1 Department of Orthopedics, Chongqing Traditional Chinese Medicine Hospital, 400021 Chongqing, China
2 Department of Clinical Laboratory, the Second Affiliated Hospital, Chongqing Medical University, 400000 Chongqing, China
3 Division of Cardiology, The First Affiliated Hospital of Chongqing Medical University, 400016 Chongqing, China
4 Department of Rehabilitation Medicine, The First Affiliated Hospital of Chongqing University of Chinese Medicine, Chongqing Traditional Chinese Medicine Hospital, 400021 Chongqing, China
5 Chongqing Precision Medical Industry Technology Research Institute, 400000 Chongqing, China
†These authors contributed equally.
Abstract
The development of biomaterials capable of accelerating bone wound repair is a critical focus in bone tissue engineering. This study aims to evaluate the osteointegration and bone regeneration potential of a novel multilayer gelatin-supported Bone Morphogenetic Protein 9 (BMP-9) coated nano-calcium-deficient hydroxyapatite/poly-amino acid (n-CDHA/PAA) composite biomaterials, focusing on the material-bone interface, and putting forward a new direction for the research on the interface between the coating material and bone.
The BMP-9 recombinant adenovirus (Adenovirus (Ad)-BMP-9/Bone Marrow Mesenchymal Stem Cells (BMSc)) was produced by transfecting BMSc and supported using gelatin (Ad-BMP-9/BMSc/Gelatin (GT). Multilayer Ad-BMP-9/BMSc/GT coated nano-calcium deficient hydroxyapatite/polyamino acid (n-CDHA/PAA) composite biomaterials were then prepared and co-cultured with MG63 cells for 10 days, with biocompatibility assessed through microscopy, Cell Counting Kit-8 (CCK-8), and alkaline phosphatase (ALP) assays. Subsequently, multilayer Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterial screws were fabricated, and the adhesion of the coating to the substrate was observed using scanning electron microscopy (SEM). In vivo studies were conducted using a New Zealand White rabbit intercondylar femoral fracture model. The experimental group was fixed with screws featuring multilayer Ad-BMP-9/BMSc/GT coatings, while the control groups used medical metal screws and n-CDHA/PAA composite biomaterial screws. Fracture healing was monitored at 1, 4, 12, and 24 weeks, respectively, using X-ray observation, Micro-CT imaging, and SEM. Integration at the material-bone interface and the condition of neo-tissue were assessed through these imaging techniques.
The Ad-BMP-9/GT coating significantly enhanced MG63 cell adhesion, proliferation, and differentiation, while increasing BMP-9 expression in vitro. In vivo studies using a rabbit femoral fracture model confirmed the biocompatibility and osteointegration potential of the multilayer Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterial screws. Compared to control groups (medical metal screws and n-CDHA/PAA composite biomaterial screws), this material demonstrated faster fracture healing, stronger osteointegration, and facilitated new bone tissue formation with increased calcium deposition at the material-bone interface.
The multilayer GT-supported BMP-9 coated n-CDHA/PAA composite biomaterials have demonstrated favorable osteogenic cell interface performance, both in vitro and in vivo. This study provides a foundation for developing innovative bone repair materials, holding promise for significant advancements in clinical applications.
Keywords
- bone tissue engineering
- composite biomaterial
- gelatin-supported BMP-9 coating
- n-CDHA/PAA
Medical metallic biomaterials have become the main choice for clinical fracture internal fixation treatment worldwide because of their high mechanical strength and biological inertness. However, due to its shortcomings such as masking effect, metal electrolytic corrosion, easy fatigue, imaging interference, poor histocompatibility, and the need for secondary surgical removal, non-metallic biomaterials have become another alternative for clinical internal fixation of fractures. Compared with metallic biomaterials, non-metallic biomaterials have lower modulus of elasticity, better biocompatibility and biodegradation properties. The interface between the biomaterial and the host bone is often the key to the success of internal fixation surgery. Ideal osseointegration requires that the bone tissue can be deposited directly on the implant surface, and that the implant is chemically bonded to the bone tissue rather than being in physical contact only, and that the implant can be long-lastingly and sufficiently biomechanically stabilized. However, the vast majority of non-metallic biomaterials studied so far only have good osteoconductive properties and insufficient osteoinductive function. In recent years, the modification of non-metallic biomaterials with surface coatings has been beneficial in promoting the formation of chemical bonding between the material and the host bone, improving the osteointegration interface condition [1]. Therefore, surface coating of nonmetallic biomaterials to make them have good osteoinductive function, so as to obtain a good material-bone interface is one of the focuses of current research on the application of nonmetallic biomaterials.
Surface coating technology, i.e., increasing the physical and chemical properties of the implant surface by certain technical means, is used to change the surface of the implant that is in direct contact with the bone tissue after implant placement, thus promoting faster and better osseointegration of the implant with the bone tissue surface or giving the implant a better biologically active function [2]. Surface coatings have been shown to significantly enhance the functional properties of hip and knee prostheses, improving hardness, wettability, elastic strain, coefficient of friction, and wear resistance [3, 4]. Among these, polyethylene coatings on non-biological material are currently the most prevalent in orthopedic clinics [5, 6]. Extensive research has been conducted on coatings for joint replacement applications, utilizing materials such as diamond-like carbon, graphite-like carbon, tantalum, and titanium nitride. These coatings have been fabricated using various techniques, including physical or chemical vapor deposition, electrodeposition, molten salt heat treatment, laser shaping, and ion implantation. It has shown that the wear rate of ceramic materials is much less than that of polyethylene, and the wear of carbon coatings is several times less than that of ceramic materials [7]. Diamond-like, graphite-like, and tantalum-coated surfaces have proven to exhibit superior mechanical properties, including reduced surface roughness, increased hardness, and improved elastic strain. These coatings also demonstrate enhanced tribological performance, resulting in lower wear rate [4]. Nevertheless, research on these nonmetallic biomaterials has mainly focused on the improvement of physical properties such as coefficient of friction, yet very little research has been conducted on the material-bone interface. Therefore, it is important to develop a surface coating that releases bioactive factors for nonmetallic biomaterials to promote their osteointegration at the interface with bone.
Bioactive factors are a class of peptides that selectively bind to specific, high-affinity cell membrane receptors, triggering a cascade of effects that regulate cell growth and other cellular processes. Currently, bioactive factors that promote new bone production are mainly focused on vascular endothelial growth factor, matrix metalloproteinase family, transforming growth factor and bone morphogenetic proteins (BMPs) [8]. Among them, BMPs are a class of biologically active proteins isolated from bone matrix, which are capable of inducing the differentiation of undifferentiated and differentiated stem cells into chondrocytes, osteoblasts, and osteoclast precursor cells to promote bone production [9, 10]. A study of 14 BMPs showed that five of them (2, 4, 6, 7, and 9) possessed potent osteogenic activity, and BMP-9 was found to have the strongest osteogenic activity [11]. Existing studies have reported that BMP-9 promotes stem cell osteogenic differentiation through various mechanisms [12], such as activating the phosphorylation of the transcription factor Smad1/5/8 to regulate the expression of downstream genes and inducing stem cell osteogenic differentiation [13]; inducing the expression of Hey1 to promote Mesenchymal Stem Cells (MSCs) osteogenesis [14]; interacting with the Notch signaling pathway to induce embryonic fibroblastic stem cells to become bone [15]; and up-regulating the up-regulation of long non-coding RNA (lncRNA) H19 induces early osteogenesis in MSCs [15]. Together, these studies suggest that BMP-9 has a role in promoting osteogenic differentiation of stem cells in vitro and in vivo, and also imply that we BMP-9 may promote fracture healing. Localized release of exogenous growth factors to promote tissue rejuvenation is an effective means of regenerative repair; however, as with other growth factors, BMP-9-coated pro-osteogenic release still suffers from uncontrollable flow, uneven distribution, abrupt release, and over piggybacking at a threshold level to maintain osteoblast proliferation and differentiation. As such, piggybacking for ordered release of the growth factor is critical.
In situ polymerization-derived nano-calcium deficient hydroxyapatite/polyamino acid (n-CDHA/PAA) is a degradable non-metallic biocomposite material. Previous study has shown that it possesses excellent biocompatibility, biomechanics, and biodegradability [16]. To evaluate its potential for internal fixation, we fabricated n-CDHA/PAA composite bio-screws and tested their efficacy in a rabbit intercondylar fracture model. Our findings revealed comparable fixation efficacy of n-CDHA/PAA bioactive screws to metallic screws, along with superior tissue compatibility. However, a significantly lower pull-out strength was observed for n-CDHA/PAA screws within the initial 4 weeks of fixation, potentially compromising internal fixation stability. Observations via SEM and immunohistochemistry revealed that this was likely due to the predominantly mechanical interlocking between the screw threads and bone tissue at the interface, lacking sufficient osteointegration strength. Enhancing the initial integration of the n-CDHA/PAA screws with bone tissue at the interface is an effective approach to mitigate this issue.
In summary, the present study was designed to design a multilayer Gelatin (GT) membrane carrying BMP-9 coating on homemade n-CDHA/PAA composite biomaterial screws, and to study the ordered release of BMP-9 biologic factors, the initial internal fixation strength, and the interface between the bio-screw material and the bone of the coated screws through in vivo and ex vivo experiments. It lays a solid theoretical and practical foundation for the future research on the interface between the coated material and bone.
Bone Marrow Mesenchymal Stem Cells (BMSc) primary cells (HUM-iCell-s011, iCell Bioscience, Shanghai, China) were passed the mycoplasma and Short Tandem Repeat (STR) certification, then cultured, passaged and frozen in liquid nitrogen for spare parts to obtain the appropriate titer of Flag-tagged BMP-9 recombinant adenovirus for transfection of BMSc cells, and Flag-tagged BMP-9 recombinant adenovirus (Ad-BMP-9) was used to transfect BMSc cells.
Cells in good growth status, after reaching
80% density, were washed with Phosphate Buffered Saline (PBS)
(10010002,
Gibco™, Waltham, MA, USA) in culture flasks, added with 0.25% trypsin digest,
incubated at 37 °C for 2 minutes, gently shaken to dislodge all cells,
centrifuged by adding 3 mL of medium, discarded the supernatant, and blown up
into cell suspension. Subsequently, the cells were counted on a hemocyte counting
plate, and the cell density was adjusted to 2
The cells were grouped after attaching to the wall. (1) Ad-vector: the cell fusion rate reached about 50%, change fresh medium 1h in advance, use empty adenovirus, infect the cells with 20 µL of adenovirus in T25 cell bottles for 48 hours, and observe the cells under the microscope after the change of the solution; (2) Overexpression group (Ad-BMP9): the cell fusion rate reached about 50%, change fresh medium 1h in advance, use Flag-tagged BMP-9 adenovirus to infect one T25 cell bottle with 20 µL for 48 hours, and observe the cells under the microscope (BLD-200, COSSIM, Beijing, China).
BMSc cells that had been transfected with Ad-BMP-9 were taken to spread 80% of
the bottom of the dish and centrifuged after trypsin termination. Remove the
supernatant, add complete medium to resuspend, and adjust the cell concentration
to 2
MG63 cells (CRL-1427, ATCC, Manassas, VA, USA) were
authenticated by STR profiling and confirmed to be free of mycoplasma
contamination. Cells were harvested by trypsin digestion, counted, and adjusted
to a density of 2
Group culture: (1) Ad-vector/BMSc/GT+MG63 cell co-culture control group; (2) Ad-BMP-9/BMSc/GT+MG63 cell co-culture group. GT was used as the control, and the proliferation and differentiation of Ad-BMP-9/BMSc/GT-promoted osteoblasts were observed by inverted biomicroscope (CKX3-SLP, OLYMPUS, Japan) morphology observation, Cell Counting Kit-8 (CCK-8), and human alkaline phosphatase (ALP) activity assay on the 1st, 5th, and 10th days, respectively.
