1 Department of Morphological Disciplines, University of Veterinary Medicine and Pharmacy in Košice, 040 01 Košice, Slovakia
2 Center of Clinical and Preclinical Research MediPark, Faculty of Medicine, P.J. Šafárik University in Košice, 040 01 Košice, Slovakia
3 Department of Microbiology and Immunology, University of Veterinary Medicine and Pharmacy in Košice, 040 01 Košice, Slovakia
Abstract
Background: The epithelia of the intestine perform various functions,
playing a crucial role in providing a physical barrier and an innate immune
defense against infections. By generating a “three-dimensional” (3D) model of
cell co-cultures using the IPEC-J2 cell line and porcine blood monocyte-derived
macrophages (MDMs), we are getting closer to mimicking the porcine intestine
ex vivo.Methods: The effect of Limosilactobacillus
reuteri B1/1 and Limosilactobacillus fermentum CCM 7158 (indicator
strain) on the relative gene expression of interleukins (IL-1
Keywords
- co-culture
- IPEC-J2
- MDM
- lactobacilli
- tight-junctions
- cytokines
The increased productivity in livestock husbandry accompanied by heightened demands on the quality of the meat produced. At the same time, the livestock industry represents an important economic activity in many countries. In large-scale farms, intensive conditions expose animals to stress, resulting in diseases and environmental decline, leading to significant economic losses. Treatment of these diseases has led to a surge in the use of veterinary drugs, fostering antimicrobial resistance and posing risks to consumers [1]. Health is closely tied to mucosal immunity and the intestinal microbiome. The intricate interactions between the host and the gut microbiome impact nutrient exchange, thereby influencing gut physiology, immunity, and morphology. On the other hand, the presence of intestinal pathogens can lead to undesirable changes in the morphology of the intestine [2]. Therefore, it is essential to promote sufficient cooperation and activation of the mucosal immune system, particularly through the production of antimicrobial proteins. Protein molecules such as lumican and olfactomedin-4 may promote intestinal homeostasis by initiating innate immune inflammatory responses that are beneficial in the early stages of enteritis and colitis [3, 4]. The mucosal surface of the intestine is formed by an epithelium, while tight junctions (TJs) exist between the epithelial cells, forming a continuous intercellular barrier, which is necessary for the separation of tissue spaces and the regulation of the selective movement of dissolved substances [5]. Both the occludin and claudin families represent TJ transmembrane proteins capable of adhesive interactions with other molecules between adhered cells, including the control of ion selectivity [6].
Consequently, there is a high demand for natural substances that have a beneficial effect on animal health, including protection against infectious diseases. Probiotic bacteria are undoubtedly among the most widely used substances of natural origin [7]. The effect of probiotics can vary depending on the properties of the specific probiotic bacteria used as well as their combination. Limosilactobacillus reuteri (L. reuteri) is among the most studied probiotic strains, which dominantly colonizes the intestines of mammals and humans. It is considered an “intestinal probiotic with prebiotic efficacy” because most of them show remarkable intestinal colonization including the secretion of bacteriocins, which also increases the expression of mucin genes, thus supporting the development and maturation of intestinal organoids and increasing the secretion of mucin itself [8]. Both animal and human studies suggest that probiotics can significantly influence the modulation of immune and inflammatory mechanisms. The use of probiotics can improve the balance of intestinal microorganisms, increase mucus secretion, and reduce the degradation of TJ proteins by reducing the presence of bacterial lipopolysaccharide (LPS) [9, 10]. Probiotic microorganisms, together with intestinal symbionts, also modulate host intestinal barrier function through their metabolites and various surface molecules [11]. In addition to this, they also promote host health by strengthening the intestinal barrier through various direct and indirect mechanisms [12]. Studying the mechanism of action for each probiotic strain is crucial. Typically, potential strains undergo microbiological testing in vitro and on two-dimensional (2D) cell lines. However, these lines lack realism in mimicking the natural reactions of the organism. Utilizing 3D animal gut models is proving to be more suitable, offering a closer simulation of the in vivo intestinal environment and facilitating a detailed study of bacteria-gut interactions. It’s important to note that live animal experiments are subject to legal restrictions in the European Union due to ethical concerns [13].
