1. Introduction
Osteoporosis is a widespread skeletal issue characterized by
reduced bone density, microstructural deterioration, and weakened bone strength,
which escalates the risk of fractures [1]. The menopausal period is the primary
stage for osteoporosis development, as women experience a decline in estrogen
levels during this phase, leading to disrupted bone remodeling, heightened
osteoclast function, and hastened bone resorption [2, 3]. In fact, postmenopausal
females are at higher risk for osteoporosis [4, 5]. However, effectively managing
osteoporosis in postmenopausal females remains a challenge in the medical field.
Therefore, investigating the pathophysiological mechanisms of osteoporosis in
postmenopausal women is of great significance for providing theoretical support
for clinical prevention and treatment strategies.
Artesunate (ART), derived from artemisinin, is predominantly employed as a
medication against malaria [6, 7]. Studies have shown that ART hinders nuclear
factor kappa B (NF-B) signaling pathway activity by impeding the
breakdown of inhibitory B (IB) and the
movement of NF-B p65 into the nucleus [8, 9]. By curbing NF-B
activity, ART diminishes the release of NF-B-driven inflammatory
cytokines like tumor necrosis factor-alpha (TNF-) and interleukin-6
(IL-6), thus demonstrating anti-inflammatory properties [10]. Although the
relationship between ART and the NF-B signaling pathway has been
extensively studied, whether ART affects the occurrence and development of
postmenopausal osteoporosis through this mechanism remains unknown.
Studies have demonstrated the pivotal involvement of NF-B in bone
formation. Specifically, blocking NF-B signaling has effectively
suppressed osteoclast differentiation and activity, thereby mitigating bone loss
[11]. Additionally, suppressing the extracellular signal-regulated kinase (ERK)/NF-B pathway has demonstrated
inhibition of osteoclast formation and activation, thereby decelerating bone loss
in ovariectomized mice [12]. In postmenopausal osteoporosis, modulating the
NF-B pathway to suppress osteoclast maturation and bone resorption has
demonstrated a certain degree of bone protection [13]. Therefore, interfering
with the NF-B pathway may potentially serve as a protective measure to
address osteoporosis in postmenopausal women. The involvement of the Notch
signaling pathway in the development and advancement of diseases is linked to its
control over various cellular functions, such as cell proliferation,
differentiation, and apoptosis [14]. Recently, it has been discovered [15] that
Notch1, as one of the main receptors in the Notch signaling pathway, plays a
crucial role in this pathway. Inhibiting the Notch signaling pathway and its
downstream target gene Hes1 can suppress the maturation and differentiation of
osteoclasts while promoting osteoblast-mediated bone formation and mineral
deposition [16]. The Notch1/Hes1 signaling pathway holds significance in
regulating bone metabolism, and ART has been demonstrated to suppress its
activation [17]. Nevertheless, whether it affects the development of
postmenopausal osteoporosis in women through the Notch1/Hes1 signaling pathway
remains uncertain.
In summary, our study suggests that ART may have therapeutic
potential for osteoporosis by inhibiting the NF-B and Notch1/Hes1
signaling pathways. Through in vitro experiments, we treated bone marrow
mesenchymal stem cells (BMSCs) with different concentrations of ART to assess its
effects on cell proliferation, osteogenic differentiation, and protein expression
related to bone metabolism. Additionally, we established a postmenopausal
osteoporosis rat model to investigate the in vivo effects of ART on bone
tissue pathology, bone density, mineral content, and inflammatory factors. Our
findings contribute to a better understanding of ART’s pharmacological activity
in diseases and provide a theoretical basis and research directions for further
elucidating the pathogenesis of postmenopausal osteoporosis in women and
exploring new treatment approaches.
2. Materials and Methods
2.1 Cell Culture and Treatment
The rat bone marrow mesenchymal stem cells (BMSCs, CP-R131,
Procell Life Science Co., Ltd., Wuhan, China) were nurtured in alpha-minimum essential medium (-MEM)
(SH30265.01B, HyClone, Logan City, UT, USA) medium augmented with 10% fetal bovine serum (FBS)
(10100147, Gibco, CA, USA) and 1% streptomycin (15140122,
Gibco, CA, USA), and incubated at 37 °C under 5% CO. Osteogenic
differentiation was induced using dexamethasone (CAS: 50-02-2, Sigma-Aldrich,
Shanghai, China), -glycerophosphate (154804-51-0, Sigma-Aldrich,
Shanghai, China) at 5 mM, and 50 µg/mL L-ascorbic acid (50-81-7,
Sigma-Aldrich, Shanghai, China). Cells received treatment with artesunate (ART,
IA1300, Solarbio, Beijing, China) at four distinct concentrations (0, 3, 6, or 12
µM) [18], along with Phorbol myristate acetate (PMA, 10
µM, P8139, Merck, Darmstadt, Germany) and Valproic acid (VPA, 2 mM, S3944,
Selleck.cn Houston, TX, USA) for 4 hours. Various treatment groups were designed:
control group, ART group, PMA group, VPA group, ART+PMA group, and ART+VPA group
to determine the effects of ART and PMA, VPA on BMSCs osteogenic differentiation.
PMA and VPA are activators of NF-B and Notch1 signaling pathways,
respectively. Before the experiment, all cells underwent short tandem repeat
(STR) identification and mycoplasma detection to ensure that the SRT
identification of all cell lines was consistent with the reference values in the
database and no signs of mycoplasma infection were detected. All procedures
followed an aseptic technique to prevent cell contamination.
2.2 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide
(MTT) Assay
Cells were distributed into a 96-well plate, each well housing 5
10 cells, and then allowed to incubate for 24 hours. Following that,
various doses of ART (0, 3, 6, 12 µM) were applied to the cells for 12 or
24 hours. After adding 20 µL of MTT solution (5 µg/mL per well,
PB180519, Procell Life Science Co., Ltd., Wuhan, China), the plate was further
incubated for 4 hours. Afterward, 150 µL of dimethyl sulfoxide (DMSO) was
introduced into each well and allowed to incubate for 15 minutes. The analysis
was conducted at 540 nm using a microplate reader.