The n-CDHA/PAA composite biomaterial discs with 30% n-CDHA mass ratio were
prepared by in situ polymerization method, and the size of the discs was
According to the experimental design the following three experimental subgroups were designed: (1) n-CDHA/PAA control group (without any coating treatment); (2) control group of composite biomaterials of n-CDHA/PAA with single-layer coated GT; and (3) experimental group of composite biomaterials of multilayered Ad-BMP-9/BMSc/GT coated n-CDHA/PAA. After co-culturing the materials of the experimental and control groups with well-grown MG63 cells for 10 days, the cell culture medium was aspirated at day 1, 5, and 10, respectively, followed by two washes with PBS. According to the protocols of the Cell Plasma Membrane Staining Kit with DiO (Green Fluorescence) (C1993S, Beyotime, Shanghai, China), an appropriate volume of green fluorescent cell membrane staining working solution was added and gently agitated to ensure uniform staining of all cells. The cells were incubated at 37 °C in the dark for 20 minutes. The cell membrane staining working solution was then aspirated, and the cells were washed three times with PBS. Finally, 37 °C pre-warmed cell culture medium was added, and the adhesion and growth of MG63 cells on the material surface were observed under an inverted biological microscope. The proliferation of MG63 cells and ALP activity were observed by CCK-8 and ALP activity analysis at three time points, respectively; and the proliferation of MG63 cells and the activity of ALP were detected by Quantitative Polymerase Chain Reaction (q-PCR) and Western Blot (WB), respectively, in the MG63 cells.
Cell proliferation of MG63 cells was assessed using a CCK-8 assay (C0038, Beyotime, China) at three time points (days 1, 5, and 10). Cells were seeded in 96-well plates, and 10 µL of CCK-8 solution was added into each well. Blank wells containing only cell culture medium and CCK-8 solution were used as zero controls. Plates were incubated in a cell culture incubator (BPH-9042, Yiheng, Shanghai) for 1 hour. Absorbance was measured at 450 nm using an enzyme marker (CMax Plus, Molecular Devices, San Jose, CA, USA).
Following the procedure described in the manual, the ALP enzyme-linked immunosorbent assay (ELISA) kit (CB12388-Hu, COIBO, Shanghai, China) was used to perform cell activity analysis of MG63 cells at three different time points (Day 1, Day 5, and Day 10). The cell suspension was diluted with phosphate-buffered saline (PBS). Cells were lysed and intracellular components were released through repeated freezing and thawing. After centrifugation for 20 minutes, the supernatant was collected. Standard wells, sample wells, and blank wells were set up accordingly. A standard curve was generated using different concentrations of the standard in the standard wells. Sample wells were loaded with 10 µL of cell lysate, followed by the addition of 100 µL of the enzyme-labeled reagent. After incubation at 37 °C for 60 minutes, the wells were washed five times with diluted washing solution. The chromogenic agent was added, and color development was allowed proceed in the dark at 37 °C for 15 minutes. Following reaction termination, the absorbance (OD value) was measured at 450 nm using an enzyme-linked immunosorbent assay reader (CMax Plus, Molecular Devices, USA).
Cell lysates were prepared from each group using Radioimmunoprecipitation Assay
(RIPA) buffer (P0013B, Beyotime, China) and centrifuged to
collect the supernatant. Total protein was extracted and stored at –80
°C for subsequent Western blotting. For Western blotting, 500 µg
of total protein from each sample was mixed with 5
Total RNA was extracted from BMSc and MG63 cells using TsingZol (TsingKe,
Beijing, China) and quantified using agarose gel electrophoresis and Nanodrop
oneC (Thermo, USA). Only RNA samples meeting quality criteria were used for
subsequent analysis. First-strand cDNA was synthesized using the GoldenstarTM RT6
cDNA Synthesis Kit Ver.2 (TsingKe, Beijing, China). The qPCR experiments were
conducted using the 2
| Primer Name | Sequences |
|---|---|
| BMP-9-F | CGCCGCAGTACATGATTGAC |
| BMP-9-R | GACCACGCTTCCTTTCAGGT |
| h-GAPDH-F | TCAAGGCTGAGAACGGGAAG |
| h-GAPDH-R | TCGCCCCACTTGATTTTGGA |
Twenty-four male New Zealand White rabbits, weighing between 2 kg to 2.2 kg, were provided by Byrness Weil biotech Ltd (Chongqin, China). The animal room maintained a 12-hour light/dark cycle, allowing free access to water and food for the experimental rabbits, with a temperature maintained between 23–25 °C. All experimental procedures and animal care were strictly conducted in accordance with the “Guide for the Care and Use of Laboratory Animals”, and were approved by the Ethical Committee of Chongqing Medical Hospital (IACUC-CQMU-2023-0067). After one week of adaptive feeding, the animals entered the experiment.
A femoral condyle fracture model was constructed in 24 New Zealand White rabbits. Anesthesia was induced by administering 3% pentobarbital sodium (1 mL/kg) via ear vein injection. The animals were positioned supine and fixed. All surgical procedures were conducted under strict aseptic conditions. A 5 cm longitudinal parapatellar incision was made, and the subcutaneous tissue, fascia, and muscles were dissected to expose the patella. After dislocation of the patella, the femoral condyle was exposed. An intercondylar femoral fracture was created using a saw. Following temporary reduction, a 2.5 mm diameter hole was drilled from the lateral side to the opposite cortex. Two different types of screws were used to fix the left and right legs of each rabbit. Specifically, the experimental group received multilayer Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterials screws. Two control groups were treated with medical metal screws and n-CDHA/PAA composite biomaterials screws, respectively.
Rabbits were sacrificed at 1, 4, 12, and 24 weeks post-surgery via intravenous
air injection. Operated knee joints were harvested and stored in liquid nitrogen
for subsequent analysis. Digital veterinary X-ray imaging systems (20111337C,
Hangzhou Zhiyuan Medical Equipment Co., Ltd., Hangzhou, China) were used to take
X-ray images of the surgical sites of the living rabbits at different time points
(kVP: 58, mAs: 4.00, mA: 320). Micro-CT in vivo imaging system for small
animals (IMAGING 100, Hefei Ruishi Medical
Technology Co., Ltd., Hefei, China) was used to observe the material-bone
interface integration at four time points and to measure the bone density
adjacent to the interface. Scanning electron microscopy (SU8100, Hitachi, Japan)
was used to observe the bone-screw interface of the rabbit knee joints in each
group (3.0 kV
The sample was fixed with 4% paraformaldehyde, decalcified with Ethylenediaminetetraacetic acid (EDTA) (DD0002, Leagene, Beijing, China), dehydrated with ethanol (64-17-5, Chuandong Chemical, Chongqing, China), transparent with xylene (1330-20-7, Chuandong Chemical, Chongqing, China), waxed in paraffin (80200-0007, Shitai, Hangzhou, China), then embedded, sliced and baked. The slices were dewaxed in xylene, dehydrated in ethanol, dipped in toluidine blue dye solution (G1032, Servicebio, Wuhan, China) for 5 min, washed, and differentiated into light blue background with 0.1% glacial acetic acid, and stopped by tap water washing. After dehydration and sealing, the slices were observed and recorded with Mshot MF53 microscope (Mingmei, Guangdong, China).
The slices were processed by Masson trichromatic staining kit (Solarbio, Beijing, China) according to the instructions. The steps are as follows: Dip the prepared paraffin slices into mordant dye solution, let them act at room temperature for one night, and rinse them with running water. The sections were stained with lapis lazuli blue staining solution for 3 min, Mayer hematoxylin staining solution for 3 min, acidic ethanol differentiation solution until the tissues turned red completely, and washed with distilled water. The slices were dyed with ponceau magenta dye solution for 10 min, treated with phosphomolybdic acid solution for 10 min, and dyed with aniline blue dye solution for 5 min. After washing the slices with weak acid solution to remove aniline blue solution, continue to drip weak acid working solution to cover the slices for 2 min. The samples were dehydrated and sealed, then observed and recorded under a microscope (MF53, Mingmei, Guangzhou, China).
The sections were processed using the Eosin-Van Gieson (EVG) staining kit (Servicebio, Wuhan, China) according to the instructions, as follows: The paraffin sections were dyed with 5:2:2 mixed EVG A, B and C solutions for 5 min, and then dyed with EVG B dye solution diluted twice to control the differentiation until the elastic fibers were dark purple and the background was grayish white under the microscope. The EVG dyes D and E were mixed at a ratio of 1:9, and the slices were dyed for 12 min and washed with water. Finally, the slices were dehydrated and sealed, and observed and recorded under a microscope (MF53, Mingmei, China).
