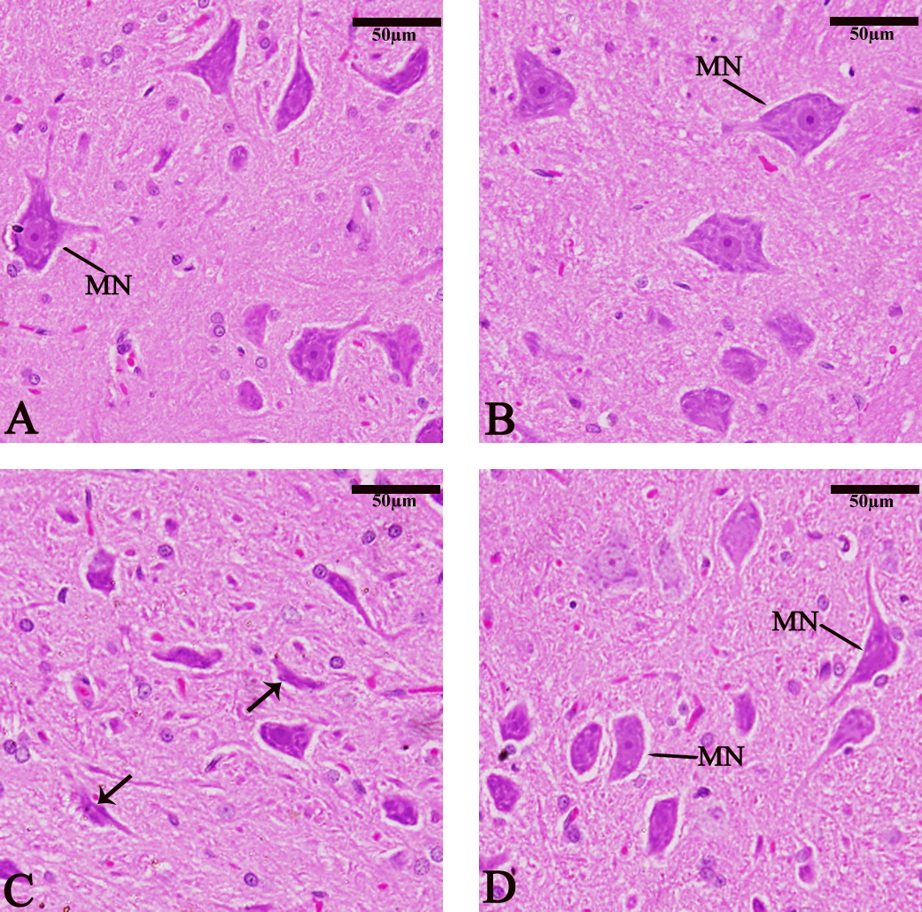 Fig. 10.
Fig. 10. Photomicrograph of sagittal sections of the medulla oblongata showing medullary neurons (MN) and pyknosis (PKC) (thin arrow). (A) Control group, (B) Chitosan group, (C) TBHQ group, (D) Chitosan and TBHQ group (hematoxylin and eosin, 400
The pathological scoring system was performed to assess the histopathological changes in the cerebral cortex, cerebellum and medulla oblongata of all groups. The pathology score was significantly increased in all brain regions of TBHQ group as compared to control, and that chitosan treatments reversed these pathological changes (Fig. 11).
