1 Department of Respiratory Medicine, Guangming Traditional Chinese Medicine Hospital of Pudong New Area, 201399 Shanghai, China
2 Department of Respiratory Medicine, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, 201399 Shanghai, China
Abstract
Background: L-Theanine, a nonproteinogenic amino acid derived from green tea, is being recognized as an anti-cancer candidate. However, it’s roles in the development of cancer chemoresistance is still unknown and the molecular mechanism is urgently to be explored. Methods: The effects of L-Theanine on lung cancer chemoresistance were validated by Cell Counting Kit-8 (CCK-8) assay, transwell assay, and in vitro tumor spheroid formation assay; the expression of proteins was detected by using polymerase chain reaction (PCR) and western blotting. RNA-sequencing (RNA-seq) and bioinformatics analysis were used to identify differentially expressed genes induced by L-Theanine. BMAL1 knockdown and overexpression were constructed by using a lentivirus-mediated transfection system. Results: L-Theanine improved the chemoresistance to cis-diamminedichloroplatinum (DDP) and inhibited stemness of DDP-resistant lung cancer cells but not non-resistant lung cancer cells. The results from RNA-seq analysis showed that STAT3/NOTCH1 pathway was a potential dominant signaling involved in L-Theanine improving the chemoresistance in DDP-resistant lung cancer. Mechanistically, L-Theanine impeded migration and stemness activation of DDP-resistant lung cancer cells via regulating the expression of STAT3/NOTCH1/BMAL1 signaling-induced stemness markers as well as inhibiting the expression levels of drug resistance-related genes. In addition, a combination of L-Theanine and Stat3 blockade synergistically improved the chemoresistance in DDP-resistant lung cancer. Conclusion: L-Theanine improves the chemoresistance by regulating STAT3/NOTCH1/BMAL1 signaling, reducing stemness, and inhibiting the migration of DDP-resistant lung cancer cells. The finding might provide some evidence for therapeutic options in overcoming the chemoresistance in cancers, including lung cancer.
Keywords
- lung cancer
- chemoresistance
- stemness
- L-Theanine
- STAT3/NOTCH1 signaling
- BMAL1
Cancers are considered to be heterogeneous entities, and it is cancer heterogeneity that leads to poor efficacy of chemotherapy, partly due to the existence of cancer stemness [1], which is believed to initiate chemotherapy resistance [2]. Conventional chemotherapy kills cancer cells but has little effect on cancer stem cells, leading to recurrent cancers more resistant [3]. Therefore, investigating the mechanism of cancer stemness-induced chemoresistance will help to improve conventional chemotherapies and enhance the overall efficacy.
About 80–85% of lung cancers are non-small cell lung cancer (NSCLC), which is the leading cause of cancer deaths worldwide. Although some patients with critical genes including EGFR, BRAF, and HER2 mutation are suitable for molecularly targeted therapies, resistance to targeted therapies inevitably occurs in these patients over time [4]. After targeted therapies, NSCLC patients usually receive systemic platinum-based chemotherapy with or without immune checkpoint inhibitors (ICIs). Cisplatin is one of the most effective chemotherapeutic agents for treating human cancers, including NSCLC. Unfortunately, a considerable percentage of patients develop resistance to cisplatin-based chemotherapy [5, 6]. Therefore, understanding the underlying mechanisms of platinum-based compounds is necessary to overcome the barriers to its clinical use in lung cancer patients. Currently, platinum drug resistance mechanisms include the following aspects: reduced intracellular platinum accumulation, inactivation of intracellular platinum, enhanced DNA damage repairment, enhanced inhibition of apoptosis in cancer cells, etc. [7, 8]. However, as mentioned above, current chemotherapeutic agents can kill most cancer cells, but their ability to kill resting cancer stem cells (CSCs) is extremely limited. Hence, effectively inhibiting CSCs may be the key to reversing chemoresistance in lung cancer.
Recently, it has been recognized that L-Theanine, one of the unique chemical components of tea, has not only anti-cancer effects but also bioregulatory effects on certain anti-cancer drugs [9, 10]. However, the roles of L-Theanine in chemotherapy resistance of lung cancer are understudied. In the study, we clarified that L-Theanine relieved the chemoresistance to cisplatin in resistant lung cancer cells; mechanistically, L-Theanine inhibited migration and reduced stemness of cis-diamminedichloroplatinum (DDP)-resistant cancer cells through regulating STAT3/NOTCH1/BMAL1 signaling.
A549, A549/DDP, NCI-H446, and NCI-H446/DDP cells used in this study were purchased from the National Authorized Cell Culture Preservation Center (Chinese Academy of Sciences, Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Grand Island, New York, NY, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, Waltham, MA, USA), 2 mM L-glutamine and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin sulfate). All cell lines were validated by STR profiling and tested negative for mycoplasma. All cells were cultured at 37 °C in a humidified incubator with 5% (v/v) carbon dioxide in the air.
Cells were cultured in a 96-well plate at a density of 1
Cell migration was examined by performing a Transwell assay. In brief, transfected or treated cells in 100 µL serum-free media were plated on the upper compartment of the chamber; meanwhile 800 µL media with 10% fetal bovine serum were added in the lower compartment of the chamber as a chemoattractant. After 6 h or 12 h of incubation at 37 °C, cells the upper chamber were carefully removed by using a cotton swab. The migrated cells on the lower surface were analyzed by crystal violet staining and photographed with a Leica DMi8 inverted microscope (Deerfield, IL, USA).
Lung cancer cells at the concentration of 10 viable cells/mL in 100 µL
media were seeded into ultra-low attachment 96-well plates (Corning) in DMEM
medium supplemented with 20 ng/mL epidermal growth factor (EGF),
10 ng/mL basic fibroblast growth factor (bFGF),
5 mg/mL insulin, and 2% B27 and incubated at 37 °C and
5% CO
Total RNA was extracted by using a Trizol reagent (Invitrogen, Carlsbad, CA,
USA). RNA-seq libraries were established with an Illumina TruSeq RNA sample prep
kit (San Diego, CA, USA) and sequenced using Illumina HiSeq 4000 (San Diego, CA,
USA) with a 150-bp paired-end sequencing strategy. Differential gene expression
analysis was performed using HISAT2 and DESeq2, and determined with the cutoff
fold change
Protein extracts from cultured cells were collected by using RIPA lysis buffer
(Beyotime, Hangzhou, China) with protease inhibitor cocktail, and protein
concentrations were determined by a Pierce BCA protein assay (Thermo Fisher
Scientific, Waltham, MA, USA). Aliquots of protein extracts were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then,
transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA). The following antibodies used in the study were listed below: rabbit
polyclonal anti-ALDH1 antibody (1:1500), rabbit polyclonal anti-ABCG2 antibody
(1:1000), rabbit polyclonal anti-Nanog antibody (1:1500) and mouse monoclonal
anti-
The lentivirus-mediated BMAL1 knockdown and overexpression system and
corresponding controls were constructed by GeneCreate (Wuhan, China).
