1 Department of Cardiothoracic Surgery, The Sixth People's Hospital of Chengdu, 610051 Chengdu, Sichuan, China
2 Department of Pain Treatment, The Second Hospital of Shanxi Medical University, 03001 Taiyuan, Shanxi, China
Abstract
Human umbilical cord mesenchymal stem cells (hUCMSCs) exhibit potent self-renewal and multilineage differentiation characteristics. They have garnered substantial attention within the domain of regenerative medicine owing to their therapeutic potential, such as in tissue repair, regeneration, immunomodulation, anti-inflammation, angiogenesis, wound healing, neuroprotection, and neuroregeneration. The process of fate determination is initiated by multiple signaling molecules. During development and tissue homeostasis, the Notch signaling pathway assumes a pivotal function in cell differentiation and the renewal of stem cells. A growing body of research has revealed that the Notch signaling pathway plays a pivotal role in hUCMSC proliferation and differentiation. The latest progress concerning the crucial functions of the Notch signaling pathway in maintaining homeostasis and determining the cell fate of hUCMSCs is summarized. Furthermore, the authors also summarized the mediators related to the Notch signaling pathway in hUCMSC differentiation, as well as the pathway alterations and mechanisms involved in hUCMSC therapy.
Graphical Abstract
Keywords
- notch signaling
- human umbilical cord mesenchymal stem cells
- cell differentiation
- cell fate decision
- stem cell therapy
The Notch signaling pathway consists of a cell-to-cell communication system that functions significantly during various physiological and developmental processes [1]. Delta-like (DLL) ligands and Notch receptors are the primary components of this pathway. DLL ligands are transmembrane ligands consisting of Delta-like-1, 3, and 4, Jagged 1 and 2, and the Notch receptors (Notch1–4) present on neighboring cells. These ligands and receptors are involved in signaling interactions between neighboring cells (Fig. 1). The binding of ligands to Notch receptors initiates a series of proteolytic cleavages, leading to the release of the Notch intracellular domain (NICD) from the cell membrane [2]. Following its release, the NICD undergoes nuclear translocation, where it forms a transcriptional activation complex with an array of coactivators, among which are Mastermind-like proteins. This assembly catalyzes the upregulation of downstream target genes (Fig. 1) [3, 4, 5].
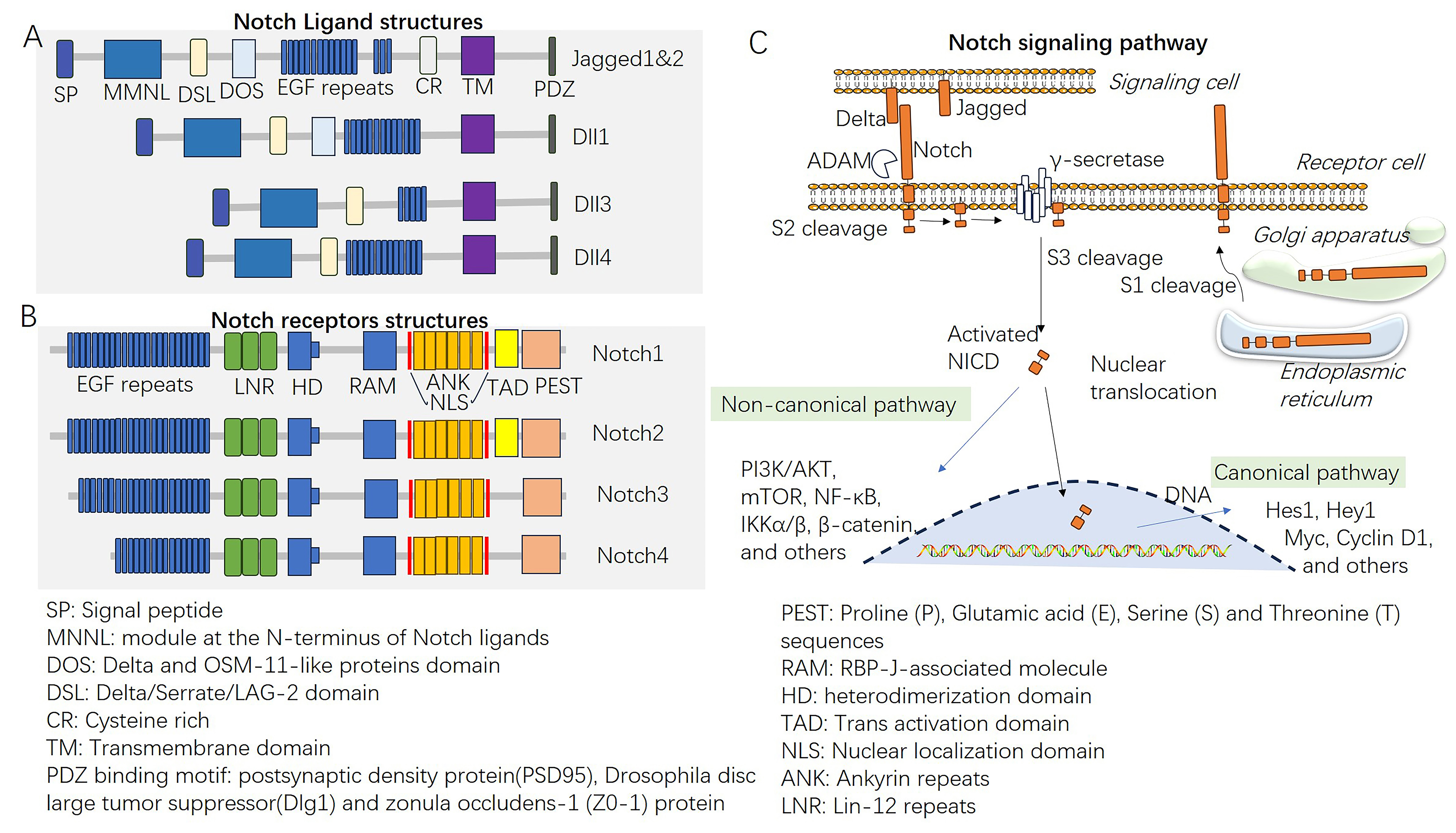 Fig. 1.