In a previous study, we demonstrated that L. reuteri B1/1 exhibited
substantial adherence to non-carcinogenic porcine-derived enterocytes (CLAB) cells, especially at a concentration of 1
The intestinal porcine epithelial cell line IPEC-J2 was obtained from J.J. Garrido from the University of
Córdoba, Spain and was authenticated using Short Tandem Repeat (STR) profiling. Epithelial cells were maintained exactly as previously
described in the study by Kiššová et al. [14]. After
reaching a 75 % monolayer confluence on culture flasks, the cells were released
and transferred to a new culture flask, as follows: cells were incubated with
EDTA (1 mmol/L; Sigma-Aldrich, St. Louis, MA, USA) for 5 min at 37 °C
with 5 % CO
Blood samples were collected aseptically from the supraorbital sinus of healthy
pigs aged 10 to 12 weeks. The pigs were of a Landrace crossbreed with The Large
White, and were housed at the Clinic of Swine of the University of Veterinary
Medicine and Pharmacy in Košice. The collection procedure adhered strictly to
animal welfare guidelines and was approved by ethics committee namely “Ethics
committee for the approval of research involving animals in accordance with the
legislative requirements applicable at the UVMP in Košice” (Ethics committee
at the UVMP in Košice, permit No. EKVP/2023-04). Blood samples were collected
in 50 mL tubes filled with 1.5 % heparin prepared in phosphate-buffered saline
(PBS). Mononuclear leukocytes (MNL) were obtained from the heparinized blood
diluted 1:1 in PBS, underlayed with 15 mL separation solution (LSM1077
“Lymphocyte Separation Medium”; PAA, Austria) in Leucosep™ tubes
(Greiner-Bio-One, Frickenhausen, Austria) and centrifuged (300
Limosilactobacillus reuteri B1/1 and Limosilactobacillus
fermentum CCM 7158 strains were grown in de Man Rogosa Sharpe (MRS) broth
(Merck) at 37 °C overnight. The strains were then centrifuged at 500
Cells were grown on semipermeable membrane inserts as mentioned above, for 15
days to allow differentiation and establish tight epithelial monolayers. Fresh
culture medium was changed every other day. On the day of the experiment, inserts
containing monolayers of differentiated IPEC-J2 cells were transferred to 12-well
plates with differentiated MDMs at the bottom of the well. Complete
antibiotic-free DMEM/F-12 medium was added to the upper and lower chambers of the
inserts. Only confluent monolayers of differentiated IPEC-J2 grown on a
semipermeable membrane for 13–15 days with transepithelial electrical resistance
(TEER)
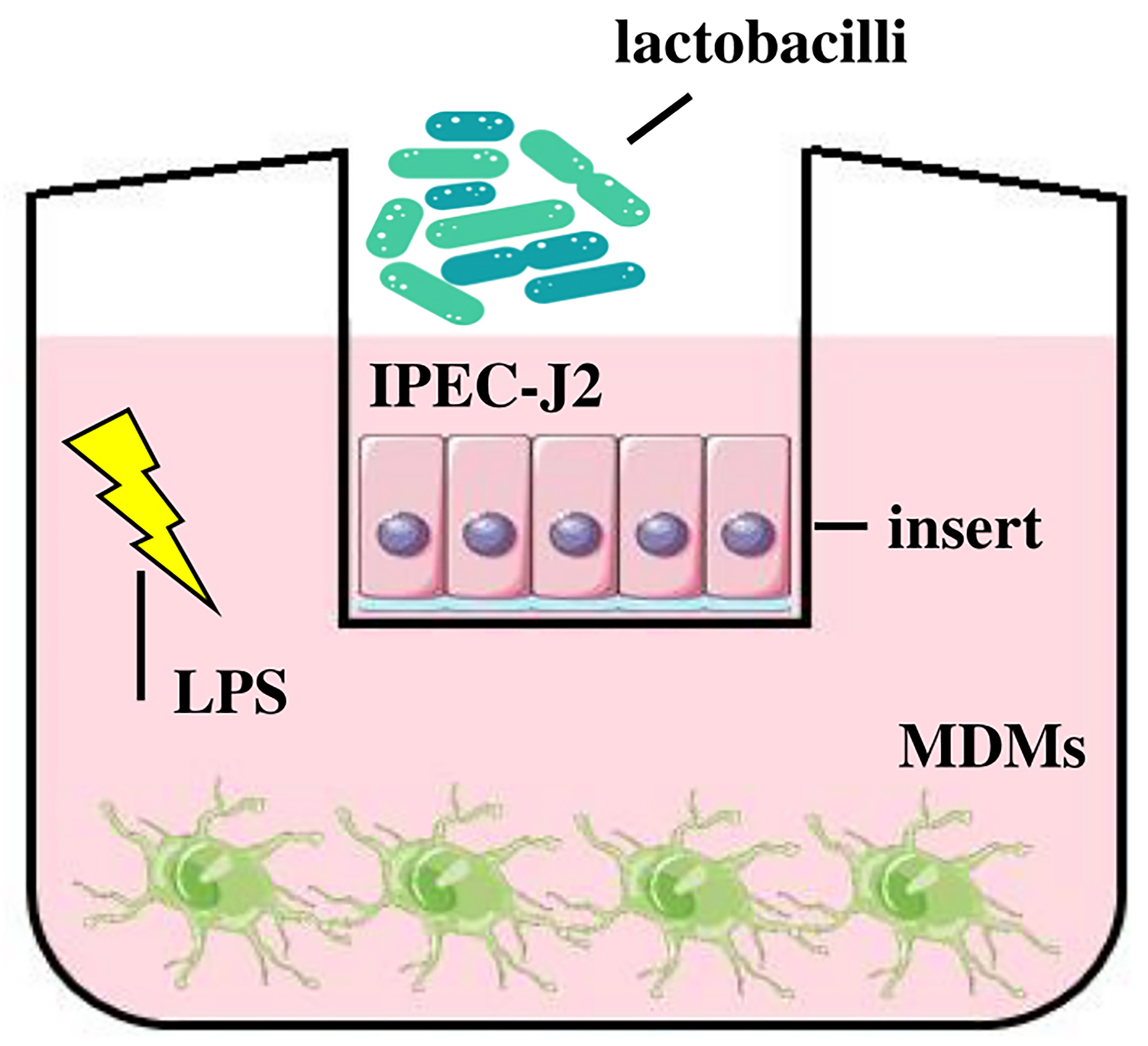 Fig. 1.