Transfection was performed using the transfection reagent Endo-FectinTM Max
transfection (Genecreate) according to the manufacturer’s instructions. Briefly,
5 µg lentivirus were diluted in 250 µL Opti-MEM media
(Thermo Fisher Scientific, Inc.), supplemented with 10 µL Endo-
Endo-FectinTM Max. After mixed softly, the mixture was stewed at room temperature
for 10–20 min. Then, the mixture was added into the cells (1
SPSS 24.0 software (SPSS/IBM, Chicago, IL, USA) was used for the statistical
analysis. Continuous variables and categorized variables were respectively
reported as mean
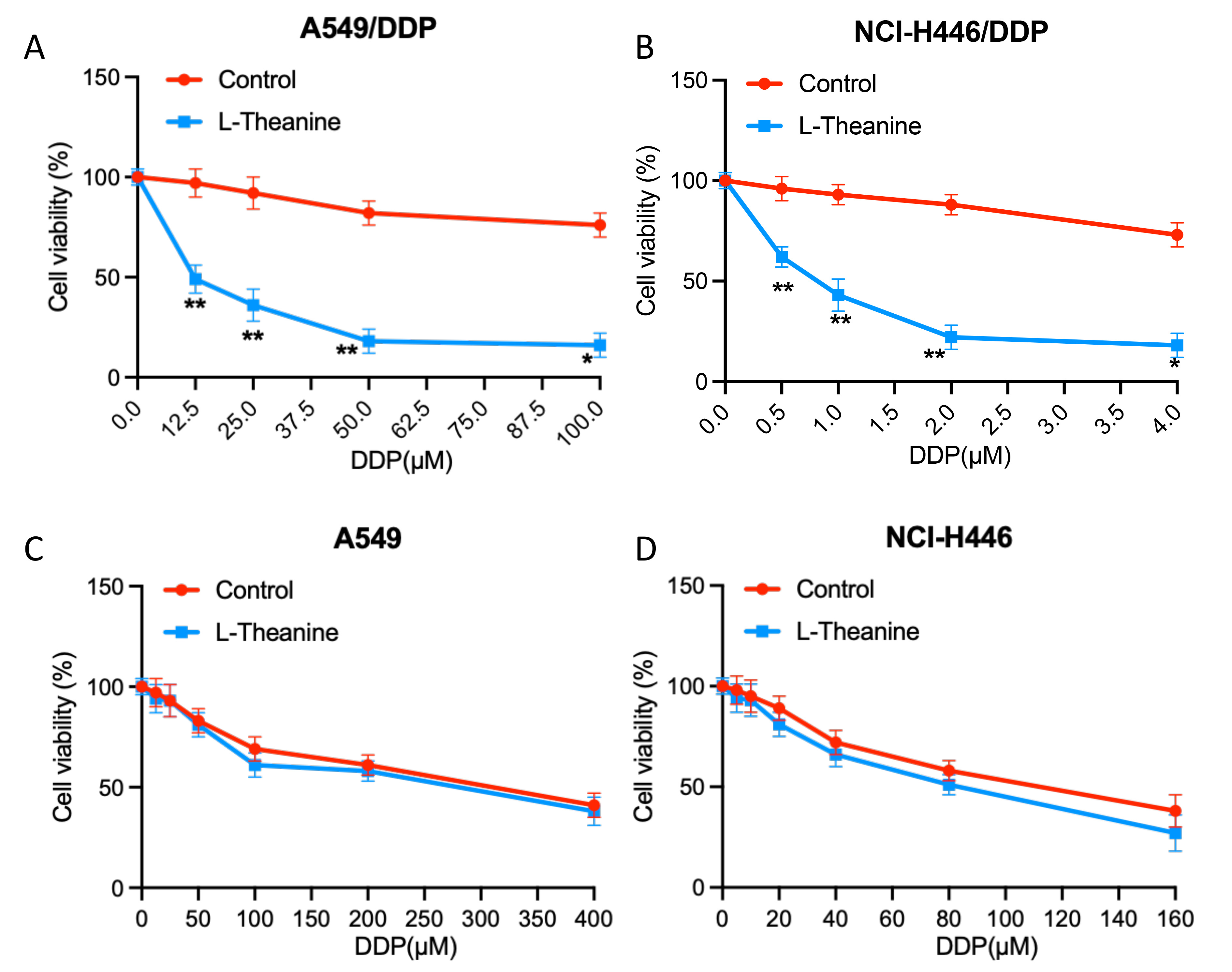
To investigate the effects of L-Theanine on the chemosensitivity of lung cancer cells, we examined the changes in the sensitivity of lung cancer cells A549 and NCI-H446 to DDP after L-Theanine treatment. It showed that L-Theanine did not lead to a significantly enhancemed sensitivity of lung cancer cells A549 and NCI-H446 to DDP (Fig. 1A,B). However, it is noteworthy that for DDP-resistant strains (A549/DDP, NCI-H446/DDP), the resistance index of cells to DDP was significantly reduced after L-Theanine treatment (Fig. 1C,D).
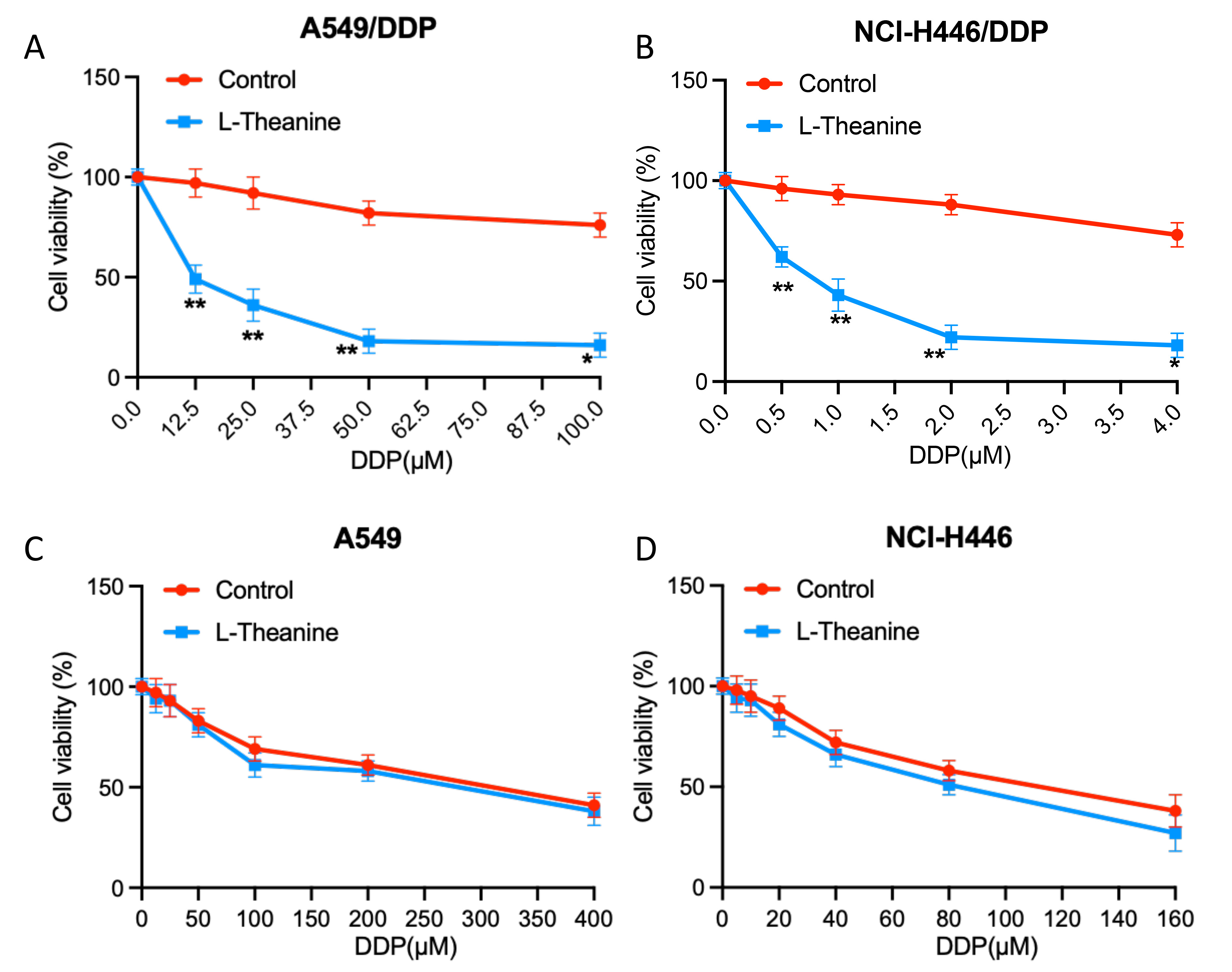 Fig. 1.