Experimental data were presented as mean
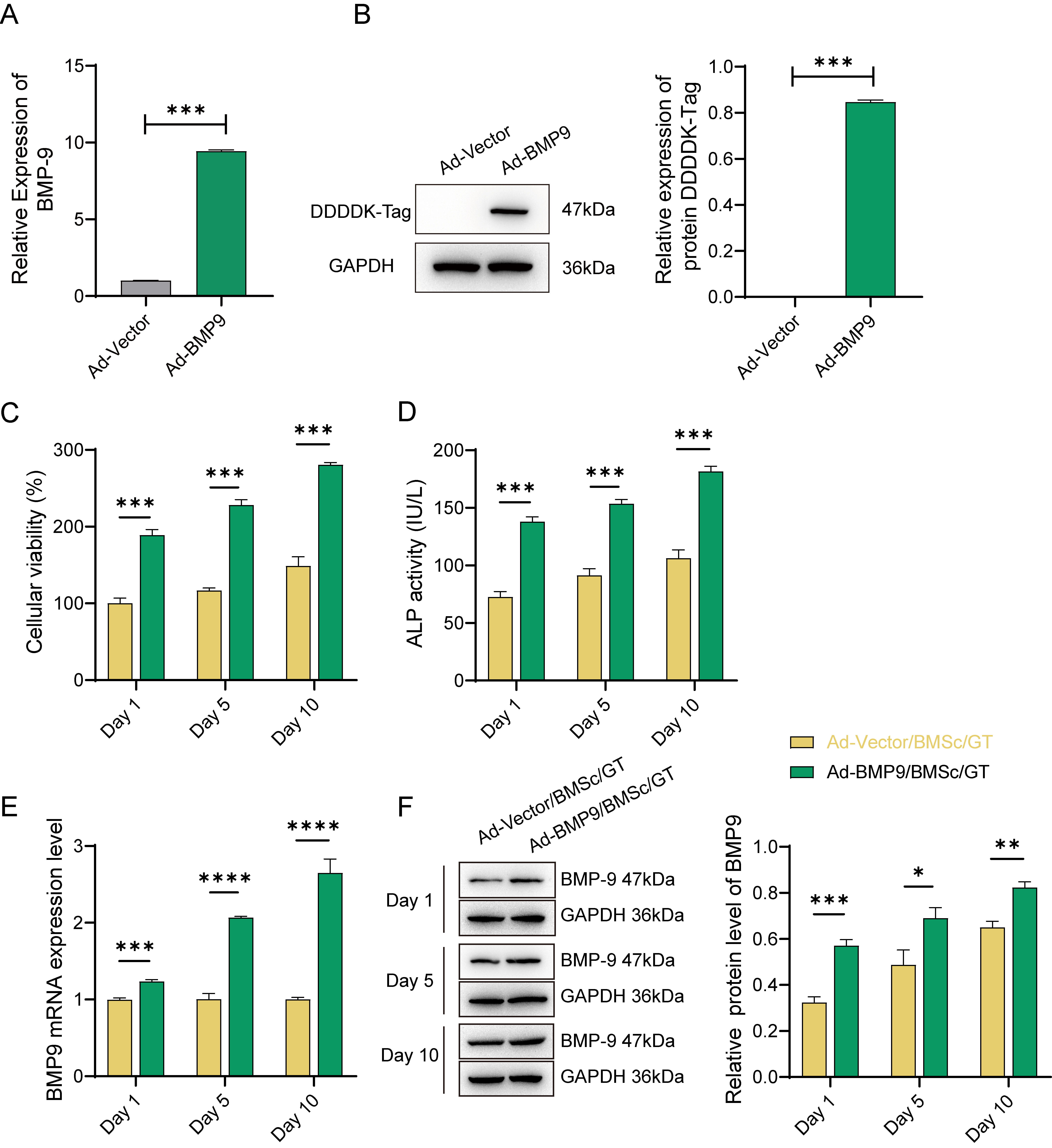
Firstly, we successfully established BMP-9
overexpressing BMSc cells by transfecting the Flag-tagged BMP-9 recombinant
adenovirus. Successful transfection of BMSc cells was confirmed by a significant
increase in both BMP-9 gene and DDDDK-Tag protein expression levels, as
depicted in Fig. 1A,B (p
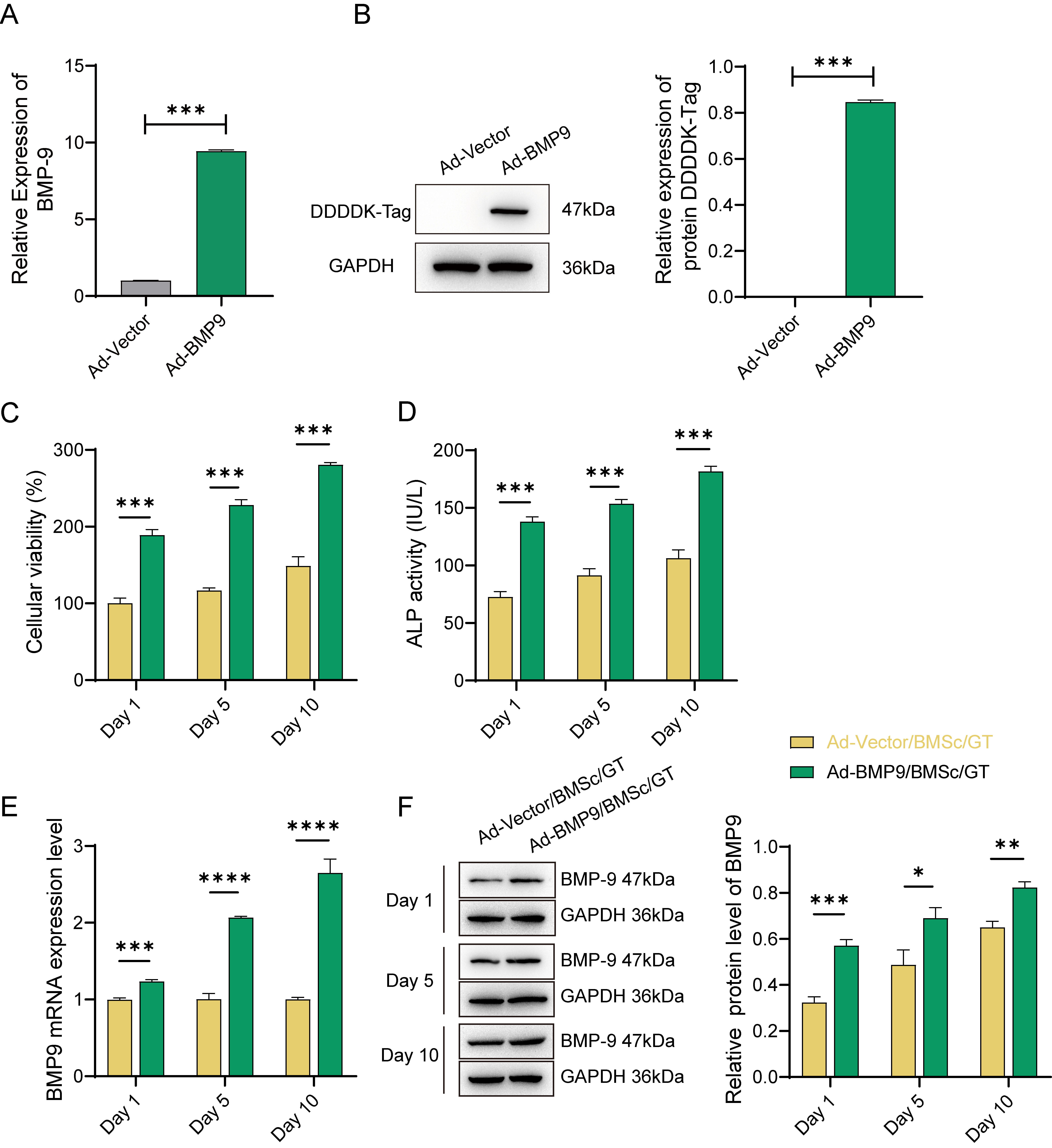 Fig. 1.
Fig. 1.
The Effect of Gelatin (GT)-Loaded Adenovirus
(Ad)-Bone Morphogenetic Protein 9(BMP-9)/Bone Marrow Mesenchymal Stem Cells
(BMSc) on the Proliferation and Differentiation of MG63 Cells. (A) Expression of
the BMP-9 gene and (B) DDDDK-Tag protein after transfection of BMSc
cells with recombinant adenovirus (Ad-BMP-9) for 48 hours. (C) Cell viability
assays performed on days 1, 5, and 10 during the 10-day co-culture of
Ad-BMP-9/BMSc/GT with MG63 cells. (D) alkaline phosphatase (ALP) activity assay.
(E) Detection of BMP-9 gene levels in cells on days 1, 5, and 10. (F)
Relative expression of BMP-9 protein in cells on days 1, 5, and 10. *, Indicates
a statistically significant difference between the two groups with
p-value
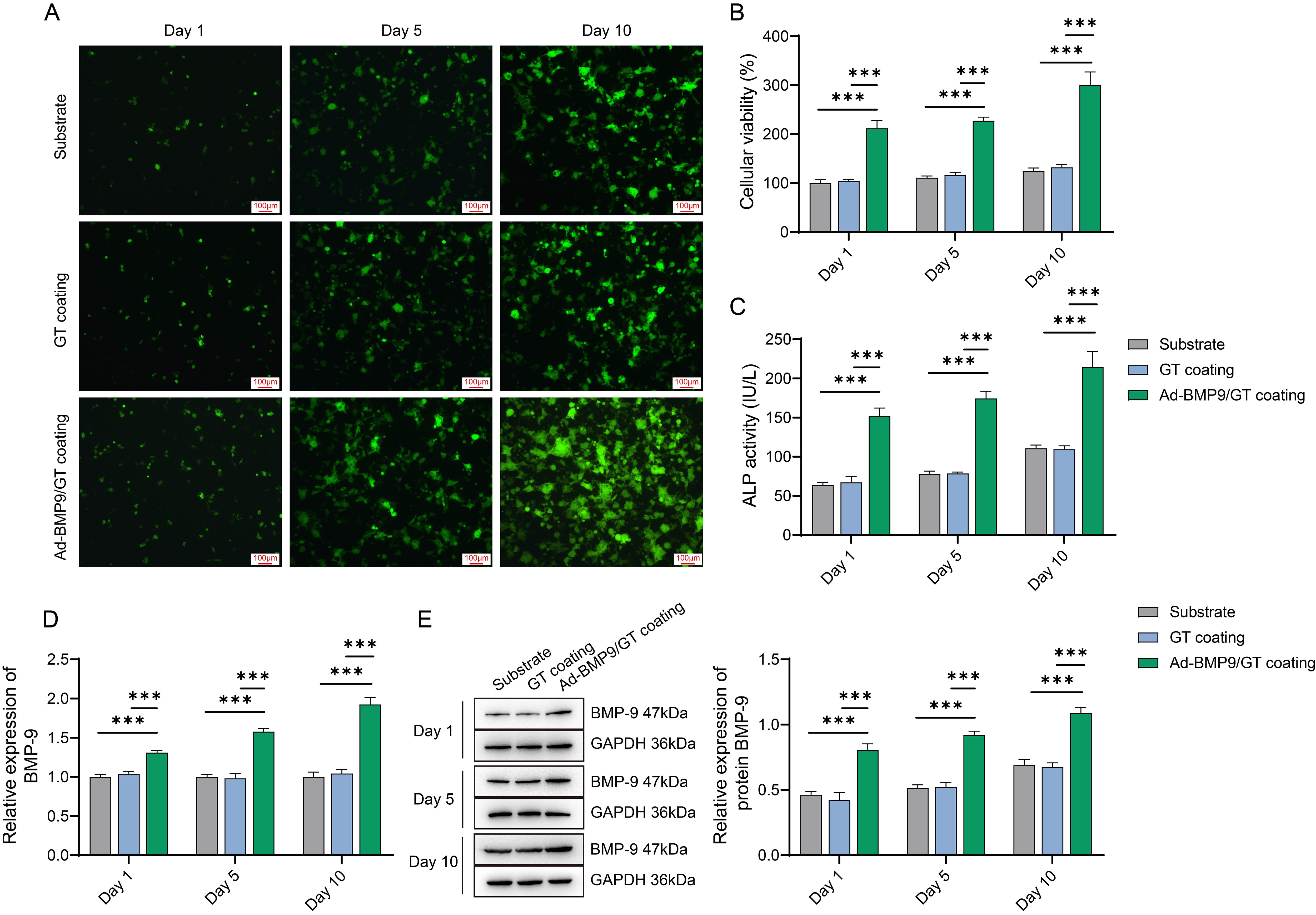
To evaluate the in vitro biocompatibility of the multilayer
Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterials, we conducted cell
cultures on the experimental group with the multilayer Ad-BMP-9/BMSc/GT coating
on n-CDHA/PAA composite biomaterials (Ad-BMP-9/GT coating), the control group
with a single layer of GT coating on n-CDHA/PAA composite biomaterials (GT
coating), and the n-CDHA/PAA control group (Substrate), with a series of
assessments performed on days 1, 5, and 10. To visually observe the growth of
cells on the material surface, we conducted microscopic observations. The results
showed that on the first day, there was not much cell growth in all groups. In
the Ad-BMP-9/GT coating group, MG63 cells exhibited good spreading and growth
morphology on the material surface, with a significant increase in cell numbers
(Fig. 2A). Concurrently, CCK-8 assay results indicated that the proliferation
capacity of MG63 cells in the Ad-BMP-9/GT coating group was significantly