Fig. 1.The Notch signaling pathway. (A,B) The structures of Notch ligands and Notch receptors. (C) Overview of the Notch signaling pathway and therapeutic targets. EGF, epidermal growth factor; ADAM, a disintegrin and metalloprotease domain; NICD, Notch intracellular domain; PI3K/AKT, phosphatidylinositide 3-kinases/AKT; mTOR, mechanistic target of rapamycin kinase; NF-
Notch signaling governs cell fate determination in various biological processes throughout development, homeostasis, and disease, and its effects on cell behavior are contingent upon the specific biological context [6]. Notably, researchers have shown pivotal roles for the Notch pathway in cell fate decisions by promoting self-renewal or differentiation of multiple stem cell populations, such as embryonic stem cells (ESCs) [7], pluripotent stem cells (PSCs) [8], hematopoietic stem cells (HSCs) [9], neural stem cells (NSCs) [10], and intestinal stem cells (ISCs) [11] (Fig. 2). Notch signaling maintains stemness by inhibiting differentiation-promoting factors and transcription factors associated with specific lineages [12, 13, 14]. In various cellular contexts, Notch has the capacity to function as both an enhancer and inhibitor of differentiation [11, 15]. The Notch pathway plays an indispensable role in maintaining stem cell niches, the specialized microenvironments that support stem cell functions. Niche components can activate or suppress Notch signaling to control stem cell behavior [16, 17]. In addition, Notch signaling is involved in tissue repair and regeneration processes by enabling stem cell activation and directing stem cell differentiation to replace damaged or lost cells. Notch signaling coordinates stem cell responses during tissue injury, facilitating tissue regeneration in various organs and tissues [18, 19, 20].
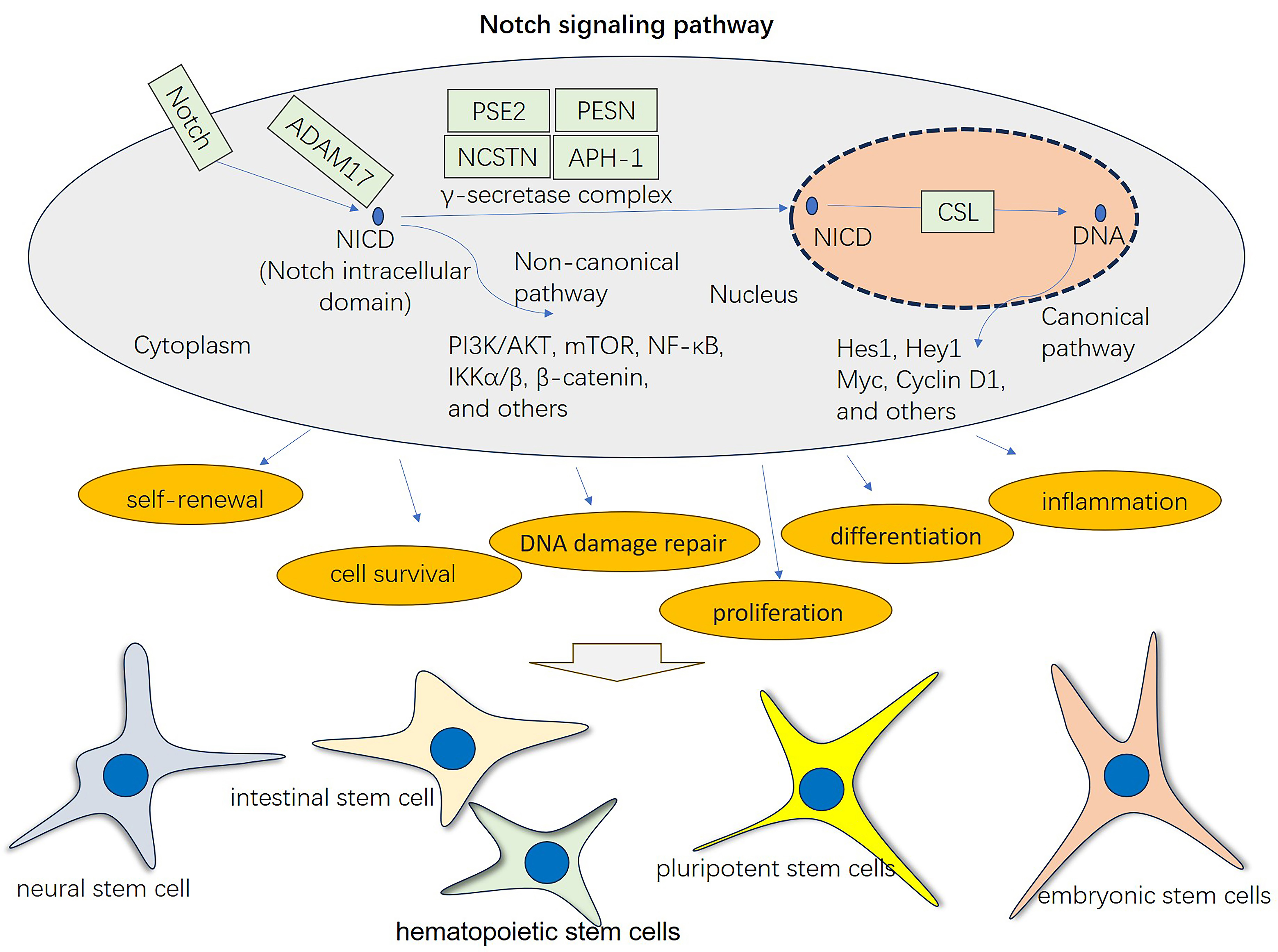 Fig. 2.
Fig. 2.The general function of the Notch signaling pathway in mediating cellular processes and stem cell differentiation. Stem cells can be differentiated into different lineages, such as neural stem cells, intestinal stem cells, hematopoietic stem cells, pluripotent stem cells, and embryonic stem cells. Notch signaling activation through the canonical pathway and noncanonical pathway has a vital role in affecting multiple biological processes, including self-renewal, cell survival, DNA damage repair, proliferation, differentiation, and inflammation. CSL refers to CBF1, Suppressor of Hairless, Lag-1.
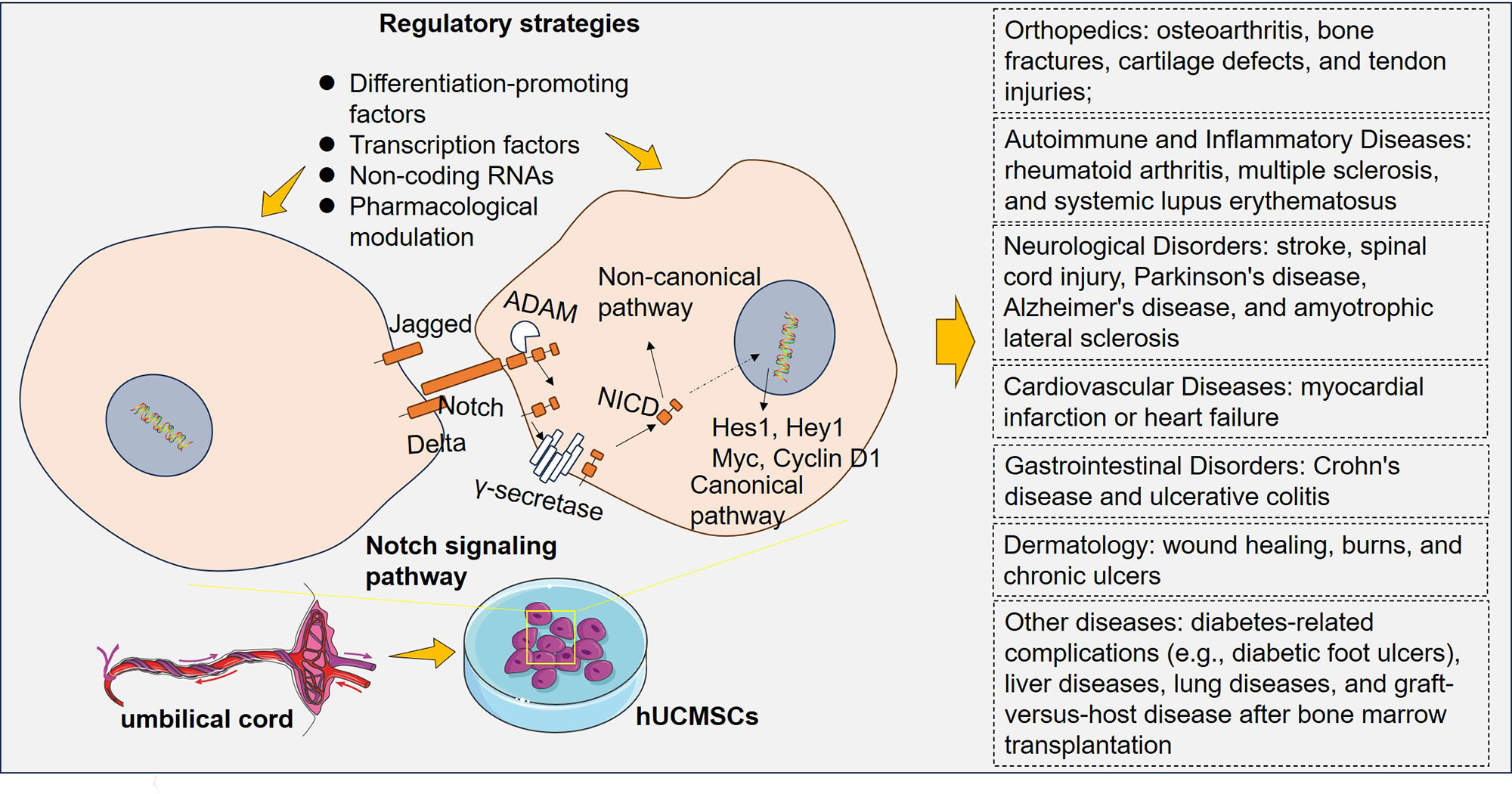