Fig. 1.L-Theanine relieves the chemoresistance to cisplatin in
resistant lung cancer cells. (A) A549/DDP cells were treated with increasing
concentrations of DDP (0–100 µM). (B) NCI-H446/DDP cells were treated with
increasing concentrations of DDP (0–4 µM). (C) A549 cells were treated
with increasing concentrations of DDP (0–400 µM). (D) NCI-H446 cells were
treated with increasing concentrations of DDP (0–160 µM). After that,
CCK-8 analysis showed the viability of the above cells following 48 h DDP
treatment. Data are presented as Mean
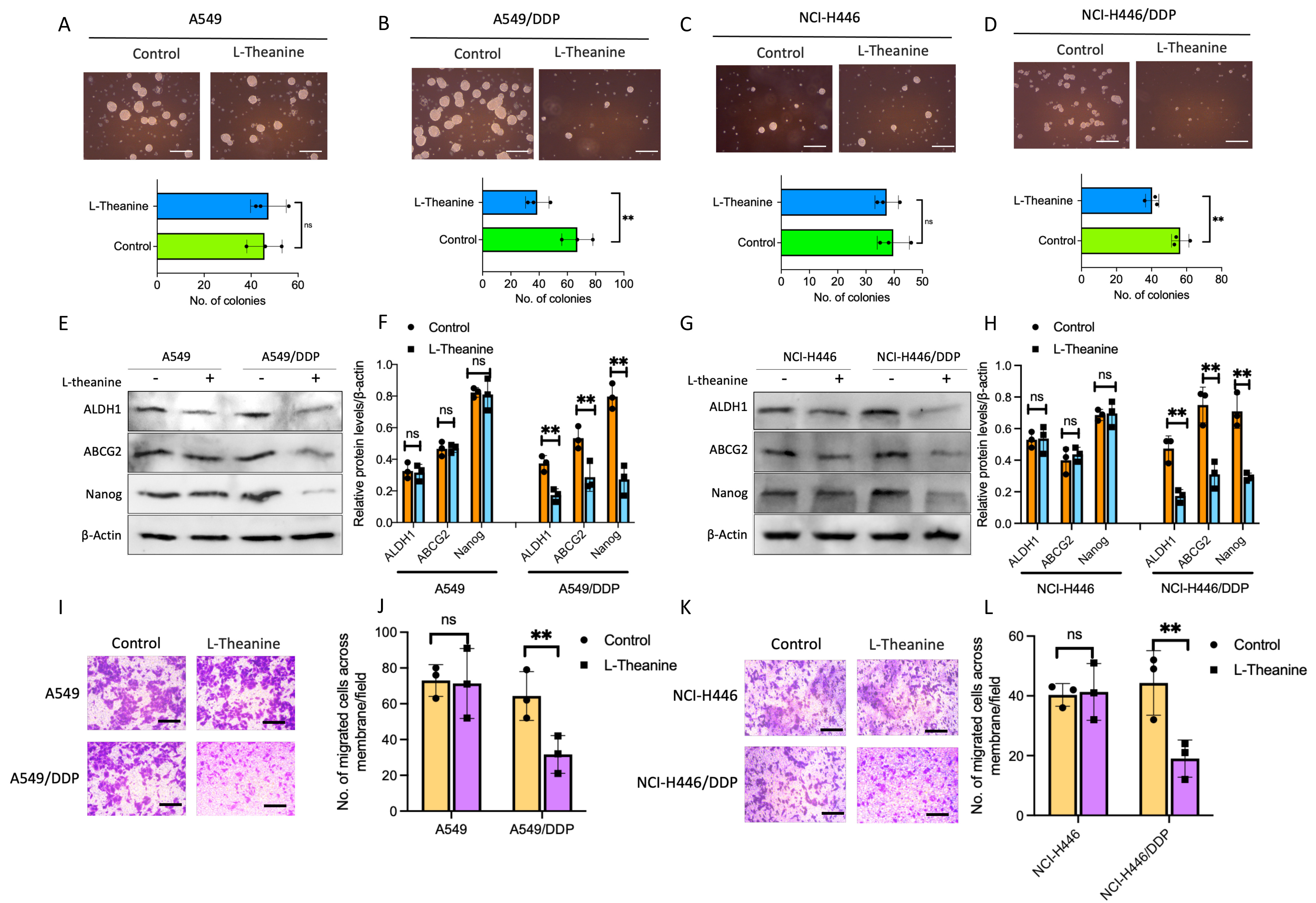
As mentioned above [2], there is a close relationship between cancer stemness and platinum-based chemotherapy resistance. To clarify whether the effect of L-Theanine on drug resistance in lung cancer is related to cancer stemness, we first examined the clone forming ability of lung cancer cells with L-Theanine treatment. The results showed that L-Theanine significantly inhibited the clone formation ability of DDP-resistant lung cancer cells; while no significant effect on the clone formation ability of non-resistant lung cancer cells (Fig. 2A–D). Subsequently, we examined the expression of stemness markers in DDP-resistant and non-resistant lung cancer before and after L-Theanine treatment. The results revealed that L-Theanine led to decreased expressions of stemness markers in DDP-resistant lung cancer cells, whereas no significant reduction in non-resistant lung cancer cells (Fig. 2E–H). In addition, L-Theanine only inhibited the invasion ability of DDP-resistant lung cancer cells (Fig. 2I–L). In summary, L-Theanine may targetedly inhibit the stemness of DDP-resistant lung cancer cells, but no significant effect on the stemness of non-resistant cells, which will be helpful for the personalized development of reversal strategies for chemotherapy-resistant lung cancer.