enhanced compared to the Substrate group, and this capacity continued to improve
over time (Fig. 2B, p
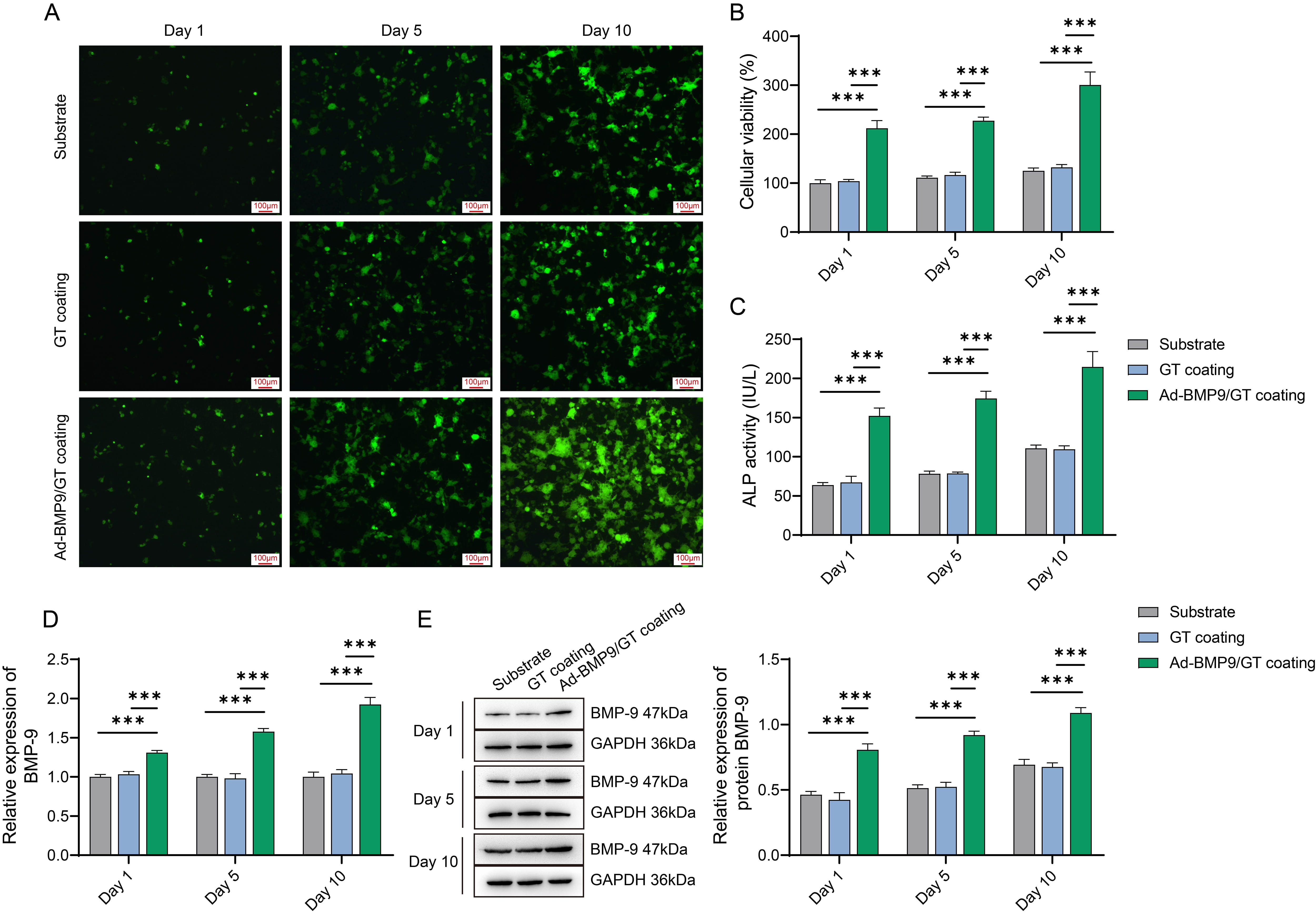 Fig. 2.
Fig. 2.
Assessment of Biocompatibility of Multilayer Ad-BMP-9/BMSc/GT
Coated nano-calcium deficient hydroxyapatite/poly-amino acid(n-CDHA/PAA) Composite Biomaterials with MG63 Cells. (A) Microscopic
observation of MG63 cell growth on the material surface at three time points.
Scale bars: 100 µm. (B) Cell Counting Kit-8 (CCK-8) assay for the
proliferation of MG63 cells at three time points. (C) ALP activity assay for the
viability of MG63 cells at three time points. (D) Quantitative Polymerase Chain
Reaction (q-PCR) detection of BMP-9 mRNA expression in MG63 cells at three time
points. (E) Western blot (WB) detection of BMP-9 protein expression in MG63 cells
at three time points. ***, Indicates a statistically significant difference
between groups with p-value
In vitro experimental results indicate that the multilayer
Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterials exhibit good
biocompatibility with MG63 cells and effectively promote the attachment,
proliferation, differentiation, and expression of BMP-9 in these cells.
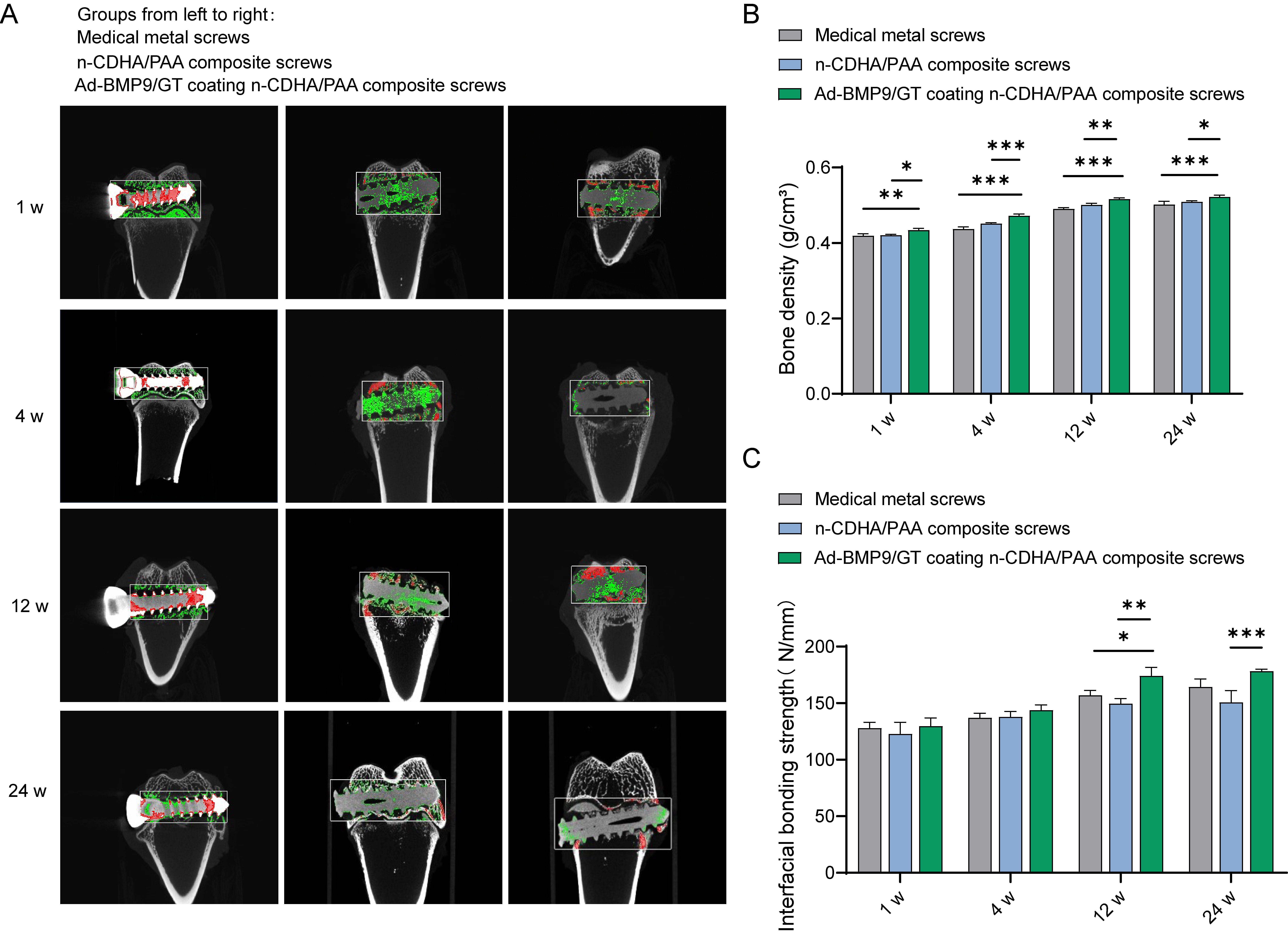
Subsequently, we constructed an intercondylar femoral fracture model in rabbits
and fixed it with Ad-BMP9/GT coated n-CDHA/PAA composite screws, medical metal
screws, and n-CDHA/PAA composite screws, followed by a series of assessments at
1, 4, 12, and 24 weeks postoperatively. Micro-CT images revealed that at one week
post-surgery, there were clear fracture gaps visible in the knee joint samples of
all groups; at four weeks post-surgery, the fracture gaps were less distinct
compared to the first week, indicating that new bone formation occurs over time;
at twelve weeks post-surgery, the fracture gaps had essentially healed, with no
obvious signs of fracture visible in the Micro-CT images, and no abnormalities in
the overall samples; the bone density of the knee joint samples in all groups
gradually increased over time, with the Ad-BMP9/GT coated n-CDHA/PAA composite
screws showing the best results at later stages (Fig. 3A,B, p
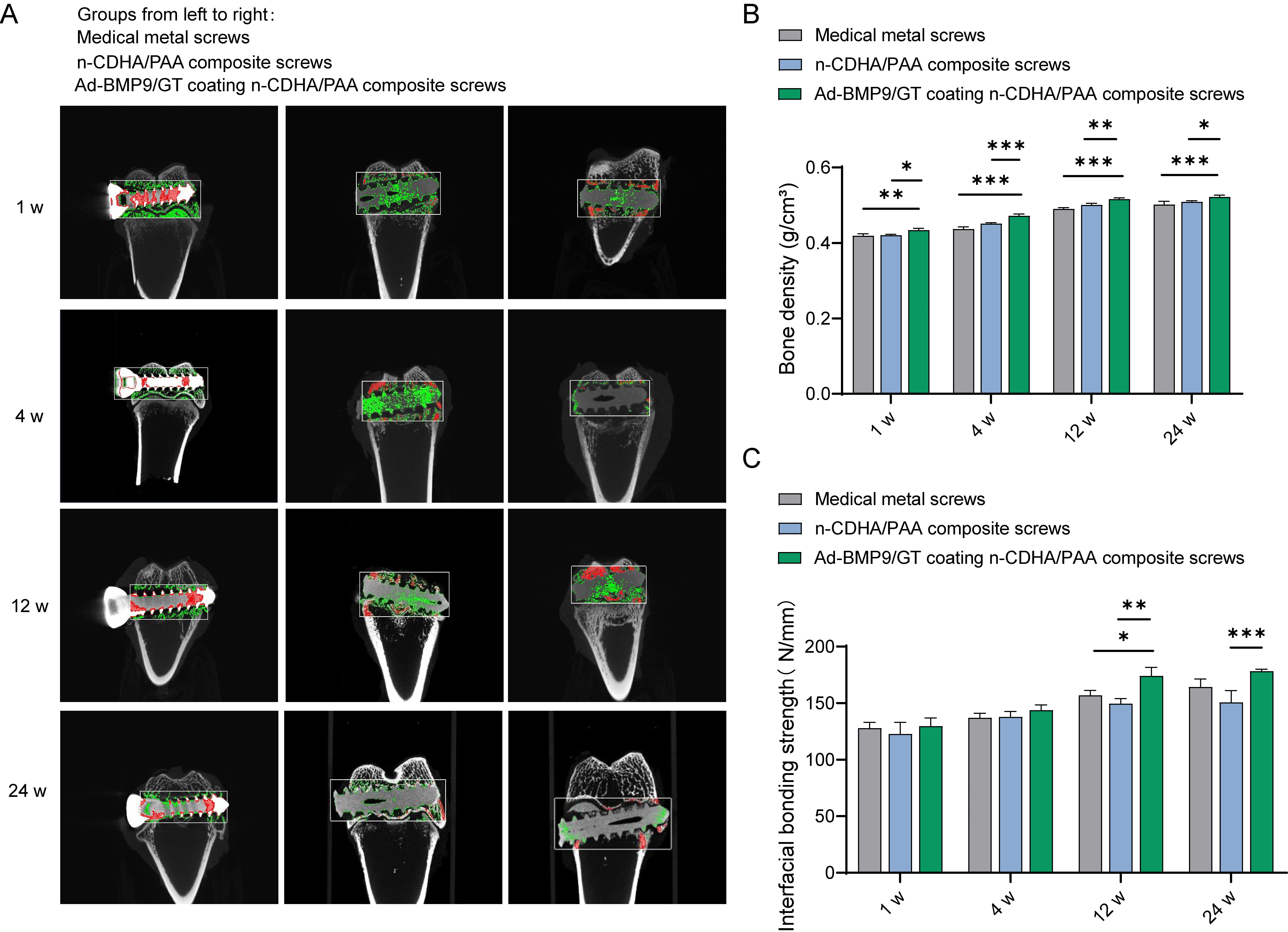 Fig. 3.
