1 Department of Physiology, Faculty of Medicine, Hacettepe University, 06230 Ankara, Turkey
Abstract
Vitamin D, a steroid hormone, acts via the vitamin D receptor expressed in various tissues, including bones, muscles, and the cardiovascular system and is associated with well-being of individuals and vitamin D deficiency is considered as a prevalent public health problem. Menopause is an important cornerstone for women, where the hormonal changes may lead to adverse health effects. Vitamin D deficiency during menopausal transition or in postmenopausal period may aggravate the health risks such as osteoporosis, sarcopenia, and cardiovascular diseases associated with menopause. This manuscript aims to provide a review of the complex interaction between vitamin D deficiency and the well-being of postmenopausal women, focusing on musculoskeletal and cardiovascular implications. Clinical studies highlight the importance of maintaining optimal vitamin D levels to decrease the risk of musculoskeletal disorders and cardiovascular diseases in postmenopausal women. However, conflicting findings regarding the effectiveness of vitamin D supplementation in reducing cardiovascular risk suggest the need for further research and a personalized approach for the chemical form of Vitamin D, dose, duration of deficiency, individual variations, and accompanying conditions. The use of vitamin D supplementation in well-evaluated patients is desirable, and help to optimize health status in postmenopausal women.
Keywords
- postmenopausal women
- Vitamin D
- musculoskeletal health
- cardiovascular health
Vitamin D is a steroid hormone, acts on key physiological processes in the
kidney, gastrointestinal tract (GIT), bones and parathyroid glands to facilitate
the absorption of calcium and phosphorus in the small intestines, decrease renal
calcium excretion, promote new bone deposition, and regulate parathyroid hormone
to maintain blood calcium and phosphorus concentrations within the narrow
physiological limits. The myriad of actions in several tissues and systems is due
to the wide tissue distribution of vitamin D receptor (VDR). Naturally, Vitamin D
exists in two forms: Vitamin D2; ergocalciferol, product of plants and/or
exposure of ergosterol to ultraviolet irradiation in vertebrates, and Vitamin
D3; cholecalciferol synthesized in vertebrates from 7-dehydrocholesterol in
the skin upon exposure to sunlight or obtained in limited quantities from
selected foods like milk, fish, and eggs. The bioactive hormone 1,25-dihydroxy
Vitamin D, or calcitriol, emerges through a dual hydroxylation process; in the
liver vitamin D is converted into 25-hydroxyvitamin D, and in the kidneys and
other tissues calcitriol is produced through a second hydroxylation step
(1-
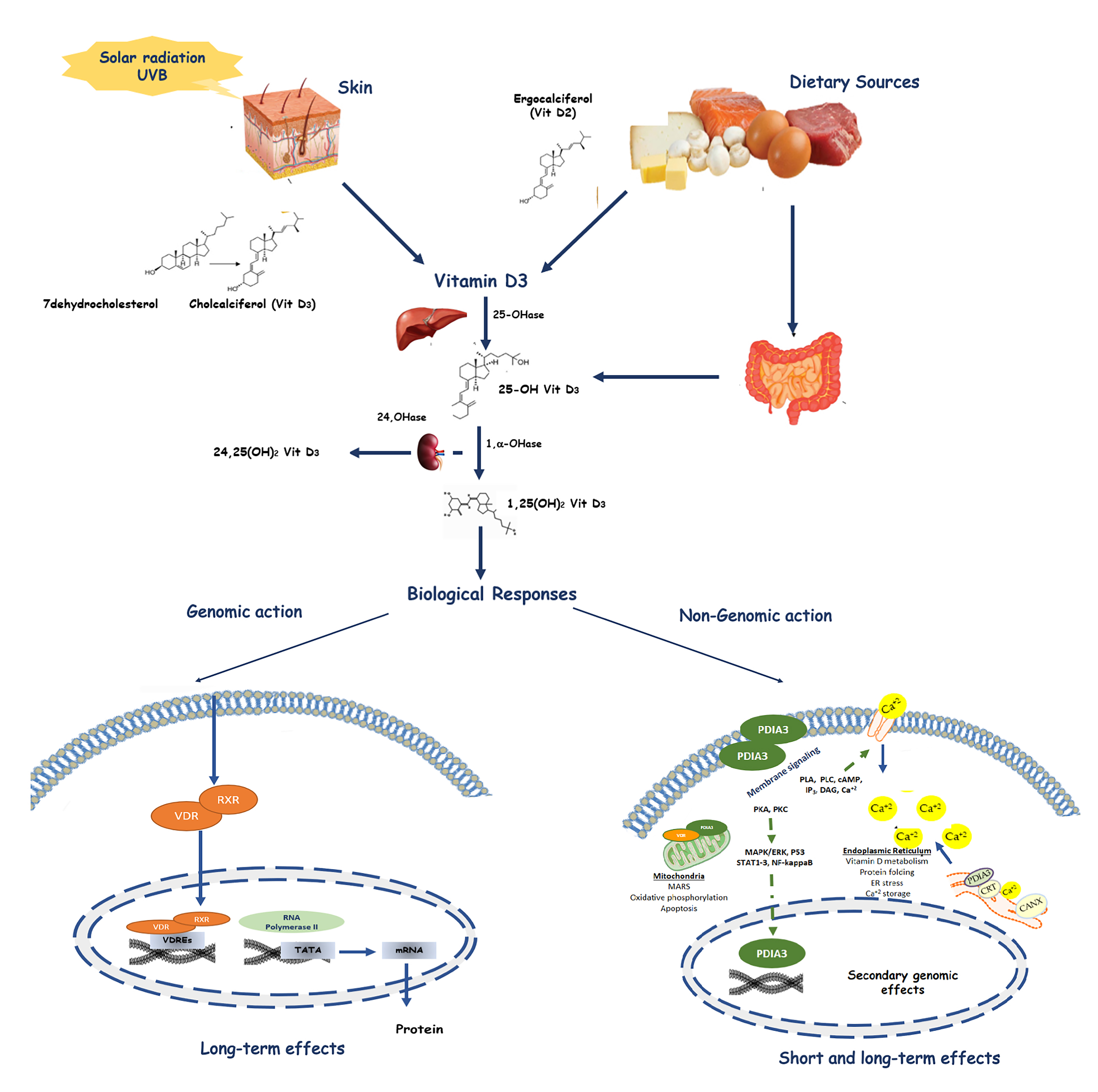
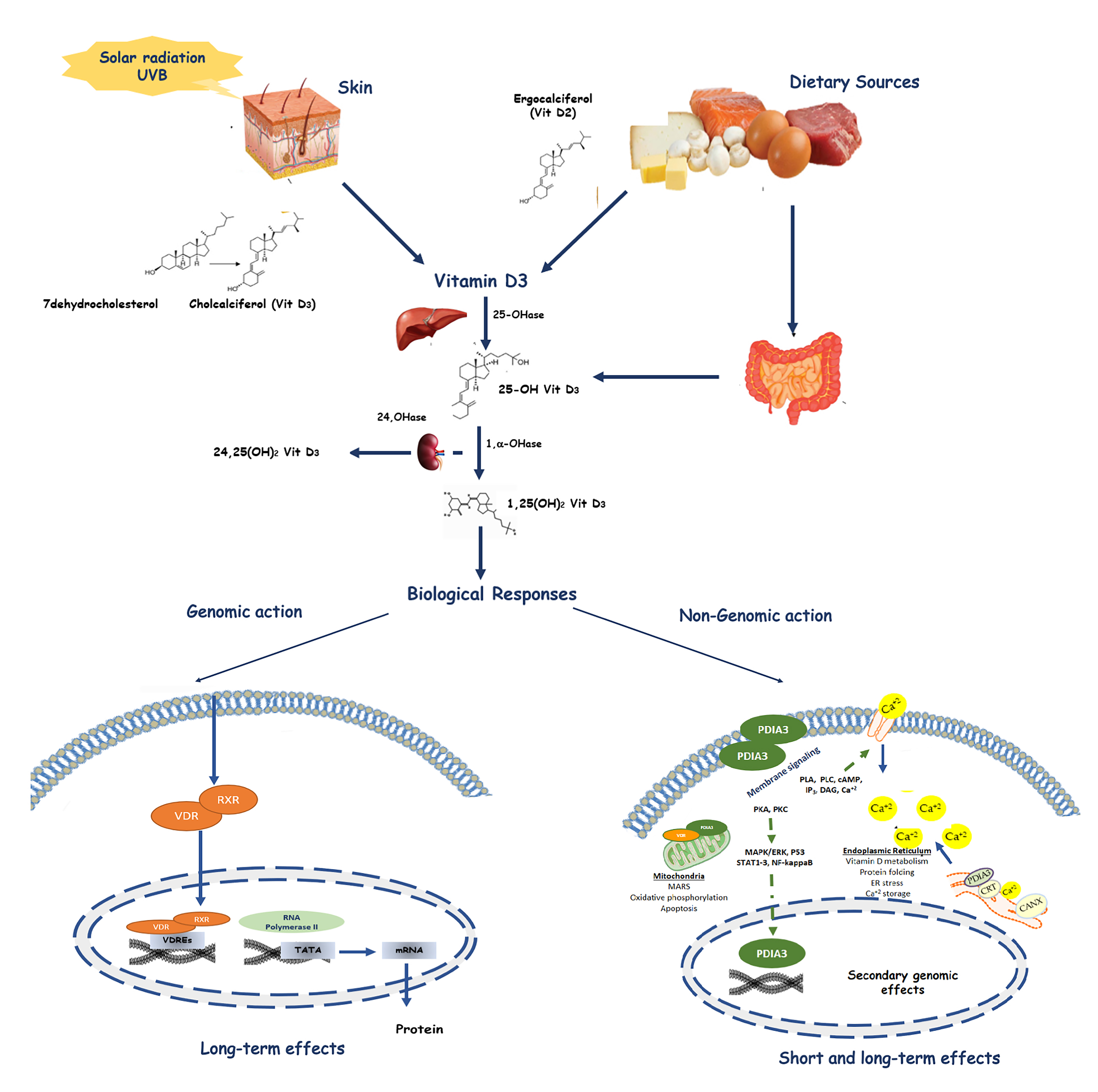 Fig. 1.