2.3 Alkaline Phosphatase Staining (ALP)
BMSCs underwent fixation in 4% paraformaldehyde (PFA, ml28498-5, Shanghai
Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) for 30 minutes, then
underwent two PBS washes. Afterward, ALP staining was conducted utilizing a
5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) staining
kit (E-BC-K091-M, Elabscience, Wuhan, China). Cells were then incubated with
BCIP/NBT in the dark for 3–10 minutes, washed in running water to stop the
staining, and rinsed twice with PBS (P1010, Solarbio, Beijing, China). The
samples were air-dried overnight, and mineralized nodules were observed under a
optical microscope (N-SIM, Nikon, Tokyo, Japan).
2.4 Alizarin Red S Staining (ARS)
Following fixation in 4% PFA for 30 minutes, BMSCs were exposed to 1 mL of ARS
staining solution (130-22-3, Sigma-Aldrich, Shanghai, China) for an extra 30
minutes at room temperature. The ARS staining solution binds to calcium ions
within the cells, creating Alizarin Red-calcium complexes, which exhibit a
vibrant red color. Following this, images were taken using an optical microscope
(N-SIM, Nikon, Tokyo, Japan). To quantify the results, each well received 1 mL of
10% cetylpyridinium chloride solution. Following incubation at room temperature
for 1 hour, the absorbance was assessed at 570 nm using a microplate reader
(Biotek, Winooski, VT, USA).
2.5 Real-Time Quantitative PCR (RT-qPCR)
RNA was isolated using the TRIzol (Sigma, St. Louis, MO, USA) and then subjected
to reverse transcription. Subsequently, PCR amplification was performed using the
SYBR Green I fluorochrome (SYBR Green) Pro Taq HS pre-mix qPCR
kit (AG11756, Accurate Biotechnology Co., Ltd., Changsha, China) according to the
manufacturer’s guideline. RT-qPCR analysis was conducted using the Applied
Biosystems (ABI) QuantStudio 5 Real-Time PCR Systems. The primer sequences for
OCN, RUNX2, OPG, RANKL, and GAPDH are listed in Table 1,
designed by Shanghai Sangon Biotech Co., Ltd (Shanghai, China). Results were
quantified employing the 2 approach.
Table 1.Primer sequences.
| Name |
ID |
Forward primer |
Reverse primer |
| OCN |
NM_013414.1 |
5-CCGTTTAGGGCATGTGTTGC-3 |
5-CCGTCCATACTTTCGAGGCA-3 |
| RUNX2 |
NM_001278483.2 |
5-CAAGGAGGCCCTGGTGTTTA-3 |
5-TTGAACCTGGCCACTTGGTT-3 |
| OPG |
NM_012870.2 |
5-CACAACCGAGTGTGCGAATG-3 |
5-AAGTGAGCTGCAGTTGGTGT-3 |
| RANKL |
NM_057149.2 |
5-AGGCTGGGCCAAGATCTCTA-3 |
5-GTTGGACACCTGGACGCTAA-3 |
| GAPDH |
NM_017008.4 |
5-GATTCCACCCATGGCAAATTC-3 |
5-CTGGAAGATGGTGATGGGATT-3 |
2.6 Western Blot (WB)
Cellular proteins were isolated using radio-immuno precipitation assay (RIPA)
buffer (P0013B, Beyotime, Shanghai, China), followed by separation via 8% sodium
dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and their transfer
onto polyvinylidene fluoride (PVDF) membranes (IPVH00005, Millipore, Billrica,
MA, USA). Afterward, the membranes underwent blocking with 5% Bovine Serum
Albumin (BSA) for 1 hour, followed by PBS washing. Subsequently, the membranes
underwent an overnight incubation at 4 °C with the respective primary
antibodies: p-IB (AP0707, 1:1000, ABclonal, Wuhan, China),
NF-B (A14754, 1:1000, ABclonal, Wuhan, China), Notch1 (A7636, 1:1000,
ABclonal, Wuhan, China), Hes-1 (A0925, 1:1000, ABclonal, Wuhan, China), GAPDH
(A19056, 1:5000, ABclonal, Wuhan, China). After washing with PBS, the membranes
underwent incubation with the corresponding secondary antibody, goat anti-rabbit
IgG (H+L) (AS014, 1:2000, ABclonal, Wuhan, China), for 2 hours at room
temperature. After three washes with tris-buffered saline Tween-20 (TBST) buffer (60145ES76, Yi Sheng
Biotechnology, Shanghai, China) for 5 minutes each, signal detection was
performed utilizing an enhanced chemiluminescence detection kit (P0018S,
Beyotime, Shanghai, China). Image J software (V1.8.0.112, NIH, Madison, WI, USA)
was used for image analysis.
2.7 Immunofluorescent Staining (IF)
Cells were seeded on coverslips and cultured until reaching
70% confluence. Following fixation with 4% PFA for 10 minutes, permeabilization
was achieved using 0.2% Triton X-100 (20107ES20, Yi Sheng Biotechnology,
Shanghai, China) for an additional 10 minutes. After overnight incubation at 4
°C, the coverslips were subjected to primary antibody treatment against
NF-B p65 (AN365, 1:500, Beyotime, Shanghai, China) and Notch1 (AF5249,
1:200, Beyotime, Shanghai, China), followed by subsequent incubation with
secondary antibodies at room temperature for 1 hour. After PBS washing, the
coverslips received staining with 0.5 µg/mL
4,6-diamidino-2-phenylindole (DAPI) (R0306S, Beyotime, Shanghai, China) for 10
minutes and were then sealed with 20 µL mounting medium.
The fluorescence intensity of cells was observed by confocal
fluorescence microscopy (Olympus, Tokyo, Japan). All the intensities of
immunofluorescence expressions were quantitatively evaluated by using Image
Pro-Premier version 9.1 (Media Cybernetics, Rockville, MD, USA).