Fig. 1.Schematic illustration of a co-culture system of IPEC-J2/MDMs.
IPEC-J2 cells representing the apical compartment were treated with either
Limosilactobacillus reuteri B1/1 or Limosilactobacillus
fermentum CCM 7158 (1
| Cell treatment | Design of experiments in co-cultures | Abbreviation |
| Control | IPEC-J2 or MDMs without treatment | - |
| LPS challenge | MDMs were induced with LPS (1 µg/mL) and incubated for 24 h | LPS |
| L. fermentum CCM7158/ LPS treatment | IPEC-J2 were cells treated with probiotic LF (1 |
LF + LPS |
| L. reuteri B1/1 + LPS treatment | IPEC-J2 were cells treated with probiotic LR (1 |
LR + LPS |
MDMs, monocyte-derived macrophages; LPS, lipopolysaccharide.
TEER was measured using an epithelial voltohmmeter EVOM2 (World Precision Instruments, Sarasota, FL, USA) connected to the STX4 electrode. The change in TEER was monitored after 3, 6, and 24 h, and individual TEER values were calculated by subtracting the value of the empty insert and multiplying it by the surface area of the membrane. TEER values at 3, 6, and 24 h were normalized to the own TEER value of the insert at 0 h. To assess the integrity of the formed IPEC-J2 cell monolayer on insert membranes, the monolayer permeability test was carried out using the fluorescent dye Lucifer Yellow (LY, Thermo Fisher Scientific, Waltham, MA, USA). Cell monolayers of differentiated epithelial cells were first washed with Hanks’ balanced salt solution (HBSS; Sigma-Aldrich), and then 500 µL of LY solution in HBSS (40 µg/mL) was added to the apical side and 1300 µL of HBSS to the basolateral side of well, followed by incubation on a shaker in the dark. After 1 h, the solution was collected from the basolateral side of the well and the fluorescence intensity was measured on a Synergy H4 hybrid plate reader (Bio-Tek Instruments, Winooski, VT, USA) using a 485 nm excitation and 530 nm emission filter. All measurements were performed in triplicate.
High-quality total RNA was isolated using the TRI reagent (Sigma-Aldrich) and then purified using RNeasy mini kit (Qiagen, Manchester, UK) exactly according to the manufacturer’s instructions. RNA purity and concentration were determined using spectrophotometer NanoPhotometer P-Class P 300 (Implen, Munich, Germany) at rations 260/280 nm 260/230 and then reversely transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. The resulting cDNA from the cells was stored at –18 °C until further use.
The qPCR analysis was performed on a Lightcycler 480 II Instrument (Roche
Holding AG, Basel, Switzerland) using LightCycler® Software
(LightCycler® Software 4.1, Basel, Switzerland) in a 10 µL
reaction volume consisting of 1
| Gene | Primer | Sequence 5 |
Reference |
| Forward | CATCACCATCGGCAACGA | [20] | |