Derived from umbilical cord tissue, human umbilical cord mesenchymal stem cells (hUCMSCs) can undergo differentiation into a wide range of cell types, encompassing osteoblasts, chondrocytes, type II alveolar cells, hepatocytes, and cardiomyocytes [21]. hUCMSCs possess immunomodulatory properties, enabling them to regulate and suppress immune responses [22]. hUCMSCs secrete a multitude of bioactive molecules, including extracellular vesicles, cytokines, and growth factors. These secreted factors can stimulate tissue regeneration, promote angiogenesis, and modulate inflammation, apoptosis, and oxidative stress [23, 24]. hUCMSC therapy holds promise in various medical fields, including tissue repair and regeneration, treating autoimmune and inflammatory diseases, neurological disorders, liver diseases, cardiovascular diseases, lung diseases, diabetic wounds, graft-versus-host disease (GVHD), and cancer [25, 26, 27, 28]. Hence, it is believed that hUCMSCs exhibit greater therapeutic efficacy than MSCs sourced from alternative tissues.
Therefore, understanding the function of Notch signaling within stem cells provides insights into stem cell biology, tissue regeneration processes, and disease pathogenesis. Throughout this review, an up-to-date overview of both established and emerging findings concerning Notch signaling during hUCMSC differentiation is provided. We also discuss potential therapeutic opportunities by regulating Notch signaling to improve hUCMSC-based therapies and develop targeted interventions.
The Notch signaling pathway involves several key mediators (Notch receptors, ligands, the
Several feedback regulators modulate and fine-tune Notch signaling [45]. For example, MyoD, which functions as a master controller of myogenesis, is a basic helix-loop-helix (bHLH) transcription factor. It plays a vital role in activating muscle-specific gene programs and driving the differentiation of myoblasts into mature muscle fibers [46]. Deletion of MyoD by CRISPR/CAS9 leads to brown adipocyte differentiation in myoblasts [47]. Overexpression of Delta1 (a Notch ligand) during early myogenesis inhibits MyoD expression and activity, thereby maintaining myogenic progenitor cells in an undifferentiated state [48]. MyoD functions as an essential mediator of Dll1 expression. Gain- and loss-of-function experiments revealed that MyoD activates the expression of the Notch ligand gene Dll1, resulting in the activation of the Notch pathway and enhanced myogenic differentiation. However, MyoD autonomously inhibits the Notch pathway within cells that express Dll1, promoting a myogenic program and facilitating differentiation [49]. Notch1 can enhance SRY-related high-mobility-group box 2 (SOX2) expression by enhancing the transcription of SOX2 and promoting the invasion of glioma stem cells (GSCs). A positivefeedback loop of the Notch1-SOX2 axis has been found to control GSC invasion [50]. The expression of Notch and Sox2 serve as promising markers for evaluating the differentiation status of NSCs [51]. Moreover, hypoxia-inducible factor 1
