1 Periyar College of Pharmaceutical Sciences, 620021 Tiruchirappalli, Tamil Nadu, India
2 Karthikeyan Child Development Unit, Sri Ramachandra Institute of Higher Education and Research, 600116 Chennai, Tamil Nadu, India
3 SRM College of Pharmacy, SRM Institute of Science and Technology, 603203 Kattankulathur, Chennai, Tamil Nadu, India
4 Faculty of Data Science and Information Technology, INTI International University, 71800 Nilai, Malaysia
5 School of Pharmacy, KPJ Healthcare University, 71800 Nilai, Malaysia
6 Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, 60115 Surabaya, Indonesia
7 Department of Medical Sciences, School of Medical and Life Sciences, Sunway University, 47500 Sunway City, Malaysia
8 PAP Rashidah Sa’adatul Bolkiah Institute of Health Sciences, Universiti Brunei Darussalam, BE1410 Gadong, Brunei Darussalam
Abstract
Literature indicates that L-carnosine may be deficient in autism spectrum disorder (ASD) children. The aim of the present study was to estimate the level of L-carnosine in plasma and correlate it with the Autism Treatment Evaluation Checklist (ATEC) and Childhood Autism Rating Scale 2nd Edition, Standard Version (CARS2-ST) scores. To measure L-carnosine level, a bio-analytical method was developed using reverse phase high- liquid chromatography and validated as per International Conference on Harmonization guidelines.
Children were supplemented with L-carnosine (10–15 mg/kg) along with standard care therapies for 2 months. Before and after supplementation, scores on the ATEC, CARS2-ST, BEARS sleep screening tool, 6-item Gastrointestinal Severity Index, and Parental Stress Scale were evaluated, and L-carnosine was measured at the end of the trial.
The calibration curve was linear in the range of 100–600 ng/mL (R2 = 0.998). The level of L-carnosine quantified was 33.7 ± 0.2 ng/mL. There was no significant difference found in any of the outcome measures (p > 0.05).
Despite the fact that L-carnosine is detectable in the blood, it was found to be ineffective in the management of ASD in children.
The study was registered in the Clinical Trial Registry-India, registration number: CTRI/2019/07/020102.
Keywords
- autism spectrum
- carnosine
- HPLC
- occupational therapy
- early childhood education
- psychological well-being
Autism spectrum disorder (ASD) is a complex developmental disability characterized by difficulties with social communication, interaction, and restricted or repetitive behaviours [1]. The rising frequency of ASD in many countries, with an estimated 0.5–1% of children affected globally and no known solution, has posed a challenge to health-care systems [2]. Conventional therapies such as occupational, speech, and behavioral therapies strive to alleviate the disorder’s symptoms, but the high cost of these therapies encourages caregivers to seek out complementary and alternative medicine (CAM) [3, 4]. People feel that CAM, particularly nutritional supplements, has a higher safety profile than pharmacological therapies; hence, its use has expanded in recent years [5]. Children with ASD may benefit from CAM alone or in combination with conventional therapy to improve their quality of life [6, 7].
L-carnosine, a dipeptide, is found in excitable tissues such as skeletal
muscles, the brain, myocardium and is made up of two amino acids,
L-carnosine has been used in the treatment of ASD children but has yielded mixed results. In children with ASD, L-carnosine has improved receptive speech, socialization, and behavior [17]. In contrast to these findings, studies have reported that L-carnosine has no effect on the severity of autism [18, 19]. Supplementing L-carnosine in ASD children decreased sleep disturbances [18]; however, no such findings were seen in a study reported by Ann Abraham et al. [19]. When provided as an adjunctive therapy to risperidone, Hajizadeh-Zaker et al. [20] observed an improvement in hyperactivity and non-compliance. Hyperactivity is one of the side effects of L-carnosine, according to Abraham et al. [21].
Researchers found lower plasma levels of L-carnosine in ASD children [22, 23, 24]. There is a lack of data on the relationship between L-carnosine plasma levels and clinical outcomes in ASD children. As a result, the present study aimed to determine whether there is a correlation between the concentration of plasma L-carnosine and its efficacy in terms of the Childhood Autism Rating Scale, Second Edition, Standard Version (CARS2-ST) and Autism Treatment Evaluation Checklist (ATEC) scores in children with ASD. The investigation also developed and validated an analytical method for estimating plasma L-carnosine in children with ASD.
A prospective, single-group interventional trial was conducted from December 2020 to July 2021 at the Karthikeyan Child Development Unit (KCDU) and VidhyaSudha, Sri Ramachandra Learning Centre for Children with Special Needs, Sri Ramachandra Institute of Higher Education and Research (SRIHER), Chennai, Tamil Nadu, India. The study comprised children aged 3 to 6 years who were diagnosed with mild to moderate ASD as per the Childhood Autism Rating Scale, Second Edition, Standard Version (CARS2-ST) [25]. Children who were on any type of chronic drug treatment, had a had a history of seizures, severe intellectual disabilities, genetic and chromosomal syndromes, or any other chronic illness were excluded.
The study enrolled the children based on the selection criteria. The children received a dose of 10–15 mg/kg of L-carnosine syrup (Cognicare, Brio Bliss Life Sciences Pvt. Ltd, Alwarpet, Chennai, Tamil Nadu, India) in 2 divided doses (one in the morning and one at night) for 2 months, in addition to their standard therapies such as occupational and speech therapy [19]. Prior to enrollment, parents were informed that they should bring their child to the hospital on day 61 after taking the morning dose to collect blood samples at the 68th minute [26]. On day 60, parents received a reminder call. We collected blood samples into 5 mL EDTA tubes. We centrifuged the blood samples at 12,800 rpm for 5 mins to separate the plasma. We transferred them into airtight containers and stored them at –20 °C until our analysis.