2.8 Establishment of Animal Model
Ovariectomy was performed on female Sprague-Dawley (SD) rats (Slack-Janda,
Changsha, China) at 6 weeks of age, weighing 180–220 g, to establish a
postmenopausal osteoporosis rat model [19, 20]. Rats resided in an environment
maintained free of specific pathogens and were randomly assigned to 7 groups (n =
5). After a 12-hour fasting period, rats received anesthesia using 2% sodium
pentobarbital (4 mL/kg, administered via intraperitoneal injection) before
undergoing ovariectomy. Sham-operated rats had equivalent volumes of periovarian
fat tissue removed. Four weeks after ovariectomized (OVX), the
following treatments were administered to OVX rats [21]: (1) In the sham-operated
group, rats received water via oral gavage (10 mg/kg/d). (2) OVX group, rats were
administered distilled water by gavage (10 mg/kg/d). (3) OVX+ART group: OVX rats
received ART treatment (10 mg/kg) [22]. (4) OVX+PMA group: OVX rats received PMA
treatment (10 mg/kg). (5) OVX+VPA group: OVX rats received VPA treatment (10
mg/kg). (6) OVX+ART+PMA group: OVX rats received both ART and PMA treatment. (7)
OVX+ART+VPA group: OVX rats received both ART and VPA treatment. PMA and VPA were
administered by gavage every 3 days for 8 weeks, while rats in the ART group
received 50 mg/kg ART by gavage every other day for 8 weeks. After 8 weeks, all
rats were euthanized in a CO chamber upon cessation of movement, breathing,
and pupil dilation, confirming death. The Institutional Animal Care and Use
Committee of Guangzhou Orthopedic Hospital approved all experimental protocols
(No.GZOH20240113), ensuring compliance with ethical guidelines for laboratory
animal care and use.
2.9 Hematoxylin Eosin Staining (HE)
After 48-hour fixation with 4% PFA, tissue samples were decalcified using a
10% Ethylenediaminetetraacetic acid (EDTA) solution for 20 days, and then
prepared into 5 µm sections. After deparaffinization in xylene for
30 min, the sections were transferred to 100% ethanol for 6 min, followed by
90% ethanol for 3 min, and 80% ethanol for 2 min. They were then rinsed with
distilled water and PBS, followed by HE staining for 10 minutes (Sigma Aldrich,
St. Louis, MO, USA) was performed. Finally, the stained sections were observed
under an optical microscope (Leica Microsystems, Wetzlar, Germany).
2.10 Determination of Bone Mineral Density
Bone mineral density (BMD) in right tibia
bone tissue was measured by dual-energy X-ray absorptiometry (DXA) using Hologic DXA
device (Hologic QDR-4500A) [23].
2.11 Determination of Bone Mineral Salt Content
The rat femur underwent drying in an oven at 105 °C until reaching a
constant weight, which was recorded as the dry weight. The dried sample underwent
ashing at 650 °C for 36 hours in a muffle furnace. Afterward, the ashed
sample was weighed as the ash weight. The mineral salt content was calculated as
the ratio of the ash weight to the dry weight.
2.12 Immunohistochemistry
The tissue samples underwent fixation with 4% PFA for 48 hours, followed by
decalcification using a 10% EDTA solution for 20 days, and then were prepared
into 5 µm sections. NF-B p65 (AN365, 1:500, Beyotime,
Shanghai, China), Notch1 (AF5249, 1:200, Beyotime, Shanghai, China) antibody was
added and incubated for 60 minutes, followed by rinsing with distilled water and
placement in PBS. Goat anti-rabbit IgG-HRP polymer (ab150077, 1:1000, abcam,
Cambridge, UK) was added and incubated for 40 minutes, followed by rinsing with
distilled water and placement in PBS. 3,3-Diaminobenzidine (DAB) chromogen (P0202
Beyotime, Shanghai, China) was applied for 3 minutes, and the reaction was
controlled under a microscope, terminated by rinsing with tap water. After
rinsing with distilled water, counterstaining was performed, and the slides were
coverslipped. The presence of yellow or brown particles in the cytoplasm and/or
nucleus was considered as positive cells.
2.13 Enzyme-Linked Immunosorbent Assay (ELISA)
In each well, 40 µL of sample diluent and 10 µL of the sample were
combined and the plate was sealed. After incubating at 37 °C for 30
minutes, the liquid was discarded from the well, and the well was washed five
times with washing solution. The solution in the well was then dried by patting.
Following that, 50 µL of enzyme-labeled reagent was added and left to
incubate for 30 minutes. Subsequently, 50 µL each of chromoe developer A
and B were added and incubated at 37 °C in the dark for 15 minutes. The
process was halted by introducing 50 µL of termination solution, and the OD
value at 450 nm was gauged. The test kits, encompassing bone gla protein (BGP,
ml002883), osteoprotegerin (OPG, ml003271), receptor activator of the nuclear
factor kappa ligand (RANKL, ml003065), TNF- (ml002859), IL-6
(ml064292), IL-1 (ml037361), were procured from Shanghai Enzyme-linked
Biotechnology Co., Ltd. (Shanghai, China).
2.14 Statistical Analysis
The experiments were conducted separately on at least three occasions, and the
outcomes are displayed as mean SD. Statistical analysis was conducted
using GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA), with
one-way analysis of variance (ANOVA) followed by the Holm-Sidak post hoc test for
multiple comparisons. A significance level of p 0.05 was considered
statistically significant. Tissue histopathology examination was analyzed using
Image-Pro Plus 6.0 software (NIH, Madison, WI, USA).
3. Results
3.1 ART Concentration-Dependently Promotes Proliferation and
Osteogenic Differentiation of BMSCs
In this study, we first treated cells with different concentrations of ART (0,
3, 6, 12 µM) to preliminarily observe the effect of the drug on cell
proliferation. In contrast to the 0 µm group, cell viability remained
relatively unchanged in the low-concentration group (3 µM) (p
0.05). However, there was a significant increase in cell viability observed in
the 6 and 12 µM groups, with the highest cell viability noted at 48 h (Fig. 1A) (p 0.05). This suggests that ART enhances the proliferation of
BMSCs in a dose-dependent manner.