Cytokines assume a pivotal function in mediating stem cell differentiation, partly by mediating the Notch signaling pathway [54, 55]. For example, IL-6-mediated Stat3 signaling participates inbasal stem cell differentiation and enhances ciliogenesis by promoting the expression of the multicilin gene and the forkhead box protein J1. Thus, the Notch pathway is inhibited after IL-6/Stat3 pathway activation [56]. Shear stress promotes the endothelial differentiation of stem cells from human exfoliated deciduous teeth, which is accompanied by elevated levels of VEGF, VEGFR2, EphrinB2, DLL4, Notch1, Hey1, and Hey2 [57], suggesting that VEGF is potentially activated. Moreover, VEGF can induce DLL4 expression and activate the Notch/DLL4 signaling pathway. VEGF may exert a positive regulatory influence on the Notch/DLL4 signaling pathway [58]. Transforming growth factor
hUCMSC differentiation involves complex processes. Many transcription factors are expressed at specific levels and are involved in the directional differentiation of hUCMSCs, encompassing the Notch signaling pathway [64]. Positive signals for Notch receptors (Notch 1 and Notch 2) and ligands (Jagged 1 and Delta-like 1) were detected in hUCMSCs. Hu YH et al. [65] reported that overexpression of Notch signaling contributed to reduced insulin gene expression, proinsulin protein expression, and insulin-positive cell percentage in hUCMSCs. Thus, Notch signaling plays a crucial role in governing the differentiation of insulin-producing cells (IPCs) from hUCMSCs, and further suppressing Notch signaling could be an effective strategy for augmenting the quantity of IPCs. However, Venkatesh K and his colleagues reported that the Notch inhibitor DAPT suppressed NSC differentiation from hUCMSCs, accompanied by repression of the Notch intracellular domain [NICD], HES, and HES1. This study suggested that the Notch signaling pathway has an essential role in the derivation of NSCs and their subsequent lineage commitment from hUCMSCs [66]. Therefore, the Notch signaling pathway has dual roles in mediating the differentiation of hUCMSCs.
The Notch signaling pathway can regulate hUCMSC differentiation via a noncanonical mechanism. For example, the overexpression of transcription factor genes (GATA-4 and Nkx 2.5) promotes hUCMSC differentiation into the cardiomyogenic lineage. Within the cardiomyogenic-induced cohort, there was notable overexpression of cardiac-specific genes, including GATA-4, Nkx-2.5, MHC, cTnI,
Recently, several transcription factors, such as SOX2 [50], SOX9 [72] and HIF-1
