1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is ranked as the fourth leading cause of
cancer-related deaths worldwide [1], and the five-year survival rate is only 9%
[2]. Surgical resection combined with postoperative neoadjuvant chemotherapy is
the primary treatment option for patients with resectable PDAC. However, several
factors, such as genetic instability, metabolic abnormalities, and
immunosuppression [3], make chemical therapy less effective, and drug resistance
has become a key factor affecting the efficacy of chemotherapeutic drugs.
Gemcitabine (GEM; 2,2-difluorodeoxycytidine), a difluoride analog of
deoxycytidine, is commonly used for the treatment of PDAC. The drug interferes
with DNA synthesis by inhibiting ribonucleotide reductase and DNA polymerase
(through diphosphate analogs) or by misincorporation of DNA to prevent chain
elongation (through triphosphate analogs) [4]. However, the
median progression-free survival for advanced PDAC treated with GEM monotherapy
is only 3.7 months [5], and long-term chemotherapy can induce drug resistance in
patients, making continued treatment difficult [6].
Chemoresistance induced by GEM is associated with several factors, including
bacteria, and metabolic reprogramming [7]. In addition, various transcription
factors, cytokines, enzymes, and signaling pathways are involved in the
development of GEM resistance [8]. The transforming growth factor-
(TGF-) superfamily comprises various conserved growth factors
[9]. TGF- has a key role in tumorigenesis and associated stem
cell genesis [10]. It is an important factor involved in GEM resistance of
tumors, such as oral squamous cell carcinoma and prostate cancer [11]. In
addition, TGF- is associated with GEM resistance in PDAC. The
inhibition of TGF- receptor I increases the susceptibility of
parental and drug-resistant pancreatic cancer cells to GEM and promotes the
apoptosis of GEM-resistant cells [12]. Porcelli et al. [11] suggested
that crosstalk between mast cells and PDAC cells reduces the survival inhibition
of GEM-dependent tumor cells by activating the TGF- signaling
pathway.
TGF- interacts with specific genes and may indirectly
influence tumor therapy. Growth factor independence-1 (GFI-1), a
cellular proto-oncogene, was originally thought to play a role in T-cell
differentiation and lymphoma [13]. Xian et al. [14] reported that
simvastatin can decrease the resistance of PDAC to GEM by inhibiting the
TGF-1/GFI-1 axis. Kruppel-like factor 4
(KLF-4)—a transcription factor containing zinc finger
structure—regulates various biological processes including the
TGF- signaling pathway [15]. KLF-4 can be targeted by
TGF-1 to regulate vascular smooth muscle cells [16].
TGF-1 regulates the transcription of zinc finger E-box-binding
homologous box (ZEB) family genes involved in the epithelial-to-mesenchymal
transition (EMT) of tumor cells. Ursolic acid targets the
TGF-1/ZEB-1 axis and consequently decreases the
invasiveness of colorectal cancer cells [17].
Here, we found that TGF- knockdown alleviated the malignant
progression of PDAC induced by GEM resistance. Notably, the expressions of
KLF-4 and ZEB-1 (downstream of GFI-1) were altered
after TGF- knockdown. Our results suggest a novel target for
ameliorating GEM resistance in PDAC to increase the efficacy of treatment. To our
knowledge, this is the first report on the role of TGF- in
mediating GEM resistance in PDAC.
2. Materials and Methods
2.1 Cell Lines and Animals
Human PDAC cell line PANC-1 was procured from American Type Culture
Collection (ATCC, Manassas, VA, USA), and MIA PaCa-2 was purchased from Wuhan Pricella Biotechnology (No. CL-0627, Wuhan, China). The cell lines used have been tested for
mycoplasma and cell STR identification. The cells were cultured in RPMI 1640
medium (HyClone, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 100
U/mL penicillin, 100 U/mL streptomycin, and 0.03% l-glutamine. The
6-week-old nude SPF Balb/c female mice were purchased from Beijing Weitonglihua
Biotechnology Co., Ltd. (Beijing, China) and were adaptively fed for 3 days. Approximately 100
µL of tumor cell suspension (10 cells/mL) was subcutaneously
injected into the axilla, and tumors were allowed to grow for 6 weeks. The tumor
volume was measured every 5 days, and the mice were treated after the experiment.
2.2 Treatment of PANC-1 Cell Line with GEM and TGF-
The cells were cultured in RPMI 1640 medium containing 50
nmol/L GEM. The state of cells was observed after 2–3 days, and the
drug-supplemented medium was replaced with the standard medium until normal cell
growth was restored. The same protocol of cell culture was repeated until the
cells were stable under GEM exposure. The concentration of GEM was gradually
increased to 2 µmol/L. Finally, 10
µg/mL TGF- was added to the medium, and other
culture conditions remained same.
2.3 Construction of GEM-Resistant Cell Lines
Actively growing PDAC-1 cells were treated with 100 times IC50 of GEM as the
induction dose and subsequently cultured with GEM for 2 h. The drug-containing
medium was removed after 2 h, cells were rinsed three times with
phosphate-buffered saline, and the standard medium was added. The dead cells were
removed by changing the medium every day. The growth of the surviving cells
resumed, and the logarithmic growth stage was observed after 15 days. The cells
were passaged three times, and the above protocol was repeated four times.
Subsequently, the duration of the GEM exposure was extended to 4 h and the above
protocol was repeated eight times. The induction lasted 6 months, and the
drug-resistant cell line PGAC-1/GEMR was obtained.
2.4 TGF- Knockdown
TGF--silencing lentivirus was purchased from Ghanghai Bolsen Biotechnology Co., Ltd. (BES-20241Ab, Shanghai, China). The adherent cells (1 10/well) were plated onto a
24-well plate. The original medium was replaced with 2 mL fresh medium containing
6 µg/mL polybrene, and the viral suspension was added to the medium. The
plate was incubated at 37 ℃ for 24 h, and the virus-containing
medium was replaced with fresh medium. The transfection efficiency was measured
after 72 h using fluorescence-activated cell sorting.
2.5 Flow Cytometry
Annexin V-PE/7-ADD apoptosis detection kit (MA0429-2;
Dalian Boglin Biotechnology Co., Ltd., Dalian, Liaoning, China) was used to detect
apoptosis. PDAC cells were treated with GEM for 72 h and suspended in 500
µL of binding buffer. Annexin V-FITC and propidium iodide (5 µL each)
were subsequently added. Macrophages were isolated from tumor tissues and the
polarization of macrophages was detected by incubating them
with F4/80 antibody (1:100; ab6640, Abcam, Waltham, MA, USA). Annexin V-FITC was detected
using the PerCP channels. The cells were kept in the dark for 15 min, and the
stained cells were analyzed using a flow cytometer (BD Biosciences, San Jose, CA,
USA).