L-carnosine (purity
The assay was performed using Reversed-Phase High-Performance Liquid
Chromatography (RP-HPLC) with Agilent 1220 Infinity II. The system contained
binary pump for supplying of solvents, an automated injector, with
Photodiode-Array (PDA) detector. Chromatographic separation was performed on
Agilent C18 column 150
Stock solutions of L-carnosine at concentration levels of
1000 µg
A series of 10 mL volumetric flaskscontained appropriate aliquots of the drug pipetted out from the standard stock solution. A set of solutions for L-carnosine of concentration 100, 200, 300, 400, 500, and 600 ng/mL was made up to the volume with the mobile phase. The preparation of triplicate dilutions of each drug concentration was doneseparately. 10 µL injections of each drug concentration were injected into the HPLC system twice separatelyfrom these triplicate solutions and chromatographed under the optimal conditions. Drug evaluation was performed with the PDA detector set at 210 nm and the peak areas were documented.
The marketed formulation of L-carnosine (syrup Cognicare) which contains 100 mg in 5 mL wasextracted with mobile phase. This wasthen diluted with mobile phase to obtain the sample solution within the Beer Lambert’s range of the drug solution. Triplicate sample solutions at concentrations 300 ng/mL, 400 ng/mL and 500 ng/mL of L-carnosine were prepared. Under the optimized chromatographic conditions, a 10 µL volume of each solution was injected into the sample injector of HPLC.
The standard solution of L-carnosine was used to determine the precision of the method. The degree of precision of the developedmethod was studied by preparing six replicates of standard solution of concentration 300 ng/mL.
Liquid-liquid extraction technique was used to extract plasma samples. A 200
µL aliquot of plasma sample in radio-immuno assay (RIA) vial was
vortexed for 10 secs and extracted on a Vibramax100
(plate shaker, Heidolph Instruments, Schwabach,
Germany) by vortex mixing 2 mL of ethyl acetate (Rankem, Haryana, India) for 4
mins for the determination of L-carnosine in plasma. The centrifugation of the
contents took place at 2000 rpm for 4 mins using a multifuge (Multifuge 3L;
Heraeus, Osterode, Germany) at 22
Changes in CARS2-ST, Autism Treatment Evaluation Checklist (ATEC), BEARS sleep
screening tool, 6-item Gastrointestinal Severity Index (6-GSI), and Parental
Stress Scale (PSS) scores at the beginning (day 0) and end of the trial (day 61)
assessed treatment efficacy. CARS2-ST, a 15 item rating scale used for the
identification and classification of severity in children with ASD was completed
by the clinical psychologist [29]. ATEC assesses the severity of ASD following
treatment and contains 77 items which are scored from 0–2 scale in the
speech/language/communication, sociability, and sensory/cognitive awareness
subscales and 0–3 scale in the health/physical/behavior subscale. ASD children
were classified as mild (20–49), moderate (50–79), and severe (
BEARS sleep screening tool is used to determine sleep problems in preschool children (2–5 years), school children (6–12 years) and adolescents (13–18 years) [32, 33]. The tool assesses the following parameters: Bedtime problems (B), Excessive daytime sleepiness (E), Awakening during night (A), Regularity and duration of sleep (R), and Snoring (S). Gastrointestinal problems were assessed using 6-item Gastrointestinal Severity Index (6-GSI) where a higher score indicated severity [34]. Children who had gastrointestinal problems at the start of the study were only included. Parental Stress Scale (PSS) is an 18-item tool to measure parent stress in relationship with their child. It ranges with a score of 18–90 with higher scores indicating high level of stress [35].
The Statistical Package for the Social Sciences (SPSS) version 22 (SPSS, Inc. Chicago, IL, USA) was used to conduct statistical analysis. Quantitative and qualitative data were expressed as mean (standard deviation) and frequency (percentage), respectively. Pre and post differences in L-carnosine therapy were compared using paired t-test. A p-value of less than 0.05 was considered statistically significant. GraphPad Prism, version 9.0.2 (Dotmatics, Boston, MA, USA) was used to study the correlation between the plasma concentration of L-carnosine and CARS2-ST and ATEC scores.
Thirty one children were recruited in the study and all of them had completed the study and their demographic characteristics are shown in Table 1.
| Variables | N = 31 | |
| Age in years, mean (SD) | 4.5 (0.5) | |
| Gender, n (%) | ||
| Male | 21 (68) | |
| Female | 10 (32) | |
| Birth order, n (%) | ||
| First | 22 (71) | |
| Second and above | 9 (29) | |
| Gestation, n (%) | ||
| Term | 26 (84) | |
| Preterm | 5 (16) | |
| Birth weight in kg, mean (SD) | 2.8 (2.1) | |
| Anthropometrics, mean (SD) | ||
| Height in m | 1.2 (2.2) | |
| Weight in kg | 18.0 (3.1) | |
| BMI in kg/m2 | 12.5 (4.7) | |
| Nutritional status, n (%) | ||
| Underweight | 6 (19) | |
| Normal | 25 (81) | |
| Autism onset, age in years, mean (SD) | 2.6 (1.2) | |
| CARS2-ST, total score, mean (SD) | 32.7 (2.2) | |
SD, Standard deviation; BMI, body mass index; CARS2-ST, Childhood Autism Rating Scale, Second Edition - Standard Version.
RP-HPLC method to measure plasma L-carnosine in ASD children was developed and validated as per the ICH guidelines with the following parameters.