Fig. 3.
Assessment of the Integration of Multilayer Ad-BMP-9/BMSc/GT
Coated n-CDHA/PAA Composite Biomaterials with Neo-tissue at Rabbit Bone Wounds.
(A)
Micro-CT imaging to observe the integration
at the composite biomaterial-bone interface at four time points (1 week, 4 weeks,
12 weeks, 24 weeks), red represents cortical bone, and green represents
trabecular bone. (B) Measurement of bone density adjacent to the composite
biomaterial-bone interface. (C) Biomechanical testing to detect the interface
binding strength between the composite biomaterials and bone tissue at 1 week, 4
weeks, 12 weeks, and 24 weeks. *, Indicates a statistically significant
difference between groups with p-value
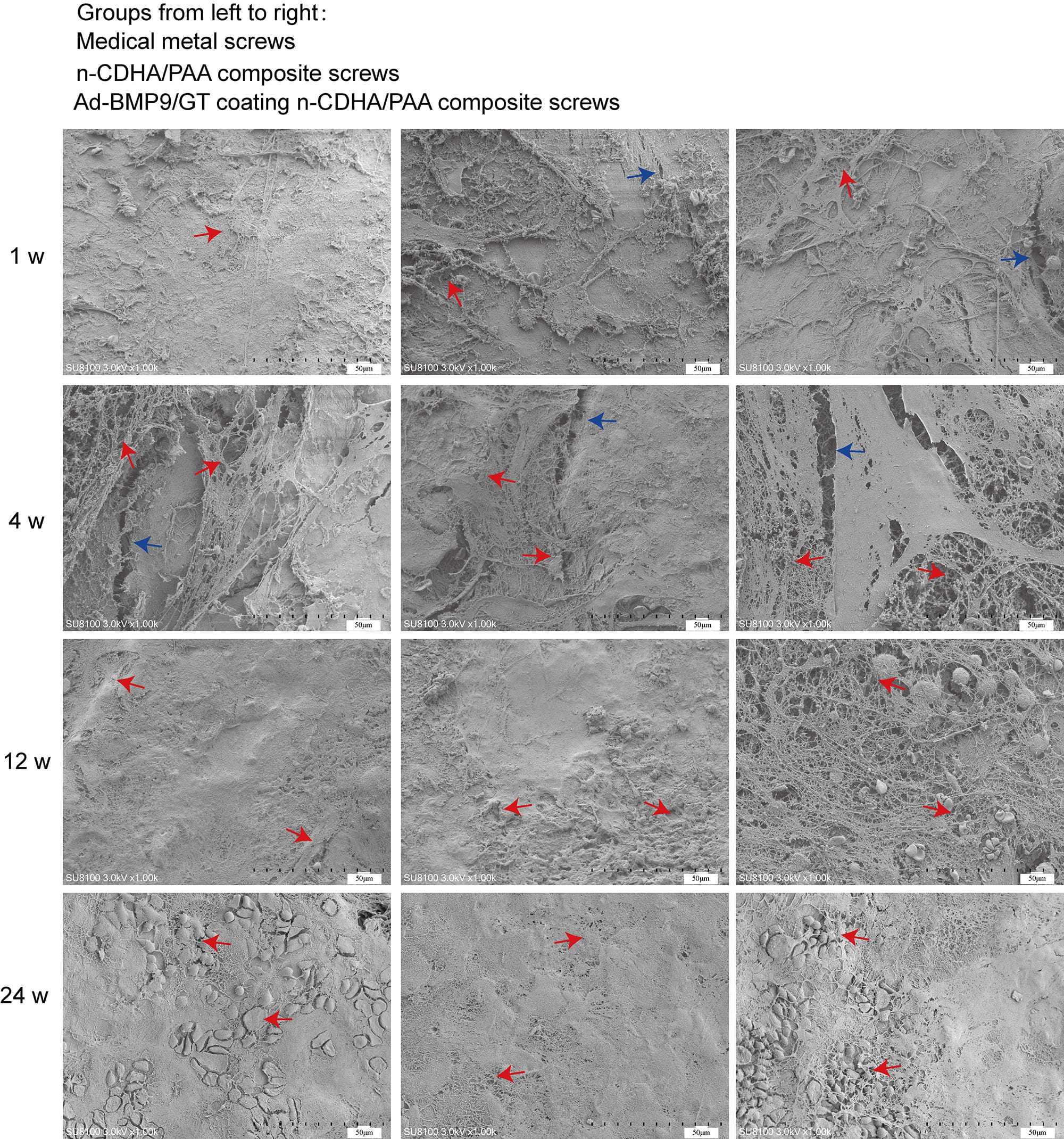
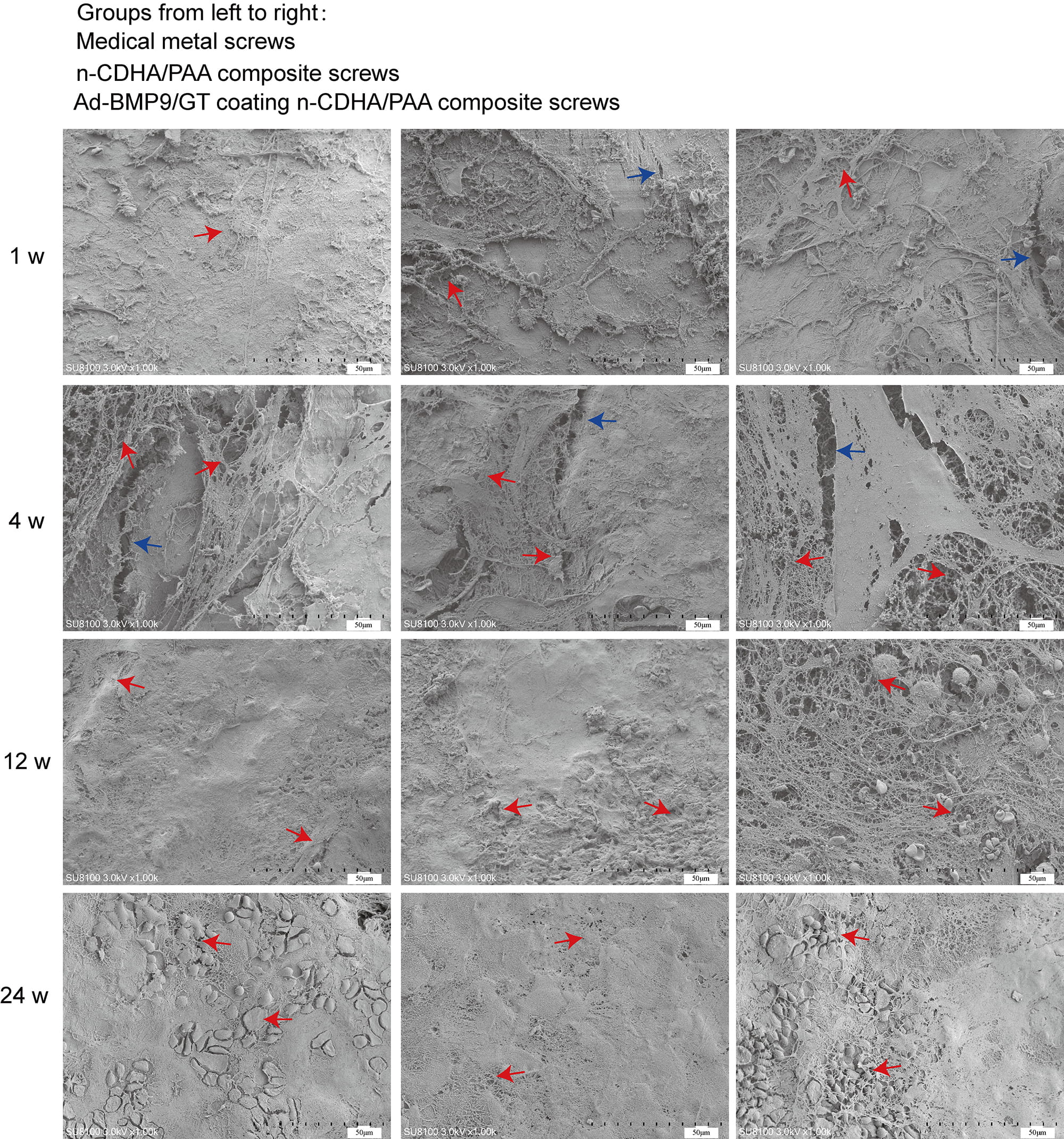 Fig. 4.
Fig. 4.
Scanning electron microscopy (SEM) to observe the interface between the composite biomaterials and bone tissue at 1 week, 4 weeks, 12 weeks, and 24 weeks. Red arrow: three-dimensional structure; Blue arrow: crack. Scale bars: 50 µm.