| Reverse | GCGTAGAGGTCCTTCCTGATGT | ||
| HPRT | Forward | AACCTTGCTTTCCTTGGTCA | [21] |
| Reverse | TCAAGGGCATAGCCTACCAC | ||
| IL-18 | Forward | CTGCTGAACCGGAAGACAAT | [20] |
| Reverse | TCCGATTCCAGGTCTTCATC | ||
| IL-1 |
Forward | GCCCTGTACCCCAACTGGTA | [22] |
| Reverse | CCAGGAAGACGGGCTTTTG | ||
| IL-6 | Forward | CCACCAGGAACGAAAGAGAG | [23] |
| Reverse | AGGCAGTAGCCATCACCAGA | ||
| IL-8 | Forward | TTATCGGAGGCCACAATAAG | [24] |
| Reverse | TGGAATAGTAGATGGAGCCA | ||
| IL-10 | Forward | TGTCATCAATTTCTGCCCTGT | [25] |
| Reverse | TCTTCATCGTCATGTAGGCTT | ||
| TLR2 | Forward | CCAGGCAAGTGGATTATTGA | [26] |
| Reverse | AAGAGACGGAAGTGGGAGAA | ||
| TLR4 | Forward | TGGTGTCCCAGCACTTCATA | [27] |
| Reverse | CGGCATGACTCCTCAGAAAC | ||
| CLDN1 | Forward | TGGTCAGGCTCTCTTCACTG | [28] |
| Reverse | TTGGATAGGGCCTTGGTGTT | ||
| OCLN | Forward | ATGCTTTCTCAGCCAGCGTA | [29] |
| Reverse | AAGGTTCCATAGCCTCGGTC | ||
| LUM | Forward | ACCTGCGTTTGTCTCATAAT | [30] |
| Reverse | ATTGTAGGAGAGATCCAGCT | ||
| OLFM-4 | Forward | GGTGATTTACGCAACTGAAG | [30] |
| Reverse | GTTTGTACTGCTTGGTATGC |
The results from the gene expression analysis are expressed as the mean and the
SD of two independent experiments which were conducted in
triplicate. GraphPad Prism 9.0.0 software (La Jolla, California, UK) was used to
determine significant differences by one-way analysis of variance (ANOVA)
followed by Dunnett’s multiple comparisons. The level of significance was set as
*p
During the course of the experiments, TEER values of the formed IPEC-J2
monolayers were measured after 3, 6, and 24 h in order to monitor the integrity
of the cell barrier (Fig. 2). As shown in Fig. 2, the initial TEER values of the
co-cultures did not differ from the control group of cells after 3 h of
incubation. TEER values of differentiated IPEC-J2 cells decreased after 6 h
(p
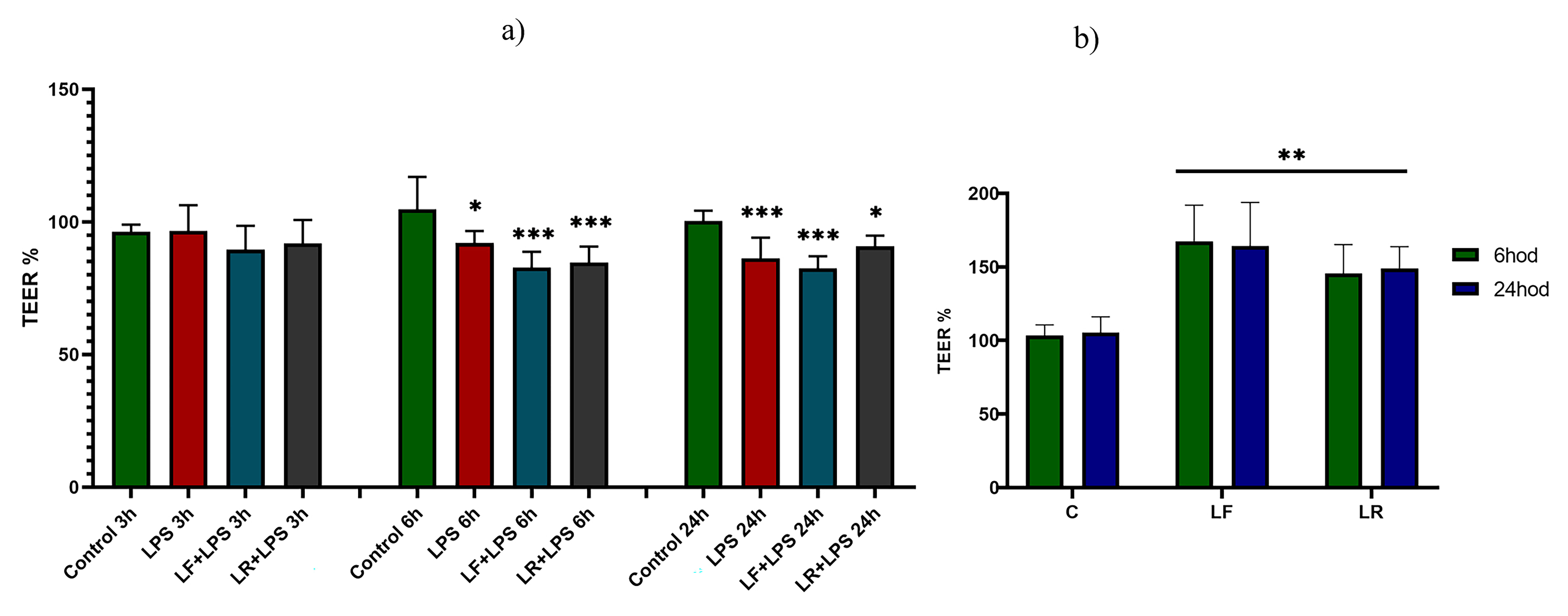 Fig. 2.
Fig. 2.Assessment of transepithelial electrical resistance. (a)
Transepithelial electrical resistance (TEER) values at 3, 6, and 24 h were
normalized to the own TEER value of insert at 0 h. (b) The effect of both
lactobacilli on the epithelial barrier enhanced integrity. TEER values measured
after 6 h and 24 h of incubation. Results of individual TEER measurements are
expressed as a percentage. The control groups represent 100 % (
Lucifer yellow represents a small molecule able to cross through the epithelial barrier by passive paracellular diffusion. Given its hydrophilic nature and small molecular size, LY can be employed to chemically assess the functionality of TJs [31]. LPS induced a significant increase in LY flux measured after 24 h. None of the lactobacilli were able to significantly attenuate the LPS-induced increase in permeability in agreement with the TEER results (Fig. 3).