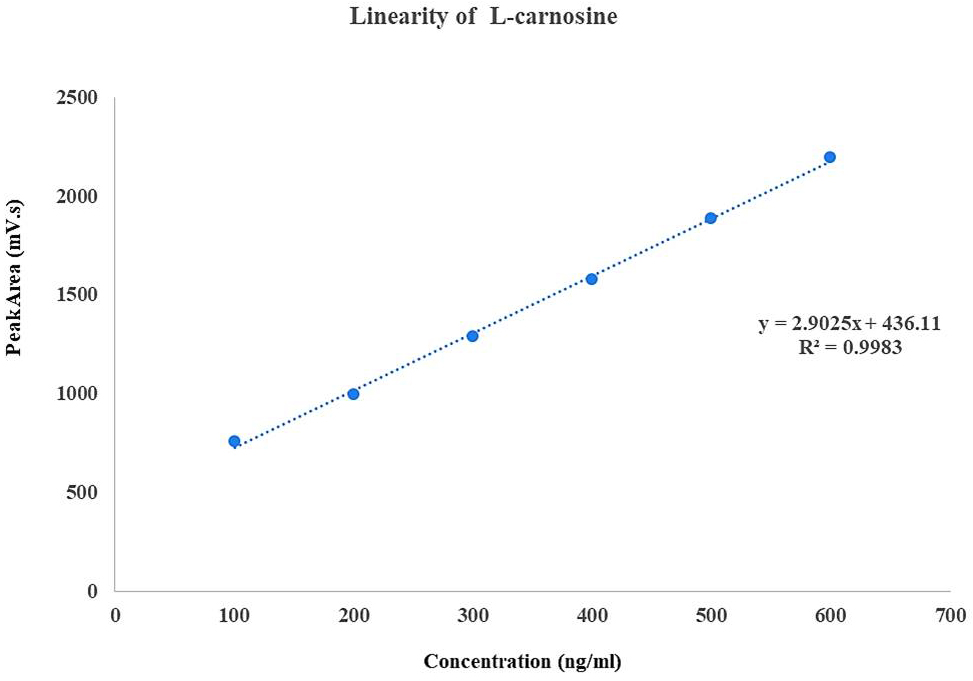
A calibration curve was plotted by taking concentration on x-axis and peak area on y-axis (Fig. 1). The method was found to be linear with concentration in the range of 100–600 ng/mL with correlation coefficient R2 = 0.998. The limit of detection (LOD) and limit of quantification (LOQ) were 3.7 ng/mL and 11.4 ng/mL respectively.
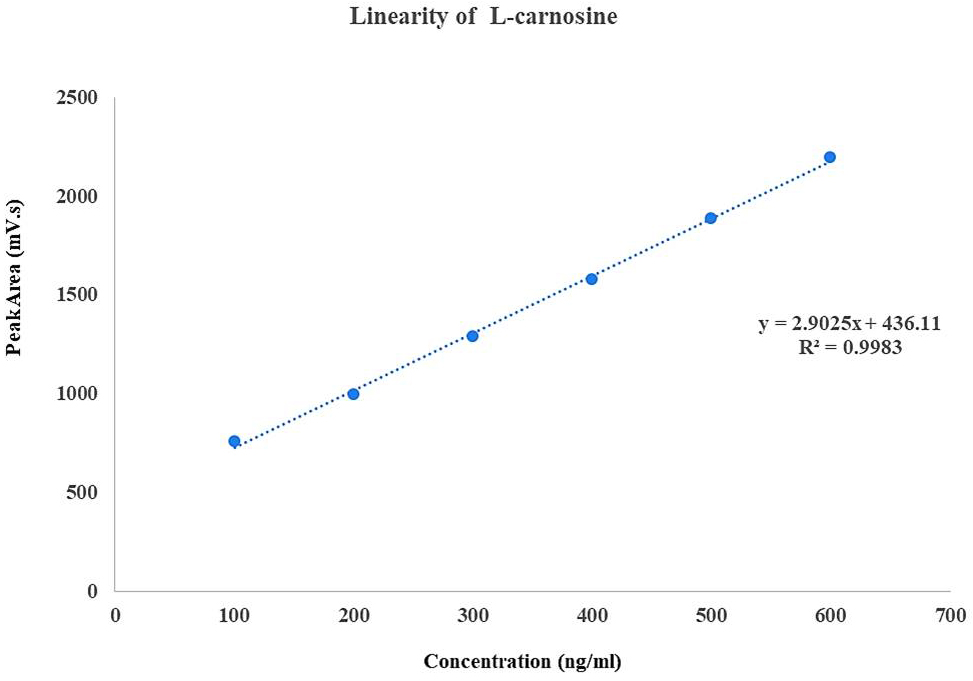 Fig. 1.
Fig. 1.
Calibration curve for L-carnosine concentration versus peak area.