2.6 CCK8 Assay
PDAC cells were plated in a 96-well plate and cultured for 12 h. The cells were
then treated with a gradient concentration of GEM (0.1, 1,10, 100, 1000, and
10,000 nM) and cultured for 48 h. Finally, the CCK-8 reagent (C0037, Beyotime
Biotechnology, Shanghai, China, 10 mL/well) was added to each well, and absorbance was
measured at 450 nm using an immunosorbent instrument (BioTek Synergy H1, Agilent, Beijing, China).
2.7 MTT Assay
MTT cell proliferation assay kit [40206ES76; Yisheng Biotechnology (Shanghai)
Co., Ltd., China] was used to detect cell proliferation. The cells were seeded
into a 96-well plate and allowed to grow for 24 h. Approximately 20 µL of
MTT (5 mg/mL) was added into each well, and the plate was incubated for 4 h. The
culture medium was then removed, and 100 µL of dimethyl sulfoxide was added
to dissolve Jiazan particles. The plate was oscillated for 2–5 min to ensure
proper dissolution of formazan, and the OD value was recorded at 570 nm using an
enzyme-label instrument.
2.8 Western Blot Analysis
Cells were lysed in RIPA buffer with a proteinase inhibitor cocktail to extract
total cellular protein. The nuclear and cytoplasmic components were separated
using nuclear and cytoplasmic extraction reagents, respectively (Thermo Fisher
Scientific, Waltham, MA, USA). The protein samples were separated on 10%
polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The
blotted membranes were then incubated with the primary antibodies against cleaved
caspase-3 (1:1000; ab32042, Abcam), caspase-3 (1:1000; ab32351,
Abcam), KLF-4 (1:1000; ab215036, Abcam),
GFI-1 (1:1000; ab21061, Abcam), and ZEB-1 (1:1000; ab203829,
Abcam) overnight at 4 °C. Finally, goat anti-rabbit IgG secondary
antibodies were added to the membranes for 4 h. The membranes were then developed
using an enhanced chemiluminescence kit (20-500-120; Shanghai Xiao Peng
Biological Technology Co., Ltd., Shanghai, China).
2.9 Statistical Analysis
Each sample was analyzed in at least 3 independent experiments and at least
three technical replicates. Data are reported as the mean SEM. One-way
ANOVA, two-way ANOVA, or two-tailed Student’s t-test were performed for
pair-wise comparisons. p-values of 0.05 or less were considered
statistically significant.
3. Results
3.1 TGF- is a Chemoresistance-Associated Gene in the
GEM-Treated Pancreatic Cancer Cell Lines
TGF- has been confirmed as a gene associated
with GEM resistance in cancer [16]; however, the mechanism of
TGF--mediated chemoresistance in PDAC remains unclear. The
protein expression of TGF- was significantly downregulated in
GEM-treated PANC-1 and MIA PaCa-2 cells, suggesting the critical role of
TGF- in this process (Fig. 1A,B and Fig. 2A,B). We then
stimulated PNAC-1 cells with TGF- to induce an EMT environment.
Western blotting results revealed that the protein expressions of the
EMT-promoting transcription factors ZEB-1 and GFI-1 (downstream
of TGF-) were significantly increased, whereas the expression
of EMT-inhibiting KLF-4 were significantly decreased in pancreatic
cancer cells (Fig. 1C–F and Fig. 2C–F). These findings suggest that
TGF- may promote EMT through its downstream transcription
factors (ZEB-1, GFI-1, and KLF-4) in GEM-treated PDAC
cells, thereby playing a role in inducing chemoresistance.
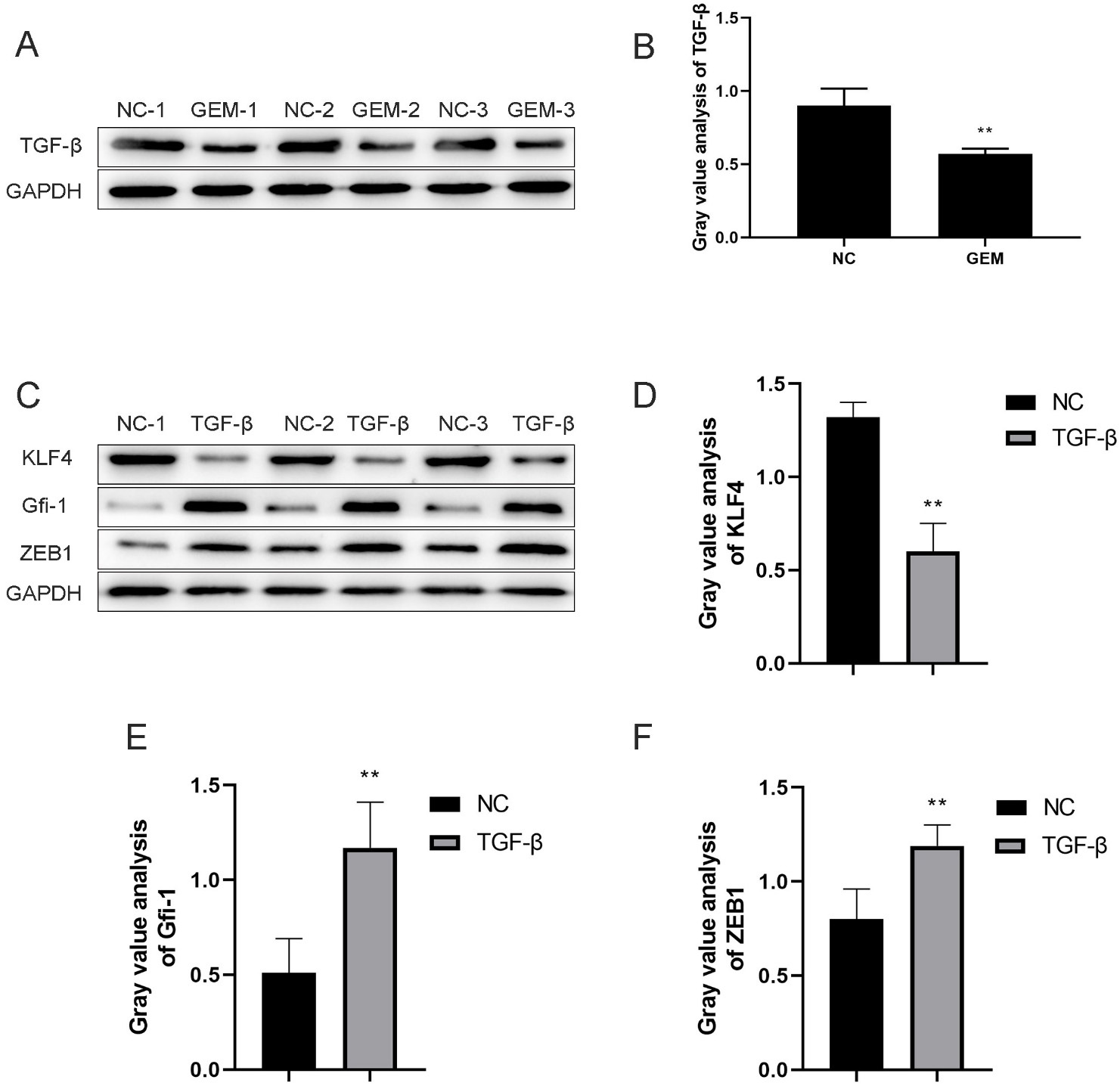 Fig. 1.