Fig. 1.
Vitamin-D metabolism and biologic affects via genomic and non-genomic pathways. VDR, Vitamin D receptor; RXR, retinoic acid receptor; VDRE, Vitamin D response element; PDIA3, via protein disulfide-isomerase A3; MARS, Mitochondrial Adaptive ROS Signaling; PLA, Phospholipase A; PLC, Phospholipase C; PKC, Protein kinase C; DAG, Diacylglycerol; IP3, Inositol trisphosphate; cAMP, cyclic adenosine monophosphate; NF-kappaB, nuclear factor kappa B; MAPK, mitogen-activated protein kinases; ERK, extracellular signal-regulated kinases; STAT1-3, Signal transducer and activator of transcription 1-3; CRT, calreticulin; CANX, calnexin; ER, endoplasmic reticulum; UVB, Ultraviolet B.
Menopause is a natural life stage, clinically defined as the lack of menstruation for one year, experienced by all women and typically occurs around the age of 50. Loss of ovarian follicular activity leads to hormonal fluctuations, particularly a decline in estrogen level accompany this transition and contribute to a spectrum of symptoms associated with women’s well-being. Moreover, the longer life span of women after menopause is reported to be with increased health problems [4]. The reason for increased morbidity and sometimes mortality in this period of time starting with menopausal transition (perimenopause) is attributed to both aging and hormonal shift from estrogen-rich to estrogen-lacking milieu [5]. The health problems during the climacteric period include vasomotor menopausal symptoms (VMS), sleep disorders, increased adiposity and change in body composition, higher risk of metabolic syndrome, neurodegenerative and cardiovascular diseases, psychogenic symptoms, increased prevalence and severity of urogenital dysfunctions, referred as genitourinary syndrome of menopause (GSM), in addition to the loss of muscle strength and coordination, sarcopenia, and osteoporosis. Menopause impairs the quality of life of women significantly where medical approaches help the management of them. The increased risk for above mentioned conditions are linked to low vitamin D, hypoestrogenism, and other age-related changes [6].
Vitamin D deficiency is a frequently emerging demographic risk, experienced during menopause [7, 8, 9]. Aging, regardless of gender, is a significant contributing factor and notably diminishes the skin’s capacity to produce vitamin D [10]. Chalcraft and colleagues demonstrated 13% reduction in cutaneous synthesis of vitamin D per decade. As, older individuals experience decreased absorption from GIT and reduced renal synthesis ability, as well [11], even though the synthetic capacity of skin is impaired, cutaneous synthesis remains the major source of vitamin D in elderly [12]. Moreover, increased adiposity during menopause due to metabolic and hormonal changes increases the risk of vitamin D deficiency and aggravate the adverse menopausal symptoms [13]. High prevalence of vitamin D deficiency, improvement of general health status and well-being with Vitamin D supplementation underlies the importance of screening Vitamin D status in postmenopausal women and investigating the potential role of vitamin D both in physiopathology and alleviating the menopausal symptoms is essential, as highlighted in studies exploring its connection with menopausal health [14].
As all nucleated cells express VDR, it is reasonable to assume that Vitamin D deficiency can be associated with dys/malfunction in all of the organs or systems of the human [3, 15]. Even though Vitamin D was advertised as the miracle molecule for every organ or disease in recent years, the causal relationships between hypovitaminosis and symptomatic or analytical findings could have been demonstrated only in some specific conditions. That’s why, we would like to briefly review the effect of sufficient Vitamin D in postmenopausal women in musculoskeletal and cardiovascular systems where improved or maintained function is achieved.