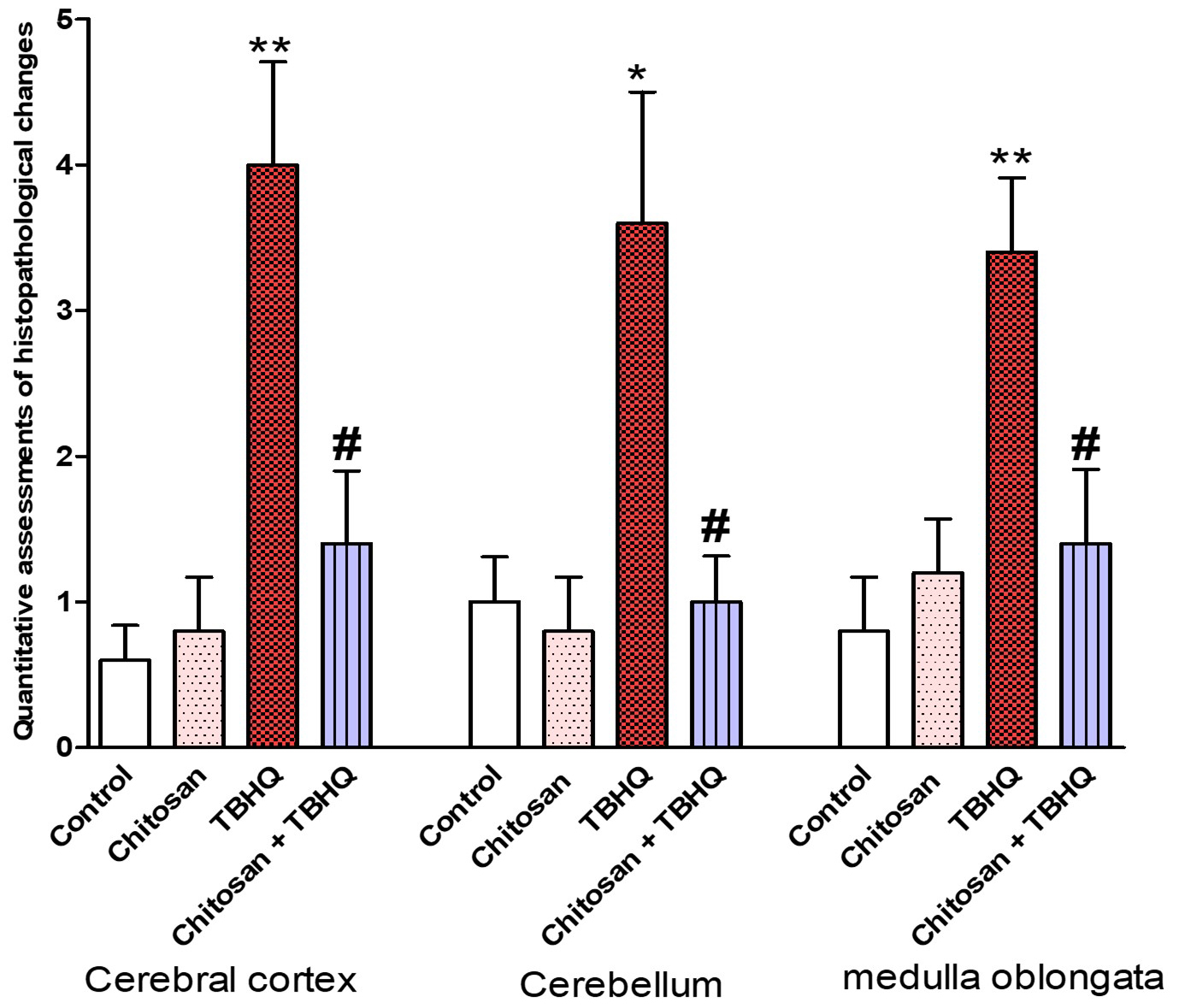 Fig. 11.
Fig. 11. Quantitative assessments of pathological anatomical changes in the three brain regions (cerebral cortex, cerebellum and medulla oblongata). The evaluation was conducted in five fields. Data are expressed as mean
Liver tissue was observed normally in the control and chitosan groups (Fig. 12A,B). In TBHQ group (Fig. 12C) observed several changes in the liver specifically hepatocellular degeneration, inflammatory cells, and hemorrhage. The TBHQ and chitosan group (Fig. 12D) exhibited protection of chitosan against TBHQ. The pathology score was significantly increased in the liver of TBHQ group compared to control, and chitosan treatments reversed these pathological changes (Fig. 12E).