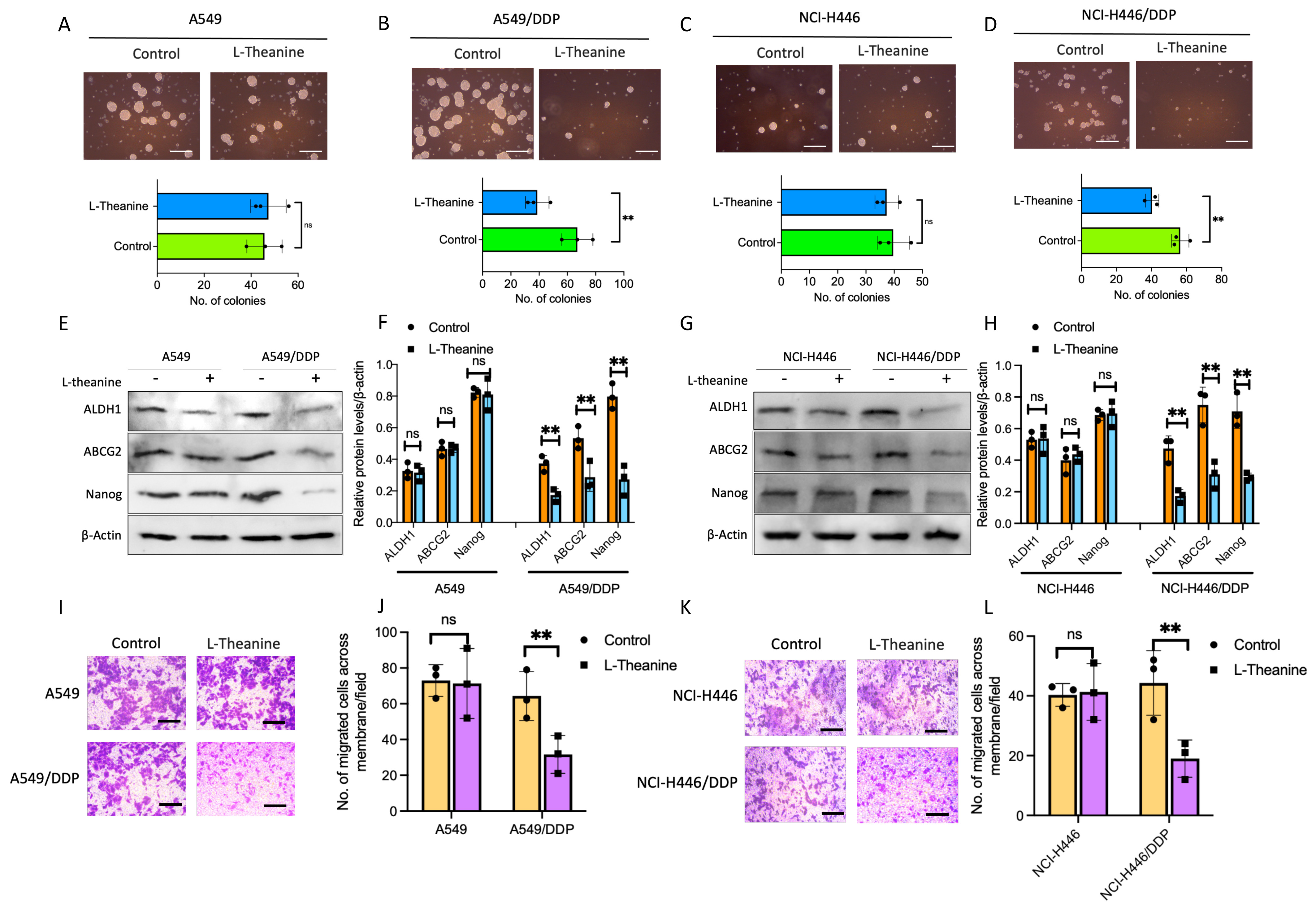 Fig. 2.
Fig. 2.L-Theanine inhibiting the stemness of chemoresistance
lung cancer cells. Tumor spheroid formation assay was applied in A549, A549/DDP,
NCI-H446, and NCI-H446/DDP cells with or without L-Theanine treatment for 14
days. The number of tumor spheres means colonies/5000 cells. Scale bar 200
µm (A–D). The levels of proteins, including ALDH1, ABCG2, and Nanog in
A549, A549/DDP, NCI-H446, and NCI-H446/DDP cells with or without L-Theanine
treatment were analyzed by a western blotting assay (E–H). The indicated cells
were treated with DDP for 14 days. Images and quantification of transwell
migration assays; cells were stained with crystal violet. Scale bar 100 µm
(I,K). The bar graphs show the statistical analysis of the number of cells cross
membranes (J,L). Data are presented as Mean
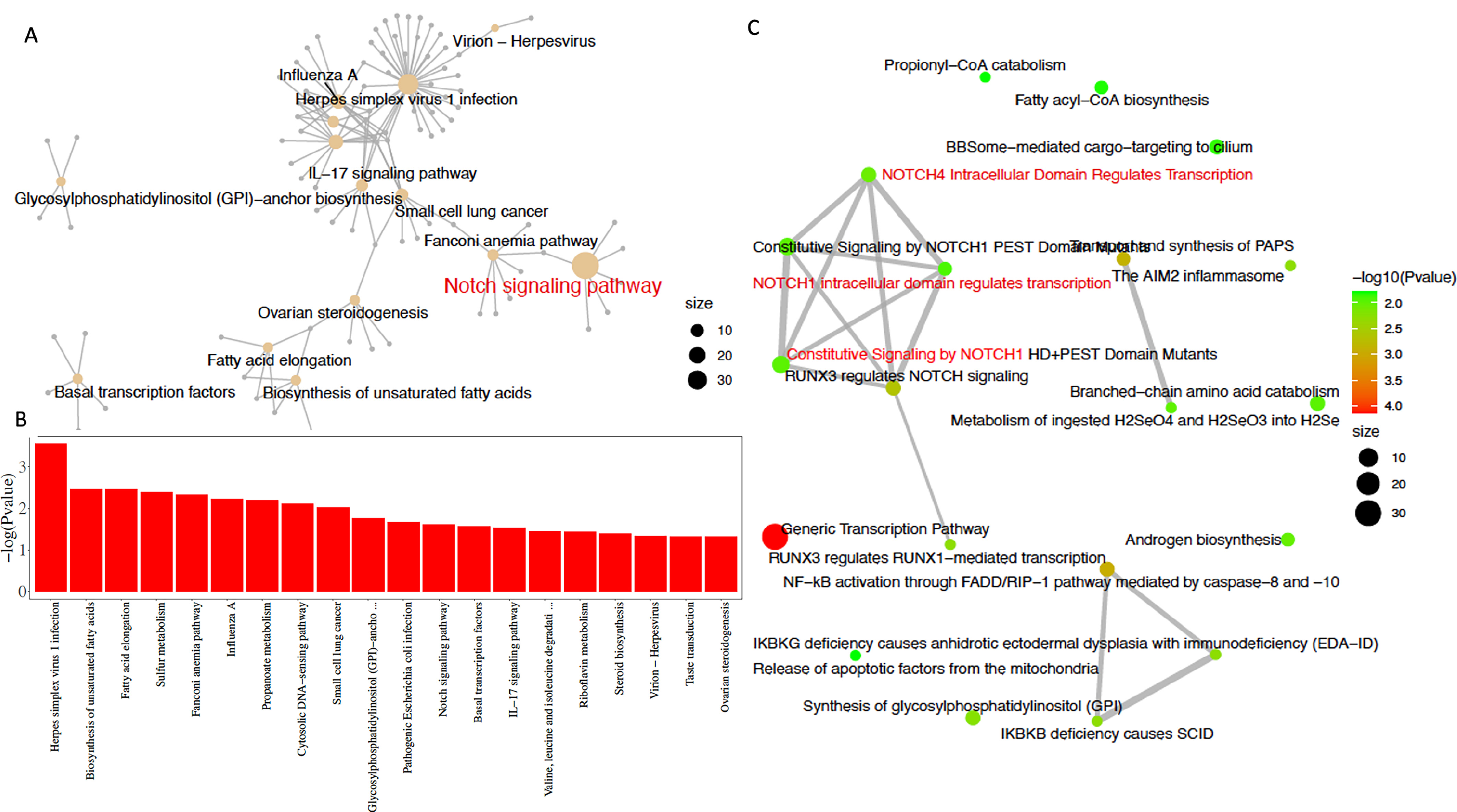
To clarify the differences in the effects of L-Theanine on DDP-resistant and non-resistant lung cancer cells, we performed a comparative analysis on the RNA-seq data from L-Theanine-treated DDP-resistant lung cancer cells and control cells. For lung cancer cells, a total of 725 differentially expressed genes were identified between the L-Theanine-treated group and the control