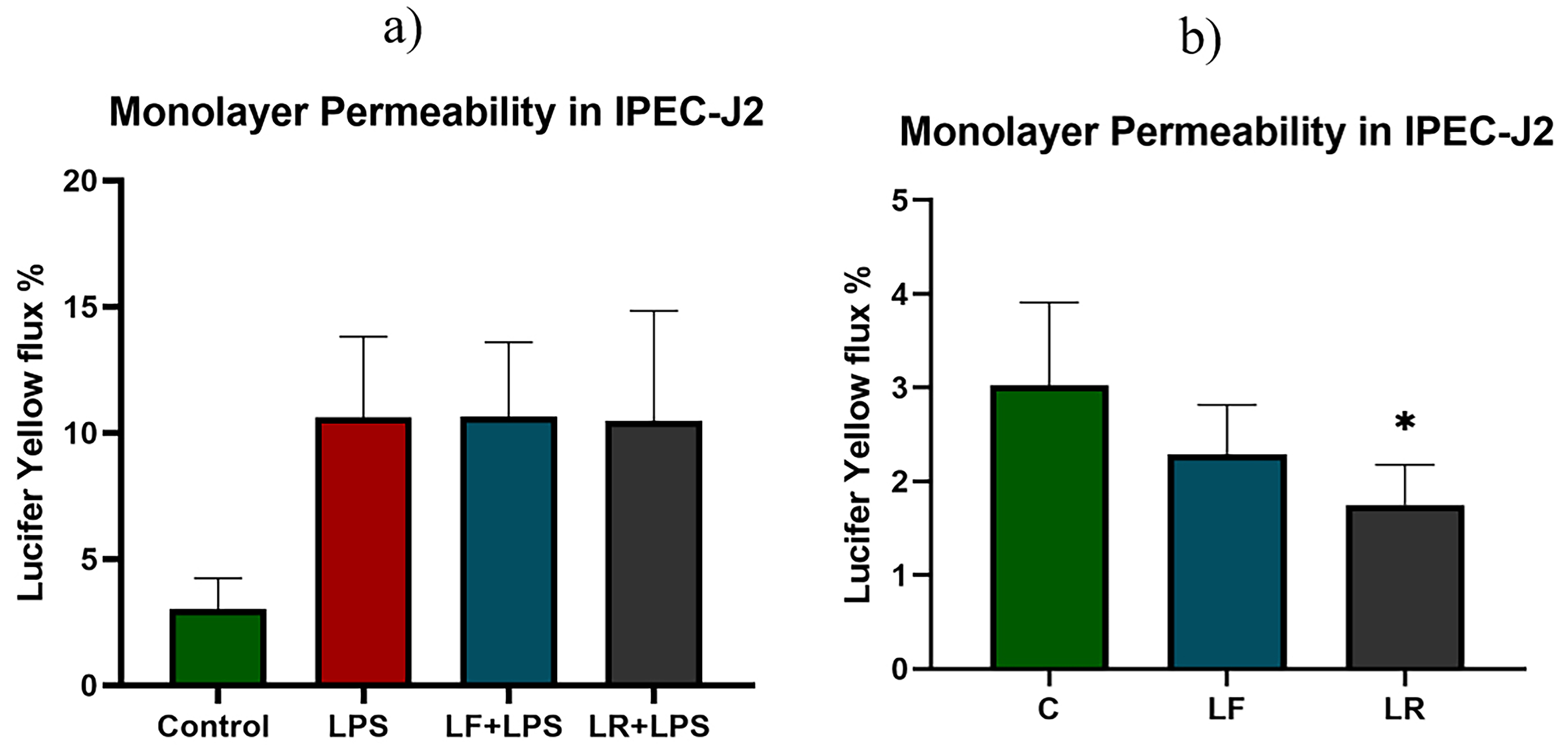 Fig. 3.
Fig. 3.Monolayer permeability test in IPEC-J2. Lucifer yellow was used
for the macromolecular permeability test of the IPEC-J2 monolayer. The monolayer
permeability test was performed in all experimental groups (a) and also in
IPEC-J2 cells incubated with probiotic isolates only (b). After incubation with
LY for 1 h, the solution was collected from the basolateral side of the well and
the fluorescence intensity was measured. The results are expressed as a
percentage. The level of significance was set as *p
The pathological effect of LPS from E.coli was evaluated in an
in vivo-like co-culture model by challenging MDMs in the basolateral
compartment. The effect of LPS was assessed by analyzing the gene expression of
important pro-inflammatory cytokines (IL-1
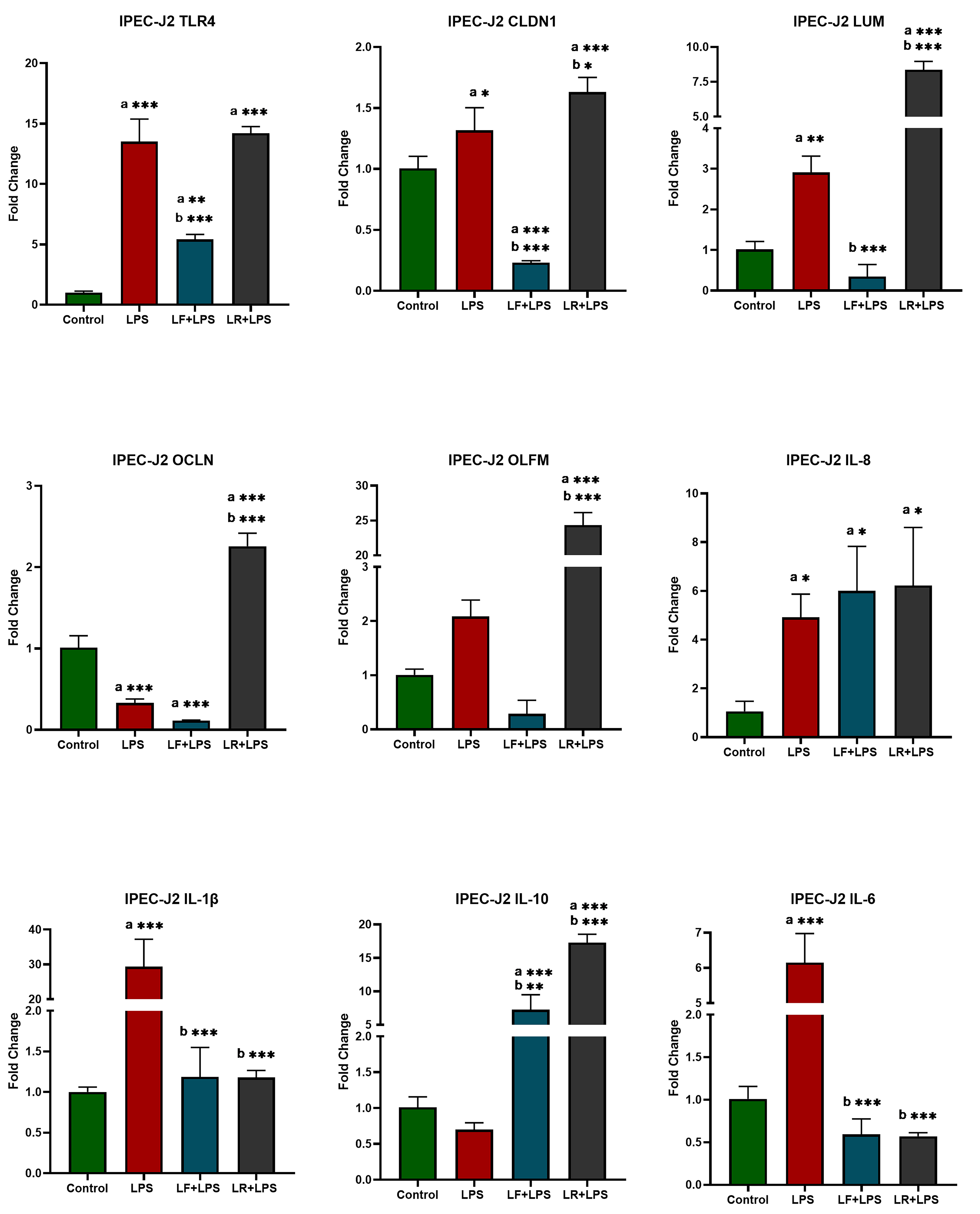 Fig. 4.
Fig. 4.Gene expression in IPEC-J2 cells. Gene expression analysis of
pro-inflammatory related genes, TJ-related genes, and Toll-like receptors in
IPEC-J2 cells. The level of significance was set as *p
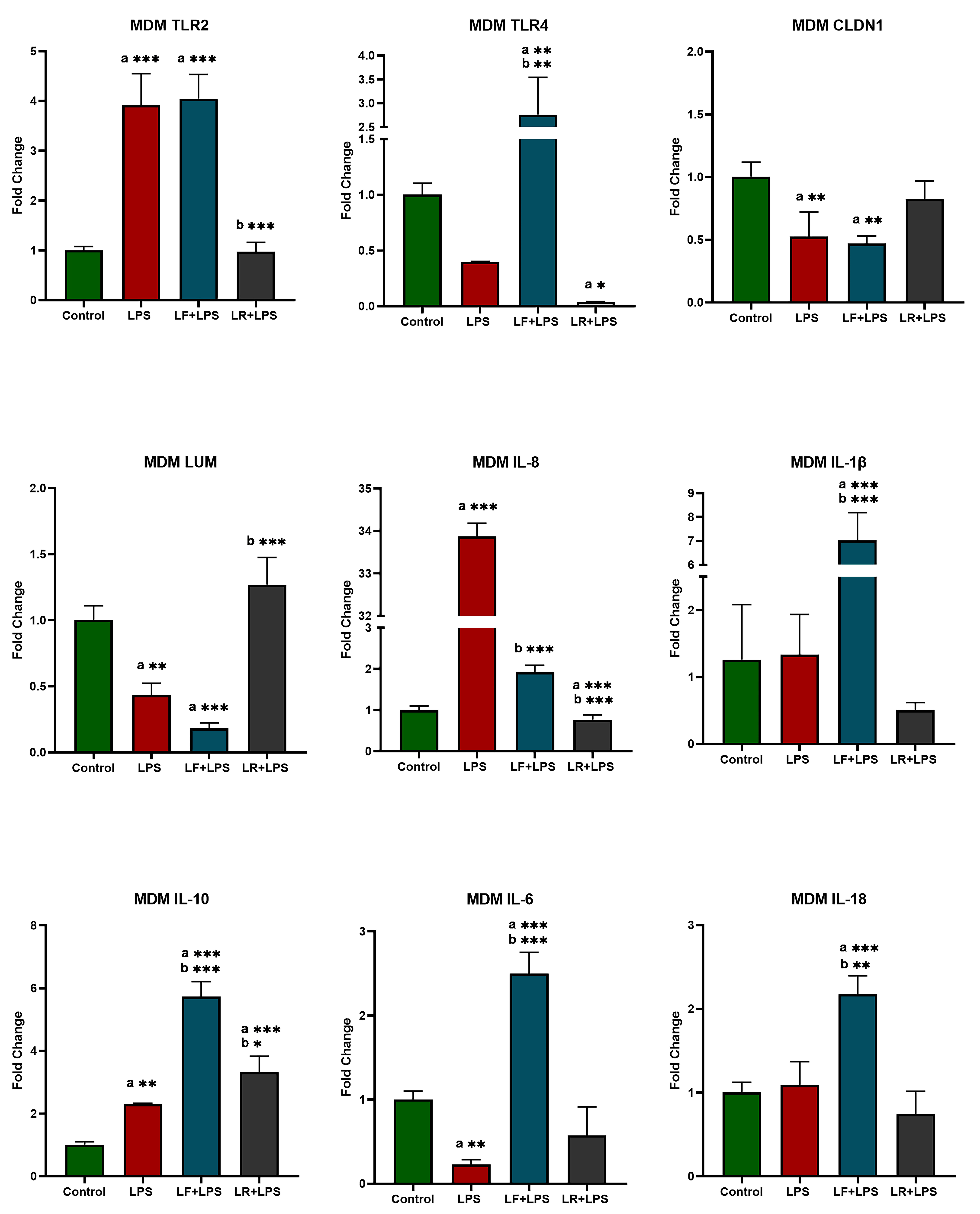 Fig. 5.
Fig. 5.Gene expression in MDMs. Gene expression analysis of
pro-inflammatory related genes, TJ-related genes, and Toll-like receptors in
MDMs. The level of significance was set as *p
The direct effect of live probiotic strains of L. reuteri B1/1 and
L. fermentum CCM 7158 was investigated on IPEC-J2 cells, representing
the apical compartment of the co-culture model. The results obtained from the
gene expression of the pro-inflammatory cytokines IL-1
As a regulatory cytokine, we also investigated IL-10 expression, which was
affected by probiotic bacterial culture of both LR and LF in both IPEC-J2 and
MDMs, with statistical significance p