The amount and percentage purity were calculated and tabulated in Table 2. The estimated percentage purity ranged from 99.79% to 99.97% which confirms the method accuracy.
| Concentration (ng/mL) | Area (mV.s) | Amount (ng/mL) | Average (ng/mL) | % purity |
| 300 | 1299.8 | 302.32 | 299.93 | 99.97 |
| 1292.2 | 300.55 | |||
| 1276.5 | 296.90 | |||
| 400 | 1568.2 | 397.49 | 399.19 | 99.79 |
| 1567.1 | 397.21 | |||
| 1589.5 | 402.89 | |||
| 500 | 1882.3 | 498.22 | 499.88 | 99.97 |
| 1887.9 | 499.70 | |||
| 1891 | 500.52 |
The intra-day and inter-day precision obtained was shown in Table 3. The standard deviation (SD) and relative standard deviation (RSD) was low which proves the precision of the method.
| N = 6 (300 ng/mL) | Concentration (ng/mL) | ||
| Day 1 | Day 2 | ||
| Morning | Evening | Morning | |
| 1 | 299.85 | 299.80 | 299.29 |
| 2 | 299.59 | 299.05 | 299.69 |
| 3 | 299.93 | 299.72 | 299.72 |
| 4 | 299.99 | 299.43 | 299.96 |
| 5 | 299.60 | 299.44 | 299.88 |
| 6 | 299.08 | 299.52 | 299.90 |
| Mean | 299.67 | 299.49 | 299.74 |
| SD | 0.33 | 0.26 | 0.24 |
| % RSD | 0.11 | 0.08 | 0.08 |
SD, Standard deviation; RSD, Relative Standard Deviation.
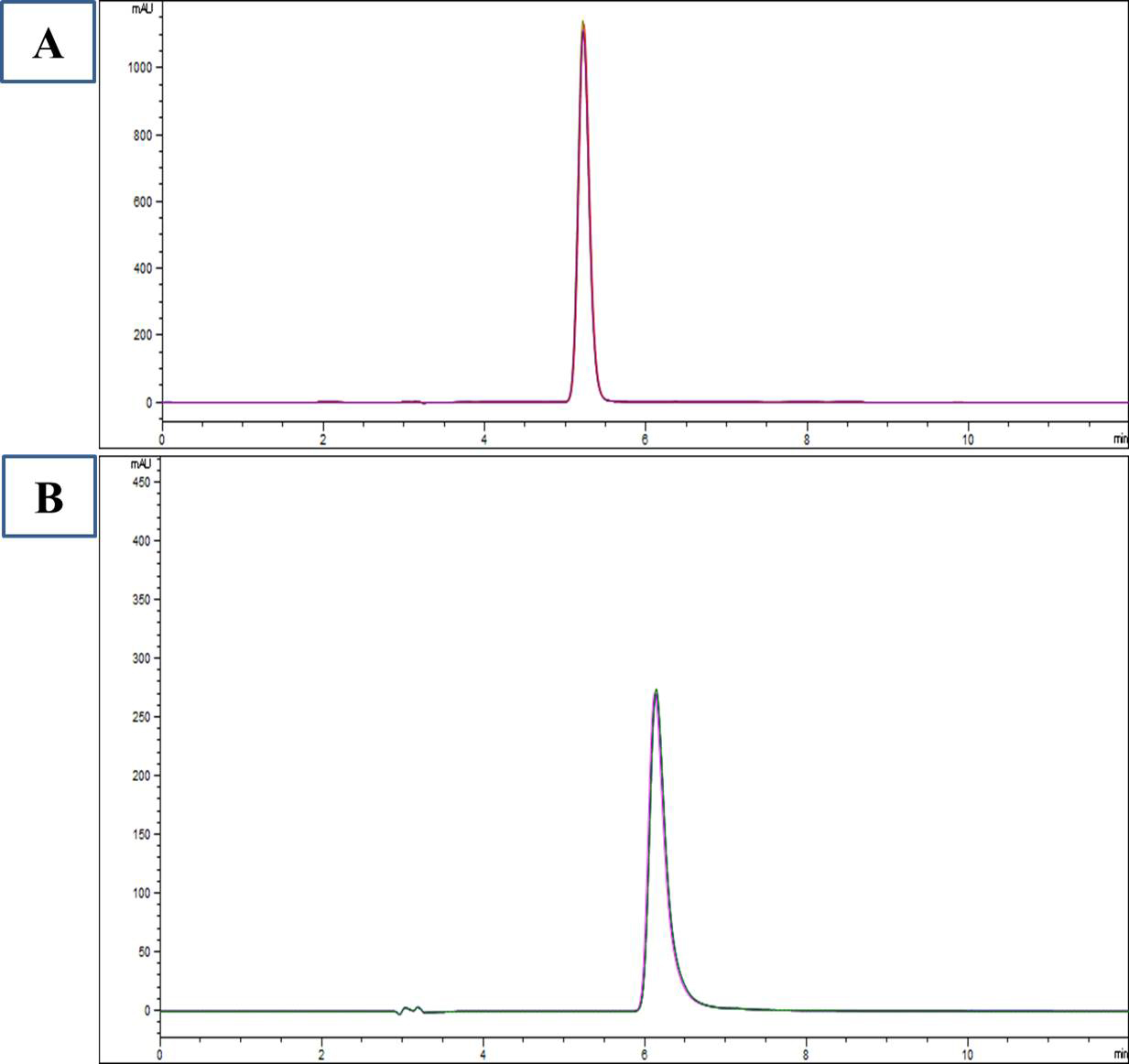
Plasma L-carnosine after supplementation of L-carnosine in ASD children was 33.7
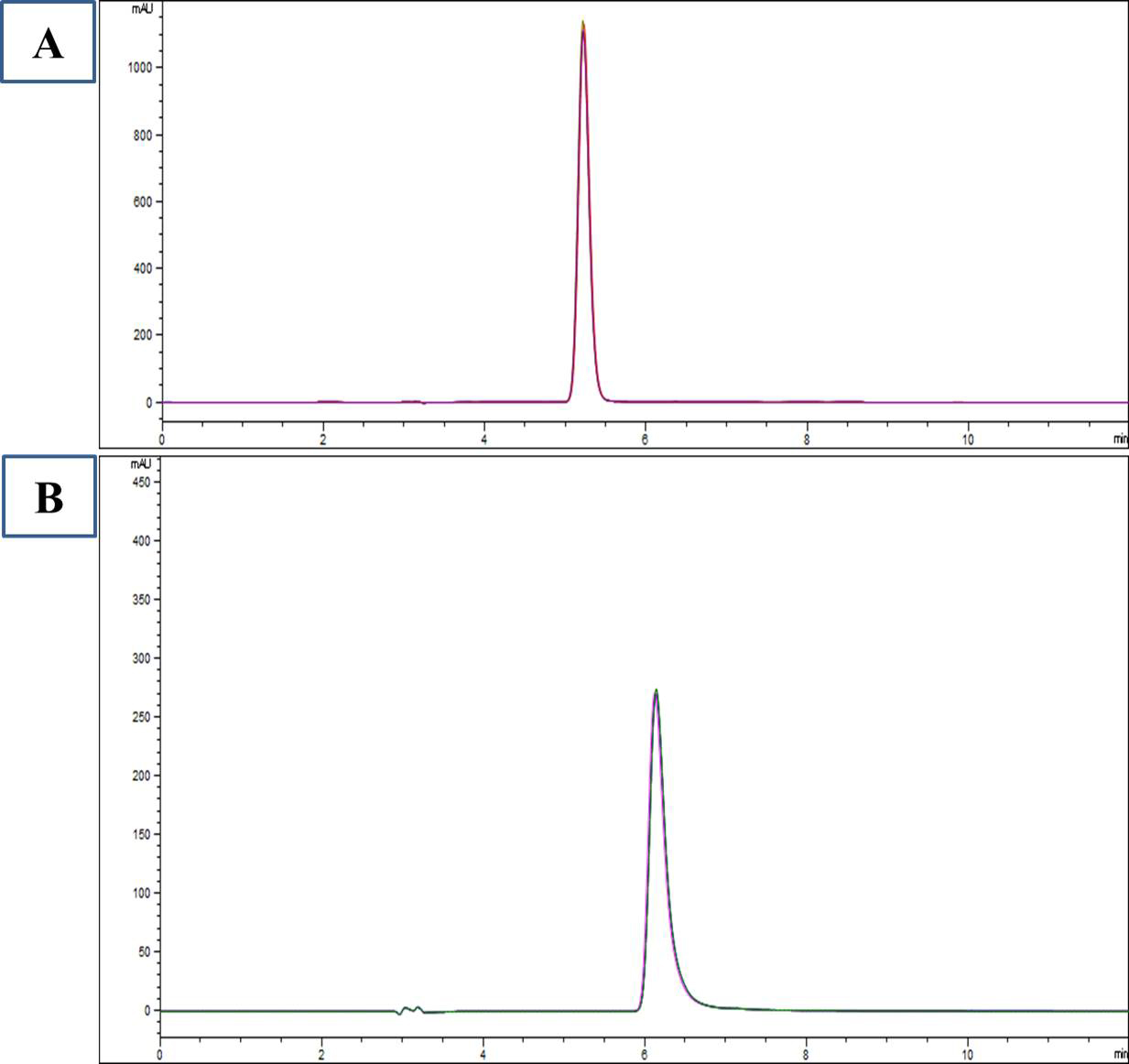 Fig. 2.
Fig. 2.
HPLC chromatogram of standard L-carnosine (A) and plasma L-carnosine after supplementation (B).