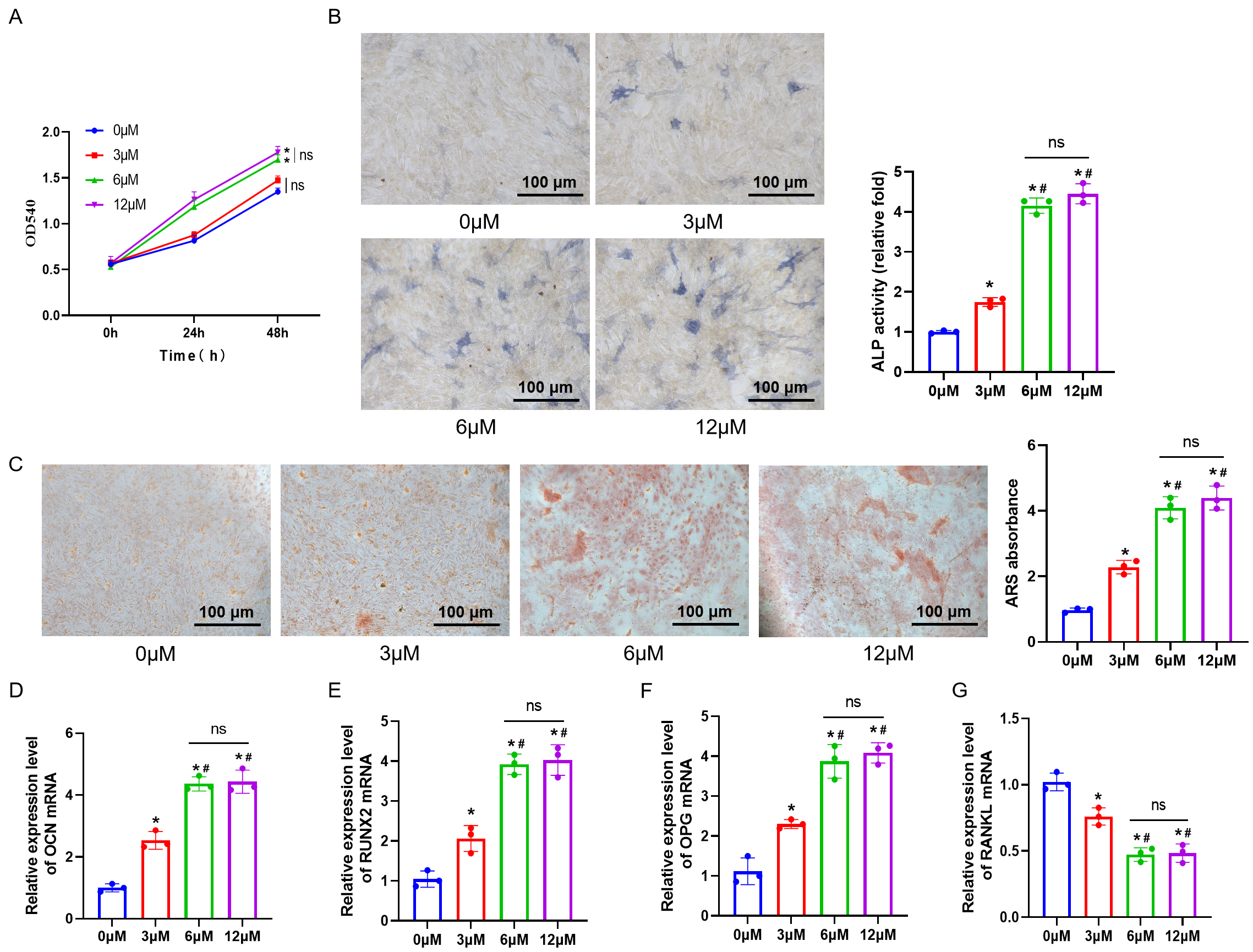 Fig. 1.
Fig. 1.
Promotion of ”bone marrow mesenchymal stem cells (BMSCs) proliferation and osteogenic differentiation
by different concentrations of Artesunate (ART). (A) Cell proliferation of BMSCs
at 0, 24, and 48 hours measured by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay. (B) Alkaline phosphatase staining (ALP) staining to detect
intracellular ALP expression and mineralization. Scale bar = 100 µm. (C) Alizarin red S staining (ARS) staining to detect cellular calcium deposition. Scale bar = 100 µm. (D–G)
Expression levels of OCN, RUNX2, OPG, and
RANKL in BMSCs measured by real-time quantitative PCR (RT-qPCR). n = 3.
ns, no statistical difference. *p 0.05 versus the 0 µM group,
#p 0.05 versus the 3 µM group. Results are shown as mean
SD.
To investigate the effects of the drug on osteogenic differentiation, ALP and
ARS staining were conducted to evaluate the effect on ALP content and bone
deposition in cells. In comparison with the 0 µM group, the staining
results revealed a significant increase in ALP activity in the 3 µM, 6
µM, and 12 µM groups, accompanied by an augmentation in the area of
calcium deposition. There was a notable increase in the expression levels of
OCN, RUNX2, and OPG, while RANKL expression
exhibited a significant decrease (p 0.05). Moreover, compared to the
3 µM group, the 6 µM and 12 µM groups exhibited stronger ALP
activity, more extensive calcium deposition areas, significantly higher
expression levels of OCN, RUNX2, and OPG, and
decreased RANKL expression (p 0.05) (Fig. 1B–G). However, the osteogenic differentiation ability of cells in the 12
µM group did not show a significant improvement over that observed in the 6
µM group (p 0.05). Overall, within a specific concentration
range, ART can progressively improve the osteogenic differentiation capacity of
BMSCs.
3.2 ART Treatment can Suppress NF-B and Notch1/Hes1
Pathway Expression in BMSCs
To explore the molecular mechanisms of ART in treating
osteoporosis, we conducted in vitro experiments to examine how varying
ART concentrations affect the expression of proteins associated with the
NF-B and Notch1/Hes1 signaling pathways (p-IB,
NF-B p65, Notch1, Hes1). The results showed that compared to the 0
µM group, the expression of p-IB,
NF-B p65, Notch1, and Hes1 in cells significantly decreased in the 3
µM, 6 µM, and 12 µM groups (p 0.05). Moreover,
compared to the 3 µM group, the expression of pathway proteins decreased in
the 6 µM and 12 µM groups (p 0.05). Moreover, in
comparison to the 3 µM group, pathway protein expression declined in the 6
µM and 12 µM groups. However, there was no notable alteration in the
expression of pathway proteins in the 6 µM and 12 µM groups
(p 0.05) (Fig. 2A–E).