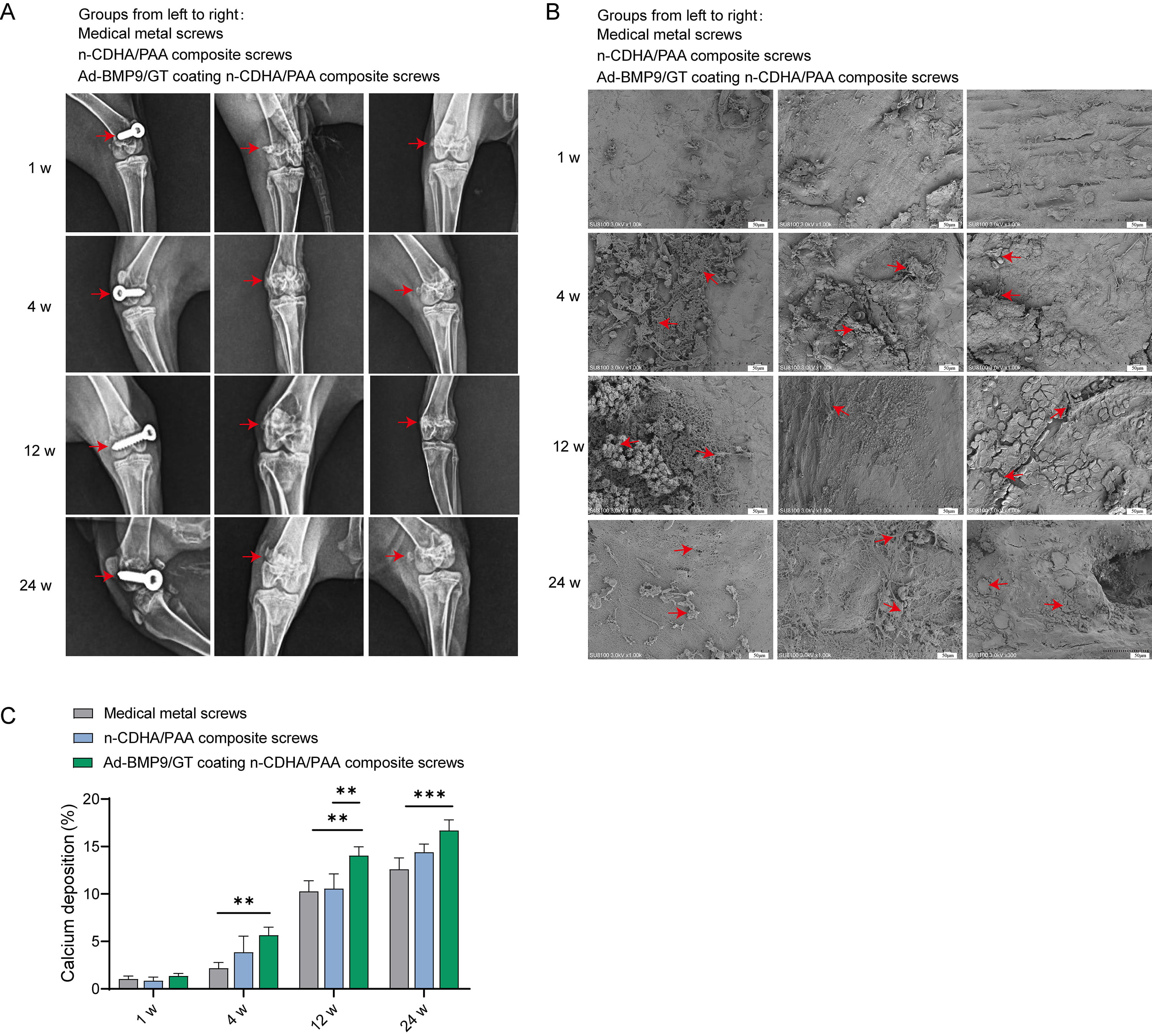
X-ray images of the surgical sites of the rabbit femur show that none of the
screws in any group exhibited loosening or detachment (Fig. 5A). Starting from
the 4th week, obvious neo-tissue attachment was observed on the surface of the
screws in all groups. By the 12th week, the neo-tissue was more tightly
integrated with the screw surfaces, with the Ad-BMP9/GT coated n-CDHA/PAA
composite screws showing the most abundant new bone tissue, visible cells closely
adhering to the material surface. By the 24th week, the surfaces of the screws in
all groups were covered with biological tissue, indicating that over time, there
was no rejection phenomenon between the biological body and the materials (Fig. 5B). Calcium deposition was significantly enhanced at the material-bone interface
in Ad-BMP9/GT coated n-CDHA/PAA composite screws compared to medical metal screws
at both 12 w and 24 w (Fig. 5C, p
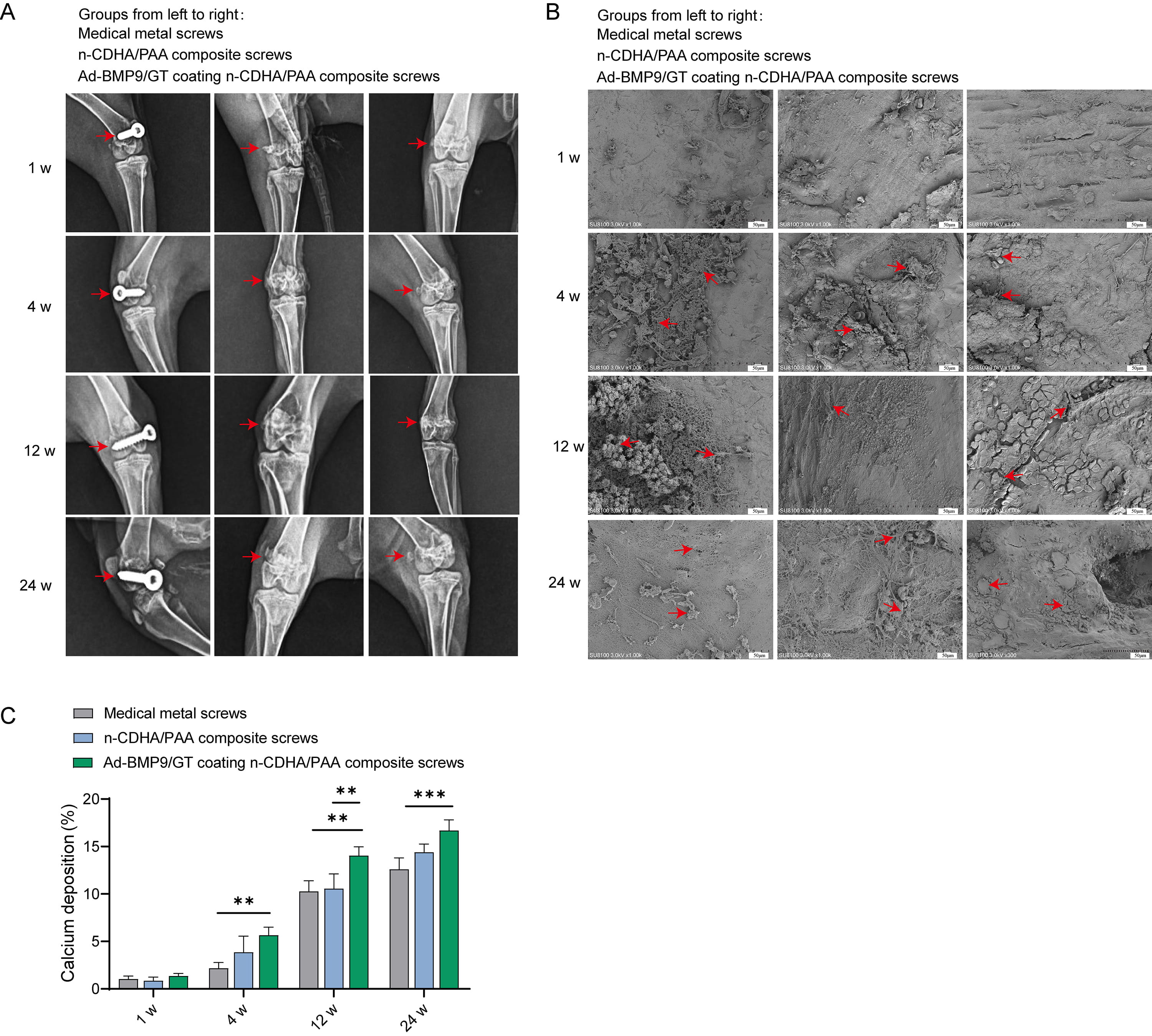 Fig. 5.
Fig. 5.
The Impact of Multilayer Ad-BMP-9/BMSc/GT Coated n-CDHA/PAA
Composite Biomaterials on Neo-tissue Formation at Rabbit Bone Wounds. (A) X-ray
imaging (anteroposterior view) of screw loosening at the surgical sites of each
group at 1 week, 4 weeks, 12 weeks, and 24 weeks post-surgery. (B) Scanning
electron microscopy (SEM) observation of the screw surface conditions after
push-out experiments at 1 week, 4 weeks, 12 weeks, and 24 weeks. Scale bars: 50
µm. (C) X-ray energy dispersive spectroscopy (EDS) analysis of calcium
deposition at the material-bone interface at 1 week, 4 weeks, 12 weeks, and 24
weeks. **, Indicates a statistically significant difference between groups with
p-value
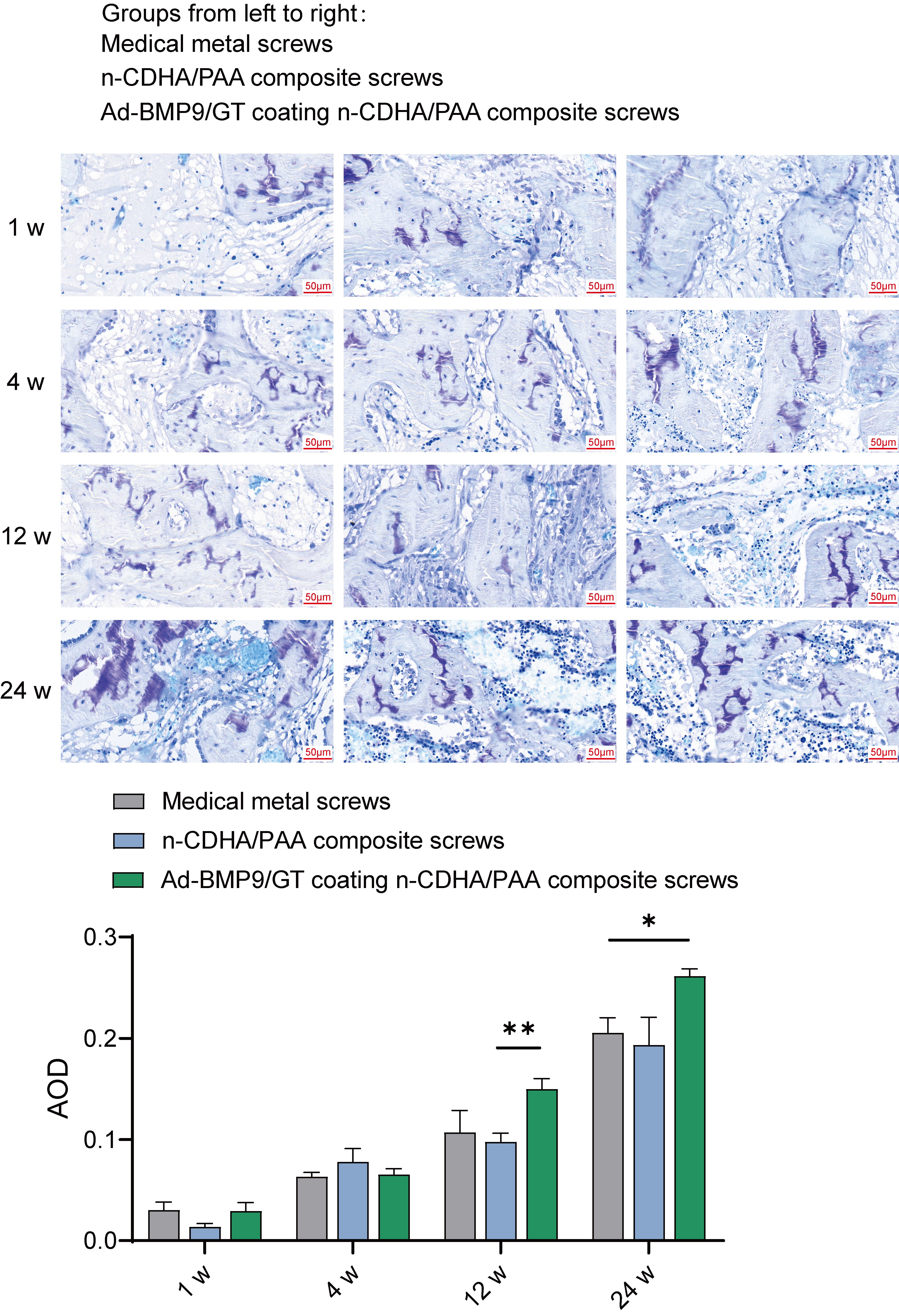
To determine the effect of Ad-BMP-9/BMSc/GT Coated n-CDHA/PAA Composite Biomaterials on chondroitin sulfate and collagen fiber growth in bone tissue, we observed rabbit bone wounds by multiple staining.