No significant change was observed before and after therapy in outcome measures
of CARS2-ST (p
| Assessments | Baseline (day 0) | End of study (day 60)* | |
| CARS2-ST | |||
| Relating to people | 1.8 (1.2) | 1.7 (1.1) | |
| Imitation | 2.2 (1.0) | 2.1 (1.3) | |
| Emotional response | 1.7 (1.1) | 1.6 (0.9) | |
| Body use | 1.9 (1.1) | 1.8 (1.0) | |
| Object use | 2.1 (1.3) | 1.9 (1.2) | |
| Adaptation to change | 2.1 (1.0) | 1.8 (1.1) | |
| Visual response | 2.2 (0.9) | 2.1 (1.1) | |
| Listening response | 1.8 (1.2) | 1.7 (0.9) | |
| Taste, smell, touch | 1.8 (1.1) | 1.6 (0.9) | |
| Fear or nervousness | 2.2 (1.3) | 2.1 (1.2) | |
| Verbal communication | 3.0 (1.1) | 2.9 (1.2) | |
| Nonverbal communication | 1.7 (1.0) | 1.6 (1.0) | |
| Activity level | 2.3 (1.4) | 2.5 (1.0) | |
| Intellectual response | 2.1 (1.6) | 1.5 (1.1) | |
| General impression | 2.4 (1.5) | 2.2 (1.3) | |
| Total CARS2-ST score | 33.2 (2.4) | 32.7 (2.2) | |
| ATEC | |||
| Speech communication | 9.5 (3.5) | 9.3 (3.2) | |
| Sociability | 10.2 (4.2) | 10.0 (3.8) | |
| Sensory/Cognitive awareness | 10.8 (3.2) | 10.5 (2.5) | |
| Health/Physical/Behaviour | 17.0 (5.8) | 16.2 (5.2) | |
| Total ATEC score | 47.5 (10.5) | 46.0 (9.0) | |
Data expressed as mean (SD); *p
CARS2-ST, Childhood Autism Rating Scale, Second Edition - Standard Version; ATEC, Autism Treatment Evaluation Checklist.