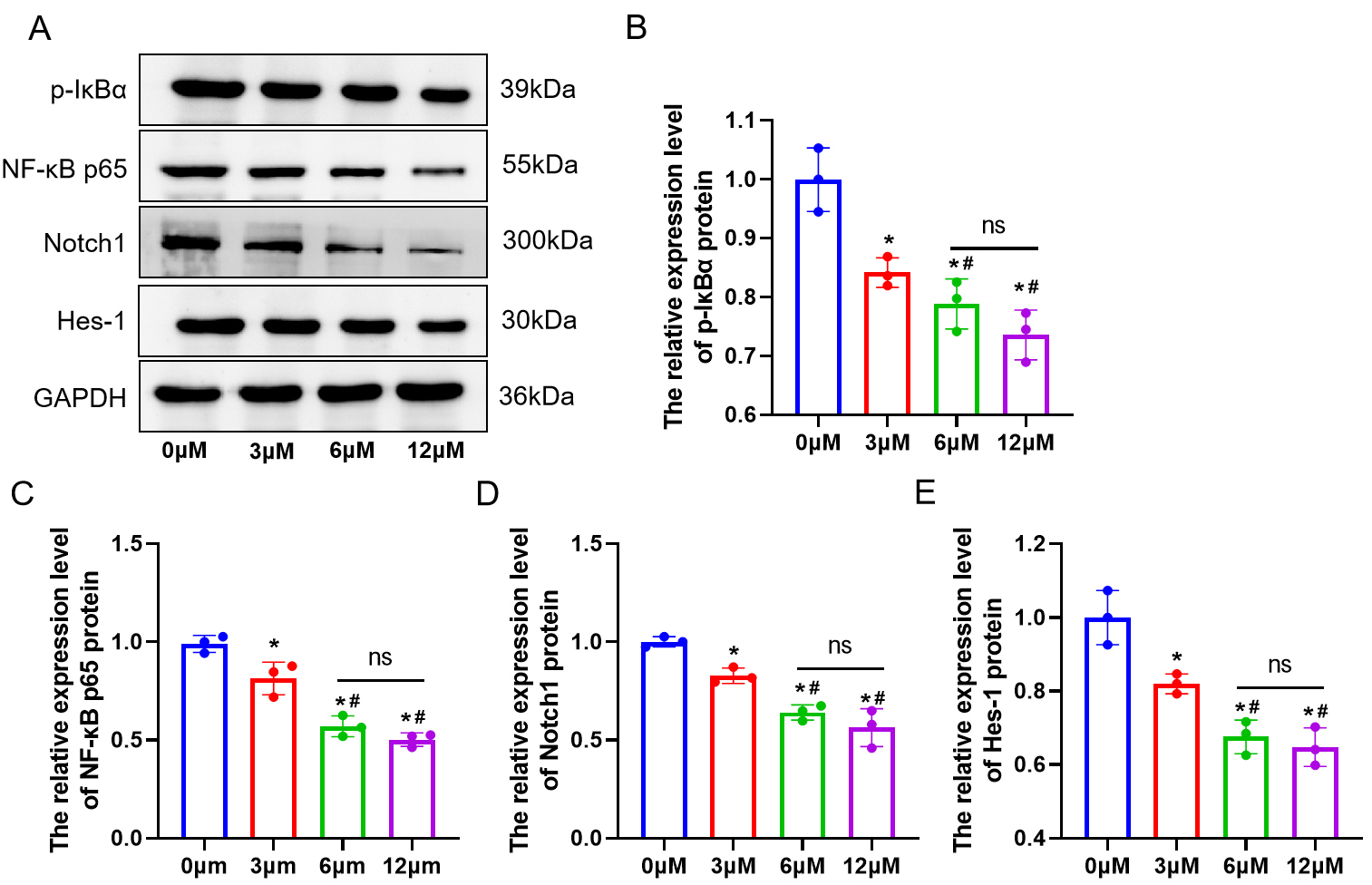 Fig. 2.
Fig. 2.
ART inhibits the expression of NF-B and Notch1/Hes1
pathway proteins in BMSCs in a concentration-dependent manner. (A–E) Western
blot (WB) analysis of p-IB, uclear factor kappa B (NF-B) p65, Notch1, and
Hes1 proteins in BMSCs. n = 3. ns, no statistical difference. *p
0.05 versus the 0 µM group, #p 0.05 versus the 3 µM
group. Results are presented as mean SD.
3.3 ART Facilitates BMSCs Osteogenic Differentiation via Suppression
of the NF-B and Notch1/Hes1 Pathways
To validate whether ART promotes osteogenic differentiation of BMSCs by
suppressing the NF-B and Notch1/Hes1 signaling pathways, we selected
the optimal concentration of ART at 6 µM for subsequent experiments and
divided the cells into control, ART, PMA, VPA, ART+PMA, and ART+VPA groups. ALP
and ARS staining results showed that ART significantly enhanced the osteogenic
differentiation of BMSCs, while PMA and VPA inhibited this differentiation (Fig. 3A,B) (p 0.05). Similarly, the increase in OCN,
RUNX2, and OPG mRNA expression after ART treatment was reversed
after co-treatment with PMA or VPA, while RANKL mRNA expression
decreased after ART treatment and increased after co-treatment with PMA or VPA
(Fig. 3C–F) (p 0.05). Immunofluorescence results
showed that ART significantly inhibited the expression of NF-B p65 and
Notch1 proteins, whereas NF-B p65 and Notch1 protein expression
increased after treatment with PMA and VPA (p 0.05). Furthermore,
compared to ART alone, co-treatment of ART with PMA or VPA resulted in increased
expression of NF-B p65 and Notch1 proteins (Fig. 3G,H) (p
0.05). In summary, our findings suggest that ART inhibits the activation of the
NF-B and Notch1/Hes1 signaling pathways, facilitating the osteogenic
differentiation of BMSCs.