Based on the metachromatic property of chondroitin sulfate, which stains
purple-red with toluidine blue, we conducted measurements of the staining
results, including the area, depth of the stained regions, and their proportions
in the total area. Statistical analysis revealed no significant differences in
chondroitin sulfate content among the groups at weeks 1 and 4. However, at week
12, the chondroitin sulfate content in the bone tissue of the Ad-BMP-9/BMSc/GT
coated n-CDHA/PAA group was significantly higher than that in the n-CDHA/PAA
group (p
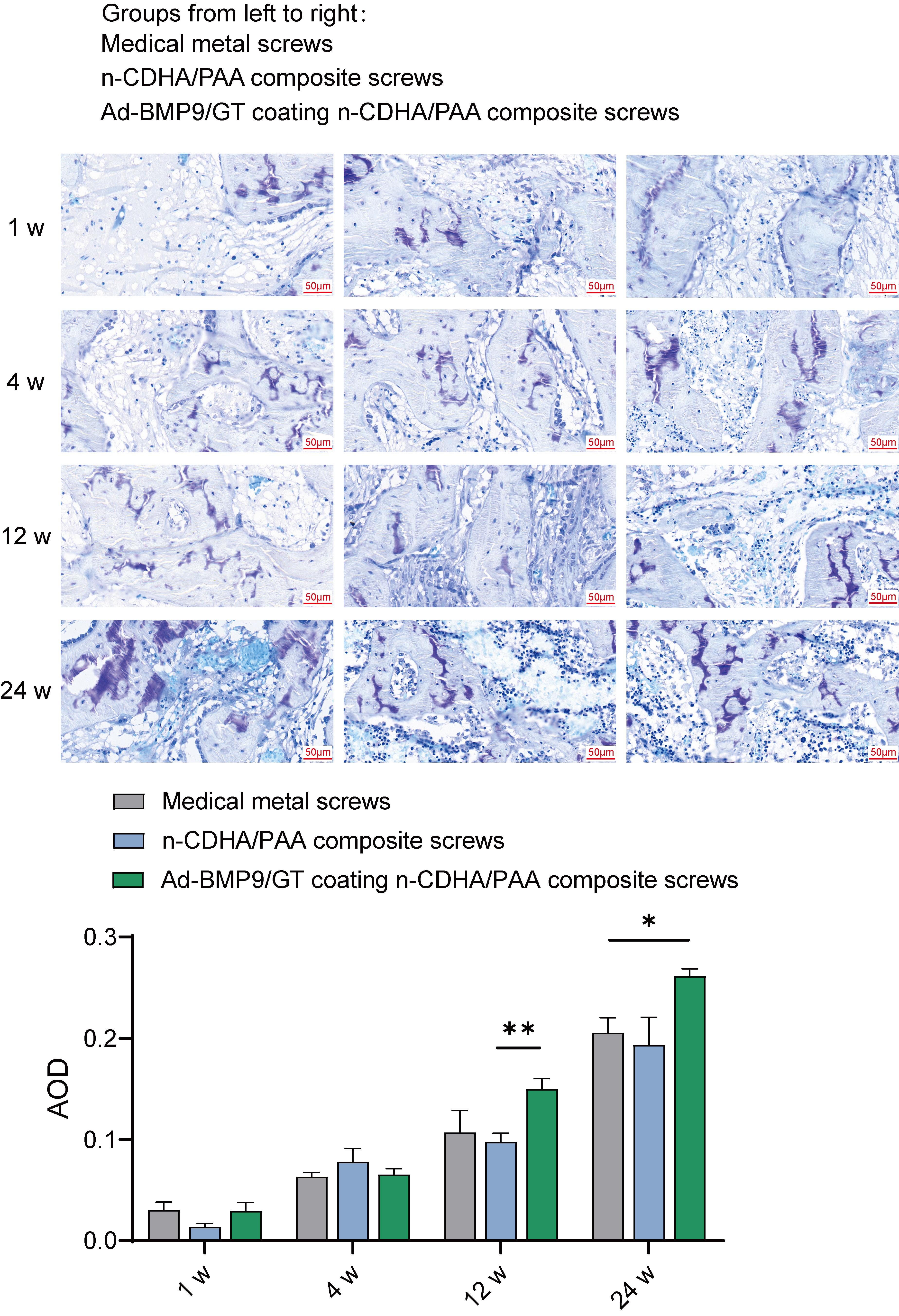 Fig. 6.
Fig. 6.
The Impact of Multilayer Ad-BMP-9/BMSc/GT Coated n-CDHA/PAA
Composite Biomaterials on Chondroitin Sulfate at Rabbit Bone Wounds by Toluidine
Blue Staining. *, Indicates a statistically significant difference between
groups with p-value
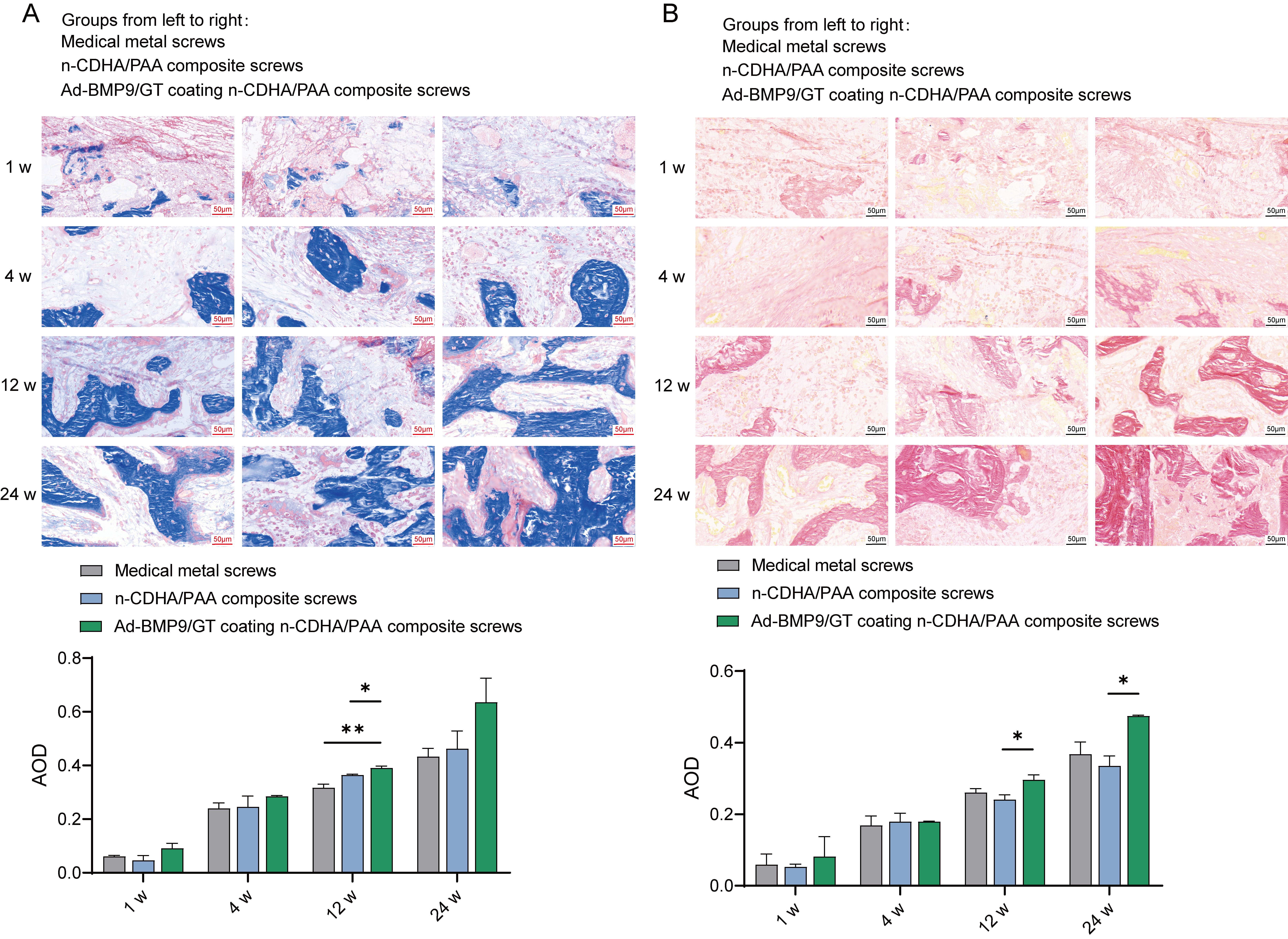
The results of Massonstaining and Verhoeff’s vangiesonstaining showed that there
was no significant difference in the growth of collagen fibers in the bone tissue
of each group at the 1th and 4th weeks, but at the 12th and 24th weeks,
Ad-BMP-9/GT Coated n-CDHA/PAA composite screws group exhibited the most abundant growth of collagen fibers in the bone tissue (Fig. 7, p
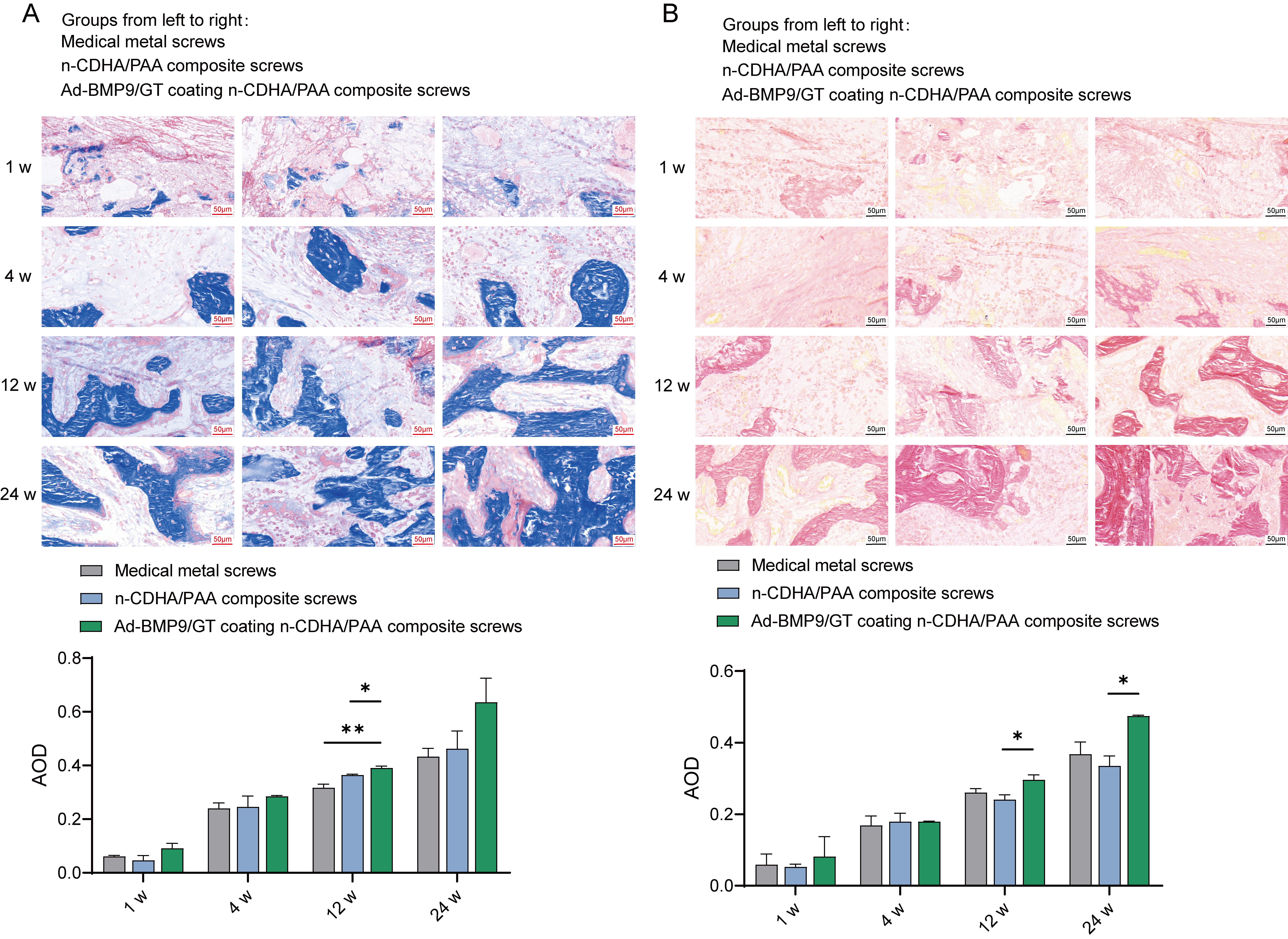 Fig. 7.