group, including 278 up-regulated genes and 447 down-regulated genes; for DDP-resistant lung cancer cells, a total of 829 differentially expressed genes were identified between the L-Theanine-treated group and the control group, including 387 up-regulated genes and 442 down-regulated genes. Relative to non-DDP-resistant cells, a total of 316 differentially expressed genes (DEGs) were identified in L-Theanine-treated DDP-resistant lung cancer cells, among 208 up-regulated genes, BMAL1 was significantly upregulated in L-Theanine-treated DDP-resistant lung cancer cells. DEGs in L-Theanine-treated DDP-resistant lung cancer cells relative to non-DDP-resistant lung cancer cells were enriched by using molecular Signature Data Base (mSigDb) markers and the Gene Ontology of Biological Processes (GO). The result showed they are statistically enriched in some biosynthesis (fatty acids, glycosyl-phosphatidylinositol, etc.), signaling transduction, transcription factors, and virus infection (Fig. 3A). Subsequent KEGG and Reactome enrichment analysis identified 239 genes enriched in cancer-related signaling pathways, including the dominant STAT3/NOTCH1 signaling pathway and the IL-17 signaling pathway (Fig. 3B,C).
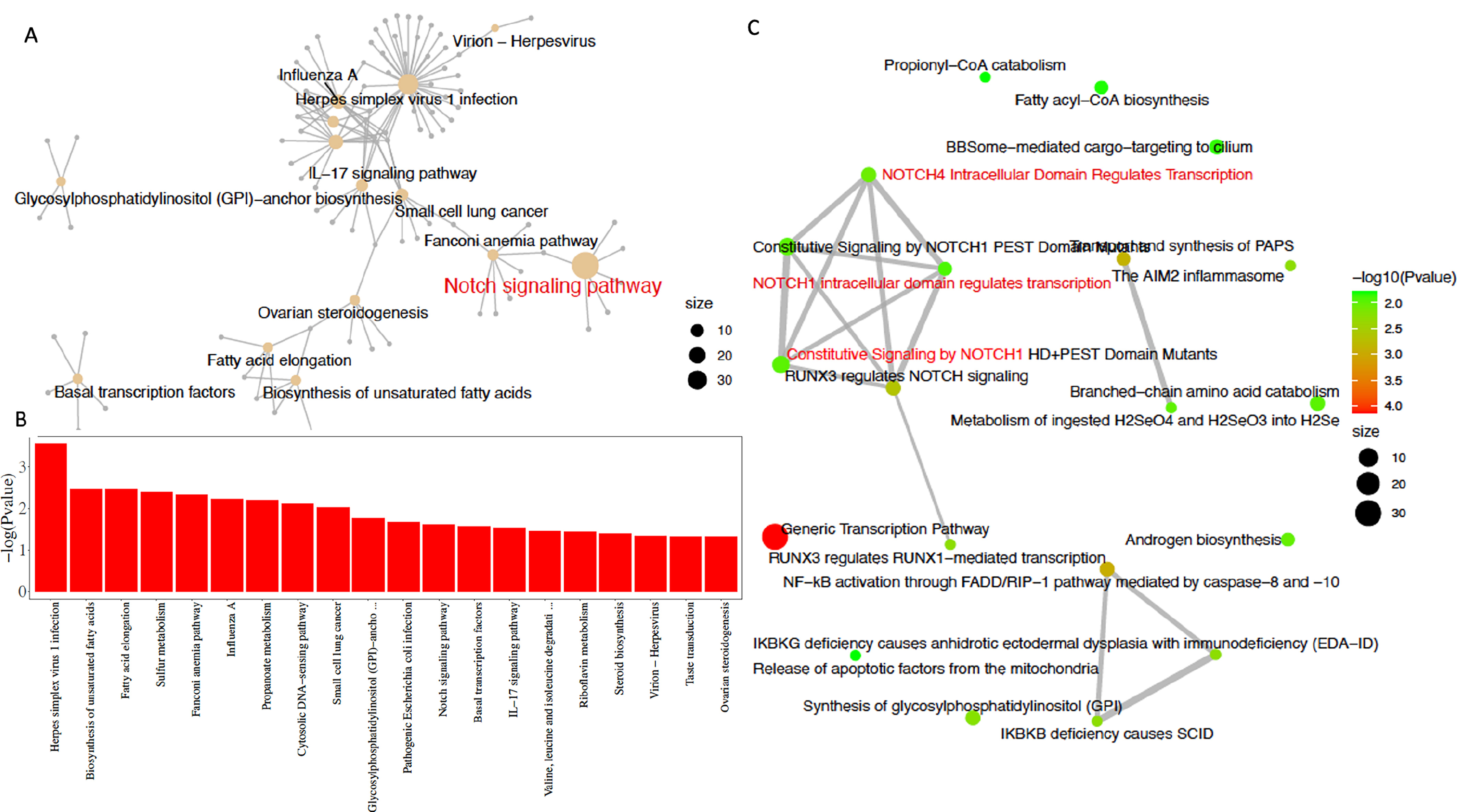 Fig. 3.