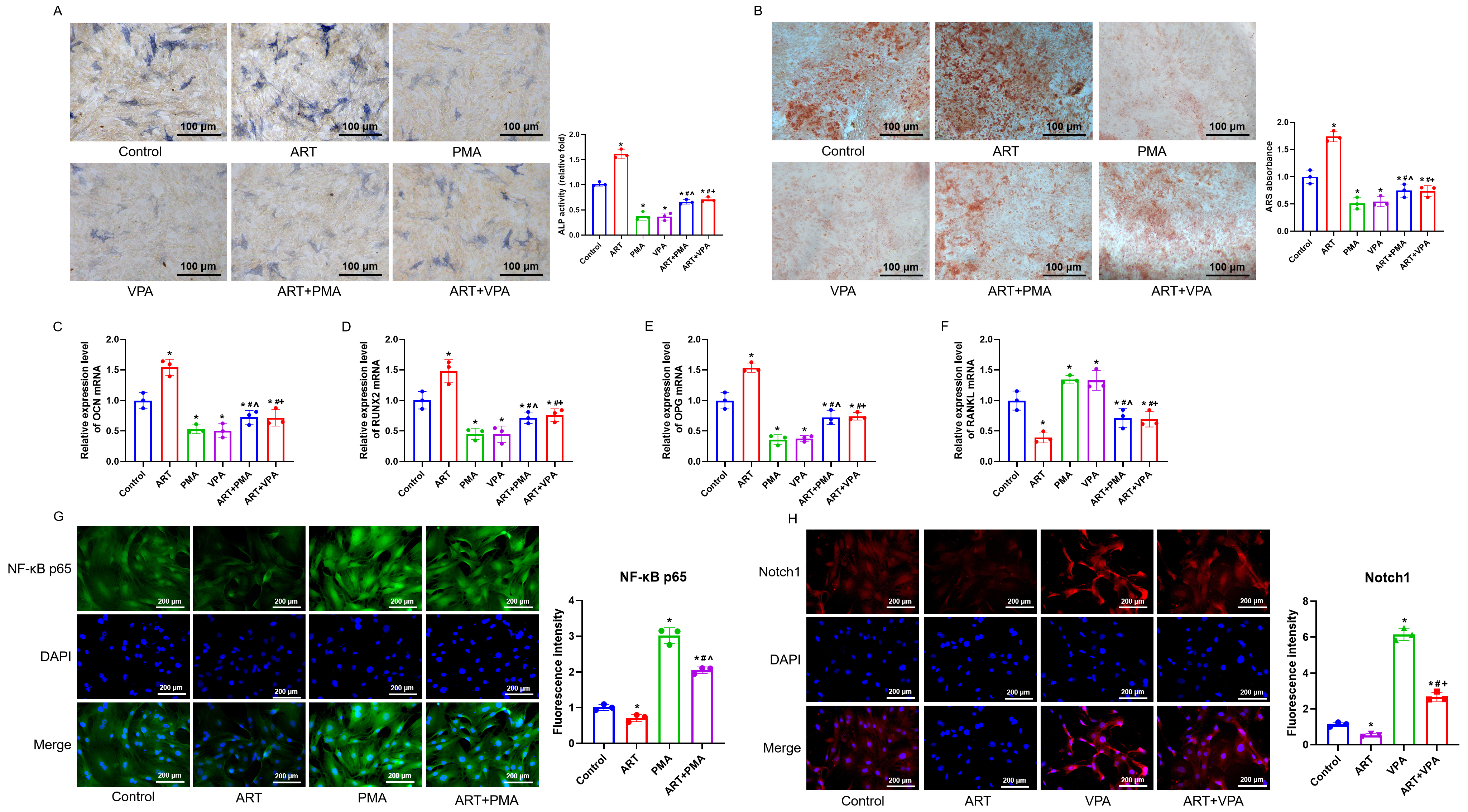 Fig. 3.
Fig. 3.
Stimulation of the NF-B and Notch1/Hes1 signaling
pathways counteracts the enhancing impact of ART on the osteogenic
differentiation of BMSCs. (A,B) ALP staining and ARS staining were performed to
detect the expression of ALP and mineralized calcium deposition, respectively.
Scale bar = 100 µm. (C–F) RT-qPCR was performed to assess the mRNA levels of
OCN, RUNX2, OPG, and RANKL in BMSCs. (G,H)
Immunofluorescence was employed for evaluating the NF-B p65 and Notch1
protein expression. n = 3. Scale bar = 200 µm. *p 0.05 versus
the Control group, #p 0.05 versus the ART group,
^p 0.05 versus the phorbol myristate acetate (PMA) group, p
0.05 versus the valproic acid (VPA) group. Results are shown as mean SD.
3.4 ART Improves Morphological Histology, and Increases
Bone Mineral Density (BMD), and Enhances Bone Mineral Content
in Osteoporotic Rats
The results of in vitro studies indicate that ART
inhibits the expression of the NF-B and Notch1/Hes1 pathways in BMSCs
and promotes their osteogenic differentiation. To verify whether this mechanism
also applies to in vivo conditions, we established a postmenopausal
osteoporosis rat model. The results showed that the bone tissues in the Sham
group did not exhibit obvious pathological changes, while rats in the Model group
suffered severe bone damage accompanied by bleeding and inflammatory cell
infiltration. In contrast to the Model group, the ART group exhibited
considerable alleviation of bone damage, characterized by decreased bleeding and
a noticeable reduction in inflammatory cell infiltration (Fig. 4A) (p 0.05). The bone tissue ALP level exhibited a significant decrease in the
Model group, whereas in the ART group, there was a significant increase compared
to the Model group (Fig. 4B) (p 0.05). Further analysis of bone
density, bone mineral content, and expression levels of inflammatory factors
(TNF-, IL-6, IL-1) in the left femur of each group of rats
revealed significant findings. Compared to the Sham group, the model group
exhibited a marked decrease in bone density and bone mineral content, alongside a
significant increase in the expression of inflammatory factors. However, in
comparison to the model group, the ART group showed a significant recovery in
bone density and bone mineral content, and a suppression of TNF-, IL-6,
and IL-1 expression (Fig. 4C–G), with statistically significant
differences (p 0.05). These results suggest that ART treatment can
partially reverse bone damage and inflammatory response in the model group rats.