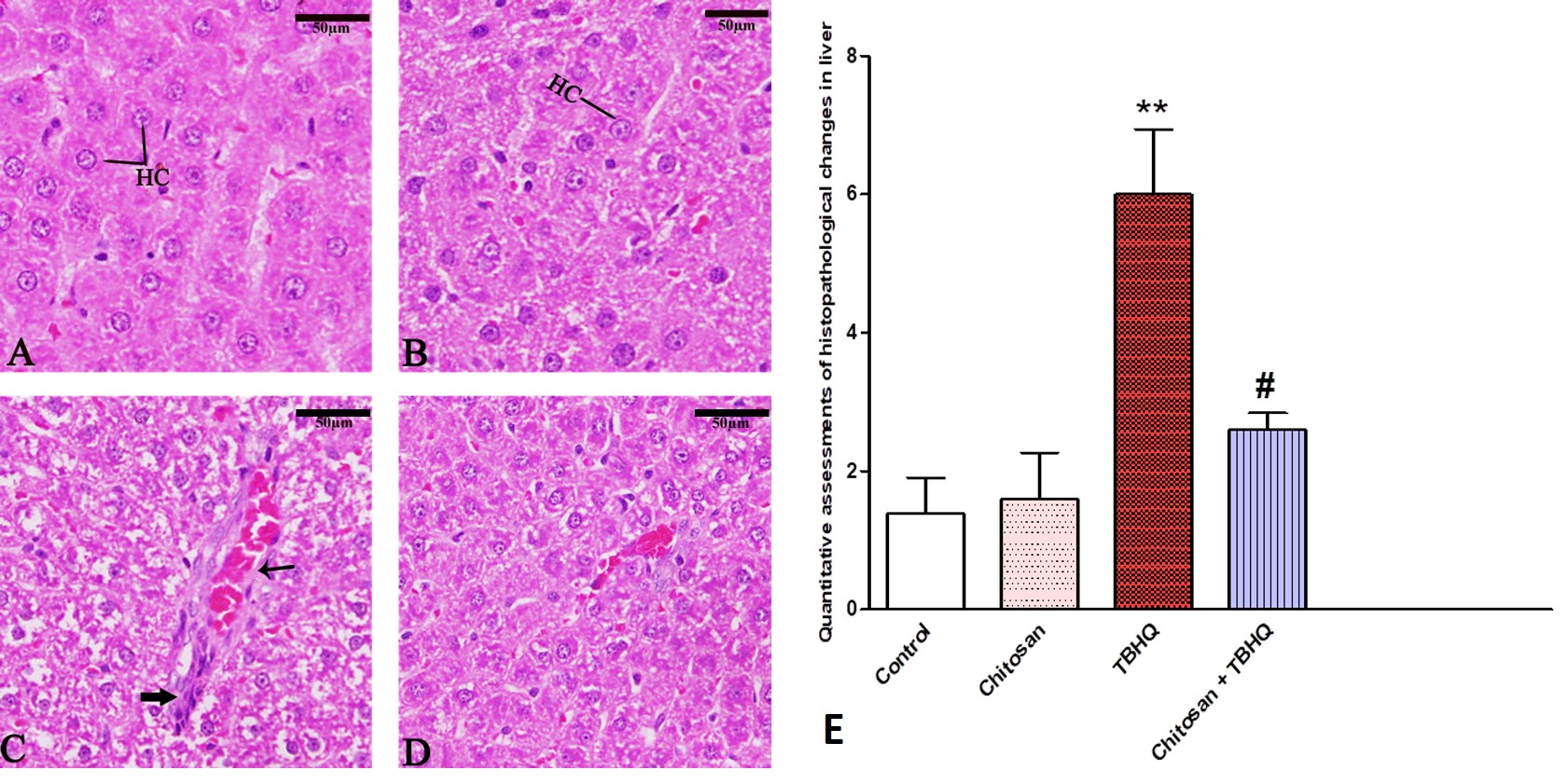 Fig. 12.