Vitamin D, as a steroid hormone shows its biological actions, by the VDR, which belongs to the nuclear receptor superfamily, and expressed in almost all tissues of the body, including the, muscles of GIT, bones, immune cells. The VDR gene is evolutionarily conserved and localized on chromosome 12q which has eight coding exons and at least six noncoding exons [16]. The most emerging human VDR protein isoform contains 427 amino acid and functions by heterodimerization with retinoic acid receptor (RXR). RXR functions as a coactivator for the target genes of vitamin D [17]. The post-transcriptional and post-translational modifications of VDR is an important contributor for its role in diverse physiological and pathological processes [18].
The active vitamin D (1,25 (OH)2D3)-VDR complex attaches to the cell’s DNA between 5000 to 20,000 spots. Some of these loci are constitutionally occupied by VDR, acting as “hotspots” for vitamin D signals. They play a key role in the response of cell to changing vitamin D levels over time. Other loci are temporarily engaged and help to fine-tune the cell’s response to vitamin D. Overall, when vitamin D activates VDR and binds to specific sites on the DNA, expressions of various genes are modulated [19]. The metabolism and biological actions of Vitamin D are summarised in Fig. 1.
Vitamin D-VDR complex regulates the direct binding of the substrate-activated VDR/RXR complex to specific DNA sequences. This complex partners with various proteins to regulate gene expression across the genome resulting in either activation or repression of transcription [20]. The VDR has two functional domains; an NH2-terminal DNA binding domain (DBD) and the COOH-terminal ligand binding domain (LBD). Binding of ligand leads to a conformational change facilitating interaction with RXR and coregulatory complexes, which is the key step for the transcription of target genes. Findings of Orlov et al. [21] suggested a cooperative and allosteric interaction between the LBD and the DBD of the VDR and its importance in regulation of gene expression.
Besides genomic effects vitamin D carry out non-genomic effects. These are mainly rapid so that exclude ranscriptional activity and modulated via protein disulfide-isomerase A3 (PDIA3). PDIA interacts with VDR directly and indirectly. These non-genomic effects regulate membrane signaling and intracellular signal molecules, endoplasmic reticulum and mitochondrial functions (Fig. 1) [18, 19, 20].
As recent data, demonstrated the mutual interaction between vitamin D and
17
In summary, the mechanism of vitamin D action is a sophisticated process involving its conversion to calcitriol, binding to the VDR, and subsequent regulation of gene expression. Through this mechanism, vitamin D maintains calcium homeostasis, supports bone health, modulates the immune system, and potentially impacts various cellular processes essential for overall health and well-being.
Of the systems effected most in postmenopausal period, skeletal muscles and bones can be listed in the first place. Osteoporosis and muscle weakness and/or sarcopenia are almost inevitable in postmenopausal women unless effective precautions are taken.
Although the expression of VDR in numerous cells and tissues suggests diverse targets for 1,25(OH)2D, the primary role of it is to regulate calcium homeostasis. Calcium homeostasis involves a coordinated effort among the bones, parathyroid gland, GIT, and kidneys to keep calcium levels in the blood within a narrow range (8.9–10.2 mg/dL). When calcium is absorbed after a meal, it triggers responses from these organs to maintain calcium homeostasis. For example, if calcium intake is low, efficiency of gastrointestinal absorption increases, urinary calcium loss decreases and calcium is freed from the bones via the regulatory mechanisms controlled mainly by parathyroid hormone (PTH) and active vitamin D. In case of persistently low calcium intake, the parathyroid gland is stimulated by low blood calcium level through the calcium sensing receptor (CaSR) and PTH production and release increases leading to a condition known as nutritional secondary hyperparathyroidism. PTH in turn, regulates the production of active vitamin D in the kidneys, to achieve calcium balance [23].
Efficient calcium absorption from the diet occurs mainly in the jejunum, and continues in the ileum and the proximal colon. Both active (energy-dependent/saturable pathway) or passive mechanisms (non-saturable pathway) are involved in gastrointestinal calcium absorption. As calcium intake increases, the absorption route shifts to non-saturable passive diffusion, allowing the efficient absorption across intestinal cells. Calcium absorption via the saturable pathway is regulated by vitamin D, and involves the upregulation of the expression of proteins involved in calcium transport, such as transient receptor potential vanilloid 6 (TRPV6) and calbindin [24]. Although it is well known that aging reduces the efficiency of calcium absorption, the exact molecular mechanisms behind this age-related decrease in calcium absorption are still not fully understood. There are studies suggesting that it is due to the decreased expression of VDR in the intestines. Additionally, the decline in sex hormone levels with age, particularly estrogen in post-menopausal women, significantly affects calcium metabolism. Estrogen deficiency, disrupts calcium absorption and reduces responsiveness to 1,25(OH)2D in the intestines. Some studies indicate that low estrogen level decreases intestinal VDR numbers, potentially explaining the reduced responsiveness to vitamin D after menopause [25]. Regarding the accumulated data, it can be stated that, both aging and hypoestrogenic environment modulates the vitamin D metabolism and function during climacteric period.