Novel mediators that affect the Notch signaling pathway have been found to regulate hUCMSC differentiation. Sera from severe burn patients (BPS) can significantly promote hUCMSC activation and proliferation through the enhancement of Notch-1 and Hes-1 expression [77]. 5-azacytidine (5-azac) is a cytidine nucleoside analog with the specific capacity to impede DNA methylation. It is widely used for inducing stem cell differentiation, such as cardiogenic differentiation of murine bone marrow-derived mesenchymal stem cells [78] and chondrogenic differentiation of metabolic syndrome-derived mesenchymal stem cells [79]. hUCMSCs that are treated with 5-AzaC can be differentiated into myocardial cells. Both Notch1 and DLL1 are promoted by 5-azac, suggesting that the DLL4-Notch signaling pathway may be pivotal for 5-azac-induced cardiomyocyte differentiation of hUCMSCs [78]. Valproic acid is an orally active HDAC inhibitor and can activate Notch1 signaling [80]. Valproic acid treatment facilitates myotube formation and cardiomyocyte differentiation of human-elicited pluripotent stem cell-derived mesodermal progenitors (cdMiPs) by activating the Notch signaling pathway [81]. Notably, valproic acid increased hepatic differentiation at the expense of adipogenic differentiation in hUCMSCs by mediating AKT and ERK activation [82], suggesting that valproic acid might affect the differentiation of hUCMSCs via the Notch signaling pathway. Melatonin is a hormone generated by the pineal gland that can activate the melatonin receptor. In an intervertebral disc degeneration model, melatonin enhanced Sirt1 expression and inhibited Notch expression, thus inhibiting M1-type macrophage polarization, oxidative stress, and proinflammatory reactions. Sirt1 can interact with NICD. Therefore, the SIRT1/Notch signaling pathway may be involved in the role of melatonin in mediating M
TGF-
Several microRNAs (miRNAs) have been found to affect hUCMSC differentiation. For example, the cotransfection of miR-106a, miR-574-3p, and miR-451 collectively prompts the differentiation of hUCMSCs into fully functional hepatocytes [95]. Notch1 has been found to negatively regulate miR-451 expression by regulating the transcription factor AP-1 in lung adenocarcinoma [96]. In another study, miR-451 was shown to act as a potent suppressor of oncogenesis in patients with Notch1-induced T-cell acute lymphoblastic leukemia [97]. MiR-410 has inhibitory effects on the direct retinal pigment epithelium differentiation of hUCMSCs [98], and upregulation of miR-410 by tangeretin was shown to lead to decreased Notch-1, Jagged1/2, Hey-1, and Hes-1 expression [99]. Therefore, miR-451 and miR-410 potentially have opposite effects on hUCMSC differentiation by regulating the Notch1 signaling pathway. Additionally, the miR-18b profile was attenuated within the placental tissues of patients with preeclampsia (PE). EV-derived miR-18b boosted the trophoblast proliferation and migration of hUCMSCs by targeting Notch2 [100]. These studies suggest that miRNAs play vital roles in hUCMSC differentiation by targeting the Notch signaling pathway, and additional miRNAs need to be identified in the future.