| Assessments | Baseline (day 0) | End of study (day 60)* | ||
| BEARS, n (%) | ||||
| Bedtime problems | ||||
| Present | 2 (6.4) | 6 (19.3) | ||
| Absent | 29 (93.5) | 25 (80.6) | ||
| Excessive daytime sleepiness | ||||
| Present | 0 | 0 | ||
| Absent | 31 (100) | 31 (100) | ||
| Awakening during the night | ||||
| Present | 2 (6.4) | 4 (12.9) | ||
| Absent | 29 (93.5) | 27 (87.0) | ||
| Regularity and duration of sleep | ||||
| Present | 3 (9.6) | 5 (16.1) | ||
| Absent | 28 (90.3) | 26 (83.8) | ||
| Snoring | ||||
| Present | 1 (3.2) | 1 (3.2) | ||
| Absent | 30 (96.7) | 30 (96.7) | ||
| 6-GSI, mean (SD) (n = 10) | ||||
| Constipation | 1.0 (0.4) | 1.0 (0.3) | ||
| Diarrhoea | 0.2 (0.1) | 0.2 (0.2) | ||
| Average stool consistency | 0.4 (0.3) | 0.4 (0.2) | ||
| Stool smell | 0.3 (0.2) | 0.3 (0.1) | ||
| Flatulence | 0.6 (0.4) | 0.6 (0.3) | ||
| Abdominal pain | 0 | 0 | ||
| Severity score | 2.5 (1.2) | 2.5 (1.1) | ||
| PSS, mean (SD) | 35.7 (5.4) | 34.2 (4.7) | ||
*p
6-GSI, 6-item Gastrointestinal Severity Index; PSS, Parental Stress Scale.
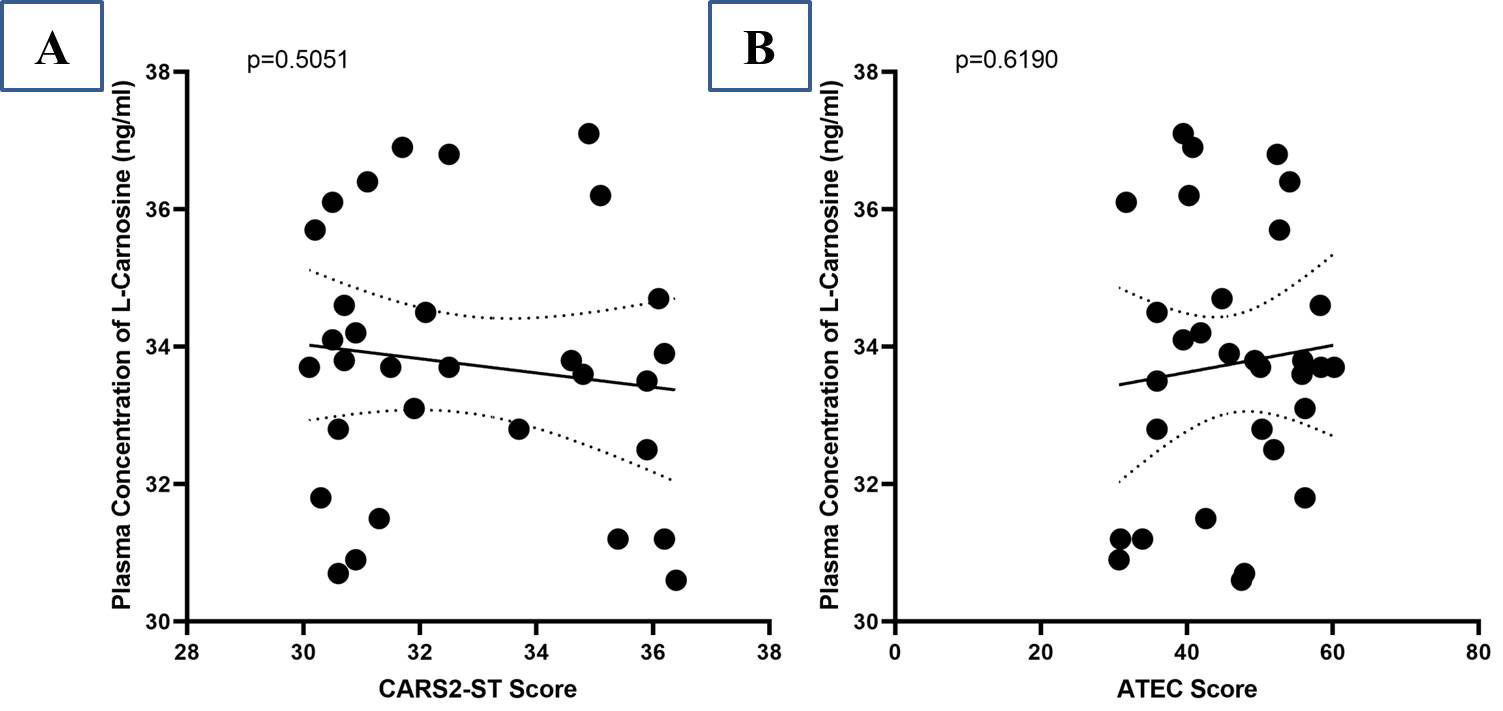
A negative correlation between the plasma concentration of L-carnosine and the reduction rate of CARS2-ST scores was found after treatment (r = –0.1244) but no statistically significant relationship was observed (p = 0.5051) (Fig. 3A). Similarly, at the termination of the study, there was no significant correlation (p = 0.6190) between L-carnosine plasma concentration and ATEC score (r = –0.0929) (Fig. 3B).