The influence of LPS from E.coli was investigated in an in
vitro co-culture model by gene expression analysis of genes related to TJ such
as claudin-1 (CLDN1), lumican (LUM), olfactomedin-4 (OLFM-4) and occludin (OCLN).
In the directly treated MDMs with LPS, the down-regulation of LUM and CLDN
(p
In LR probiotic-treated group of IPEC-J2 cells, an up-regulation of mRNA levels
for genes encoding OLFM, LUM and CLDN1 was detected (p
TLR4 mRNA levels were up-regulated in the LPS-induced group in the case of
IPEC-J2 cells (p
In the body, the intestinal epithelium performs a number of important functions such as digesting and absorbing nutrients, providing a physical barrier, and shielding the body from the challenging conditions of the gut lumen. The epithelial barrier ensures selectivity, preventing the entry of a potentially harmful luminal contents by rejection, while facilitating the controlled absorption and secretion of significant amounts of solutes and water in a specific direction. Individual epithelial cells are linked through a network of intercellular junctions - TJs, which are of particular significance in defining the properties of the paracellular barrier and its selectivity [32]. To determine barrier strength, TEER is commonly measured in in vitro cell models and permeability to paracellular markers such as the enzyme horseradish peroxidase, inulin, or mannitol is assessed [33]. Our results showed that a basolateral challenge in the form of LPS had an effect on the reduction of TEER values already after 6 h of application. The fact that basolateral LPS challenge leads to a significant decrease in TEER was also demonstrated by Wine et al. [34], who found that the basolateral aspect of the T84 cell line by