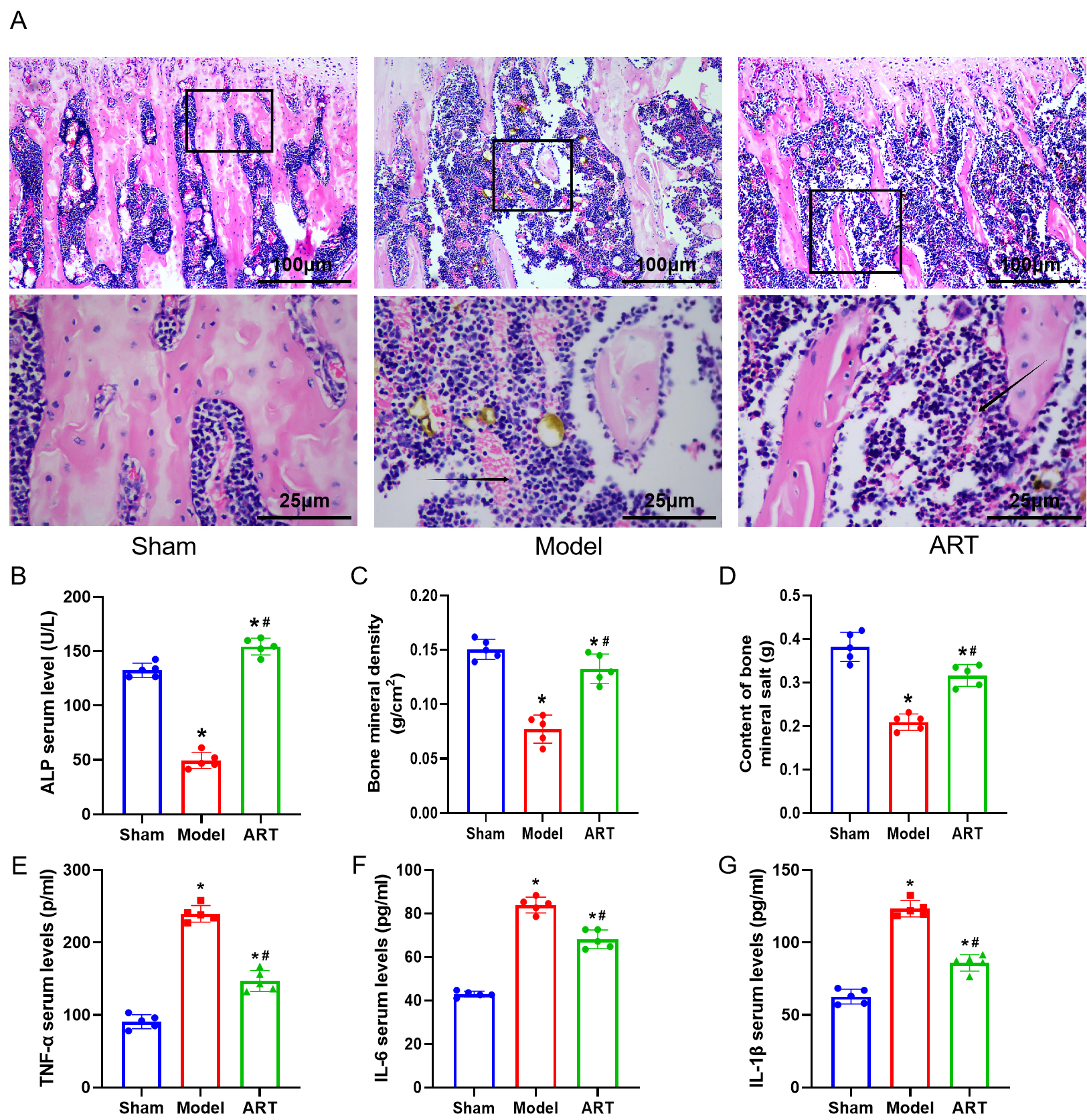 Fig. 4.
Fig. 4.
ART improves morphological histology,
increases BMD, and enhances bone mineral content in
osteoporotic rats. (A) Hematoxylin Eosin (HE) staining for morphological changes
in bone tissue. Scale bar = 100 or 25 µm. (B) Expression levels of ALP. (C)
Measurement of bone mineral density. (D) Measurement of bone mineral content.
(E–G) Enzyme-linked immunosorbent assay (ELISA) analysis to measure the concentrations of tumor necrosis factor (TNF)-, Interleukin (IL)-6, and
IL-1 in rat serum. The arrow indicates the site of infiltration of
inflammatory cells. n = 5. *p 0.05 versus the Sham group,
#p 0.05 versus the Model group. Results are shown as mean
SD.
3.5 ART can Inhibit NF-B and Notch1/Hes1 Signaling Pathway
and Reduce the Levels of Serum Inflammatory Factors and Bone Metabolism Related
Factors in OVX Rats
In comparison with the Sham group, the Model group showed a
notable rise in NF-B p65 and Notch1 protein expression. Additionally,
there were increased levels of inflammatory cytokines (TNF-, IL-6, and
IL-1) and bone metabolism-related factors (BGP, RANKL) and decreased OPG
expression (Fig. 5A,B) (p 0.05). Following ART treatment, the
expression of NF-B p65 and Notch1 decreased, accompanied by reduced
levels of inflammatory cytokines and BGP, RANKL, and increased OPG expression.
The addition of NF-B and Notch1 pathway activators PMA or VPA led to a
notable elevation in NF-B p65, Notch1, inflammatory cytokines, BGP, and
RANKL expression, coupled with a decrease in OPG expression (Fig. 5C–E)
(p 0.05). After combined treatment with ART and PMA or VPA, the
expression of NF-B p65, Notch1, inflammatory cytokines, and bone
metabolism-related factors decreased (Fig. 5F–H) (p 0.05). These
findings suggest that ART may alleviate inflammation and bone metabolism levels
in OVX rats by inhibiting the NF-B and Notch1/Hes1 signaling pathways.