Fig. 1.
TGF- is a chemoresistance-associated gene in
the GEM-treated PANC-1 cell line. (A,B) Expression of
TGF- was detected using western blotting after PANC-1
cells were treated with GEM. (C–F) Protein expression levels of KLF-4,
GFI-1, and ZEB-1 after TGF- induction and
their quantitative analysis. **p 0.01,
n = 3.
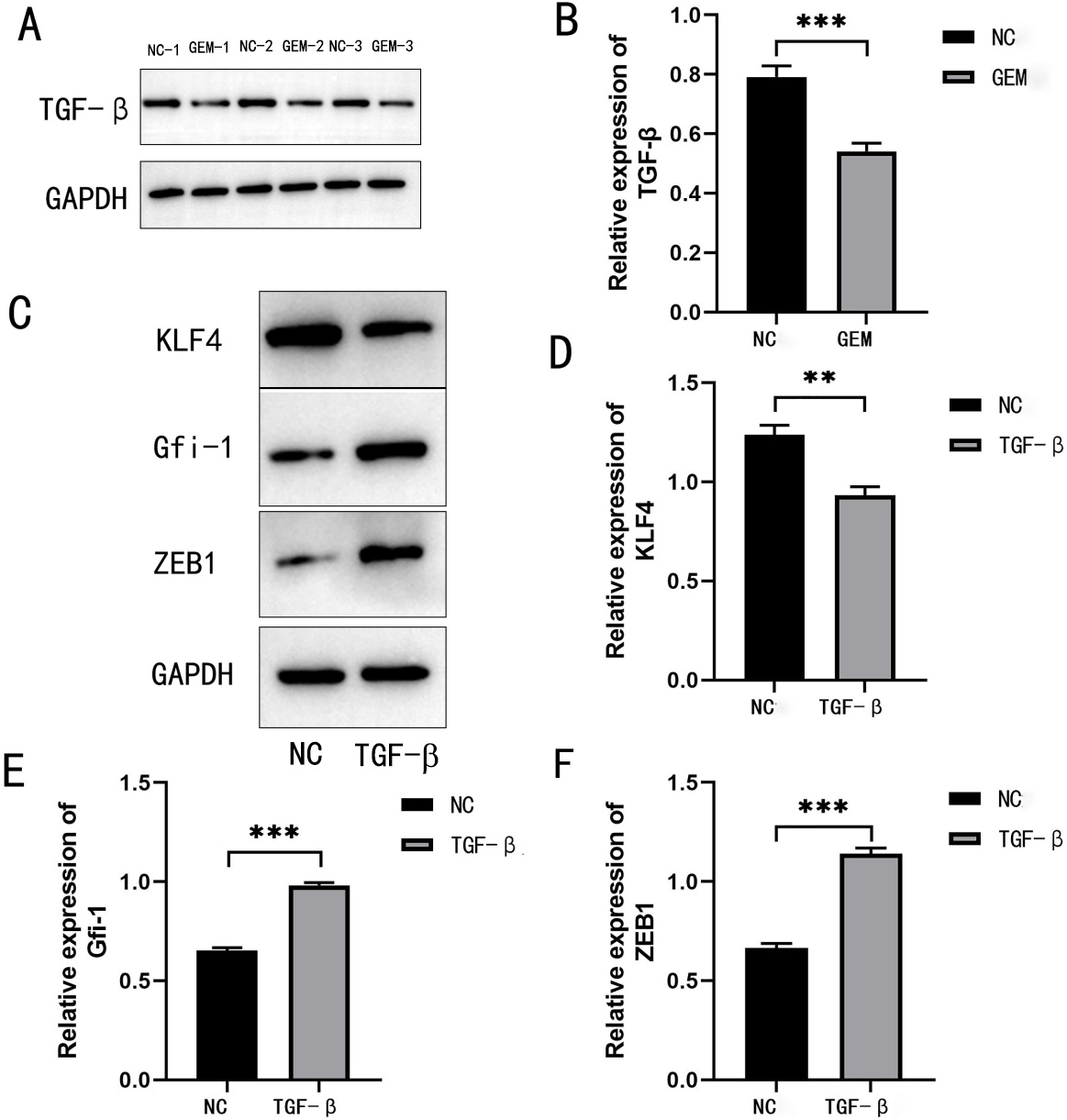 Fig. 2.
Fig. 2.
TGF- is a chemoresistance-associated gene in
the GEM-treated MIA PaCa-2 cell line. (A,B) Expression of TGF-
was detected using western blotting after MIA PaCa-2 cells were treated with GEM.
(C–F) Protein expression levels of KLF-4, GFI-1, and
ZEB-1 after TGF- induction and their quantitative
analysis. **p 0.01, and
***p 0.001,
n = 3.
3.2 TGF- Knockdown Ameliorates GEM Resistance in PDAC
Cells
We constructed PANC-1 and MIA PaCa-2 cell lines with GEM resistance and
TGF- knockdown. Compared with the control group (GEM-shNC), the
cell proliferation was significantly decreased at 24
(p 0.01), 48
(p 0.001), 72
(p 0.05), and 96 h
(p 0.05; Fig. 3C) after
TGF- knockdown in the GEM-shTGF- group.
Compared with the control group, the apoptosis of cells was significantly
increased after TGF- knockdown in the
GEM-shTGF- group
(p 0.001; Fig. 3A,B). In addition,
protein expression levels of the ZEB-1, GFI-1, and
KLF-4 transcription factors were also compared between the two groups.
Compared with the control group, the protein expressions of ZEB-1
(p 0.01) and GFI-1
(p 0.05) were significantly
downregulated, whereas the expression of KLF-4 was significantly
upregulated after TGF- knockdown in the
GEM-shTGF- group
(p 0.01; Fig. 3D–G).