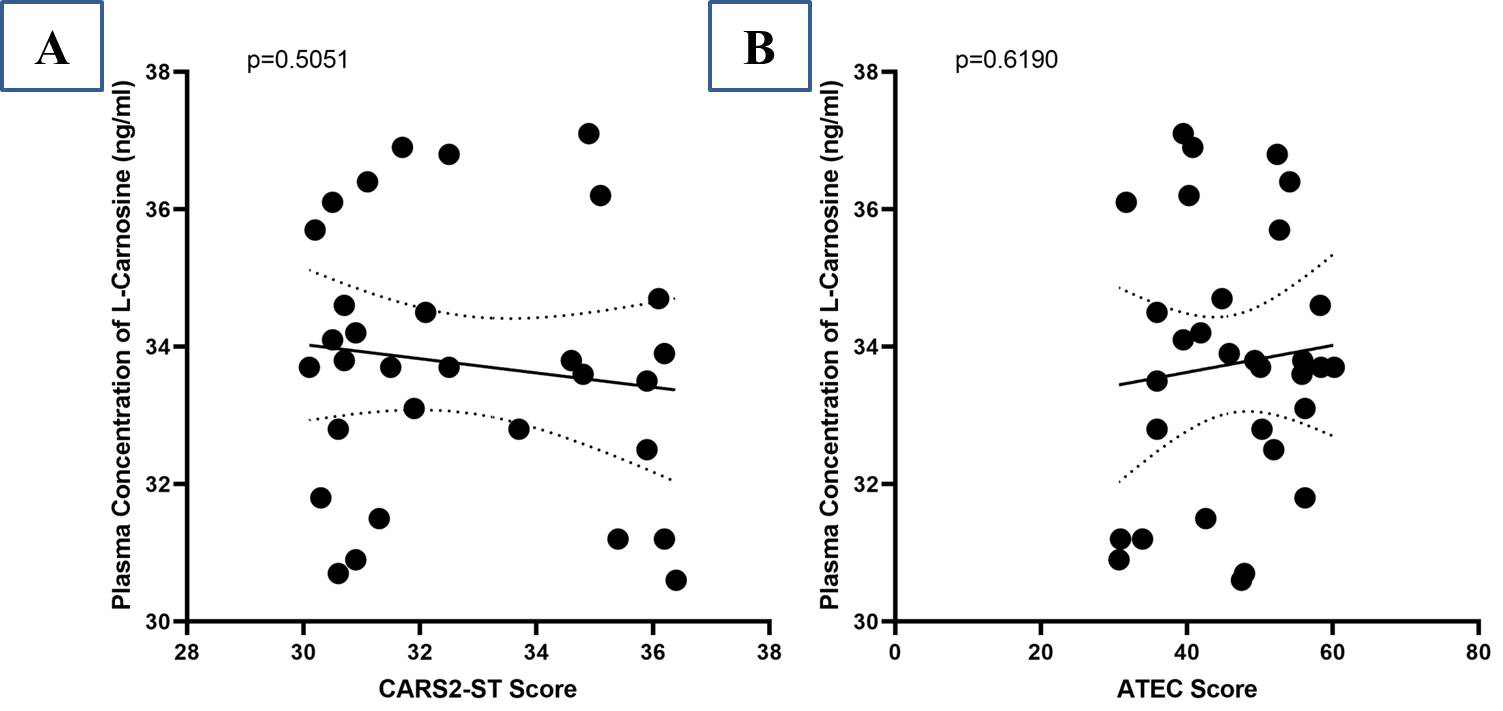 Fig. 3.
Fig. 3.
Correlation between plasma concentration of L-carnosine and CARS2-ST score (A); Autism Treatment Evaluation Checklist (ATEC) score (B).
Thirty one ASD children with an age range of 3–6 years were enrolled in this study. Males were more compared tofemales, which are concordant with other studies [19, 36, 37] (Table 1). Children with ASD usually experience amino acid deficiencies due to food selectivity, impaired digestion or low protein content foods in diet [38, 39]. Diet containing meat has been proposed to be the main source of histidine dipeptides [40, 41]. Research indicates that L-carnosine is found to be deficient in children with ASD compared to neurotypical children [22, 23, 24]. This has prompted the use of L-carnosine in ASD. Literature reports improvement of ASD symptoms upon supplementation with L-carnosine but the level of circulating carnosine is presumably low in these studies [17, 20].
A simple, fast, and accurate bio-analytical method for the measurement of plasma L-carnosine using RP-HPLC was developed and validated (Tables 2,3). Different methods have been used for the estimation of L-carnosine, such as ion-exchange chromatography [22], high-performance liquid chromatography (HPLC) [42], and liquid chromatography-mass spectroscopy (LC-MS) [41], which require expensive detectors such as mass spectroscopy, complex sample extraction procedures such as solid-phase extraction, and large retention times. The proposed method, when compared to other published methods, permitted rapid analysis of samples with a shorter runtime of 12 mins and a retention time of 5–6 mins and used less complex procedures such as liquid-liquid extraction (Fig. 1).
The study revealed plasma levels of L-carnosine after supplementation with L-carnosine but resulted in no improvement in ASD symptoms as measured by CARS2-ST and ATEC (Fig. 2). Autism severity in terms of CARS2-ST and ATEC scores had no significant changes, which is consistent with the results of Mehrazad-Saber et al. [18] and Ann Abraham et al. [19] (Table 4). BEARS sleep screening tool and 6-GSI had no significant changes before and after supplementation with L-carnosine, which is comparable with the results of Ann Abraham et al. [19]. Parental stress, as measured by PSS, had no significant changes with L-carnosine supplementation (Table 5). There was no significant correlation between plasma concentrations of L-carnosine and CARS-2ST scores as well as ATEC scores (Fig. 3).