Fig. 12. Photomicrograph of Sagittal sections in rat’s liver showing hepatocyte (HC), Hemorrhage (Thin arrow) inflammatory cells (Thick arrow). (A) Control group, (B) Chitosan group, (C) TBHQ group. (D) Chitosan and TBHQ group. Sections are stained with hematoxylin and eosin. 400
Photomicrographs of H&E-stained sections in the rat kidney showed normal structure in glomeruli and renal tubules in control and chitosan groups group (Fig. 13A,B). In (Fig. 13C) TBHQ group-exposed rats showed inflammatory cells around the glomerulus and between renal tubules, Also, damage to the renal tubes was observed. in addition, to hemorrhage between renal tubules. The TBHQ+Chitosan (Fig. 13D) markedly prevented TBHQ-induced kidney injury in rats. The pathology score was significantly increased in the kidney of TBHQ group compared to control, and chitosan reduced these changes (Fig. 13E).
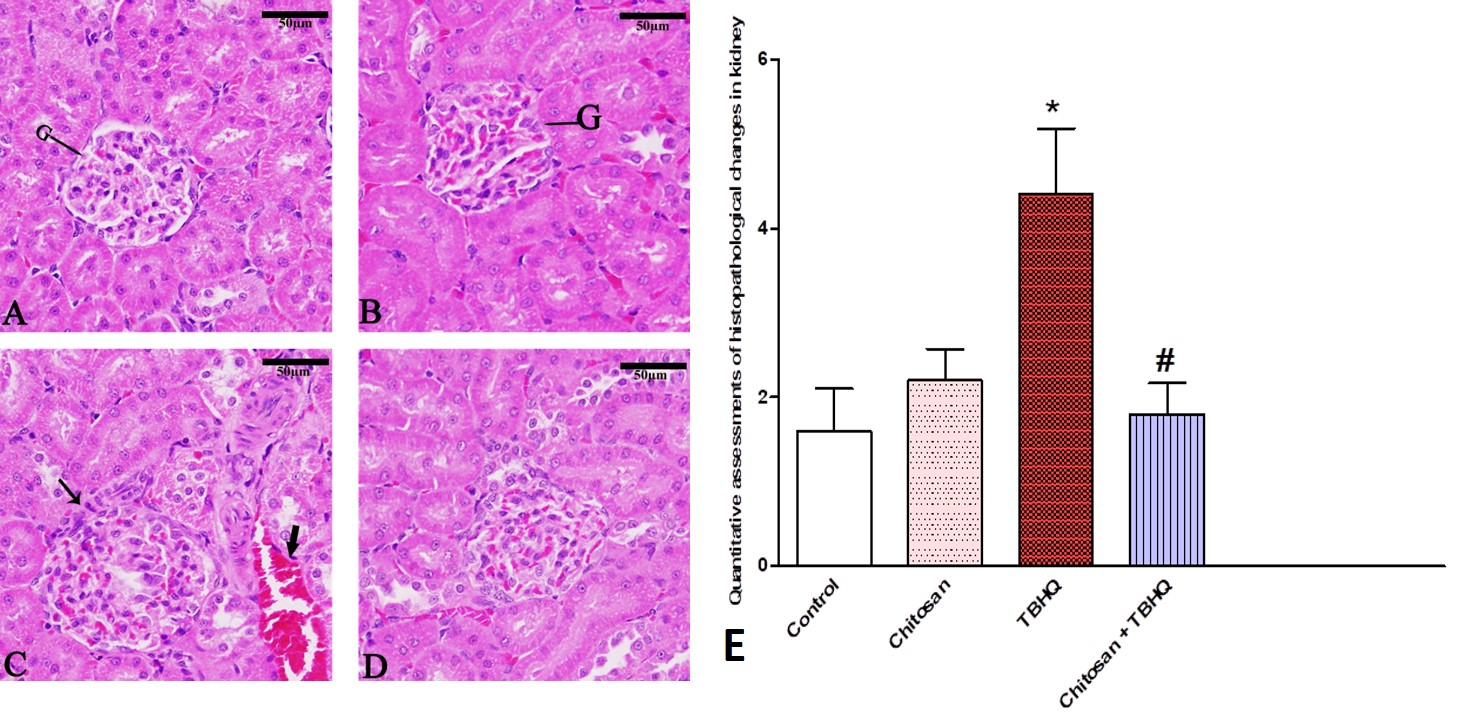 Fig. 13.