Osteoporosis is one of the most common age-related chronic diseases characterized by a gradual decrease in bone mineral density and increased risk of susceptibility of fractures, in women. Some factors such as aging, decreased estrogen level in the postmenopausal period, lack of uptake and synthesis of vitamin D, immobilization are blamed in pathogenesis of osteoporosis [26]. Among all these risk factors, vitamin D deficiency is given more emphasis due to its crucial role in maintaining calcium balance and supporting bone health. In bone tissue, calcitriol stimulates the expression of genes involved in differentiation and activity of the osteoclast cells responsible for bone resorption, stimulating the breakdown of bone, releasing calcium and phosphate into the circulation. Vitamin D also promotes the differentiation of bone laying osteoblastic cells. Overall bone turnover is facilitated. In case of hypovitaminosis, above mentioned effects are lost and resulting secondary hyperparathyroidism contributes to reduced bone mass and increased risk of osteoporotic fractures [27].
Estrogen as an anabolic hormone, is pivotal in maintaining skeletal muscle size as well as its functions. Postmenopausal women often experience reduced muscle mass and strength. This fact leads to skeletal muscle loss, known as sarcopenia, which is associated with decreased physical performance, increased risk of falls, and overall reduced quality of life in menopausal women. Understanding and addressing the multifaceted relationship between menopause and musculoskeletal diseases are crucial for developing effective strategies to promote healthy aging in women and has received significant attention in recent literature [23]. Numerous randomized controlled trials have examined the impact of vitamin D supplementation on bone health, fractures, and related parameters. Sanders et al. [28], investigated the effect of annual high-dose oral vitamin D supplementation in postmenopausal women on preventing falls and fractures among the elderly. Similarly, Zhu et al. [29] embarked on a comprehensive five-year randomized controlled trial, focusing on the effects of both calcium and vitamin D supplementation. This parallel research initiative specifically observed its impact on hip bone mineral density in elderly Australian women. The choice of the hip as a focal point is of particular significance, considering its susceptibility to fractures and its importance in overall bone health assessment. The outcomes of Zhu et al.’s study [29] not only shed light on the immediate effects of supplementation but also offered crucial insights into the long-term implications of administering calcium and vitamin D supplements on bone health in the aging population. These results for short [30] and long term [31] vitamin D supplementation was also supported by others specifically for bone mineral density (BMD). They reported increased trabecular bone scores in the trochanter and the neck of the femur [30, 31]. Together, these independent studies significantly contribute to our knowledge about the possible advantages and long-term effects of interventions aimed at improving bone health and maintaining strong skeleton among postmenopausal women. Vitamin D effectively prevents bone fracture in postmenopausal women as it improves bone mineral density and muscle size and function [13].
In contrary, a significant number of studies have failed to demonstrate any improvement with in BMD or fracture incidence with vitamin D supplementation [32, 33, 34]. This is attributed to the differences in pre-treatment vitamin D status of the participants [35], the postmenopausal women who has already normal or high plasma vitamin D concentrations don’t benefit from the supplementation, where as in the ones with low plasma vitamin D levels significant improvements are observed [36].
Throughout life, sex steroids and their receptors play a crucial role in bone
development and remodeling. During puberty, the last growth acceleration occurs
in both genders where sex steroid hormones and growth hormone act together. After
the linear growth is completed the mineral density and bone turnover is
controlled by estrogen in women and androgens in men via conversion to estrogen
in addition to other factors involved in calcium homeostasis. Estrogens play a
significant role in bone remodeling by decreasing the number and activity of
osteoclasts. Estrogens affect various cell types, such as monocytes, marrow
stromal cells, and osteoblasts, regulating the production of cytokines that
modulate osteoclast activity. This regulation includes decreased production of
certain cytokines (Interleukin (IL)-1, Tumor Necrotizing Factor (TNF)-
Carefully evaluating accumulated data and previous meta-analysis on the results of vitamin D supplementation on skeletal system during post-menopausal period, it is concluded that correct form, dose and duration of supplementation helps to provide bone health and attenuate associated health risks. On this basis we reviewed the literature for different forms and durations of supplementation.