Western blotting results revealed that
PANC-1/GEM-sh-TGF- induced the expression of cleaved
and total caspase-3 (Fig. 4). Another piece of corroborating evidence found the
same thing, compared with the control group, the apoptosis of drug-resistant MIA
PaCa-2 cells was significantly increased after TGF- knockdown
in the GEM-shTGF- group
(p 0.001; Fig. 5A,B).
PANC-1/GEM-sh-TGF- induced the expression of cleaved
and total caspase-3 in PANC-1/GEM-sh-TGF- induced the
expression of cleaved and total caspase-3 (Fig. 5C–E). Compared with the control
group (GEM-shNC), the cell proliferation was significantly decreased at 24
(p 0.01), 48
(p 0.001), 72
(p 0.05), and 96 h
(p 0.05; Fig. 6A) after
TGF- knockdown in the GEM-shTGF- group of
drug-resistant MIA PaCa-2 cells. Compared with the control group, the protein
expressions of ZEB-1 (p 0.01)
and GFI-1 (p 0.05) were
significantly downregulated, whereas the expression of KLF-4 was
significantly upregulated after TGF- knockdown in the
GEM-shTGF- group
(p 0.01; Fig. 6B–E). Therefore,
TGF- knockdown can inhibit the proliferation, promote
apoptosis, and suppress EMT, thereby ameliorating GEM resistance in pancreatic
cancer cells.
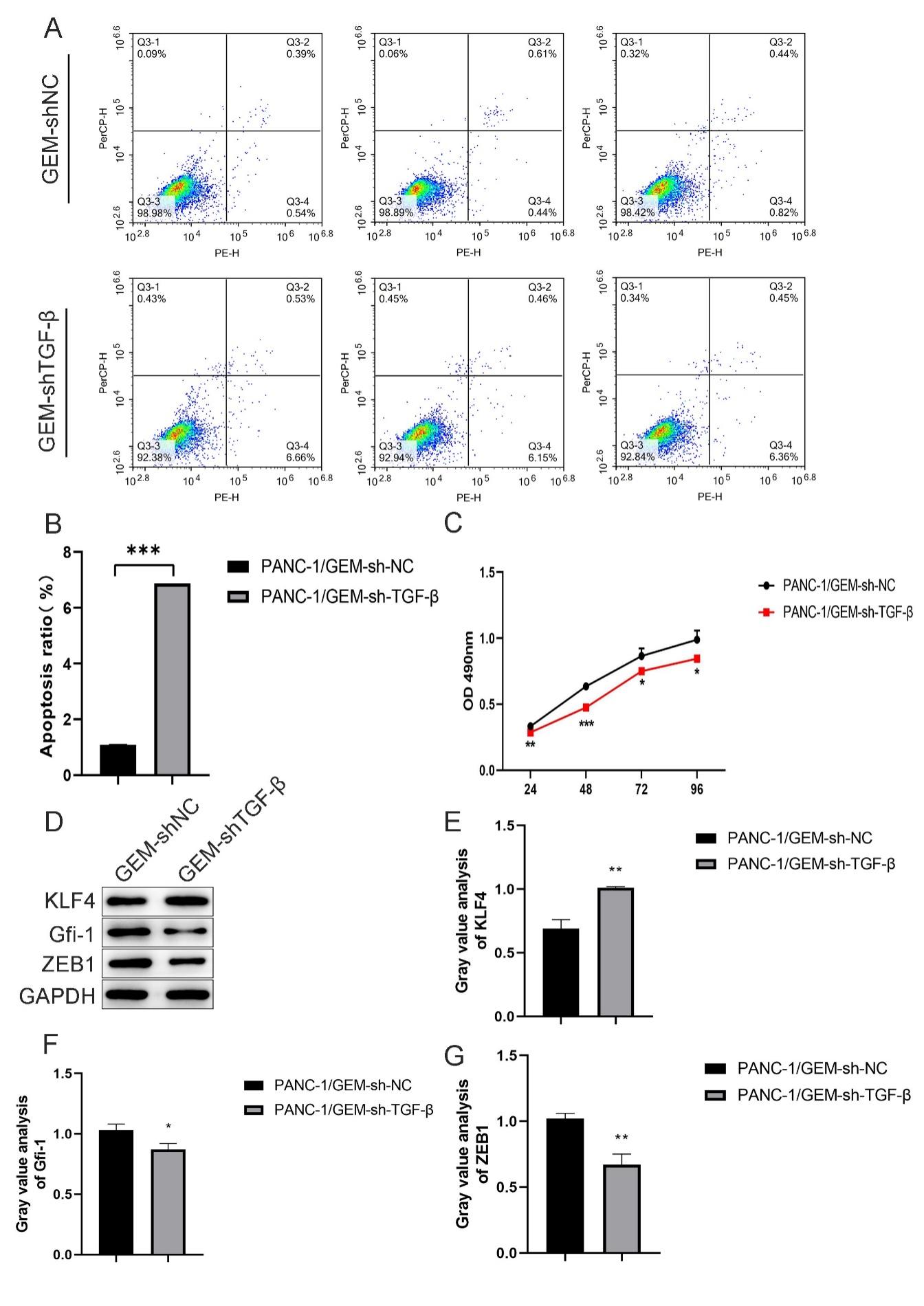 Fig. 3.
Fig. 3.
TGF- knockdown ameliorates GEM resistance in
PANC-1 cells. (A,B) Flow cytometry was performed to detect the
percentage of apoptotic drug-resistant PANC-1 cells after
TGF- knockdown. (C) CCK-8 proliferation assay was performed to
detect the proliferation of drug-resistant PANC-1 cells after
TGF- knockdown. (D–G) Western blotting was performed to detect
the percentage of apoptotic cells after TGF- knockdown. Protein
expression and quantification of KLF-4, GFI-1, and
ZEB-1. *p 0.05,
**p 0.01, and
***p 0.001;
n = 3.
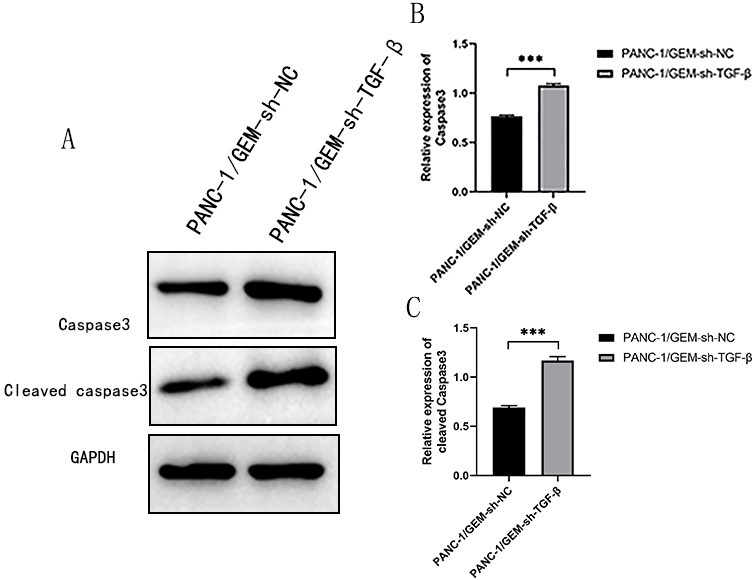 Fig. 4.