Fig. 7.
The Impact of Multilayer Ad-BMP-9/BMSc/GT Coated n-CDHA/PAA
Composite Biomaterials on Collagen Fiber Growth at Rabbit Bone Wounds. (A)
Masson staining. (B) Verhoeff’s van gieson staining. *,
Indicates a statistically significant difference between groups with
p-value
Bone tissue engineering has emerged as a promising field for addressing bone defects and promoting bone regeneration. The ideal osseointegration requires that bone tissue can be directly deposited onto the implant surface, form some kind of chemical bond with the bone tissue, and also promote rapid cell differentiation and growth (Osteoinduction) [17]. Current methods typically involve the use of biomaterials, growth factors, and stem cells [18]. In recent years, the field of bone tissue engineering has made progress in the design of biomaterials and cell therapies. Nanomaterials, due to their large surface area, are conducive to the adhesion of cells, proteins, etc., and have good mechanical properties and excellent biocompatibility, which have been widely used in bone tissue engineering [19]. At present, the nanocrystalline structure of hydroxyapatite, which is close to the crystal structure and size of human bone tissue, is well synthesized and studied, capable of forming a strong osteogenic bond with bone tissue [20]. We have previously developed n-CDHA/PAA composite biomaterials, which have been confirmed to have good biocompatibility and biomechanics both in vitro and in vivo [21, 22]. However, to promote faster bone healing and rapid differentiation and growth, it is still necessary to improve the biocompatibility, osteoconductive properties, and osteogenic potential of these materials.
The incorporation of growth factors (GF) such as BMPs, Fibroblast Growth Factors
(FGFs), Insulin-like Growth Factors I/II (IGF I/II), and Transforming Growth
Factor-
Stem cells have shown great potential in repairing bone defects, with Bone
Marrow Stromal Cells (BMSCs) being an ideal source for bone defect repair due to
their ease of accessibility, strong self-renewal capacity, and prominent
osteogenic differentiation ability [27]. Additionally, studies have confirmed the
ability of BMSCs to effectively repair bone defects in animal models [28]. In
this study, we first successfully constructed BMSCs with overexpression of the
BMP-9 gene. Compared to the GT group, GT-supported Ad-BMP-9/BMSCs could
effectively promote the proliferation and differentiation of MG63 cells by
continuous release of BMP-9. Therefore, we speculate that constructing BMSCs with
overexpression of the BMP-9 gene and combining them with GT delivery
could effectively promote the repair of bone defects. BMPs belong to the
Transforming Growth Factor-
Our study presents a novel method for the sustained delivery of BMP-9 and enhanced bone-biomaterial integration through a multi-layer coating system. We developed a composite biomaterial with multi-layer Ad-BMP-9/BMSc/GT coatings on n-CDHA/PAA and found it to provide an excellent environment for cell adhesion. Over time, the proliferation capacity of MG63 cells and the expression levels of BMP-9 in the Ad-BMP-9/GT coating group continuously improved, indicating that this coating can promote cell differentiation, endowing them with greater osteogenic potential, similar to the results of Wang et al. [32] and Shi et al. [33]. Our method utilizes a multi-layer GT-based system combined with BMP-9 expressing BMSc cells, offering several advantages. GT is a natural biomaterial, and its use helps to enhance biocompatibility and reduce the risk of inflammatory responses [34]. The multi-layer coating system allows for controlled and sustained release of BMP-9, thereby promoting bone formation. Additionally, incorporating BMSc cells overexpressing BMP-9 in the coating facilitates the integration of cells with the biomaterial, promoting bone regeneration and bone-biomaterial integration. These results offer a potential strategy for bone tissue engineering, with the expectation of enhancing the bioactivity of bone repair materials.
Internal fixation screws are commonly used to stabilize strong metaphyseal fractures, particularly in the femoral or humeral shaft [35]. Our previous research demonstrated the excellent biocompatibility and biodegradability of n-CDHA/PAA composite material when fabricated into internal fixation screws for an articular fracture animal model [22]. This study evaluated the bone repair effects of multilayer Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterials (experimental group) compared to conventional metal screws and n-CDHA/PAA composite screws (control group) by constructing an intercondylar femoral fracture model in rabbits. Micro-CT images showed that the Ad-BMP9/GT coated n-CDHA/PAA composite screws group exhibited faster fracture gap healing and higher bone density, demonstrating excellent osseointegration capabilities. Biomechanical testing results indicated that Ad-BMP9/GT coated n-CDHA/PAA composite screws showed advantages in fixation strength, especially after long-term fixation, where their fixation strength was significantly higher than that of traditional medical metal screws. This suggests that the presence of multilayer coatings enhances the binding ability of the screws with the surrounding bone tissue. More importantly, the Ad-BMP9/GT coated n-CDHA/PAA composite screws group formed dense neo-tissue at the bone interface 12 weeks post-surgery, presenting a clear three-dimensional reticular structure. This indicates that the multilayer coatings on the composite biomaterial screws contribute to the promotion of bone wound repair and recovery. The potential application of this technology lies in improving the success rate of fracture repair, reducing postoperative complications, and possibly shortening recovery time, which is of significant importance to the clinical practice of orthopedic surgery.
Traditional bone repair materials often struggle to form a strong bond with bone tissue, leading to suboptimal repair outcomes [36]. Regeneration of bone tissue is a critical step in the promotion of bone repair by implants; it enhances the fixation and stability of the implant, improves biocompatibility, promotes the long-term functionality of the implant, and reduces the risk of infection [37]. Our results indicate that from 4 weeks post-surgery, noticeable neo-tissue attachment was observed on the surfaces of screws in all groups, and chondroitin sulfate and collagen fibers appeared to increase. By the 12th week, the neo-tissue was more tightly integrated with the screw surfaces, particularly in the Ad-BMP9/GT coated n-CDHA/PAA composite biomaterial screws group, where the new bone tissue was the most abundant and closely adhered to the material surface, and chondroitin sulfate and collagen fibers showed significant growth. By the 24th week, the surfaces of the screws in all groups were covered with biological tissue, indicating no rejection phenomena between the material and the biological entity, demonstrating excellent biocompatibility. Moreover, the multi-layer coating helps to promote the calcification process, thereby enhancing the regenerative and reparative capacity of bone tissue. X-ray imaging showed that no screws in any group exhibited loosening or detachment at various time points post-surgery, indicating that the fixation performance of the screws in all tested groups was satisfactory and met clinical requirements.
The multi-layer Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterials developed in this study can sustain the release of BMP-9, promote bone tissue regeneration, and effectively enhance the integration of neo-tissue at the bone wound with the material surface, significantly improving the bioactivity of bone repair materials. Compared to traditional bone repair materials, this approach exhibits greater osseointegration capacity and better bone repair outcomes. These findings are of significant clinical application value and have promising prospects for the development of new orthopedic repair materials, especially in the repair of complex fractures and the treatment of bone defects. While our research results are encouraging, it is also necessary to acknowledge certain limitations: the study followed up with animals for 24 weeks, which is relatively short for assessing the long-term biocompatibility and degradation of the multi-layer coating system. Further long-term studies are required to investigate the long-term effects of this method. Further investigation of the biodegradation products and their potential impact on surrounding tissues is essential to ensure long-term safety and efficacy. Additionally, exploring the optimal number of layers, thickness, and composition of the coating can further enhance the efficacy of this system. To successfully apply this multi-layer coating system to clinical practice, comprehensive pre-clinical studies are needed, including large animal models and assessments of safety and efficacy in clinical settings.
This study, based on multilayer Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterials, systematically assessed their potential in promoting bone tissue regeneration. Through in vitro and in vivo experiments, we verified the significant effects of this novel biomaterial in enhancing MG63 cell adhesion, promoting MG63 cell proliferation and differentiation, and accelerating fracture healing. Notably, the Ad-BMP-9/BMSc/GT coated n-CDHA/PAA composite biomaterial screws demonstrated excellent osseointegration capabilities and biomechanical performance in a rabbit intercondylar femoral fracture model. These results indicate that the multilayer coating system not only effectively releases the growth factor BMP-9 to promote bone formation but also improves the integration of the biomaterial with surrounding bone tissue, thereby enhancing the bioactivity of bone repair materials and their potential for clinical application. This study provides strong scientific evidence for the development of innovative bone repair materials and lays a solid foundation for their clinical application. Future work will continue to explore the broad application and promotion of this multilayer coating system in the repair of complex fractures and the treatment of bone defects.
The datasets generated during the current study will be available form the corresponding author upon a reasonable request.
QY and YL: substantial contributions to the conception or design of the work, and drafted the manuscript; RW, LD and AH: the acquisition and analysis of data for the work; DZ and ZD: interpretation of data for the work, and reviewing the manuscript. All authors contributed to and approved the final manuscript. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All animal experiments were performed in accordance with the guidelines of the Chongqing Medical Hospital Animal Care and Use Committee (IACUC) and were approved by the Ethical Committee of Chongqing Medical Hospital (IACUC-CQMU-2023-0067).
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
This study was supported by the General Project of Chongqing Municipal Natural Science Foundation (cstc2021jcyj-msxmX0832) and the Doctor Through Train Project of Chongqing Science and Technology Commission (CSTB2022BSXM-JCX0072).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.