Fig. 3.Involvement of STAT3/NOTCH1 signaling in L-Theanine relieving chemoresistance in lung cancer. Different expressed genes in L-Theanine-treated DDP-resistant lung cancer cells relative to control cells were statistically enriched in molecular Signature Data Base (mSigDb) markers and in the Gene Ontology of Biological Processes (GO) (A), which are statistically enriched for genes associated with biosynthesis (fatty acids, glycosylphosphatidylinositol, etc.), signaling transduction, transcription factors and virus infection (B); subsequent Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome enrichment analysis identified 239 genes enriched in cancer-related signaling pathways, including the STAT3/NOTCH1 signaling pathway and the IL-17 signaling pathway (C).
Based on the analysis of transcriptomics data, we further explored the effect of L-Theanine on STAT3/NOTCH1 signaling in lung cancer cells. The results showed that L-Theanine treatment resulted in a significant decrease in phosphorylated STAT3 and Notch1 levels in DDP-resistant lung cancer cells, which was not observed in lung cancer cells (Fig. 4A–D). It was also observed that BMAL1 expression was significantly reduced in L-Theanine-treated DDP-resistant lung cancer cells, which was relatively not considerably altered in L-Theanine-treated lung cancer cells (Fig. 4E–H). It has been reported that STAT3/NOTCH1 signaling activation in cervical cancer can upregulate BMAL1 expression [11], which is the dominant changed gene in our transcriptomics data. To further clarify the regulation of BMAL1 expression induced by STAT3/NOTCH1 signaling in lung cancer cells, we treated DDP-resistant lung cancer cells with Stattic, a specific inhibitor of STAT3. It was found that BMAL1 protein expression was significantly reduced when phosphorylated STAT3 was inhibited considerably, suggesting that the expression of BMAL1 was affected by STAT3/NOTCH1 signaling. In addition, the expression of cell stemness markers was significantly reduced (Fig. 4I–L), after the effective knockdown of BMAL1 in DDP-resistant lung cancer cells (Fig. 4M); meanwhile the clone formation and invasive migration ability of cells were also significantly inhibited after BMAL1 knockdown (Fig. 4N–Q). The above results suggest that L-Theanine can inhibit BMAL1 expression in DDP-resistant lung cancer cells by regulating STAT3/NOTCH1 signaling, inhibiting cancer stemness.
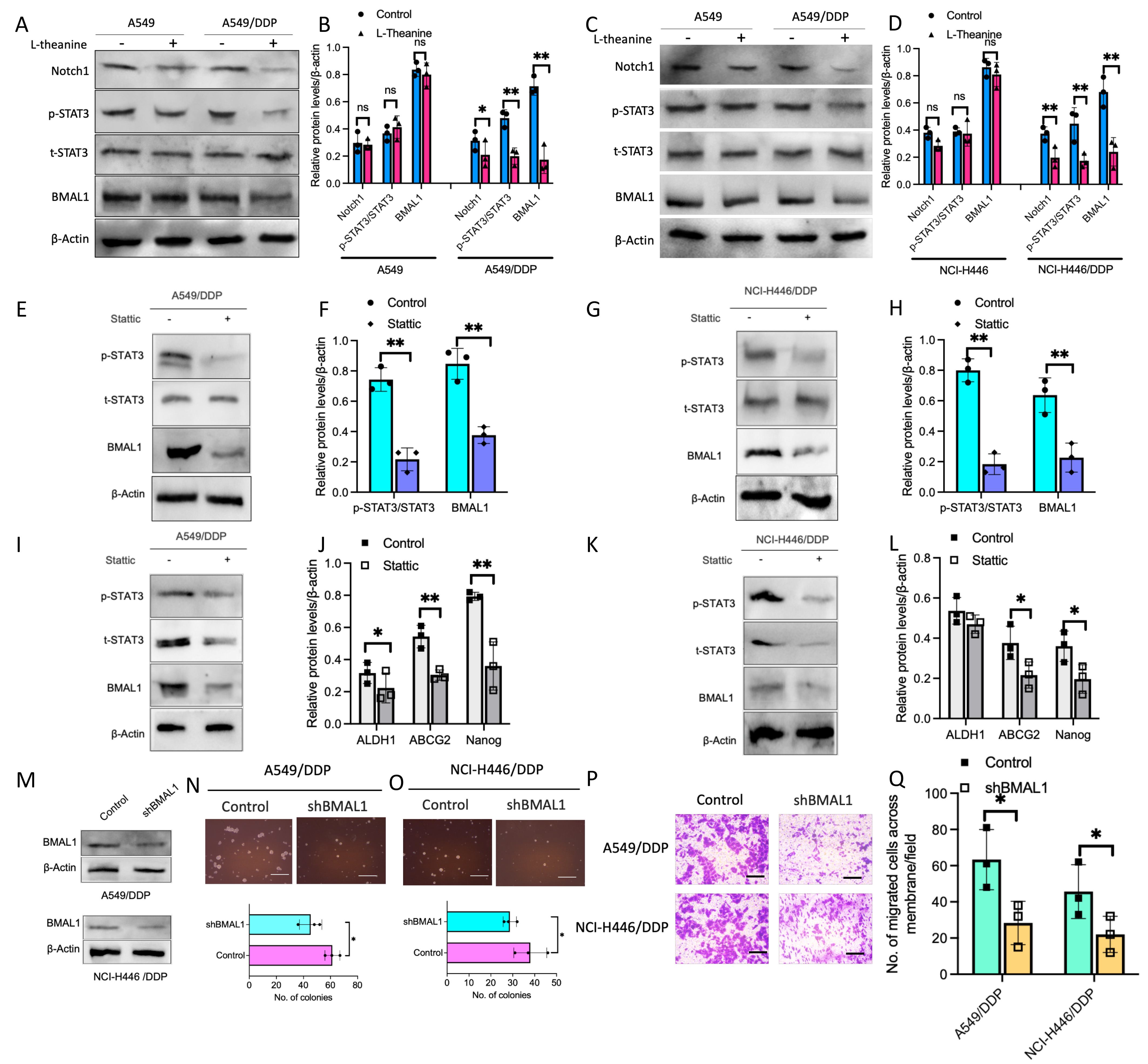 Fig. 4.