Animal studies have shown L-carnosine supplementation ameliorates deficits in social recognition in CD157KO mice. Supplementation with L-carnosine improved social deficits in CD157KO mice through an increase in oxytocin concentration in cerebrospinal fluid [43]. The carnosinase enzyme is not expressed in rodents, which may be the reason for the stability of L-carnosine in rats [41, 44]. However, studies conducted on children with ASD have shown inconsistent results [21]. This may be due to the presence of the carnosinase-1 enzyme, which degrades L-carnosine in humans. Ghodsi et al. [45] reported no change in oxidative stress markers, i.e., advanced glycation and lipoxidation end products (AGEs and ALEs), upon supplementation with L-carnosine in ASD children, which may be due to the hydrolysis of carnosine by carnosinases or rapid absorption into tissues.
Various doses of L-carnosine used in other studies are summarized in Table 6 (Ref. [17, 18, 19, 20, 45]). A high dose of L-carnosine (60 mg/kg) has shown contrasting results in two studies with the presence of plasma carnosine up to 1 hour after supplementation [46] as well as no detection of carnosine in plasma [41, 47, 48] supplemented 4 g and 450 mg of carnosine, respectively, in healthy adults but were not able to detect L-carnosine in plasma. This may be due to methodological variations, varying doses, and differences in population. Serum carnosinase activity increases with age up to about 15 years and was undetectable in newborns [49, 50], which may explain the presence of plasma L-carnosine in children aged 3–6 years in our study. Low enzyme activity would have resulted in a detectable plasma concentration of L-carnosine in children with ASD.
| Authors (Country) | Participants (M/FM) | Mean age in years | Study design | Sample (intervention/control) | Intervention (total daily dose) | Duration |
| Ann Abraham et al. 2020 (India) [19] | 63 (43/20) | 4.2 | RCT | 63 (32/31) | L-carnosine (10–15 mg/kg) + OT/ST | 2 months |
| Chez et al. 2002 (USA) [17] | 31 (21/10) | 7.45 | RCT | 31 (14/17) | L-carnosine (800 mg) | 8 weeks |
| Ghodsi et al. 2019 (Iran) [45] | 36 (27/9) | 8.92 | RCT | 36 (18/18) | L-carnosine (500 mg) | 2 months |
| Hajizadeh-Zaker et al. 2018 (Iran) [20] | 42 (35/7) | 8.24 | RCT | 42 (21/21) | L-carnosine (800 mg) + Risperidone (1 mg/day for patients weighing |
10 weeks |
| Mehrazad-Saber et al. 2018 (Iran) [18] | 43 (31/12) | 8.59 | RCT | 43 (21/22) | L-carnosine (500 mg) | 2 months |
ASD, Autism spectrum disorder; M, Male; FM, Female; RCT, Randomised Controlled Trial; OT, Occupational Therapy; ST, Speech Therapy.
To the best of our knowledge, this study is the first to correlate plasma concentrations of L-carnosine with clinical outcomes in children with ASD. The levels of plasma L-carnosine did not produce a statistically significant effect on autism severity as measured by CARS2-ST and ATEC scores before and after supplementation. Hence, there was no correlation between plasma concentration and clinical outcome. This study had a few limitations as it had a small number of participants recruited from a single center. Pharmacokinetic analysis was not performed due to ethical concerns about children being a vulnerable population. The correlation of plasma L-carnosine with the severity of ASD could not be done as we chose only children with mild to moderate ASD. In spite of this, the developed method was able to quantify the L-carnosine level in ASD children. Further studies need to be conducted with varying doses of L-carnosine as well as measurements of serum carnosinase activity in ASD children.
L-carnosine supplementation led to a detectable plasma concentration of L-carnosine but resulted in no significant change in the core symptoms of ASD as measured by changes in CARS2-ST and ATEC scores.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
DAA, RMG, conceived and drafted the study protocol. DAA, UN, assisted with subject recruitment and data collection. MK conducted the estimation of L-carnosine. DAA, KWG, CST, CA, LCM, RMG was involved in manuscript preparation. KWG, CST, LCM, CA involved in literature review, conceptualization, data analysis. RMG and VTM provided data analysis and interpretation. All authors critically revised the manuscript and gave final approval. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics approval was obtained from Human IEC of Sri Ramachandra Institute of Higher Education and Research (ethical clearance number: IEC/19/JUN/151/39) and the study was registered in the ClinicalTrial Registry-India (CTRI/2019/07/020102). The study was conducted as per the 2018 amendment of the International Conference on Harmonization Good Clinical Practice (ICH-GCP) guidelines. The patients or their families/legal guardians gave written informed consent before the commencement of the study.
The authors acknowledge Sri Ramachandra Founder-Chancellor Shri N.P.V. Ramasamy Udayar Research Fellowship and Brio Bliss Life Science Pvt. Ltd., Chennai, India for providing free samples and testings. We are grateful to the children and parents for their participation in this study during the COVID-19 pandemic.
This work was supported by the Research Accelerator Grant Scheme from Sunway University (GRTIN-RAG-DBS-12-2024; GRTIN-RAG-DADTP-02-2024).
The authors declare no conflict of interest. Despite they received free samples and testings from Sri Ramachandra Founder-Chancellor Shri N.P.V. Ramasamy Udayar Research Fellowship and Brio Bliss Life Science Pvt. Ltd., Chennai, India, the judgments in data interpretation and writing were not influenced by this relationship.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.