Notch signaling pathway dysregulation is associated with several diseases and disorders. Aberrant Notch signaling can contribute to cancer development and progression, cardiovascular diseases, neurological disorders, autoimmune diseases, and developmental abnormalities [101, 102]. Interestingly, hUCMSC therapy affects both the canonical and noncanonical Notch signaling pathways (Fig. 3). For example, hUCMSC transplantation improves liver functions in rats with acute-on-chronic liver failure through the inhibition of Notch and Stat1/Stat3 signaling [103]. Activating Toll-like receptor-3 (TLR3) with poly (I:C) (a ligand of TLR3) enhanced the therapeutic efficacy of hUCMSCs against colitis. Poly (I:C) treatment promoted the expression of prostaglandin E
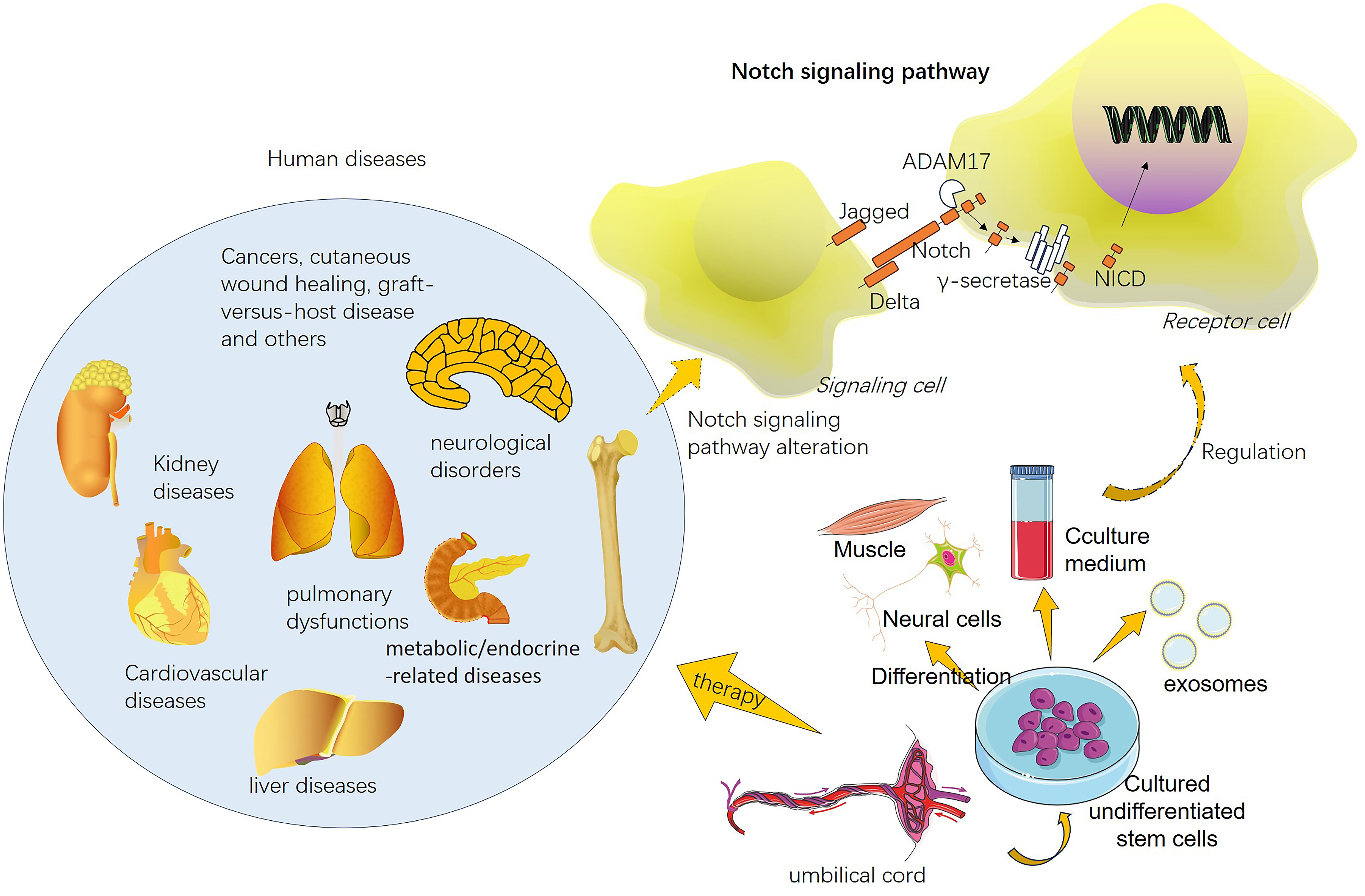 Fig. 3.