Fig. 4.Involvement of BMAL1 in the regulation of lung cancer cells
stemness as the downstream of STAT3/NOTCH1 signaling. The protein levels of
Notch1, BMAL1, and p-STAT3/t-STAT3 in A549, A549/DDP, NCI-H446, and NCI-H446/DDP
cells with or without L-Theanine treatment were detected by western blotting
(A–D). Moreover, the protein levels of BMAL1, p-STAT3/t-STAT3, and
stemness-related factors, including ALDH1, ABCG2, and Nanog in A549, A549/DDP,
NCI-H446 and NCI-H446/DDP cells with or without static treatment were analyzed by
a western blotting assay (E–L). After BMAL1 knockdown (M), tumor sphere
formation assay was performed in A549/DDP and NCI-H446/DDP cells with or without
shBMAL1 knockdown for 14 days. The number of tumor spheres means colonies per
5000 cells. Scale bar 200 µm (N,O). In addition, images and quantification
of transwell migration assays were presented in A549/DDP and NCI-H446/DDP cells
with or without shBMAL1 knockdown. Scale bar 100 µm (P,Q). Error bars
indicate SEM; n = 3. *p
Based on the above findings, we further explored the possibility of reversing drug resistance in lung cancer cells by combining L-Theanine with STAT3 blockade. Firstly, the changes in the cellular resistance index of DDP-resistant lung cancer cells to DDP after L-Theanine combined with STAT3 blockade treatment were investigated. The results showed that the combination treatment synergistically reduced the resistance index of DDP-resistant lung cancer cells relative to monotherapy (Fig. 5A,B). Secondly, it was also found that L-Theanine combined with STAT3 blockade treatment significantly inhibited the clone formation ability and the invasive migration ability of DDP-resistant lung cancer cells (Fig. 5C,D). Finally, L-Theanine combined with STAT3 blockade treatment effectively reduced the expression levels of stemness markers in DDP-resistant lung cancer cells (Fig. 5E–H). These results suggest that L-Theanine combined with STAT3 blockade synergistically inhibits the stemness of DDP-resistant lung cancer cells, thus overcoming platinum-based chemotherapy resistance in lung cancer cells.
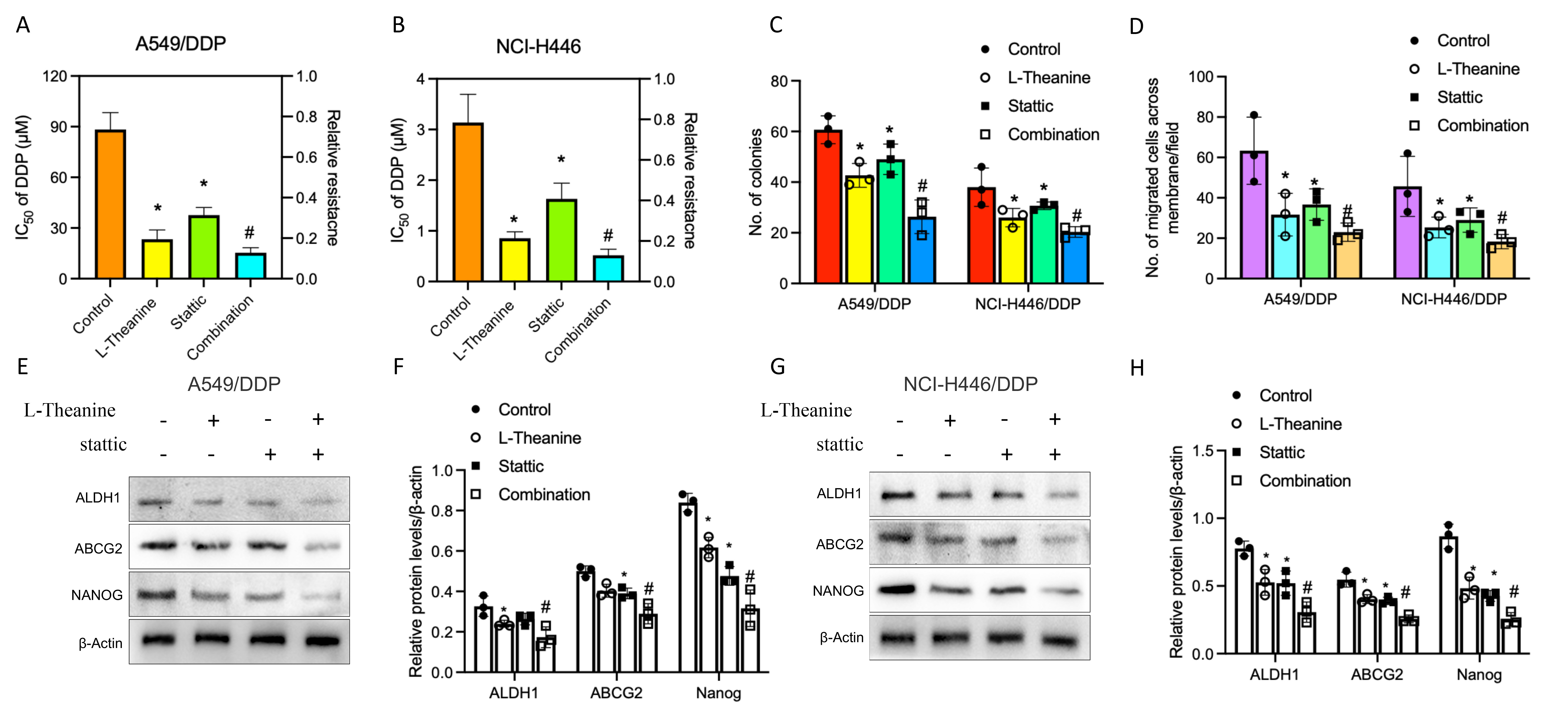 Fig. 5.
Fig. 5.Combination of L-Theanine and STAT3 blockade improved
synergistically the chemoresistance in lung cancer. The changes in the cellular
resistance index to DDP after L-Theanine combined with STAT3 blockade treatment
of A549/DDP and NCI-H446/DDP cells (A,B). Moreover, number of colonies per 5000
A549/DDP and NCI-H446/DDP cells was quantified (C); meanwhile, number of migrated
cells (A549/DDP and NCI-H446/DDP cells) was calculated (D). In addition, the
levels of proteins including ALDH1, ABCG2, and Nanog in A549, A549/DDP, NCI-H446,
and NCI-H446/DDP cells with or without combination treatment were analyzed by
western blotting (E–H). Error bars indicate SEM; n = 3. * vs control,
p
Theanine is a unique secondary metabolite of tea, one of the non-protein-free amino acids with the highest content. In recent years, with the application of modern biotechnology, the pharmacological mechanism of theanine has been widely studied [12]. At present, many studies have reported that theanine and its synthetic derivatives can induce apoptosis and inhibit growth, invasion, and metastasis of cancer cells; moreover, theanine can also enhance the activity of certain anticancer drugs and have specific effects on alleviating the adverse effects, which plays a vital role in the development of cancer chemotherapy [13, 14]. However, the roles of L-Theanine in chemoresistance is still to be explored. In the study, we revealed for the first time that L-Theanine plays an essential role in DDP-resistant lung cancer cells but not parental lung cancer cells; mechanistically, L-Theanine was demonstrated to inhibit stemness of DDP-resistant lung cancer cells by regulating Notch1/STAT3/BMAL1 signaling. These findings suggest a potential value of L-Theanine in overcoming chemoresistance in lung cancer.