Fig. 13. Photomicrograph of Sagittal sections in rat’s kidney showing glomeruli and renal tubules, Hemorrhage (Thick arrow), and inflammatory cells (Thin arrow). (A) Control group, (B) Chitosan group, (C) TBHQ group. (D) Chitosan and TBHQ group. 400
TBHQ exhibits diverse actions under experimental conditions and can have harmful effects on all living organisms upon excessive intake. Our study used a TBHQ dose of 1500 mg over 3 weeks, according to previous studies [4, 34], which categorized TBHQ as a class 4 compound: oral fatal dose for mice of 300–2000 mg/kg body weight. According to the USFDA and Chinese National Standards, the maximum allowable amount of TBHQ in food is 200 mg/kg. Our results showed that TBHQ has damage effects in rat organs, similar to that reported by Wang et al. [2], suggesting that excessive intake of TBHQ may have adverse effects on human health. Vishakha et al. [8] suggested that chitosan reduces the potential toxicity of TBHQ as a food additive. Abd El-Hack et al. [11] have reported that chitosan is a polysaccharide derived from chitin, an abundant natural polymer found in the exoskeletons of crustaceans, insects, and arthropods.
This study explored the effect of TBHQ in a male rat model. No deaths were recorded in the TBHQ or control groups. Exposure to TBHQ increased the body weight of male rats. These findings are consistent with those of Wang et al. [35] and Zhu et al. [36], who suggested that TBHQ increased body weight, reduced blood glucose metabolism, and increased serum insulin levels. Our study showed that chitosan decreased the body weight of male rats. These findings align with those of Anraku et al. [9], who indicated that chitosan is a safe and effective dietary supplement that can help people lose weight by improving calorie balance through lowering the amount of dietary fat absorbed.
Behavioral effects are closely linked to the degree of neuronal dysfunction in Purkinje cells, which is known to have a harmful pathophysiological impact on the brain of rats, reflected in the interruption of normal learning and memory [37, 38]. In this study, we evaluated the protective effects of chitosan on learning and memory. Our findings are in accordance with those of Yu et al. [14], who showed that the chitosan extract can treat brain disease and that the blood–brain barrier formed by tight junctions of endothelial cells, pericytes, and astrocytes, allows only hydrophobic molecules weighing
The MWM test is a universal method for testing cognitive function in rodents. Rats are able to swim during the test after training period as a behavioral marker to reach the platform, and the total distance travelled represents their thigmotactic behavior. This makes them indispensable for studying aging, neurodegenerative diseases, and effects of therapeutic drugs [40]. Rats exposed to TBHQ showed impaired cognitive function and spent a long time and distance finding the hidden platform. Therefore, further research is required in this area. While treatment with TBHQ and chitosan improved functional recovery, similar to that reported by Yu et al. [14], chitosan such as COS promoted peripheral nerve regeneration, partially restored neuron damage, improved early neuro reflex behavior, reduced the cerebral infarction volume, and attenuated nerve cell degeneration. In addition, it enhances the recovery of damaged sensory and motor functions in the brain.
Short- and mid-term memories were evaluated using the PAL behavioral task [41]. Our study showed cognitive dysfunction, such as memory and attention impairment, anxiety, and other mood disorders, after exposure to TBHQ. In addition, the TBHQ group spent more time in the dark than other groups. Short- and mid-term memory consolidation were impaired by TBHQ because latency represents the memory, and the darkroom stay time indicates memory consolidation. Over the past 20 years, chitosan nanoparticles have achieved considerable maturity in treating brain diseases. Many types of chitosan nanoparticles have been shown to improve the therapeutic efficacy of different brain diseases owing to their biocompatibility, biodegradability, and low toxicity. Overall, chitosan-based nanoparticles are excellent carriers for treating brain diseases [14].