Fig. 4.
Downregulating TGF- promotes apoptosis of
PANC-1 cells. (A) Western blotting was performed to detect the
expression of caspase-3 and cleaved caspase-3. (B) Caspase-3 and (C) cleaved
caspase-3 quantification using Western blotting.
***p 0.001;
n = 3.
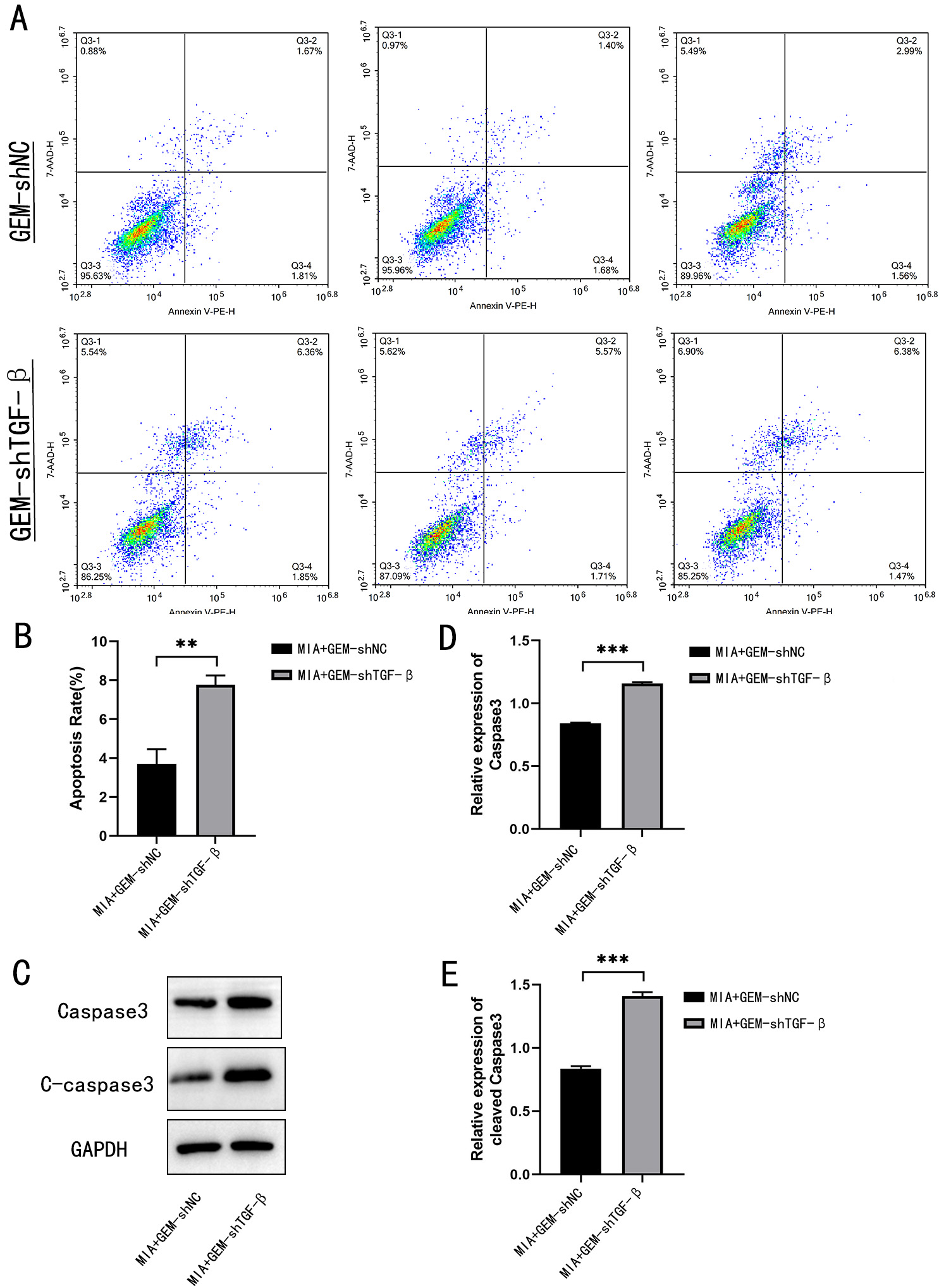 Fig. 5.
Fig. 5.
TGF- knockdown promotes apoptosis of MIA
PaCa-2 cells. (A,B) Flow cytometry was performed to detect the percentage of
apoptotic drug-resistant MIA PaCa-2 cells after TGF- knockdown.
(C) Western blotting was performed to detect the expression of caspase-3 and
cleaved caspase-3. (D) Caspase-3 and (E) cleaved caspase-3 quantification using
western blotting. **p 0.01,
***p 0.001;
n = 3.