Fig. 3.Human umbilical cord mesenchymal stem cells (hUCMSCs) exert therapeutic effects on both the canonical and noncanonical Notch signaling pathways. hUCMSCs have been applied in the treatment of multiple human diseases, such as kidney diseases, cardiovascular diseases, pulmonary diseases, liver diseases, metabolic/endocrine-related diseases, neurological disorders, and cancers. hUCMSC therapy can mediate the activation of Notch signaling in receptor cells via canonical or noncanonical pathways.
MSCs extracted from adipose, umbilical cord, and placental tissues exhibit differences in the secretion of specific factors. The three MSCs abundantly secreted insulin-like growth factor-binding protein (IGFBP)-4, IGFBP-3, tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, IGFBP-6, monocyte chemoattractant protein-1, and granulocyte colony-stimulating factor. Vis-à-vis A-MSCs and P-MSCs, hUCMSCs secrete elevated cytokines, such as HGF, TNF-IR, and TGF-
Stem cell-derived conditioned medium (CM) treatment has been regarded as a practicable approach to overcome limitations such as tumorigenic potential and low survival rates of transplanted cells [116]. CMs consist of soluble proteins such as cytokines, chemokines, enzymes, cell adhesion molecules, signaling and signal transduction proteins, and growth factors; nucleic acids such as DNA, RNA fragments, and microRNAs; and lipids and extracellular vesicles such as apoptotic bodies, exosomes, and microvesicles. The complex combination of soluble components and vesicular elements is known [117, 118]. hUCMSC-derived microvesicles/exosomes show promising therapeutic effects against diseases [119, 120]. Notch signaling has been found to play vital roles in hUCMSC-derived microvesicle/exosome therapy (Fig. 4). For example, exosomes derived from hypoxia-preconditioned MSCs (HP-MSC-DEs) heightened the self-renewal capacity and long-term clonogenic potential of hUCMSCs. Jagged-1 shuttled by HP-MSC-DE stimulates the Notch pathway in hUCMSCs [121]. Extracellular vesicles derived from hUCMSCs can alleviate silica-induced epithelial‒mesenchymal transition (EMT) in fibrosis miR-26a-5p from hUCMSC-derived extracellular vesicles attenuated the activation of the Adam17/Notch signaling pathway, thus mitigating EMT in silica-elicited pulmonary fibrosis [122]. Chen X et al. [123] reported that miR-146a-5p delivered by hUCMSC-derived exosomes significantly relieved bleeding and inflammation and diminished M1 macrophage polarization in a diffuse alveolar hemorrhage (DAH) mouse model. Notch1 was targeted and negatively regulated by miR-146a-5p. Thus, hUCMSC-derived exosomes reversed Notch1 signaling pathway-induced DAH.
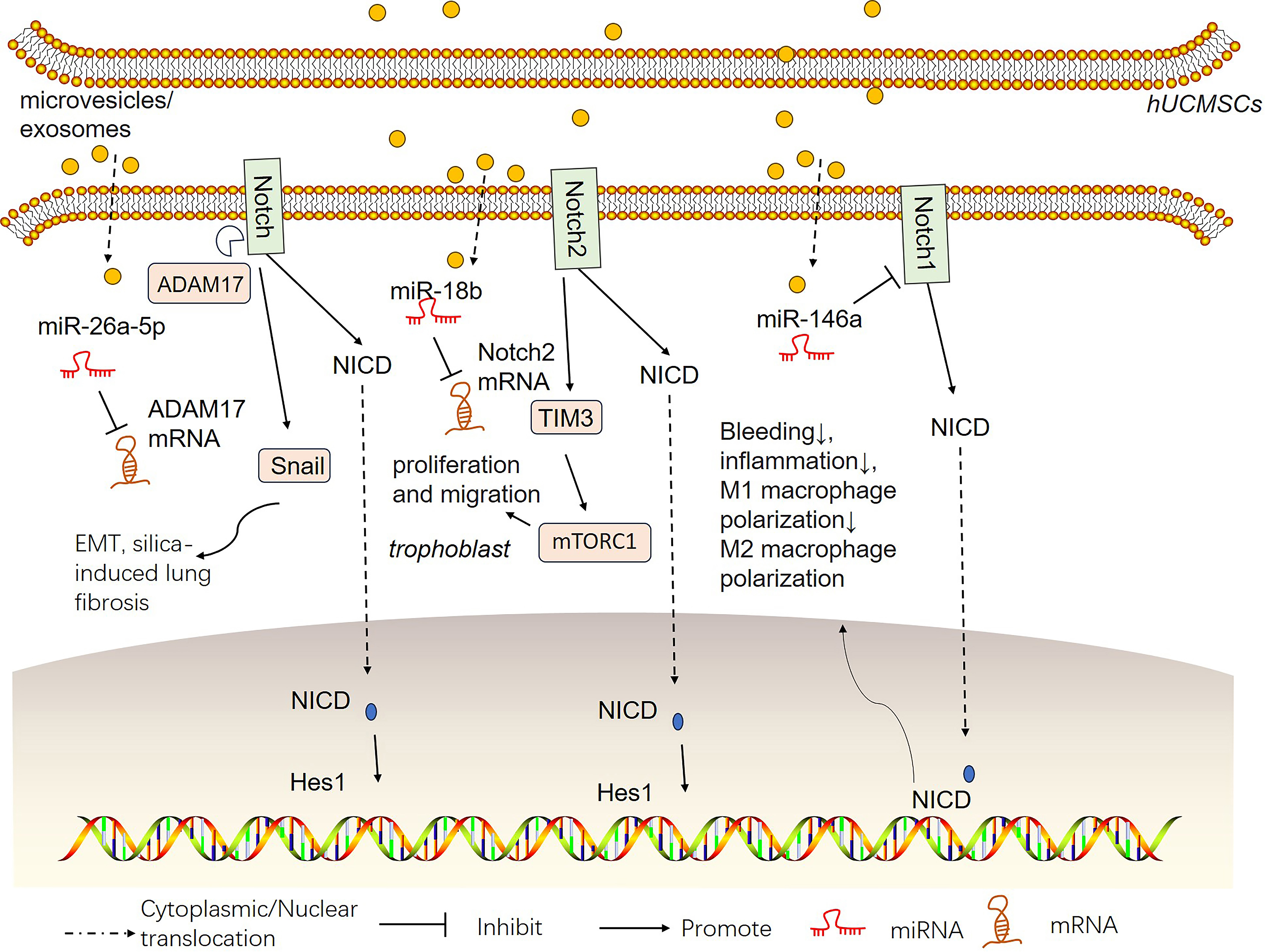 Fig. 4.
Fig. 4.hUCMSC-derived microvesicles/exosomes affect the Notch signaling pathway in different pathological environments. hUCMSCs can produce microvesicles or exosomes. microRNAs (miRNAs) shuttled by these proteins have therapeutic effects on different pathological environments.