The data from the National Health and Nutrition Examination Survey (NHANES), a cross-sectional study was conducted on 2058 participants adjusted for various factors, and the results revealed that individuals with moderate (50–74 nmol/L) and high (greater than 75 nmol/L) 25(OH)D levels exhibited lower odds ratio for osteoporosis in total femur, femoral neck, and lumbar spine compared to the ones with low serum vitamin D concentrations (below 50 nmol/L). The protective effect of high 25(OH)D levels was particularly notable in postmenopausal women aged 65 and older. The findings suggest that maintaining adequate vitamin D levels may reduce the risk of osteoporosis in postmenopausal women, emphasizing the importance of monitoring serum 25(OH)D levels for osteoporosis prevention [37]. An 18-year study of more than 70,000 postmenopausal women associated reduced risk of osteoporotic hip fracture with adequate Vitamin D intake in postmenopausal women [38].
Schaafsma and colleagues evaluated the effects of vitamin D3 and vitamin K1 supplementation on plasma vitamin D level and carboxylated osteocalcin (a marker of bone turnover) on Dutch postmenopausal women with both normal and low bone mineral densities. The results showed significantly increased serum 25-(OH) vitamin D level and improved percentage of carboxylated osteocalcin. Implementing beneficial outcome of their protocol for maintaining bone health in this population [39]. A review written by Capozzi et al. [40], highlighted the significance of vitamin K2 as an effective and safe treatment for bone loss, contributing to the structural integrity of osteocalcin. The potential use of vitamin K2 alone or in combination with other medications to preserve bone quality and strength, particularly in postmenopausal women and individuals with secondary osteoporosis is considered as an important insight to enhance the synergistic effects of calcium and vitamin D on bone health.
Other studies investigating supplementation of vitamin D alone or in combination with calcium, reported better BMD values and decreased risk of fracture with this combinational approaches [41, 42, 43].
Even though, all these results indicate positive impact on skeleton when appropriate patients are selected, the approach should be personalized for each postmenopausal patient who is candidate for supplementation, as the excess vitamin D might adversely affect bone; decrease BMD and increase risk of fracture [41].
Postmenopausal women, facing a decline in estrogen levels, are susceptible to decreased bone density and increased fracture risk. Additionally, this period introduces increased risks of metabolic illnesses such as cardiovascular diseases, diabetes mellitus, hyperlipidemia, and emotional symptoms associated with menopause. Mei et al. [44] reviewed the effects of Vitamin D on skeletal muscle, cardiovascular health, genitourinary system, cancer, and emotional well-being in postmenopausal period. Regarding its actions on immune function, adipokine production, anti-proliferative effects on tumor cells, regulation of genitourinary symptoms, they concluded that Vitamin D supplementation is safe, accessible and inexpensive approach in improving the overall health in menopausal women.
Low estrogen concentration in the postmenopausal period is reported as the main cause of the rapid decline in skeletal muscle mass and muscle strength [45, 46]. Besides low estrogen; age, reduced physical performance, low testosterone, low vitamin D [47, 48, 49] are linked low skeletal muscle mass in women. In addition, muscle strength and skeletal muscle performance are also associated with vitamin D and serum estradiol [50] in postmenopausal women.
VDR is expressed in skeletal muscle, and the physiological role of vitamin D is
to regulate the proliferation and differentiation of myoblasts. The skeletal
muscle strength and function, as well as balance is improved [51]. On the other
hand, individuals with insufficient Vitamin D levels experience myalgia, reduced
muscle strength and physical performance. When the relationship between muscle
function and muscle strength was evaluated in postmenopausal women, the women
with Vitamin D levels
Comprehensive overview of the proposed mechanisms underlying the vitamin D/VDR axis’s role by Bollen et al. [53] in sarcopenic muscle atrophy, put forward that both genomic and non-genomic effects of active vitamin D and VDR play crucial roles in maintaining skeletal muscle function. In vitro studies have highlighted the relationship of vitamin D/VDR in regulating key processes contributing to sarcopenic muscle atrophy, including proteolysis, mitochondrial function, cellular senescence, and adiposity.
A research conducted by Garcia-Alfaro et al. [54] investigated factors associated with muscle strength in postmenopausal women under 65 years of age who had normal vitamin D status and identified dynapenia, a condition characterized by muscle weakness, in 12.2% of women, a weak negative correlation between grip strength and age, moreover linked the menopausal duration with elevated risk of dynapenia.