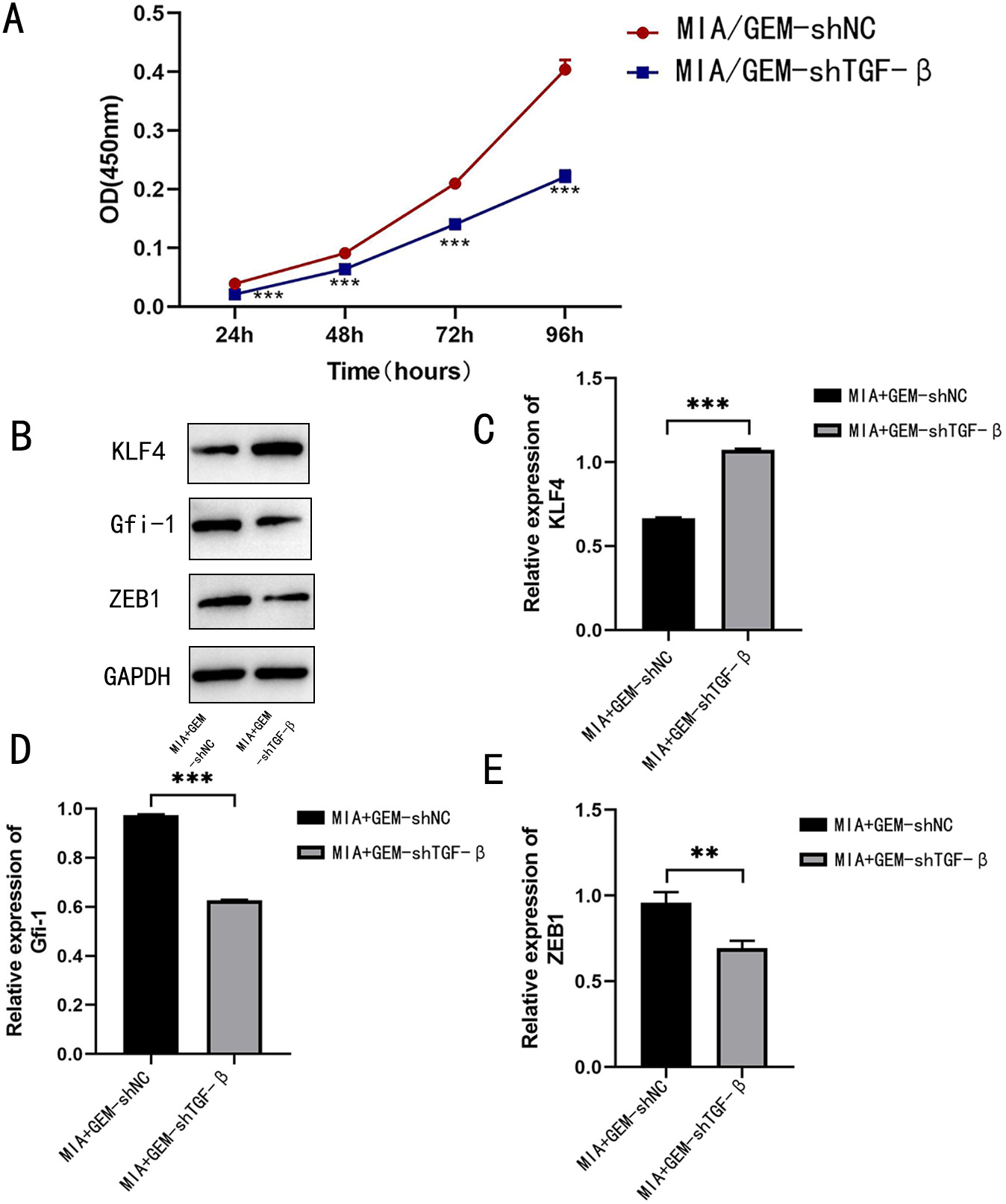 Fig. 6.
Fig. 6.
TGF- knockdown ameliorates GEM resistance in
MIA PaCa-2 cells. (A) CCK-8 proliferation assay was performed to detect the
proliferation of drug-resistant MIA PaCa-2 cells after TGF-
knockdown (B–E) Western blotting was performed to detect the percentage of
apoptotic cells after TGF- knockdown. Protein expression and
quantification of KLF-4, GFI-1, and ZEB-1.
**p 0.01, and
***p 0.001;
n = 3.
3.3 TGF- Knockdown Helps Alleviate GEM Resistance in
Pancreatic Cancer Mice
According to Fig. 7D, GEM-resistant PANC-1 cells transfected with the TGF- knockdown plasmid could stably inhibit the expression of TGF- in mice, indicating that we successfully constructed the TGF- knockdown plasmid. We subcutaneously injected
sh-TGF--transfected PANC-1 cells in nude mice to
examine the effect of silencing TGF- expression on tumor
formation, the tumor formation in nude mice is shown in Fig. 7A. The tumor volume
and mass were the highest in the GEM-resistant group (GEM-shNC). However, the
tumor volume (p 0.05; Fig. 7B) and
mass (p 0.01; Fig. 7C) were decreased
in the TGF--knockdown group (GEM-shTGF-
group). In addition, protein expression levels of ZEB-1, GFI-1,
and KLF-4 were compared among the three groups (Fig. 7D–H). Compared
with the GEM-resistant group, the protein expressions of ZEB-1
(p 0.01) and GFI-1
(p 0.05) were significantly
downregulated, whereas the expression of KLF-4 was significantly
upregulated (p 0.01) after
TGF- knockdown in the GEM-shTGF- group. These
findings were similar to those of cell line experiments. Therefore,
TGF- knockdown ameliorates GEM resistance in a pancreatic
cancer mouse model by inhibiting EMT in cancer cells.
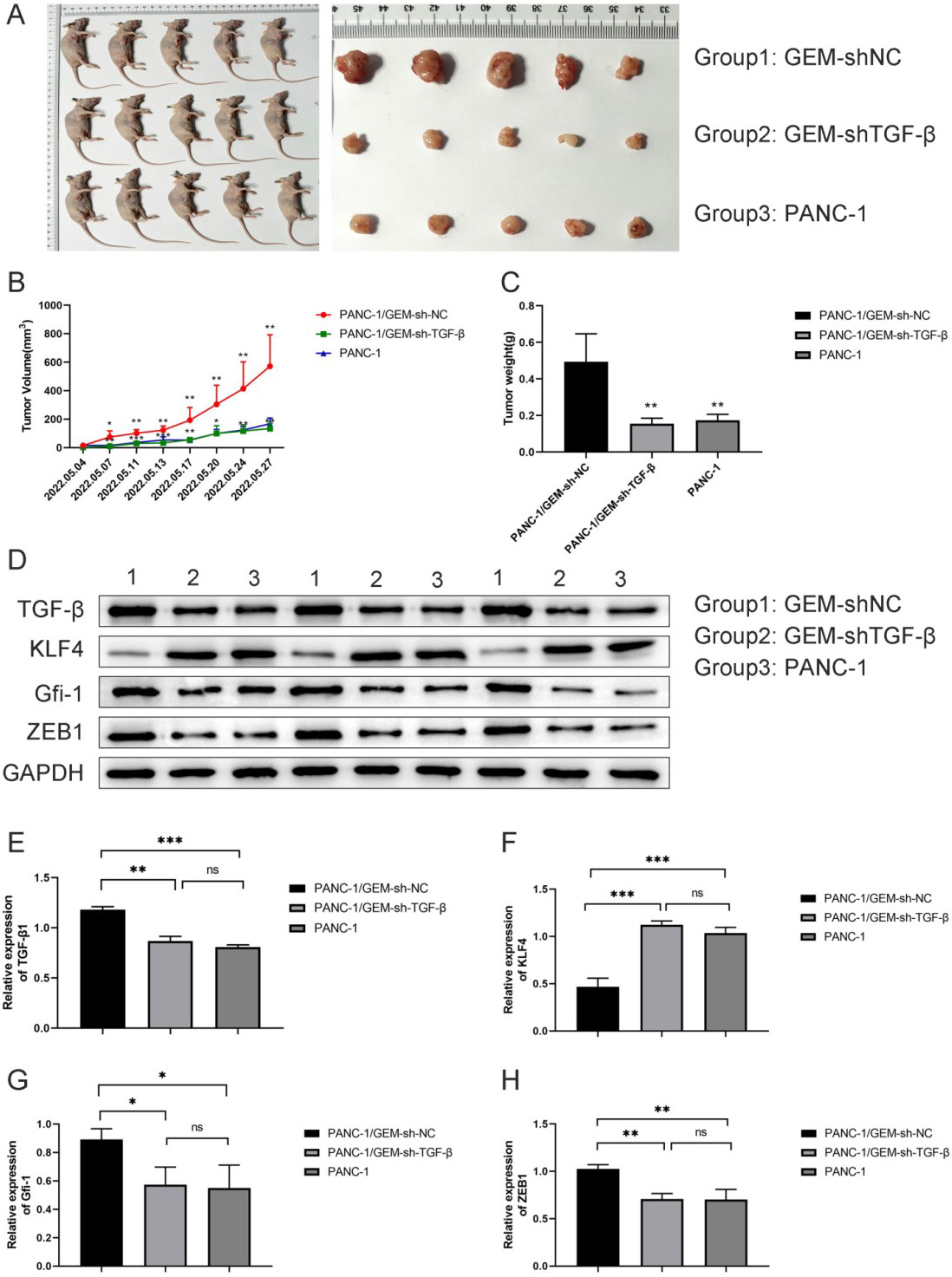 Fig. 7.