infection with invasive E. coli (O157:H7) led to a significant decrease in TEER values. This reduction was more significant after basolateral application compared to the apical compartment/application of E. coli. There is evidence that other pathogens, such as C. jejuni, enter intestinal epithelial cells through? the basolateral membrane, highlighting the importance of this finding [35].
Furthermore, it is well known that the intestinal barrier function is to some extent supported by TJ proteins [36]. Epithelial cell TJs are comprised of numerous junctional molecules, including claudins, occludins and zonula occludens, which regulate the paracellular permeability of various macromolecules, ions, and water between neighboring cells. Occludin is a key transmembrane protein in the TJs, and both occludin and claudin-1 play essential roles in maintaining the intestinal permeability and barrier function of the TJs. There is evidence that LPS-induced inflammation disrupts the integrity of intestinal epithelial cells and TJs [37]. Disruption of TJ integrity leads to immune cell activation and inflammatory processes in the affected tissues [38]. Consistent with the previous statement, in our study, basolateral application of LPS to MDMs affected the expression of the gene encoding OCLN which resulted in a significant down-regulation in IPEC-J2 cells representing the apical compartment. Similar results were reported by Zhao et al. [25], who observed a decrease in mRNA levels for the gene encoding OCLN after treating IPEC-J2 cells with bacterial LPS. Similarly, in the work of Wu et al. [37], a down-regulation of the expression of genes encoding the proteins occludin and claudin-1 was observed by applying LPS at a concentration of 1 µg/mL.