A meta-analysis by Zhang et al. [55] included 13 studies involving postmenopausal women over 60 years old. The participants were given with varying doses of vitamin D2 (2800 IU–210,000 IU) or vitamin D3 (7000 IU–100,000 IU) for varying durations (3–24 months). They concluded that vitamin D improves muscle strength by its role in calcium absorption and muscle tissue repair [55, 56].
Dzik and Kaczor [51] investigated the mechanisms by which vitamin D influences skeletal muscle function, focusing on oxidative stress, energy metabolism, and anabolic processes. They reported that deficiency in vitamin D is linked to oxidative stress in skeletal muscle, influencing mitochondrial function and contributing to the development of muscle atrophy [51]. The research on that field emphasizes the importance and benefits of maintaining adequate vitamin D levels in postmenopausal women for musculoskeletal system [51, 52, 53, 54]. The reduction in the production and circulation of estrogen, a naturally occurring antioxidant during menopause contributes to oxidative stress in skeletal muscle, inhibiting muscle protein synthesis and leading to mitochondrial dysfunction. Vitamin D and estrogen deficiency are involved in the loss of muscle mass and strength in the postmenopausal period, collectively [57].
The research performed by Kirk et al. [58], aimed to explore into the relationship between osteoporosis, the intensity of sarcopenia, BMD in older adults residing in the community. Using various measures, including dual-energy X-ray absorptiometry (DEXA) for BMD assessment, the researchers screened for osteoporosis and sarcopenia in 484 community-dwelling older adults, predominantly women with a median age of 76 years. The prevalence of osteoporosis escalated from 47.6% in non-sarcopenic individuals to 65.5% in probable sarcopenia and 78.1% in those with confirmed sarcopenia. Adjusted models revealed that osteoporosis was linked to an increased risk of confirmed sarcopenia. Although individuals with confirmed sarcopenia exhibited a higher number of fragility fractures, this association became nonsignificant in adjusted models. The findings suggest a notable association between osteoporosis and sarcopenia severity, emphasizing the importance of screening for sarcopenia in individuals with low BMD [58].
In conclusion, these studies collectively contribute to our understanding of the definite relationship between Vitamin D and musculoskeletal health, offering insights into potential preventive measures for osteoporosis, sarcopenia, and related fractures in postmenopausal women. These diseases lead to various musculoskeletal issues and diminished quality of life in menopausal women. Additionally, some researchers have explored the impact of vitamin D deficiency on skeletal muscle function, revealing potential links to oxidative stress and muscle atrophy. Examination of these multifaceted connections and understanding the complicated relationship between menopause and skeletal muscle health is crucial for developing effective strategies to promote healthy aging in women. Screening for factors such as sarcopenia and osteoporosis in individuals with low bone mineral density becomes essential for comprehensive health management in aging populations.
During menopause, the risk and occurrence of cardiovascular diseases (CVD) increase dramatically. This health risk is evident for a long period of time and explained by losing the beneficial effects of estrogen during menopause. In recent years, the relationship between Vitamin D and cardiovascular health particularly in postmenopausal women has become a focal point of research. The pioneering studies relates the cardioprotective role of estrogens mainly to the arterial vasodilation [59, 60], however a myriad of mechanism at the genomic and nongenomic levels are shown to be involved [61].
The vasorelaxant effect of estrogen is mediated via endothelial and vascular
smooth muscle cells, by biologically active estrogens with Er
With the onset of menopause decreasing estrogen results in slowed down basal
metabolic rate [52, 65]. The reduction of appetite-suppressing effect of estrogen
via estrogen alpha receptors (ER
Estrogen deficiency modulates intra-cellular calcium homeostasis, cyto-skeleton and extra-cellular matrix of cardiomyocytes and facilitates left ventricular diastolic dysfunction, which is commonly seen in postmenopausal women with heart failure (HF) proceeds with preserved ejection fraction [68]. Estrogen has been shown to be crucial in cardiomyocyte remodeling, as well [69]. Postmenopausal estrogen deficiency is significantly engaged in dysregulation of calcium signaling and mitochondrial functions, main mechanisms considered in CVD [70, 71].
The significant “female advantage” for CVD is lost with menopause and undoubtedly related to lack of estrogen but we should be aware that it is not the unique molecule involved in cardiovascular health and the role of vitamin D, exerting important biological actions, beyond its classical skeletal effects should not be overlooked [72].