In this context, we have outlined the latest developments pertaining to the role of the Notch signaling pathway in preserving homeostasis and dictating the cellular fate of hUCMSCs, the factors influencing hUCMSC differentiation through the Notch signaling pathway, and the changes and mechanisms of the Notch signaling pathway in hUCMSC therapy. In the future, there is a need for a more extensive understanding of the biological role of Notch signaling in hUCMSC therapy.
Stem cell-based therapies offer new prospects for addressing a range of challenging-to-treat ailments. Compared with other stem cell sources, hUCMSC therapy is considered relatively safe and ethical. Nonetheless, comprehensive research and carefully structured clinical trials are imperative to gain a comprehensive grasp of the therapeutic efficacy, ideal dosage, long-term safety, and effectiveness of these agents [124, 125]. Moreover, there is a need to maintain cell viability and function during continuous cell passaging [126]. Efficient and controlled differentiation of hUCMSCs into specific cell lineages is challenging. The optimization of differentiation protocols and identification of factors that stimulate lineage-specific differentiation need to be further investigated to improve therapeutic outcomes. Therefore, the differentiation of hUCMSCs holds great promise for future advancements in regenerative medicine and tissue engineering. Further advancements in the differentiation of hUCMSCs are expected, which require continued research, optimization of protocols, and exploration of novel applications.
The Notch signaling pathway is critical for deciphering the intricate mechanisms underlying its functions in cell fate decisions, development, and disease. The interplay among these mediators determines that cellular responses and disease outcomes are influenced by Notch signaling. Mediating the Notch signaling pathway through pharmacological targeting and genetic targeting holds the potential for manipulating hUCMSC behavior and directing their differentiation for various applications in regenerative medicine and disease modeling. However, challenges remain since therapeutic interventions targeting Notch signaling might lead to significant toxicity in vivo. Additionally, nonspecific intervention via Notch signaling can yield contrasting outcomes in terms of disease management due to the presence of various Notch mediators within hUCMSCs. For instance, poly (I:C) treatment activates TLR3 and enhances the adipogenic differentiation capability [127] and immunosuppressive properties [104] of UCMSCs by activating the Notch1 signaling pathway. The transcription factor NF-
WHX, JXY, LY and CY conceptualized this review. WHX drafted the manuscript. YZ, YQC, XP, and CY generated the figures. WHX reviewed and edited the language. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors read and approved the final manuscript. All authors contributed to editorial changes in the manuscript.
Not applicable.
Not applicable.
This study is supported by the Key R&D Project in Sichuan Province in 2023 (No. 2023YFS0062).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.