Fig. 7.
TGF- knockdown helps alleviate GEM resistance
in pancreatic cancer mice. (A) Pancreatic cancer mouse model was constructed by
subcutaneously injecting GEM-resistant PANC-1 cells in mouse axilla. (B)
Tumor volume and mass (C) measurements in each group. (D–H) Expression and
quantification of the KLF-4, GFI-1, and ZEB-1 proteins
in tumor tissues using western blot analysis. ns means no difference
significance, *p 0.05,
**p 0.01, and
***p 0.001;
n = 3.
3.4 TGF- Knockdown Regulates the
Polarization of Macrophages
M2-type macrophages (tumor-associated macrophages) produce several cytokines
that promote the survival, angiogenesis, and metastasis of malignant tumor cells
to maintain tumor growth [18]. Qiaofei Liu reported that TGF-
regulates the M0/M2 polarization of macrophages in pancreatic cancer [19].
Therefore, we detected the percentage of M2 macrophages in tumor tissues obtained
from the three groups of nude mice. The percentage of M2 macrophages was the
highest in the GEM-resistant group. However, the percentage of M2 macrophages was
significantly decreased after the knockdown of TGF- in the
GEM-resistant group (p 0.001; Fig. 8A,B). These findings suggested that TGF- knockdown ameliorates
GEM resistance in pancreatic cancer mice by decreasing the polarization of
macrophages to the M2 phenotype.
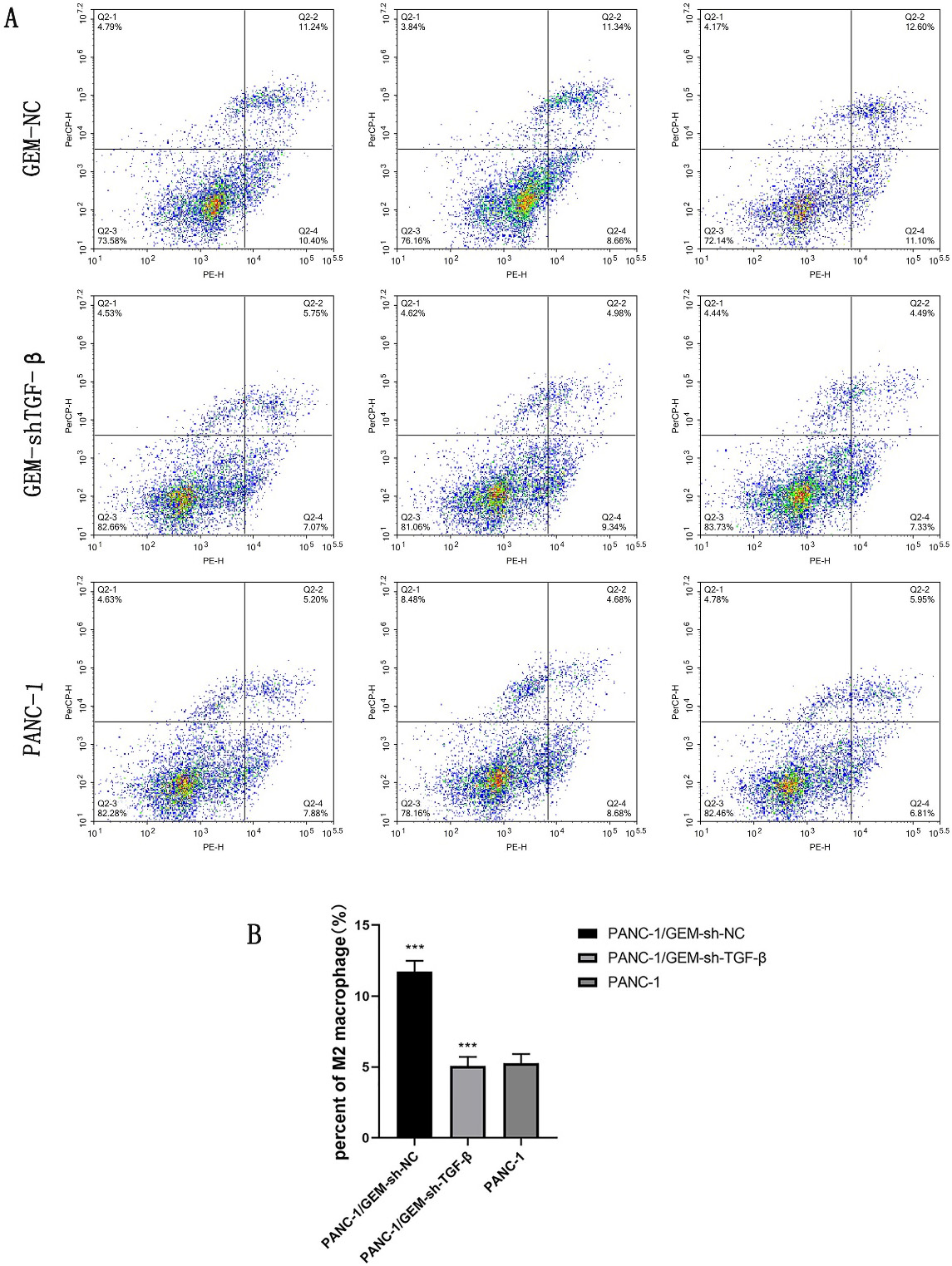 Fig. 8.
Fig. 8.