Rats exposed to TBHQ had TBHQ accumulation in vital organs, including brain, liver, and kidney. This accumulation causes generation of free radicals such as ROS. The increased reactive species can cause morphological changes, impaired biological macromolecules, and reduced antioxidants defense. Crustaceans, insects, and certain bacteria all contain chitosan, the second most abundant biopolymer in nature. Chitosan increases a variety of biological functioning, including cellular responses, antioxidant activity, and macrophage function [10]. Chitosan is an antioxidant agent that supports the idea that an oligopolysaccharide will act as an antioxidant against cell toxicity [15].
In our study, we selected the brain, liver, and kidneys as the primary organs of interest due to their susceptibility to oxidative stress. Of all organs, the brain is most affected by oxidative stress owing to its high lipid content and oxygen utilization [42]. The present study analyzed brain enzymes (Ach, AchT, and AchE), antioxidants, and oxidative stress in the cerebral cortex and hippocampus. Cholinergic neurons are widely distributed in the CNS. Rats exposed to TBHQ had Ach and AchT decline, inconsistent with the findings of Huang et al. [43] who reported that Ach as one of the most important neurotransmitters in the central cholinergic system, besides increasing central ACh levels, can enhance memory ability and comprehensively improve brain function. TBHQ-treated rats exhibited decreased AChE activity in the brain. Similar results have been reported previously [21, 43] that AChE is a significant biological component of the membrane that contributes to its integrity. Liu H et al. [44] who reported that oxidative stress might be responsible for the decreased activity of the AChE in the diaphragm. Inhibiting ACh degradation in the CNS by inhibiting AChE alleviates learning and memory impairments and hydrolyzes Ach to choline and acetate in cholinergic brain synapses and neuromuscular junctions. In addition, our study identified brain enzyme–marked resistance to the effects of chitosan on TBHQ. TBHQ exposure affects cell viability by generating ROS and other reactive species via enzymatic conversion. This enzymatic and non-enzymatic electron shuffling indicated that TBHQ may be present in its fully reduced form (hydroquinone), oxidized form (tBBQ), and as a semiquinone anion radical, contributing to the observed carcinogenic effects of tBHQ [18].
liver and kidneys as the most prominent target organs for their toxicity and oxidative stress. The liver and kidney are greatly liable to toxicity due to their role in the metabolism of most xenobiotics such as drugs and foreign compounds [42]. Our results showed that TBHQ had a strong effect on the brain, liver and kidney caused a decline in antioxidant enzyme and non-enzyme activities, including those of SOD and GSH. In addition, MDA levels, representing oxidative stress such, increased in the brain. Our experimental data are in agreement with those of Alqahtani and Albasher [42] who found a marked increase in hepatic MDA concentrations and a significant reduction in enzymatic and non-enzymatic antioxidants and markers. SOD, Catalase (CAT), and glutathione peroxidase (GPx) form the first line of defense against ROS, and a decrease in their activities contributes to oxidative stress in tissues. GSH is a key non-enzymatic antioxidant that binds to and eliminates oxidative agents. Decrease in GSH levels has been associated with changes in the oxidative stress response [45]. GSH is necessary for ROS detoxification in brain cells. In neurological disorders, it was found that an impaired GSH system in the brain is associated with oxidative stress [46]. SOD is an antioxidant that eliminates singlet oxygen and converts superoxide radicals into hydrogen peroxide. CAT effectively promotes hydrogen peroxide decomposition, which prevents lipid peroxidation [25]. GSH and GPx catalyze the decline of lipid peroxides and hydrogen peroxide, whereas glutathione reductase advances the NADPH-driven reduction of oxidized GSH [47]. The depletion of these antioxidants and particles can lead to the accumulation of hydrogen peroxide, which can impair neuronal cell integrity [48]. Chitosan supports antioxidant activities (SOD and GSH) and reduces oxidative stress (MDA), which is in accordance with the findings of Li et al. [7], who reported that chitosan is effective in improving antioxidant activity, which in turn improves its antitumor activity.