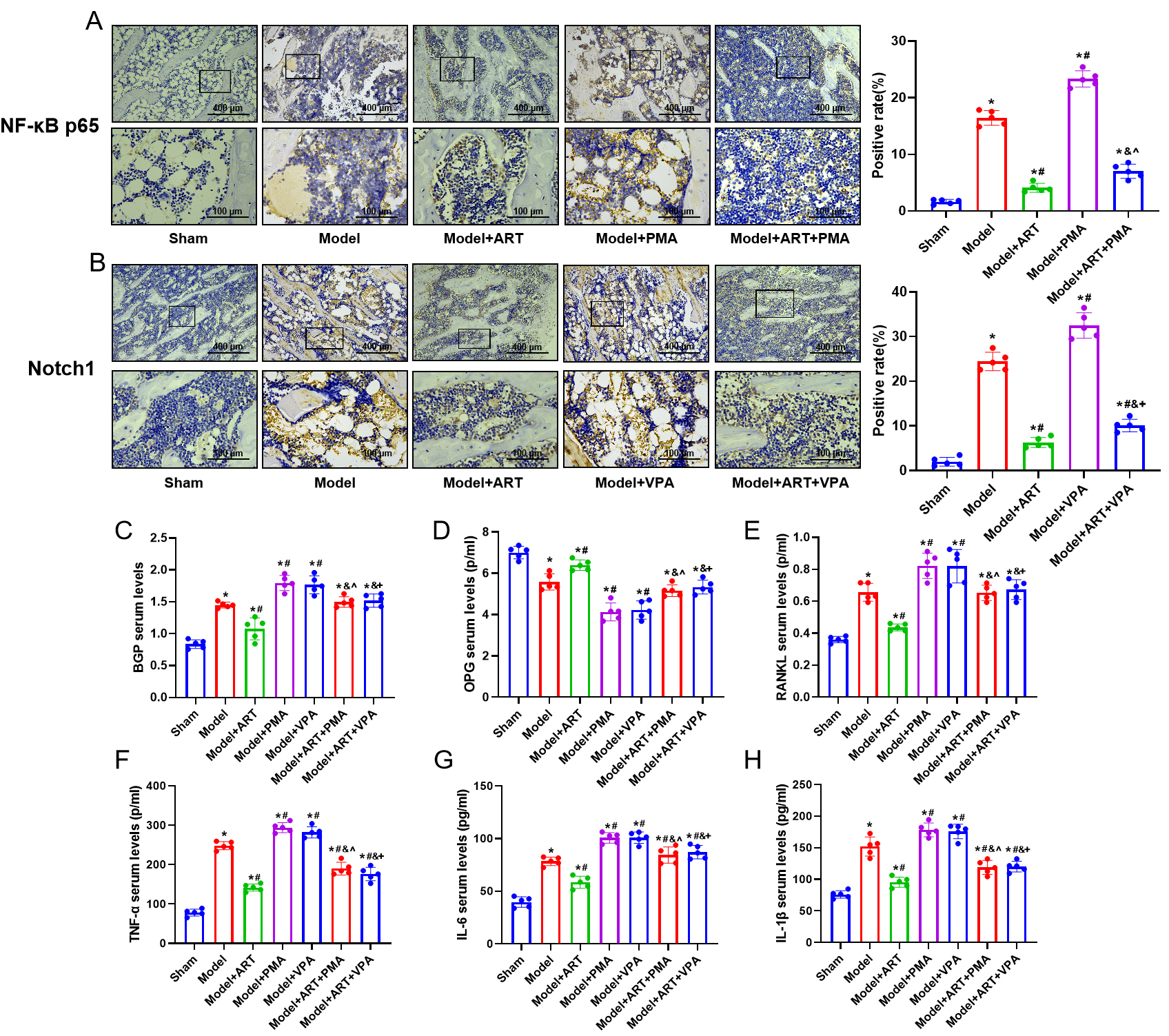 Fig. 5.
Fig. 5.
ART attenuates the expression of NF-B and Notch1
pathway proteins, inflammatory factors, and bone metabolism-related factors in
ovariectomized (OVX) rats. (A,B) Immunohistochemical analysis of NF-B
p65 and Notch1 protein expression in rats. Scale bar = 400 or 100 µm. (C–E)
ELISA assay for the levels of BGP, OPG, and RANKL in rat serum. (F–H) ELISA
analysis to measure the concentrations of TNF-, IL-6, and IL-1
in rat serum. n = 5. *p 0.05 versus the Sham group,
#p 0.05 versus the Model group, &p
0.05 versus the ART group, ^p 0.05 versus the PMA
group, p 0.05 versus the VPA group. Results are shown as mean
SD.
4. Discussion
The prevalence of osteoporotic fractures affects approximately 200 million
individuals globally, with its incidence increasing with age and posing
significant challenges due to associated secondary health issues. Osteoporosis,
particularly postmenopausal osteoporosis, emerges as a primary contributor to
fracture susceptibility among older women, leading to elevated morbidity,
mortality, and substantial economic burdens [24, 25]. Bone is a living tissue that
is constantly renewed to maintain the integrity of the whole living structure as
old bone breaks down and new bone remodels. Osteoclasts dissolve or absorb bone,
while osteoblasts produce bone and inhibit osteoclast activity. Bone mass and
mineral density accumulate from birth to adulthood but decline with age, which is
more pronounced in postmenopausal women. The decrease in bone density increases
the risk of osteoporosis and fractures. Hence, osteoporosis after menopause
stands as the predominant skeletal ailment among older women, emerging as a
primary contributor to fracture susceptibility. Patients tend to have high
morbidity and mortality as well as the economic cost of treatment is high
[26, 27].
Postmenopausal osteoporosis arises due to a multitude of
factors, including estrogen deficiency, dysregulated autophagy, heightened
apoptosis, and reactive oxygen species (ROS) elevation [28]. Among them, estrogen deficiency causes changes
in osteocytes and increases TNF secretion and the sensitivity of osteocytes to
IL-1. In addition, the lack of estrogen triggers the production of RANKL, which
is a potent stimulator of osteoclast formation and an inhibitor of
osteoprotegerin. OPG can increase the biological activity of RANKL and bone
resorption, leading to bone loss [29]. Tao et al. [30] found that
RANKL-induced osteoclast formation was inhibited by regulating inflammation and
NF-B signaling pathways. In wild-type mice, Wei H et al. [31]
demonstrated that the inhibition of nuclear factor kappa B (NF-B)
pathways can significantly promote bone formation and inhibit bone absorption
[32]. Yoshida et al. [33] discovered that overactivation of the
Notch1/Hes1 signaling pathway could result in increased osteoclast activity,
hastening bone loss.
Our research indicates that ART can induce osteogenic differentiation of bone
marrow mesenchymal stem cells, thereby enhancing their osteogenic potential and
promoting bone regeneration. In both in vivo and in vitro
experiments, ART demonstrates inhibition of the NF-B and Notch1/Hes1
signaling pathways. It promotes the expression of bone metabolism-related factors
in cells and tissues while reducing the levels of inflammatory factors in the
serum of ovariectomized rats.
However, it’s important to note that ART’s modulation of signaling pathways may
potentially lead to enhanced osteoclast activity, suggesting the need for further
investigation into its precise mechanisms of action. Nevertheless, our study
provides valuable insights into ART’s therapeutic effects on osteoporosis,
particularly its impact on the NF-B and Notch1/Hes1 pathways, laying
the groundwork for future research in this area.
In conclusion, this study elucidates the potential therapeutic effects of ART on
osteoporosis by ameliorating symptoms through the regulation of NF-B
and Notch1/Hes1 signaling pathways. It provides a foundation for the prevention
and treatment of osteoporosis, paving the way for new directions in future
research within this field.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5.