Platinum-based drugs have always been used in precision therapy and immunotherapy for many years, and one of the research mainstays is the resistance mechanisms, which have been extensively addressed in preclinical models [15]. Lung cancer is with high morbidity and mortality worldwide. When the targeted therapeutic drugs become resistant, platinum-based chemotherapy is still the most important therapeutic modality. Unfortunately, the clinical observations find that chemotherapy is more effective in its early application in lung cancer patients but inevitably becomes resistant in the later stage. In recent years, L-Theanine has been verified to suppress proliferation and migration and induce apoptosis via inhibiting VEGFR/EGFR or NF-kB signaling in lung cancer [16, 17, 18]. However, the association between L-Theanine and lung cancer chemotherapeutic resistance is still unknown. Hence, in the study, we tried to address the issue and demonstrated that L-Theanine led to a significant decrease in the resistance index of DDP-resistant lung cancer cells (A549/DDP, NCI-H446/DDP) but not in non-resistant cells (A549 and NCI-H446). The result indicated that L-Theanine can only affect the resistance phenotype of DDP-resistant lung cancer cells, which suggests the potential application of L-Theanine in lung cancer patients with chemoresistance as well as the critical value of L-Theanine in overcoming the lung cancer chemoresistance.
It has been recognized that inhibition of cancer stemness can reduce the drug resistance of cancer cells [19]; in fact, the evolution of cancer stemness and the development of drug resistance interact with each other [20]. Intriguingly, we found L-Theanine can significantly decrease the expression of cancer stemness-related markers in DDP-resistant lung cancer cells but not in non-resistant lung cancer cells. The finding indicates that L-Theanine-induced inhibition of cancer stemness may reduce the progression, but not the formation, of drug resistance in lung cancer cells, which will help to design a precise intervention strategy against cancer stemness for reversing platinum-based chemoresistance in lung cancer.
However, how is L-Theanine involved in regulating DDP-resistance in lung cancer? For this reason, we performed a transcriptomics-based comparative analysis and found that compared to lung cancer cells without DDP-resistance, L-Theanine-mediated DEGs in DDP-resistant lung cancer cells were enriched in DNA damage, stemness regulation, and ABC transporter, etc. which consistent with three main mechanisms, including high drug transporter expression, strong DNA repair ability, and recruitment of a protective niche, in drug resistance of cancer cells [21, 22]. Moreover, KEGG analysis on RNA-seq data showed that STAT3/NOTCH1 signaling is the dominant pathway. Recent studies have revealed that the aberrant activation of STAT3/Notch1 signaling plays a critical role in the interaction between cancer stemness and therapy resistance [23, 24]; targeting STAT3/NOTCH1 signaling may inhibit the development of cancer stemness and reverse platinum-based chemoresistance [25, 26]. Notably, in the study, L-Theanine significantly inhibited the activation of STAT3/NOTCH1 signaling in DDP-resistant lung cancer cells; meanwhile, the specific inhibition of STAT3/Notch1 signaling significantly decreased the formation of tumorspheres, the migration and the resistance index of DDP-resistant lung cancer cells as well as reduced the expression of BMAL1, a top DEG in the transcriptomics analysis above.
The BMAL1 gene, also known as the ARNTL gene, is a critical component in the molecular circadian oscillator of mammalian life activity [27]. In recent years, a large number of studies have shown that the BMAL1 plays a vital role in carcinogenesis, development, therapeutic efficacy, and prognosis; however, effects of BMAL1 on cancers is highly complex in different cancers. BMAL1 can affect cancer development through other mechanisms, even in the same type of cancer, and play different roles in different microenvironments [28, 29]. Hence, further elucidation of the relationship between the BMAL1 and cancers may lead to a deeper understanding of the mechanisms of cancer genesis, dissemination, metastasis and provide theoretical bases for developing new therapeutic strategies. Recent studies have revealed that BMAL1 can promote chemoresistance of various cancers, including colon cancer, pancreatic cancer, adrenocortical carcinoma etc. [30, 31, 32]. Notably, based on the studies above indicating BMAL1 as a driver factor of chemoresistance, we demonstrated that L-Theanine inhibited the expression of BMAL1 in DDP-resistant lung cancer cells in the work; furthermore, our results also suggested BMAL1 knockdown downregulated the expression of lung cancer stemness-related markers and inhibited the formation of tumorspheres, consistent with some studies [33, 34]. Altogether, a novel L-Theanine-STAT3/NOTCH1-BMAL1 regulatory axis, which connects chemoresistance with cancer stemness, was established. The potential application value of the regulatory axis was explored and identified by using the combination of L-Theanine and STAT3 blockade in DDP-resistant lung cancer cells, which shows L-Theanine combined with STAT3 blockade synergistically inhibits the stemness of DDP-resistant lung cancer cells and thus overcomes platinum-based chemotherapy resistance in lung cancer cells.
In summary, our study demonstrated that L-Theanine impedes the progression, not the formation, of chemoresistance in lung cancer and inhibits the stemness by regulating the STAT3/Notch1-BMAL1 axis. The finding might provide research evidence for developing a novel therapeutic strategy for reversing chemoresistance in lung cancer.
NSCLC, Non-small Cell Lung Cancer; CSC, Cancer Stem Cell; ICI, Immune Checkpoint Inhibitors; DDP, cisplatin (cis-diamminedichloroplatinum); GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PCR, Polymerase Chain Reaction; CCK-8, Cell Counting Kit-8; DNA, Deoxyribonucleic Acid; DMEM, Dulbecco’s Modified Eagle’s Medium; EGF, Epidermal Growth Factor; bFGF, basic Fibroblast Growth Factor; SDS-PAGE, Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis; RIPA, Radioimmunoprecipitation Assay; DEGs, Differentially Expressed Genes; BMAL1, Brain and Muscle Arnt-like 1; STAT3, Signal Transducer and Activator of Transcription 3; NOTCH1, Notch homolog 1; EGFR, Epidermal Growth Factor Receptor; BRAF, B-Raf proto-oncogene; HER2, Human Epidermal Growth Factor Receptor 2; ALDH1, Aldehyde dehydrogenase 1; ABCG2, ATP-binding cassette, sub-family G, member 2.
The data will not be shared as the data are considered property of Shanghai Pudong New District Guangming Hospital, which does not allow sharing of data with third party. Nevertheless, the data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
WJ and CZ conceived and designed the study. WJ, LS, HY and ZD, ML contributed to the experiments and WJ, LS, HY contributed to data analysis. WJ, LS, HY and ZD, ML wrote the manuscript. CZ supervised the study. All authors contributed to the revision. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This work was supported by Pudong new area Science, Technology and Economy Commission, Shanghai (No: PKJ2022-Y28).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.