On the other hand, strong adhesion provides the initial interaction of
probiotics with intestinal epithelial cells, which is key for LAB strains to
exert their beneficial effects on host health. Adhesion to intestinal cells
limits the presence of potential pathogens, thereby providing protection to
intestinal epithelial cells (IECs) [39]. The genus Limosilactobacillus
(līmōsus, Lat. - slimy), which also includes the strains
L. reuteri and L. fermentum, is characterized by the ability of
most strains in this genus to produce exopolysaccharides (EPS) from sucrose to
promote biofilm formation on intestinal epithelia [40]. In this study, L.
fermentum CCM 7158 was used as an indicator LAB strain because it is included in
the Czech Collection of Microorganisms (CCM) and its properties have been
extensively studied in previous studies under both in vitro and
in vivo [41, 42, 43]. An interesting finding in our work is that simultaneous
treatment of MDMs with LPS and treatment of IPEC-J2 with the probiotic strain
L. fermentum CCM 7158 led to the down-regulation of mRNA for CLDN-1 and
OCLN in intestinal cells. However, when L. reuteri B1/1 was used, the
mRNA levels for the above-mentioned genes were significantly increased compared
to the control group of cells. Similarly, genes encoding the antimicrobial
proteins LUM and OLFM-4, which are also involved in maintaining the integrity of
the intestinal epithelial layer [3], were significantly up-regulated in the
IPEC-J2 cells treated with L. reuteri B1/1. In light of these results,
we could suggest that L. reuteri B1/1 may act in a stimulatory manner on
intestinal epithelia. Similarly, in our recent study, we observed the stimulatory
effect of L. reuteri B1/1 on non-carcinogenic porcine-derived
enterocytes (CLAB) at both concentrations used (10
At the same time, enterocytes are an important component of the highly regulated
communication network that provides protection to the intestinal mucosa, their
ability to recognize microorganisms via pattern recognition receptors (PRRs) is
essential [45]. The best-known of these PRRs are the Toll-like receptors (TLRs).
Interaction of microorganisms with TLRs either leads to activation and triggering
of the signaling pathway or, conversely, blocks its activation through the
mechanism of negative regulators [46]. Structural components of the LAB cell
wall, as well as the EPS, can be produced and released into the surrounding
environment and are capable of interacting with TLRs in the gut [47]. In our
previous study with the IPEC-J2/moDCs co-culture model, we demonstrated the
ability of EPS derived from L. reuteri Biocenol™ to increase mRNA
levels in dendritic cell monocultures [16]. In the present work we used live
lactobacilli, where by treating apically deposited IPEC-J2 cells with L.
fermentum CCM 7158, we observed an increase in gene expression for the gene
encoding the TLR4 receptor not only in directly treated enterocytes but also in
basolaterally deposited MDMs. Although the gene encoding TLR2 could not be
captured in IPEC-J2 due to the reduced gene expression levels, the expression of
this gene was increased in MDMs. This up-regulation in basolaterally deposited
macrophages correlates with the detected increased expression of the
pro-inflammatory cytokines studied (IL-1
Numerous studies have demonstrated that LPS disrupts the structural integrity of
the intestinal epithelia, triggers inflammation, and leads to the release of
inflammatory cytokines such as IL-1
In our current study, using the porcine intestine in vitro model by
co-cultivating IPEC-J2 and MDMs, we investigated the immunomodulatory potential
of a recently isolated LAB strain L. reuteri B1/1 and the indicator LAB
strain L. fermentum CCM 7158 on the inflammatory response to LPS
challenge. An in vitro immunoprotective potential of L. reuteri
B1/1 was suggested as it was able to suppress the enhanced inflammatory response
of both cells used to LPS challenge. On the contrary, L. fermentum CCM
7158 increased the mRNA levels of pro-inflammatory cytokines (IL-18, IL-6, and
IL-1
All data from this study are included in the manuscript or are available on request from the first or corresponding author.
Conceptualization, ZK, VK, JS and DM; Methodology, Data curation, ZK and JS; Formal analysis, Writing - Original draft preparation, ZK and VK; Supervision, DM; The cell cultures were prepared by ZK, and JS; Writing - Review and Editing ZK and VK; The management for research activity planning and the financial support for the project leading to this publication was performed by VK and DM. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors contributed to editorial changes in the manuscript.
The procedure of blood sampling was performed in accordance with the guidelines for animal welfare and was approved by the Ethics Committee for the approval of research involving animals by the legislative requirements applicable at the UVMP in Košice with permit No. EKVP/2023-04.
We are grateful to assoc. prof. Jaroslav Novotný, DVM, PhD, from the Clinic of Swine, from the University of Veterinary Medicine and Pharmacy in Košice, for providing blood samples from which the monocyte-derived macrophages were isolated.
This work was funded by the Slovak Research and Development Agency under the contract no. (APVV-21-0129) and the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic VEGA 1/0098/22.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