Light microscopic examination of three brains sections (cerebral cortex, cerebellum, and medullary neurons), liver and kidney revealed extensive cell changes due to toxicity in rats, which caused morphological changes such as dilation of blood vessels, necrosis, pyknosis, and NCH. Our experimental findings do not align with those of Sargazi et al. [49], who reported that TBHQ treatment could enhance the antioxidant status in the brain and heart tissues of rats with chronic toxicity of diazinon due may be different doses of TBHQ or different toxins. While the current study showed that chitosan significantly protected the brain, liver and kidney in rats, there were no significant changes in the morphology of chitosan rats. Our experimental findings are in accordance with those of Thilagar and Samuthirapandian [10] who reported that chitosan significantly protected the gills, liver, and intestines. There were no significant changes in the morphology or antioxidants in chitosan rats indicating its non-toxic nature.
Cognitive and behavioral impairments observed in TBHQ-treated rats appear to be directly associated with increased oxidative stress, particularly within the hippocampus and cortex, which are central to memory and learning functions. TBHQ exposure significantly elevates MDA levels—a key marker of lipid peroxidation—and decreases antioxidant defenses such as GSH and SOD in the brain. These changes disrupt neuronal homeostasis and increase susceptibility to neuroinflammation, resulting in neural damage that impairs cholinergic transmission pathways. Consequently, rats exposed to TBHQ demonstrate cognitive deficits, evidenced by prolonged escape latency and greater swim distance in the MWM test, and reduced latency to enter the dark room in PAL tasks, signifying memory impairments [11, 37, 42].
Additionally, histological analysis reveals that oxidative stress from TBHQ exposure causes degeneration in Purkinje cells, a critical component for motor coordination, further contributing to observed behavioral deficits [37, 38]. This study also highlights chitosan’s protective antioxidant effects, which appear to counteract TBHQ-induced oxidative damage by restoring SOD and GSH levels, reducing MDA, and stabilizing enzyme activities essential for cognitive functions [5, 14, 15]. Chitosan’s neuroprotective impact is reflected in improved behavioral outcomes, as rats in the chitosan-treated group showed normalized escape latency and platform crossings in MWM and PAL tests compared to TBHQ-only treated rats. These findings suggest that chitosan could be a promising therapeutic agent for oxidative stress-related neurodegenerative disorders by mitigating oxidative damage and preserving cognitive functions.
Cholinergic components, including ACh, AChE, and ChAT, play a crucial role in neuroprotection and neurodegenerative diseases such as Alzheimer’s. Oxidative stress occurs due to an imbalance between ROS and the body’s antioxidant defenses, leading to cellular damage, particularly in neurons. AChE, the enzyme responsible for breaking down ACh, is sensitive to oxidative stress, which can either inhibit or overstimulate its activity. This disruption causes an imbalance in ACh levels, contributing to cognitive decline and memory impairment, as observed in Alzheimer’s disease [50, 51, 52]. Oxidative stress can also reduce ChAT activity, thereby impairing ACh synthesis, further exacerbating cholinergic dysfunction [50]. Antioxidant parameters, such as SOD and CAT, can mitigate oxidative damage by scavenging free radicals, preserving the activity of AChE and ChAT, and supporting normal cholinergic transmission. Studies indicate that the administration of antioxidants can significantly counteract the cholinergic dysfunction caused by oxidative stress, protecting cognitive functions [50, 53]. Previous research has shown chitosan’s general antioxidant properties, but its impact on behavior and cognition under neurotoxic conditions is less understood. This research specifically addresses the effects of TBHQ-induced neurotoxicity, providing valuable insights into oxidative stress mechanisms associated with a common food preservative [42, 43]. This study advances the field by providing a detailed evaluation of cognitive outcomes through behavioral assessments, including the MWM and PAL tests, to assess memory and learning impairments induced by TBHQ. Findings indicate that chitosan not only protects brain cells but also preserves cognitive function, supporting its neuroprotective efficacy from a functional perspective [14, 20, 54].