Vitamin D, a hormone with diverse physiological functions, has gathered attention for its potential implications in the possible connections between Vitamin D levels and the risk of cardiovascular conditions such as coronary artery disease (CAD), heart failure, and stroke among postmenopausal women and revealed a higher occurrence of CAD in patients with vitamin D insufficiency when compared to the patients with adequate vitamin D levels. They suggested that evaluating vitamin D status should be considered in postmenopausal women as a potential contributing factor to CAD [73, 74, 75]. In support of these results when the impact of vitamin D supplementation on lipid profile; a risk factor for CAD, was evaluated in postmenopausal women, the significant improvement was noted, such that, lowered triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C) levels and increased high-density lipoprotein cholesterol (HDL-C) levels. The duration, dose and chemical form of the vitamin D supplementation and BMI of the patients modulates the results [76]. For example; a year-long study compared monthly calcifediol and cholecalciferol treatments in 303 postmenopausal women with vitamin D deficiency. Both maintained stable vitamin D levels by the fourth month, but calcifediol was more effective in sustaining levels at the 12th month and vitamin D levels significant decreased when supplementation is discontinued. The study not only confirmed the effectiveness and safety of long-term monthly calcifediol treatment in vitamin D-deficient postmenopausal women, demonstrating its faster onset of action compared to cholecalciferol [77], but also attracts attention to the fact that chemical form and the time and persistence of treatment are also crucial for cardiovascular health benefits in postmenopausal women.
In opposition to prior investigations proposing a connection between vitamin D
levels and cardiovascular disease (CVD) risk, a 5-year randomized,
placebo-controlled trial study reported contrasting findings, challenging the
previously suggested association. They explored the impact of vitamin D3
supplementation on the occurrences of CVD and cancer among 2495 older adults (men
Data from cross-sectional studies on 2000 morbidly obese individuals in Norway [79] and approximately 4000 obese and diabetic patients in India [80] linked lower vitamin D levels with visceral obesity and higher CVD risk in men and not in women. However, low vitamin D level was associated with the severity of the CVD [81]. When evaluating the relation of vitamin D to disease states other determinants i.e., body fat, comorbidities, UV exposure, dietary habits, physical activity level and hormone supplementation should also be considered.
Despite data controversy on sex-related difference in basal vitamin D and unsubstantiated remarks regarding D level and heart health in females and males, the need to improve vitamin D status related to cardiovascular health in the general population is unequivocally recognized [82].
The Vitamin D associated mechanisms involved in the development, progression and
output of CVD are mainly explained by expression of VDR and
1
Vitamin D increases intracellular calcium concentration of cardiomyocytes and
increases contractility [88]. Vitamin D stimulates calcium-binding protein
synthesis, activates adenylate cyclase, voltage-dependent calcium channels
activation and calcium shuttling between cytoplasm and sarcoplasmic reticulum
[89]. In addition, the emerging data suggests an anti-inflammatory role via
modulation of key players in CVD: inhibiting TNF-
A recent study suggested a synergistic role of vitamin D and estrogen in postmenopausal women with metabolic syndrome. Their results show that low vitamin D levels increases the CVD risk in women with hypoestrogenism, as documented by significant negative correlation between estrogen and metabolic syndrome in women with hypovitaminosis [93].
Regarding cardiovascular system in postmenopausal women estrogen and vitamin D may exhibit synergistic and/or independent effects in vascular smooth muscles, endothelium or cardiomyocytes [94]. As the estrogen and vitamin D driven pathways interact [69], maintaining sufficient vitamin D levels would help to overcome the results of estrogen deficiency.
While there are many question marks about the potential benefits of vitamin D in
postmenopausal period, it is quite clear that vitamin D supplementation is not a
miracle treatment for all diseases or all individuals. Evaluating various
clinical trials, cross-sectional studies or meta-analysis we can conclude that
beneficial effects of vitamin D are pronounced, in individuals with low (20–30
ng/mL) or insufficient (
Even the right person is chosen for supplementation, there are other issues that should be included into the equation in postmenopausal women such as; dose duration, chemical form and/or accompanying molecules to be given, follow up and assessment of treatment, body weight and body fat amount, the physical activity etc. [36].
Regarding the fact that the Vitamin D deficiency deepens worldwide towards insufficiency, this condition when combined with hypoestrogenic milieu of climacteric period results in increased risk of musculoskeletal and cardiovascular diseases. Although, majority of the clinical and epidemiological studies report a close association between low vitamin D levels and high incidence of various diseases, controversies still present about the role of vitamin D in postmenopausal period. In efforts to clarify these, the chemical form, duration of deficiency as well as treatment, individual differences, such as Body Mass Index (BMI), menopausal year and accompanying conditions should be included in evaluation of the patients.
HK and BP equally participated in preparation of the manuscript. HK and BP made substantial contributions to the conceptualization, design and preparation of the manuscript. Both authors have been involved in drafting and reviewing the manuscript critically for important intellectual content. Both authors have read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.