TGF- knockdown regulates the polarization of
macrophages to the M2 phenotype. (A,B) Percentage of M2-type
macrophages after TGF- knockdown detected using flow cytometry;
***p 0.001;
n = 3.
4. Discussion
The activation of the TGF- signaling pathway enhances the
progression of pancreatic cancer and promotes GEM resistance; however, the
specific mechanism has not been clarified [13, 20]. TGF- can
regulate tumor invasion and metastasis by regulating the EMT signaling pathway.
In addition, it can directly promote the proliferation and inhibit the apoptosis
of tumor cells, thereby regulating the malignant progression of cancer. Lou
et al. [21] reported that naringin downregulates the mRNA and protein
levels of EMT markers by inhibiting the TGF-1/Smad3 signaling
pathway in pancreatic cancer cells. This downregulation inhibits the activity of
cancer cells and reverses their resistance to GEM. TGF-1
secreted by tumor-associated fibroblasts upregulates ATF4 expression in PDAC
cells and induces pancreatic cancer progression (proliferation, colony formation,
and migration) and GEM resistance [10]. BRAP inhibits the proliferation,
migration, and self-renewal of glioma stem cells [22]. Reserpine has potential
therapeutic value in inhibiting DNA repair, cell proliferation, and invasion
while inducing cell apoptosis by regulating the TGF- signaling
[23]. LINC00665 is overexpressed in gastric cancer cells, and the activation of
gastric cancer cell lines was inhibited by the TGF- signal
after knocking down the LINC00665 gene. Moreover, apoptosis was promoted in
cancer cells [24]. In addition, the authors indicated that downregulating
TGF- can inhibit cell proliferation and promote cell apoptosis
in GEM-resistant pancreatic cancer cell lines. The volume and weight of
transplanted tumors were markedly decreased after TGF-
downregulation, suggesting that the downregulation of TGF- can
ameliorate GEM resistance in mice.
Components of the TGF- signaling pathway are expressed in most
liver cancer cells, and the activation of this pathway promotes cell migration
and invasion. Regulating the expression of KLF-4 can block
TGF- signal transduction [25]. KLF-4 depletion
inhibits the mesenchymal characteristics of stem cells and
TGF-1 pathway activation, whereas the overexpression of
KLF-4 can activate the phosphorylation of TGF-1,
expression of Smad 2/3 and Snail, and restore the stem cell and mesenchymal
phenotype [26]. The TGF- signaling pathway promotes the
expression of IL-7R and the differentiation of CD8+ T cells through
downstream GFI-1 and plays a regulatory role in the immune
microenvironment of tumors [27]. ZEB-1 is an EMT marker gene, which can
significantly promote the metastasis and progression of pancreatic cancer [28].
In addition, it acts as an oncogene to promote the activation of pancreatic
cancer [29].
Several studies on the three downstream transcription factors of
TGF-, namely KLF-4, GFI-1, and ZEB-1
have been reported in recent years. The clinical manifestations of head and neck
squamous cell carcinoma are closely related to EMT, and TGF-1
promotes tumor progression through the EMT pathway by downregulating the
expression of anti-EMT factor KLF-4 [30]. Downregulation of the
GFI-1 transcription factor driven by TGF- promotes
Th17-cell differentiation and subsequently promotes tumor growth [31].
ZEB-1, a downstream transcription factor of TGF-,
plays a key role in EMT and tumor metastasis. Consistent with these findings, our
results also revealed that the protein expressions of ZEB-1 and
GFI-1 were significantly increased, whereas the expression of
KLF-4 was significantly decreased in a TGF--induced
environment. Moreover, the knockdown of TGF- upregulated
KLF-4 expression and downregulated ZEB-1 and GFI-1
expression in GEM-resistant pancreatic cancer cell lines or mouse models with
transplanted tumors. Therefore, TGF- may be involved in GEM
resistance in pancreatic cancer through the EMT pathway by regulating the three
downstream transcription factors (ZEB-1, GFI-1, and
KLF-4).
Conditioned medium treated with gemcitabine can promote the infiltration,
growth, and M0/M2 polarization of macrophages in pancreatic tumors, thus forming
an immunosuppressive microenvironment. Simultaneous blocking of
TGF-1 and GM-CSF improves the efficacy of chemotherapy by
decreasing the concentration of the M2-polarized tumor-associated macrophages and
inducing CD8+ T cells in mice with normal immunity [13]. Notably, our study also
indicated that the knockdown of TGF- ameliorated GEM resistance
in pancreatic cancer mice by inhibitng the M0/M2 polarization of macrophages.
Furthermore, the stability of HIF-1 regulated by mucin 1 mediates
metabolic reprogramming of PDAC, and targeting HIF-1 or neopyrimidine
biosynthesis and combining this approach with GEM therapy can significantly
reduce the tumor burden. PDAC tumors with high mucin-1 levels responded to
TGF--neutralizing antibodies, leading to a substantial decrease
in tumor growth. However, tumors with low mucin-1 levels did not respond to
TGF--neutralizing antibodies. However, we did not explore the
mechanism of mucin-1-mediated resistance to gemcitabine, and our future studies
will focus on this aspect.
5. Conclusions
This study revealed that the knockdown of TGF- inhibits EMT,
suppresses the proliferation and promotes the apoptosis of drug-resistant cancer
cells, and decreases the polarization of macrophages to the M2 phenotype, thereby
ameliorating the GEM resistance in pancreatic cancer cells.
Abbreviations
PDAC, pancreatic ductal adenocarcinoma; TGF-, transforming
growth factor-; GFI-1, growth factor independence-1;
KLF-4, Kruppel-like factor 4; ZEB-1, zinc finger E-box-binding
homologous box-1.
Availability of Data and Materials
All data analysed during this study are included in this published
article. Analysed data of flow cytometry could be found in Supplementary Material. Further enquiries can be directed to the corresponding author.
Author Contributions
XW contributed to the conception of the study. XW, ZZ and WS designed the study. CQ, RG and SS participated in data collection. XX and JG performed data analysis, prepared the figures and tables. XW and WS wrote the manuscript. ZZ and JG do the writing – review and supervised the project. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Experiments of 4–5 weeks SPF Balb/c female nude mice were been reviewed and
approved by the Animal Protection and Use Committee of Shandong Provincial
Hospital Affiliated to Shandong First Medical University. Approval number:
NO.2019039.
Acknowledgment
Not applicable.
Funding
This work supported by Chen Xiao-Ping Foundation for the Development of Science and
Technology of Hubei Province [CXPJJH12000001-2020304] and Foundation research
project of Qinghai province [2021-ZJ-719].
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5. Fig. 6.
Fig. 6. Fig. 7.
Fig. 7. Fig. 8.
Fig. 8.