Additionally, the research expands the focus to multiple organs, showing how chitosan mitigates TBHQ toxicity in the liver, kidney, and brain, which highlights its potential for broader therapeutic application in oxidative stress-related disorders [49]. Biochemical analysis of oxidative markers such as MDA, GSH, and provides mechanistic insights, underscoring chitosan’s effectiveness in restoring antioxidant defenses and reducing cellular damage [2, 11]. This comprehensive approach sets the stage for future clinical applications of chitosan in mitigating neurotoxicity and organ damage from oxidative stress.
The present study confirmed the susceptibility of the brain, liver, and kidney to the toxic effects of oral TBHQ exposure in male rats. Our findings further validated previous results showing similar effects on the brain using female rats [55]. This study examined the protective and therapeutic efficacy of chitosan in enhancing tissue regeneration following TBHQ-induced damage in rat brains liver and kidney. Based on its proven properties, chitosan is a promising material for the removal of TBHQ and for improving the health of rats. Therefore, chitosan can be used to protect against TBHQ damage through its antioxidant (GSH and SOD) and anti-inflammatory effects, ability to reduce oxidative stress (MDA) and brain enzyme levels (Ach, AchE, and AchT), and behavioral tests (MWM and PAL). However, a limitation of the current study was the low solubility of chitosan in aqueous media. Although a wide range of chitosan derivatives with absorption properties and health benefits exist, the choice of the most suitable type remains under investigation. The limited behavioral assessments (Only memory-related tests were used), and the Absence of cellular and molecular pathway analysis. This study has great scope for improvement and is based on a large number of promising results. We envision chitosan as a promising material with significant potential in drug delivery and various biomedical applications.
MWM, Morris Water Maze; PAL, Passive Avoidance Learning; CMC, Carboxymethylcellulose; TBHQ, Tert-butylhydroquinone; PBS, Phosphate-buffered Saline; H&E, Hematoxylin and Eosin; Ach, Acetylcholine; AchE, Acetylcholinesterase; AchT, Choline Acetyltransferase; SOD, Superoxide Dismutase; MDA, Malondialdehyde; GSH, Total Glutathione; ROS, Reactive Oxygen Species; ELISA, Enzyme-linked Immunosorbent Assay; CNS, Central Nervous System; PYC, Pyramidal Cells Distribution; PKC, Pyknosis; NCH, Neurocyte Chromatolysis; PCL, Purkinje Cell Layer; IGL, Internal Granular Layer; ML, Molecular Layer; MN, Medullary Neurons; USFDA, United States Food and Drug Administration; H2O2, Hydrogen Peroxide; SD, Standard Deviation; HUVECs, Human Umbilical Vein Endothelial Cells.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization, GA and WSAQ; methodology, SA, MMA, FA, WSAQ, and GA; software, SA, GA and WSAQ; validation GA and WSAQ; formal analysis, SA, MMA, FA, WSAQ, and GA; investigation, GA and WSAQ; resources, GA; data curation, SA, MMA, FA, WSAQ, and GA; writing—original draft preparation, SA, MMA, FA, WSAQ, and GA; writing—review and editing SA, MMA, FA, WSAQ, and GA; visualization, GA and WSAQ; supervision, GA; project administration, GA; funding acquisition, GA. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The animal study protocol was approved by the Institutional Review Board of KING SAUD UNIVERSITY (protocol code KSU-SE-22-88).
The authors extend their sincere appreciation to the staff of this research support project (King Saud University, Riyadh, Saudi Arabia). The authors thank Prince Naif Health Research Center, Investigator support Unit for the language editing service provided.
This research was funded by King Saud University Research Support Project, grant number RSP-2024/95.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.













